Abstract
Carbon steels containing ferrite-pearlite microstructures weaken dramatically when pearlite dissolves into austenite on heating. The kinetics of this phase transformation, while fast, can play a role during dynamic, high temperature manufacturing processes, including high speed machining, when the time scale of this transformation is on the order of the manufacturing process itself. In such a regime, the mechanical strength of carbon steel can become time-dependent. The present work uses a rapidly-heated, high strain rate mechanical test to study the effect of temperature and time on the amount of pearlite dissolved and on the resulting transient effect on dynamic strength of a low and a high carbon (eutectoid) steel. Measurements indicate that the transient effect occurs for heating times less than about three seconds. The 1075 steel loses about twice the strength compared to the 1018 steel (85 MPa to 45 MPa) owing to its higher initial pearlite volume fraction. Pearlite dissolution is confirmed by metallographic examination of tested samples. Despite the different starting pearlite fractions, the kinetics of dissolution are comparable for the two steels, owing to the similarity in their initial pearlite morphology.
Introduction
Introducing new materials into manufactured products more cheaply and rapidly is the goal of the Materials Genome Initiative (MGI) [1]. Traditional and high speed machining are and will remain an important manufacturing method for these newly discovered materials, particularly for high-volume part production. Enabling better predictive models that can inform best practices for efficient machining in both known and new material systems is an important step to realizing the promise of the MGI.
Machining subjects the workpiece material to extremely high strain rates, very large plastic strains, high temperatures and rapid heating from a combination of plastic work and friction at the tool/chip interface. This extreme environment escapes most normal laboratory dynamic testing techniques, due in part to the speed with which thermal excursions occur. Recognition of the difficulty in reproducing machining conditions with a materials test has led several researchers to attempt extracting material properties directly from machining tests via inverse methods [2–3]. In general, the mechanical behavior under rapid heating can differ from usual test conditions that employ gradual furnace heating due to the numerous possible time-dependent metallurgical processes that can affect structural response of the material, such as grain growth, recrystallization, dynamic strain ageing, and/or phase transformations. These factors require special testing methods to study how rapid thermal excursions can introduce time-dependence to the dynamic mechanical behavior. For this reason, NIST developed a rapid heating Kolsky bar method with the capability to probe non-equilibrium mechanical response by rapidly heating and rapidly mechanically testing specimens using direct electrical current to heat the specimen [4].
In this paper, we use the rapid heating Kolsky bar method study the time-dependent mechanical behavior of plain carbon steel. In particular, we focus on the effect of pearlite dissolution under rapid heating on dynamic strength. Pearlite (cementite plus ferrite) dissolves into austenite at high temperatures, leading to a weakened structure as the carbon becomes less effective at impeding dislocations when present interstitially within austenite than as cementite plates within pearlite. This reaction can be important in carbon steel machining, and results in the formation of a “white layer” on the workpiece [5]. The kinetics of pearlite dissolution under rapid heating have been studied extensively and described by various researchers with high speed dilatometry, microstructural analysis and more recently in situ X-ray diffraction [6]. Pearlite dissolution is the first stage of the austenitization reaction in carbon steels, and is followed by the second stage where the remaining ferrite transforms to austenite as carbon diffuses from the original pearlite colonies through the rest of the microstructure. The first stage is quite rapid compared to the second because of the much shorter diffusion distances involved: the inter-lamellar distances in pearlite are at least an order of magnitude smaller than the average ferrite grain size [7]. Pearlite decomposition is known to depend on heating rate, alloy composition and pearlite morphology [8–9]. A number of different models have been developed to predict pearlite dