Abstract
Hypertension-associated endothelial dysfunction is largely due to the exaggerated vasoconstrictor generation by cyclooxygenase-2 (COX-2). COX-2 is induced under inflammatory condition. Demethoxycurcumin (DMC) is a major component of Curcuma longa L, which possesses anti-inflammatory action. This study aimed to examine whether DMC protects endothelial function in hypertension by modulating COX-2. Changes in isometric tension showed that in vivo and ex vivo treatment with DMC rescued the attenuated endothelium-dependent relaxations (EDRs) and elevated endothelium-dependent contractions (EDCs) in the renal arteries of SHR, which were also corrected by acute usage of the COX-2 inhibitor celecoxib. The restoration of renovascular activity by DMC was accompanied by the normalization of COX-2 expression. The enhanced COX-2 expression observed in the renal arteries of hypertensive patients was suppressed by incubation of excised arteries with DMC for 12 hrs. In the renal arteries of Wistar-Kyoto rats (WKY), DMC prevented the endothelial dysfunction caused by angiotensin II. The reduction in the generation of nitric oxide (NO) and expression of eNOS phosphorylation (Ser1177) in human umbilical vein endothelial cells caused by angiotensin II (Ang II) were restored by DMC or celecoxib. Our findings suggest that DMC may decrease COX-2 expression and improve endothelial function in hypertension.
1. Introduction
Healthy endothelium is essential to maintain normal vascular tone by releasing vasodilators and vasoconstrictors [1, 2]. Endothelial dysfunction, owing to the imbalance between dilating and constricting factors, is often encountered in the common setting of cardiovascular risk factors such as hypertension [3].
Disturbed COX-2 metabolism contributes largely to the development of hypertension-associated endothelial dysfunction through overgeneration of vasoconstrictors [4]. Under physiological conditions, a minimal amount of COX-2 is expressed in the vascular wall. A striking upregulation of COX-2 is observed in the inflamed vascular tissue, which was also documented extensively in hypertension [5]. Experimental and clinical findings have implicated the striking generation of COX-dependent endothelium-derived contracting factors in the initiation and development of endothelial dysfunction in hypertension. Vasoconstrictors and reactive oxygen species (ROS) produced by COX-2 result in disturbed endothelial dysfunction [6].
Animal studies have provided solid evidence that COX-2 inhibitors can prevent cardiovascular disease [3]. The growing recognition of the critical role of COX-2 in the pathogenesis of endothelial function, and the reported serious side effects of COX-2 inhibitors make the development of a new anti-inflammatory drug urgent.
Plants are the main sources of naturally occurring anti-inflammatory agents. The rich source and potential safety make plant-derived inflammatory agents advantageous over synthetic compounds. Many extracts from plants possess anti-inflammatory activity either directly or indirectly. Demethoxycurcumin (DMC) is a natural derivative of curcumin, a major component of Curcuma longa L [7]. The anti-inflammatory and anticancer effects of curcumin have been widely demonstrated, but its medical utility has been limited because of rapid plasma clearance. The growing evidence of the stronger effectiveness and higher stability of DMC compared with curcumin indicates DMC as an attractive and promising replacement for curcumin in the treatment of cancer and inflammation-associated diseases [8]. Several studies have also suggested the anti-inflammatory action of DMC [9]. In addition, a recent study showed the beneficial action of DMC in pulmonary hypertension by inhibiting the PDE5 [10]. However, it remains uncertain whether DMC can provide benefits against endothelial dysfunction in hypertension, depending on its anti-inflammatory action. The aim of the present study was to test whether DMC protects renovascular function, and if so, whether COX-2 serves as a downstream effector.
2. Methods
2.1. Animals and Treatment Protocols
The animal experimental protocols in the present study were approved by the animal care and use committees of the Southeast University and were conducted according to the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. Thirty-week-old male Wistar-Kyoto rats (WKY) and spontaneous hypertensive rats (SHR) were supplied by the Laboratory Animal Services Centre, Southeast University. All the animals were housed in a temperature-controlled room (22–24°C) with a 12-h light/dark cycle and supplied with standard diet and water. The rats were assigned to one of the following three groups: (1) WKY control, (2) SHR treated with vehicle dimethylsulfoxide (DMSO; SHR + vehicle), and (3) SHR treated with DMC at 10 mg/kg per day (SHR + DMC) by intraperitoneal injection for 3 weeks.
2.2. Blood Pressure Measurement
Blood pressure was measured using the tail-cuff electrosphygmomanometer system (AD Instruments, Sydney, Australia). Blood pressure measurement was performed after the rats remained stable and quiescent. The average of 3 readings was considered the systolic blood pressure of each animal.
2.3. Preparation of Human Arteries
The use of human renal arteries in the present study was approved by the Clinical Research Ethics Committee of the Southeast University. The arteries were obtained from normotensive and hypertensive patients undergoing radical nephrectomy with their consent. The patients received radical nephrectomy for renal carcinoma. Hypertension was defined as systolic pressure above 140 mmHg and diastolic pressure above 90 mmHg. The mean age of the six hypertensive patients was 52 years (ranging between 51 and 69 years) and that of the six normotensive patients was 55 years (ranging between 48 and 70 years). Renal arteries from hypertensive patients were cultured for 12 hrs in the presence or absence of DMC (10 μmol/L) in Dulbecco's Modified Eagle Medium (DMEM, GIBCO, Gaithersburg, MD, USA) with the addition of 10% fetal bovine serum (FBS, GIBCO) and 1% penicillin/streptomycin (GIBCO) at 37°C.
2.4. Preparation of Animal Renal Arteries
Normotensive WKY rats and SHR were sacrificed by carbon dioxide inhalation. After careful dissection, intralobal renal arteries were placed in Krebs solution of the following composition: (mmol/L) 119 NaCl, 4.7 KCl, 2.5 CaCl2, 1 MgCl2, 25 NaHCO3, 1.2 KH2PO4, and 11 D-glucose. After removal of the surrounding connective tissue, the arteries were cut into 4–6 ring segments of ~1.6 mm length for functional assessment of endothelial function in a Multi Myograph System (610M, Danish Myo Technology A/S, Aarhus N, Denmark). Briefly, the artery was fixed to the jaws of the myograph by two stainless steel wires, which passed through the arterial lumen. The myograph chamber was filled with 5 mL Krebs solution that was continually supplied with 95% O2 and 5% CO2 and kept at a pH level of ~7.4 at 37°C. After being subjected to an optimal resting tension of 2.5 mN, the arteries were equilibrated for 60 min for the following experiments.
2.5. Isometric Tension Assessment
After the contraction evoked by 1 μmol/L phenylephrine was stable, concentration-dependent relaxations induced by increasing concentrations of acetylcholine (ACh 0.003–10 μmol/L) were recorded. Endothelium-dependent relaxation (EDR) was described as the percentage reduction in phenylephrine-induced contraction. When endothelium-dependent contraction (EDC) was tested, the arteries were preincubated with NG-nitro-L-arginine methyl ester (L-NAME, 100 μmol/L) to block the vasodilating effect of nitric oxide (NO). Cumulative concentrations of ACh (0.03–100 μM) were added to trigger contractions. ACh-induced tension changes in the renal arteries were expressed as the tension recorded divided by the tension evoked by 60 mmol/L KCl. COX-2 inhibitor celecoxib (3 μmol/L), when used, was added for 30 min prior to the addition of ACh.
2.6. Western Blot Assay
After treatment, renal arteries or human umbilical vein endothelial cells (HUVECs) were homogenized in RIPA lysis buffer (1 μg/mL leupeptin, 5 μg/mL aprotinin, 100 μg/mL PMSF, 1 mmol/L sodium orthovanadate, 1 mmol/L EGTA, 1 mmol/L EDTA, 1 mmol/L NaF, and 2 mg/mL β-glycerophosphate). The homogenates were then centrifuged at 20000g for 20 min at 4°C after incubation on ice. The supernatants were harvested and stored at −80°C. Before western blot assay, the protein concentration was determined using the Lowry method (Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of protein samples were electrophoresed on a 10% SDS-polyacrylamide gel and then transferred onto an immobilon-P polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA, USA). Nonspecific binding sites were blocked by 5% nonfat milk or 1% bovine serum albumin (BSA) in 0.05% Tween-20 phosphate-buffered saline (PBST) for 1 h and then probed overnight at 4°C with primary antibodies against COX-2, P-eNOS (Ser1177) or eNOS (Abcam, Cambridge, MA, USA) at a dilution of 1 : 500. The membranes were washed three times in PBST before incubation with the appropriate secondary antibodies at a 1 : 3000 dilution for 1 h at room temperature. Finally, the membranes were washed and developed with an enhanced chemiluminescence detection system (ECL reagents, Millipore, Billerica, MA, USA) and exposed to X-ray films. Equal protein loading was confirmed using a housekeeping anti-GAPDH antibody (Ambion, Austin, TX, USA).
2.7. Immunofluorescence Microscopy
Changes in the expression of COX-2 in the renal arteries from hypertensive patients after DMC administration for 12 hrs were visualized using immunofluorescence microscopy. The arteries were embedded in OCT compound (Sakura Finetek, the Netherlands), snap frozen, and cut into 10-μm-thick cryostat sections, which were then fixed in 4% paraformaldehyde for 30 min, and treated with 0.05% Triton X in PBS for 1 min. The sections were blocked with 5% normal donkey serum for 1 h at room temperature and incubated with primary antibodies against COX-2 (Abcam, Cambridge, MA, USA) overnight at 4°C. After several washes in PBS, the sections were incubated with the appropriate Alexa Fluor 546 IgG (Invitrogen, Molecular Probes, California, USA) for 1 h at room temperature. After coverslipping, the sections were observed under an Olympus Fluoview FV1000 confocal microscope. Images were first acquired with the Olympus Fluoview software (version 1.5; FV10-ASW1.5) and then merged using the SPOT advanced software (Version 4.6, Diagnostic Instruments, Sterling Heights, MI, USA).
2.8. Confocal Microscopy for Real-Time NO Production in Endothelial Cells
HUVECs were cultured in the endothelial cell growth medium (Lonza, Basel, Switzerland), which was supplemented with 20% fetal bovine serum and 1% penicillin/streptomycin (GIBCO). Endothelial cells were plated on coverslips. Upon treatment with angiotensin II (Ang II; 1 μmol/L) with or without DMC for 12 hrs, cells were treated with 1 mmol/L DAF-FM diacetate (Molecular Probes, Invitrogen, Carlsbad, CA), a fluorescent probe used to detect intracellular changes in NO, for 10 min at room temperature. Then, the cells were excited at 495 nm with an emission wavelength of 515 nm, with the use of the Olympus confocal system (Tokyo, Japan). The A23187 (1 μmol/L) induced real time changes in NO generation were recorded at 1-min intervals for 20 min. A23187 serves as a calcium ionophore to stimulate the phosphorylation of eNOS. Results were expressed as a ratio of the fluorescence relative to the initial intensity (time 0 before the use of A23187).
2.9. Chemicals
ACh, phenylephrine, celecoxib, and DMSO were purchased from Sigma-Aldrich. Ang II, losartan, and A23187 were purchased from Tocris Bioscience (Avonmouth, UK), and DMC was purchased from Chengdu Mansite Pharmaceutical Company, China (>98% purity). ACh, phenylephrine, and Ang II were dissolved in distilled water, and others were prepared in DMSO.
2.10. Statistical Analysis
The data are expressed as mean ± SEM of four to six experiments. Data were analyzed using one-way ANOVA followed by Bonferroni post hoc tests whenever appropriate (GraphPad Software, San Diego, California). P values less than 0.05 were accepted to indicate statistically significant differences.
3. Results
3.1. In Vivo Treatment with DMC Reduces Blood Pressure of SHR and Improves the Deteriorated Renovascular Endothelial Function
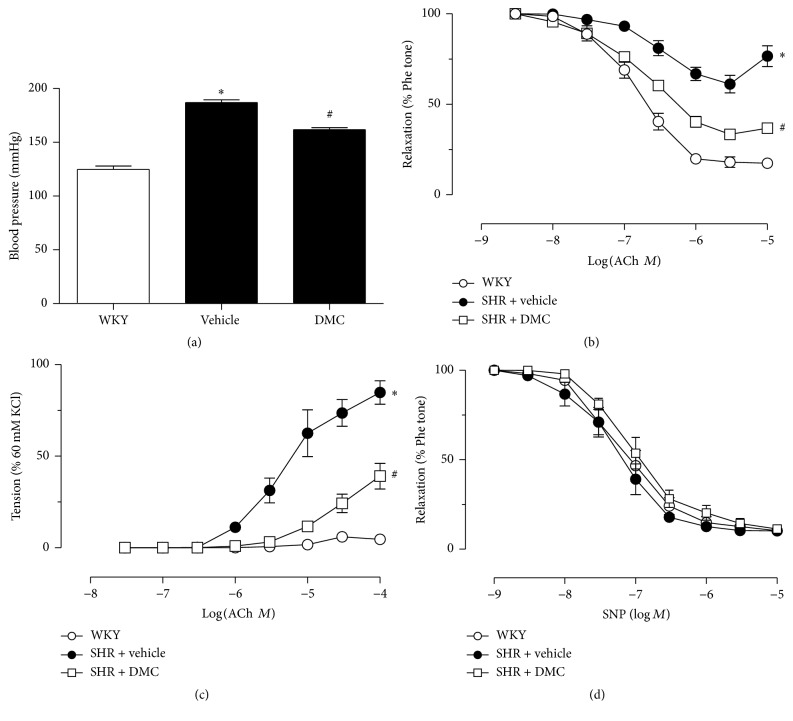
The elevated blood pressure in SHR was reduced after 3-week treatment with DMC (10 mg/kg/day) via intraperitoneal injection (187.0 ± 2.5 mmHg before treatment versus 161.8 ± 1.8 mmHg after treatment, P < 0.05; Figure 1(a)). Blunted ACh-induced relaxation and pronounced ACh-induced contractions were observed in the renal arteries of the vehicle-treated SHR compared with the normotensive WKY controls (Figures 1(b) and 1(c)). Chronic treatment with DMC enhanced the relaxations and inhibited contractions. In contrast, sodium nitroprusside-induced relaxation was not changed in hypertension (Figure 1(d)).
Figure 1.
In vivo treatment with DMC reduces blood pressure in SHR and improves the deteriorated renovascular endothelial function. WKY rats exhibited blood pressure of 124.8 ± 1.8 mmHg. Higher blood pressure in SHR was reduced after 3-week treatment with DMC (downregulation of 13.48% after treatment, P < 0.05; (a)). Blunted ACh-induced relaxation and pronounced ACh-induced contractions were observed in the renal arteries of vehicle-treated SHR compared to the normotensive WKY controls ((b)-(c)). Chronic oral treatment with DMC (10 mg/kg/day for 3 weeks) enhanced the relaxations and inhibited contractions. Relaxation induced by SNP remained similar among the three groups. Data are means ± SEM of 7-8 experiments. ∗ P < 0.05 versus WKY; # P < 0.05 SHR + vehicle.
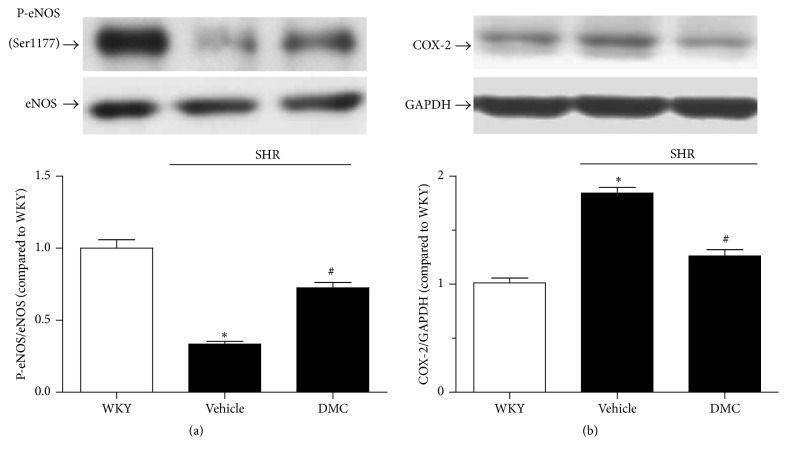
3.2. In Vivo Treatment with DMC Corrects the Downregulation of P-eNOS (Ser1177) Expression and the Upregulation of COX-2 in the Renal Arteries Isolated from Vehicle-Treated SHR
Western blot assays revealed decreased P-eNOS (Ser1177) expression and elevated COX-2 expression in the renal arteries from vehicle-treated hypertensive animals. The observed changes in these proteins were normalized by chronic treatment with DMC (Figures 2(a) and 2(b)).
Figure 2.
In vivo treatment with DMC corrects the downregulation of P-eNOS expression and the upregulation of COX-2 in the renal arteries isolated from vehicle-treated SHR. Western blot assay reveals decreased P-eNOS (Ser1177) expression and elevated COX-2 expression, which were corrected by DMC treatment for 3 weeks ((a)-(b)). Data are means ± SEM of 5-6 experiments. ∗ P < 0.05 versus WKY; # P < 0.05 SHR + vehicle.
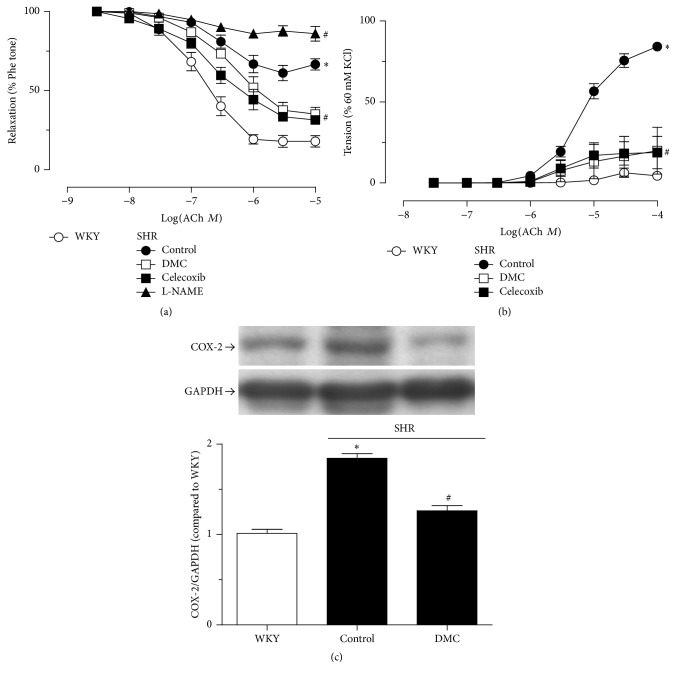
3.3. In Vitro Treatment with DMC Enhanced the Attenuated Endothelium-Dependent Relaxation and Inhibited the Augmented Endothelium-Dependent Contraction in the Renal Arteries of SHR, by Reducing the Elevated COX-2 Expression
Renal arteries from SHR exhibited attenuated EDR induced by ACh (Figure 3(a)). Furthermore, ACh induced contractions in the presence of the nitric oxide synthase (NOS) inhibitor L-NAME, whereas such contractions were small in the renal arteries of WKY rats (Figure 3(b)). In vitro exposure to 10 μmol/L DMC for 12 hrs corrected the attenuated relaxation and the elevated contractions, with similar effects afforded by the acute use of the COX-2 inhibitor celecoxib (Figures 3(a) and 3(b)). In addition, acute treatment with L-NAME abolished EDR. The improvement in endothelial function by treatment with DMC for 12 hrs was followed by the reduction in COX-2 expression (Figure 3(c)).
Figure 3.
Ex vivo DMC treatment enhances the attenuated endothelium-dependent relaxation and decreases the elevated endothelium-dependent contractions in the renal arteries from SHR, reducing the upregulated COX-2 expression in the renal vascular wall. Renal arteries from WKY rats showed high relaxation and small contractions evoked by Ach, whereas renal arteries from SHR showed impaired relaxation and huge contractions ((a)-(b)). Treatment of cultured renal arteries from SHR with DMC (10 µmol/L) for 12 hrs was followed by improved relaxation and inhibited contractions ((a)-(b)). Compared to the renal arteries from WKY, the arteries from SHR exhibited elevated COX-2 expression and DMC (10 µmol/L) treatment for 12 hrs reversed the higher COX-2 expression (c). Data are means ± SEM of 5-6 experiments. ∗ P < 0.05 versus WKY; # P < 0.05 versus SHR control.
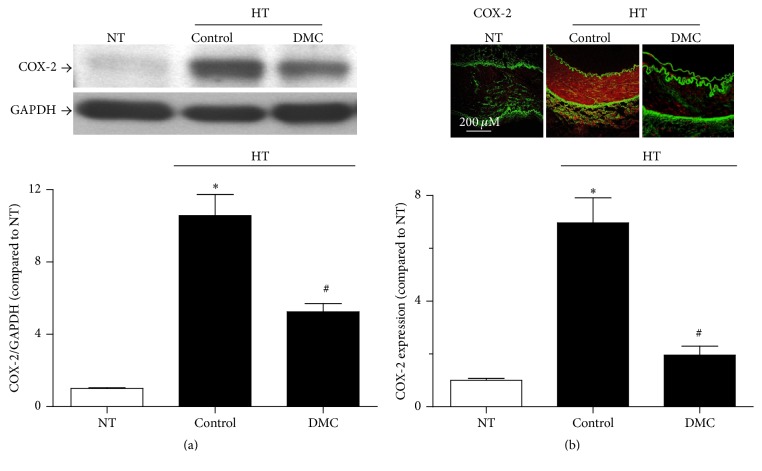
3.4. Ex Vivo Treatment with DMC Decreases the Elevated Expression of COX-2 in Renal Arteries from Hypertensive Patients
Western blot measurement (Figure 4(a)) and immunofluorescence assessment (Figure 4(b)) detected higher expression of COX-2 in the renal arteries from hypertensive patients compared to those of the normotensive patients. The enhanced COX-2 expression was depressed by treatment with DMC for 12 hrs (Figures 4(a) and 4(b)).
Figure 4.
In renal arteries from hypertensive patients ex vivo treatment with DMC for 12 hrs normalizes the altered expression of COX-2 observed by western blot assay and immunofluorescence microscopy. The renal arteries from hypertensive patients exhibited altered COX-2 expression levels by western blot measurement (a) and immunofluorescence microscopy (b). The elevation of COX-2 expression was corrected with the addition of DMC for 12 hrs ((a)-(b)). Yellowish-green autofluorescence represents elastin in the internal and external elastic laminae (b). Higher level of arterial COX-2 expression, suggested by the intense reddish orange color, was reduced by DMC. Photomicrographs are representative images from experiments performed on samples from four to five different patients. The scale bar indicates length (200 μm). Data are means ± SEM of 5-6 experiments. ∗ P < 0.05 versus NT; # P < 0.05 versus HT control.
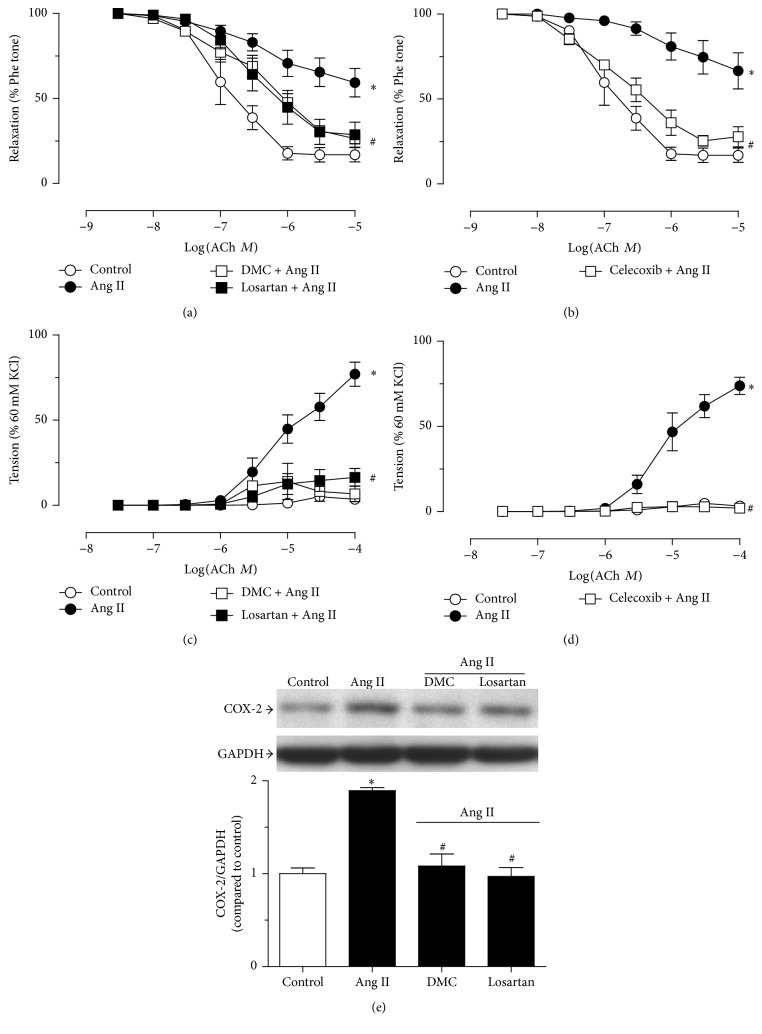
3.5. In Vitro DMC Preadministration in WKY Renal Arteries Prevents Ang II-Induced Renovascular Dysfunction, Normalizing the Increased COX-2 Expression
Incubation with Ang II (100 nmol/L) for 12 hrs resulted in impaired ACh-induced relaxation and increased EDC (Figures 5(a) and 5(c)). Pretreatment with DMC and celecoxib prevented the impairment in EDR and abolished the contractions (Figures 5(b) and 5(d)). Ang II also promoted the overexpression of COX-2 and the elevated COX-2 expression was abrogated by preincubation with either DMC or Ang II type 1 receptor blocker losartan (3 μmol/L) (Figure 5(e)).
Figure 5.
Combined exposure to DMC prevents Ang II-induced vascular dysfunction in the renal arteries from WKY, with normalization of increased COX-2 expression. Ang II (100 nmol/L, 12 hours) results in impaired relaxations to ACh and unmasked contractions to ACh in the renal arteries from WKY rats ((a) and (c)). Combined administration of DMC (10 µmol/L) rescued the impaired vascular function and COX-2 inhibitor celecoxib offered similar protection ((b) and (d)). The overexpression of COX-2 by Ang II was also reversed by coincubation with DMC (e). Data are means ± SEM of 5-6 experiments. ∗ P < 0.05 versus control; # P < 0.05 versus Ang II.
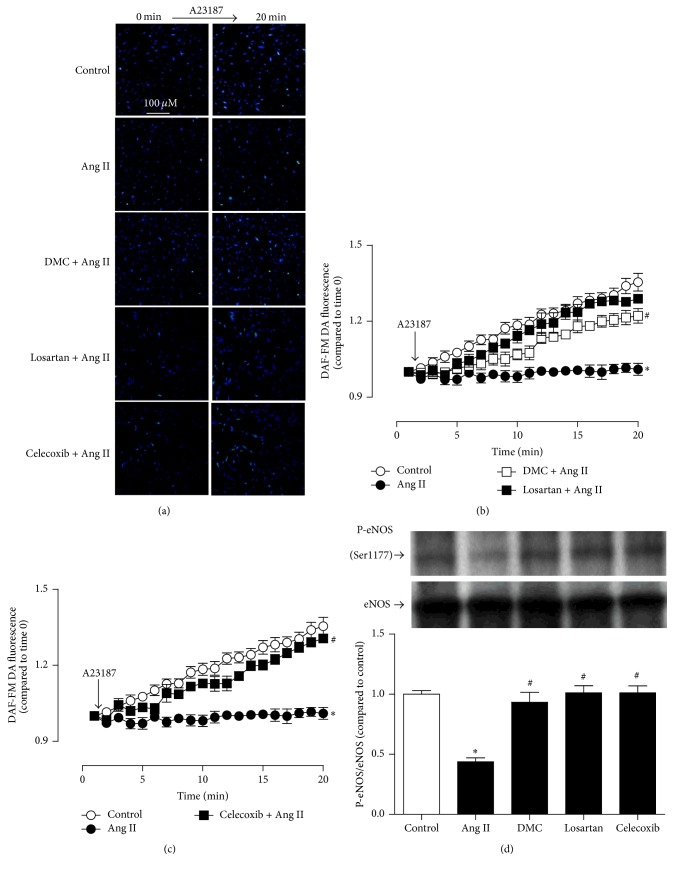
3.6. DMC Augments Nitric Oxide Generation in HUVECs
Treatment with Ang II (1 μmol/L) for 12 hrs led to a decrease in the A23187-stimulated NO production (Figure 6(a)). The presence of DMC, losartan (3 μmol/L) or celecoxib (3 μmol/L) restored the NO generation (Figures 6(b) and 6(c)). Similarly, the addition of DMC, losartan, or celecoxib reversed the downregulation of P-eNOS (Ser1177) expression in cells treated with Ang II for 12 hrs (Figure 6(d)).
Figure 6.
The effect of DMC treatment on nitric oxide generation in HUVECs Ang II (1 µmol/L) treatment for 12 hrs reduced the A23187-evoked NO production (a). Cotreatment with DMC, losartan or celecoxib restored the NO generation ((b)-(c)). Consistently, P-eNOS expression was downregulated in 12 hrs-Ang II-treated cells, which was reversed in the presence of DMC, losartan, or celecoxib (d). Data are means ± SEM of 5-6 experiments. ∗ P < 0.05 versus control; # P < 0.05 versus Ang II.
4. Discussion
Although the anti-inflammatory and anticancer activities of DMC have been reported, its effect on vascular activity is unknown. Our present study provides evidence showing the beneficial effect of DMC on the renovascular function in hypertension. Acting as an activating site, the phosphorylation sites 1177 on eNOS were used as a marker in endothelium dysfunction [11, 12]. Our major findings include (1) intraperitoneal injection of DMC in vivo for 3 weeks lowered blood pressure in the SHR. The attenuated EDR and elevated EDC in renal arteries from hypertensive animals were reversed by treatment with DMC for 3 weeks; (2) matched with the changes in vascular activity, reduced P-eNOS (Ser1177) expression in SHR renal arteries was increased by treatment with DMC in vivo for 3 weeks; (3) western blot assay and immunofluorescence measurement exhibited the upregulation of COX-2 in the renal arteries of hypertensive animals and patients, which was inhibited by DMC in vivo administration for 12 hrs; (4) in the renal arteries from normotensive patients, in vitro treatment with DMC prevented the Ang II-induced upregulation of COX-2 and the subsequent delayed renovascular function; (5) in vitro treatment with DMC reversed the reduction of NO production and P-eNOS (Ser1177) expression in HUVECs exposed to Ang II for 12 hrs.
The occurrence of endothelial dysfunction in hypertension has been well documented. COX-2 is positively responsible for the endothelial dysfunction under hypertensive conditions [6, 13]. This is related to the excessive production of vasoconstrictors and ROS derived from overactivation of COX-2 [14]. These agents disturb EDR and facilitate contraction. The pivotal role of COX-2 upregulation in the development of endothelial dysfunction in hypertension is being increasingly recognized. Here, we also demonstrated that it is likely that the proinflammatory enzyme COX-2 is a major contributor to endothelial dysfunction in hypertension, as evidenced by the protective action of COX-2 inhibitor and the upregulated COX-2 expression in SHR. Subsequent observations revealed the beneficial effect of DMC against endothelial dysfunction through downregulation of COX-2 expression.
COX-2 has been described as “inducible” owing to its upregulation under inflammatory conditions. Inhibition of COX-2 activity by celecoxib reverses the attenuation of vascular function [15, 16]. In addition to the functional studies, western blot assay and immunofluorescence measurement confirmed the positive involvement of COX-2 in the disordered renovascular function in hypertension, not only in animal but also in human specimen. In contrast, no change was detected in COX-1 expression (data not shown). The positive contribution of COX-2 to endothelial dysfunction lends credence to the concept that inhibition of COX-2 activity, or decreasing its abnormal over expression, could be a target to alleviate impaired vascular function. The serious side effects of these agents, owing to thorough depletion of COX-derived prostaglandins due to COX-2 activity inhibition [17], which led to the withdrawal of one of the widely used COX-2 inhibitors, Vioxx, from the market, suggest that remedies targeting the enhancement of COX-2 expression will be a more attractive and promising choice [18].
In this study, it was observed that in vitro treatment with DMC for 12 hrs normalized the blunted EDR and the augmented EDC in hypertension, reducing the upregulated COX-2 expression. Furthermore, previous studies using cancer cells and N9 microglial cells validated the safety of DMC at 10 μmol/L [19, 20]. In vivo treatment with DMC had the effect to reduced IL-1β in the ischemic brain. Additionally, recent evidence indicated that DMC serves as a phosphodiesterase-5 inhibitor and delayed the development of pulmonary hypertension [10]. Our study showed the lower-blood pressure effect of DMC. In Kruangtip et al. findings, they exhibited the direct specifically dilating effect of DMC on pulmonary artery by inhibiting PDE5, which inactivate guanosine cyclic monophosphate (cGMP) via hydrolysis. PDE accumulation has been proposed in the development of pulmonary artery hypertension (PAH), indicating the potentially anti-PAH effect of DMC.
As a key agent in the pathogenesis of hypertension, Ang II contributes largely to renovascular dysfunction and kidney dysfunction [21]. Mimicking hypertension in which circulating levels of Ang II were enhanced, renal arteries subjected to Ang II challenge showed augmented expression of COX-2. The impaired EDR, pronounced EDC, and excessive COX-2 expression were also normalized by cotreatment with DMC. Coupled with the upregulation of COX-2, lowered renovascular function was detected, which was restored when exposed to DMC. This result reinforced the beneficial action of DMC in vascular function in hypertension. Further experiments using HUVECs also showed that DMC normalized the reduction of NO and P-eNOS expression in Ang II–treated cells.
Endothelial dysfunction is recognized as an early feature in vascular disease. The assessment of endothelial function is essential in the evaluation of treatment effect in vascular disease [2, 22]. Notably, endothelial dysfunction is of significant use to predict the cardiovascular events due to its precedence of more serious cardiovascular attacks [23]. Hence, the present study offered solid evidence concerning the benefits of DMC against renovascular dysfunction. With regard to the inhibitory effect on COX-2 expression in the renal arteries of SHR, the effect of DMC may originate from the inhibition of NF-κB, which may secondarily downregulate COX-2 expression. A limitation of the present study is how DMC modulates COX-2 expression and whether the effect of DMC is dose-dependent.
5. Conclusion
In conclusion, the present study demonstrated for the first time that COX-2 is at least a downstream effector in the protection of DMC on renovascular function in hypertension. In addition, these findings provide constructive evidence for the development of DMC for therapeutic applications.
Acknowledgments
This work was supported by the Natural Science Foundation of Jiangsu Province [BK20141345].
Competing Interests
The authors declare that no conflict of interests exist.
References
- 1.Endemann D. H., Schiffrin E. L. Endothelial dysfunction. Journal of the American Society of Nephrology. 2004;15(8):1983–1992. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- 2.Felmeden D. C., Lip G. Y. H. Endothelial function and its assessment. Expert Opinion on Investigational Drugs. 2005;14(11):1319–1336. doi: 10.1517/13543784.14.11.1319. [DOI] [PubMed] [Google Scholar]
- 3.Martínez-Revelles S., Avendaño M. S., García-Redondo A. B., et al. Reciprocal relationship between reactive oxygen species and cyclooxygenase-2 and vascular dysfunction in hypertension. Antioxidants and Redox Signaling. 2013;18(1):51–65. doi: 10.1089/ars.2011.4335. [DOI] [PubMed] [Google Scholar]
- 4.Versari D., Daghini E., Virdis A., Ghiadoni L., Taddei S. Endothelium-dependent contractions and endothelial dysfunction in human hypertension. British Journal of Pharmacology. 2009;157(4):527–536. doi: 10.1111/j.1476-5381.2009.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartner A., Cordasic N., Goppelt-Struebe M., Veelken R., Hilgers K. F. Role of macula densa cyclooxygenase-2 in renovascular hypertension. American Journal of Physiology - Renal Physiology. 2003;284(3):F498–F502. doi: 10.1152/ajprenal.00136.2002. [DOI] [PubMed] [Google Scholar]
- 6.Wong W. T., Tian X. Y., Chen Y., et al. Bone morphogenic protein-4 impairs endothelial function through oxidative stress-dependent cyclooxygenase-2 upregulation: implications on hypertension. Circulation Research. 2010;107(8):984–991. doi: 10.1161/circresaha.110.222794. [DOI] [PubMed] [Google Scholar]
- 7.Kunwar A., Barik A., Sandur S. K., Priyadarsini K. I. Differential antioxidant/pro-oxidant activity of dimethoxycurcumin, a synthetic analogue of curcumin. Free Radical Research. 2011;45(8):959–965. doi: 10.3109/10715762.2011.571681. [DOI] [PubMed] [Google Scholar]
- 8.Pae H.-O., Jeong S.-O., Kim H. S., et al. Dimethoxycurcumin, a synthetic curcumin analogue with higher metabolic stability, inhibits NO production, inducible NO synthase expression and NF-κB activation in RAW264.7 macrophages activated with LPS. Molecular Nutrition & Food Research. 2008;52(9):1082–1091. doi: 10.1002/mnfr.200700333. [DOI] [PubMed] [Google Scholar]
- 9.Sandur S. K., Pandey M. K., Sung B., et al. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28(8):1765–1773. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- 10.Kruangtip O., Chootip K., Temkitthawon P., et al. Curcumin analogues inhibit phosphodiesterase-5 and dilate rat pulmonary arteries. Journal of Pharmacy and Pharmacology. 2015;67(1):87–95. doi: 10.1111/jphp.12302. [DOI] [PubMed] [Google Scholar]
- 11.Bird I. M. Endothelial nitric oxide synthase activation and nitric oxide function: new light through old windows. Journal of Endocrinology. 2011;210(3):239–241. doi: 10.1530/joe-11-0216. [DOI] [PubMed] [Google Scholar]
- 12.Durán W. N., Breslin J. W., Sánchez F. A. The NO cascade, eNOS location, and microvascular permeability. Cardiovascular Research. 2010;87(2):254–261. doi: 10.1093/cvr/cvq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong S. L., Lau C. W., Wong W. T., et al. Pivotal role of protein kinase Cδ in angiotensin II-induced endothelial cyclooxygenase-2 expression: a link to vascular inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(5):1169–1176. doi: 10.1161/atvbaha.110.216044. [DOI] [PubMed] [Google Scholar]
- 14.Sirker A., Zhang M., Shah A. M. NADPH oxidases in cardiovascular disease: insights from in vivo models and clinical studies. Basic Research in Cardiology. 2011;106(5):735–747. doi: 10.1007/s00395-011-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Widlansky M. E., Price D. T., Gokce N., et al. Short- and long-term COX-2 inhibition reverses endothelial dysfunction in patients with hypertension. Hypertension. 2003;42(3):310–315. doi: 10.1161/01.HYP.0000084603.93510.28. [DOI] [PubMed] [Google Scholar]
- 16.Chenevard R., Hürlimann D., Béchir M., et al. Selective COX-2 inhibition improves endothelial function in coronary artery disease. Circulation. 2003;107(3):405–409. doi: 10.1161/01.cir.0000051361.69808.3a. [DOI] [PubMed] [Google Scholar]
- 17.Becker R. C. COX-2 inhibitors. Texas Heart Institute Journal. 2005;32(3):380–383. [PMC free article] [PubMed] [Google Scholar]
- 18.Marnett L. J. The COXIB experience: a look in the rearview mirror. Annual Review of Pharmacology and Toxicology. 2009;49:265–290. doi: 10.1146/annurev.pharmtox.011008.145638. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L., Wu C., Zhao S., et al. Demethoxycurcumin, a natural derivative of curcumin attenuates LPS-induced pro-inflammatory responses through down-regulation of intracellular ROS-related MAPK/NF-κB signaling pathways in N9 microglia induced by lipopolysaccharide. International Immunopharmacology. 2010;10(3):331–338. doi: 10.1016/j.intimp.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Ni X., Zhang A., Zhao Z., Shen Y., Wang S. Demethoxycurcumin inhibits cell proliferation, migration and invasion in prostate cancer cells. Oncology Reports. 2012;28(1):85–90. doi: 10.3892/or.2012.1783. [DOI] [PubMed] [Google Scholar]
- 21.Mehta P. K., Griendling K. K. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. American Journal of Physiology—Cell Physiology. 2007;292(1):C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 22.Reriani M. K., Lerman L. O., Lerman A. Endothelial function as a functional expression of cardiovascular risk factors. Biomarkers in Medicine. 2010;4(3):351–360. doi: 10.2217/bmm.10.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mudau M., Genis A., Lochner A., Strijdom H. Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovascular Journal of Africa. 2012;23(4):222–231. doi: 10.5830/cvja-2011-068. [DOI] [PMC free article] [PubMed] [Google Scholar]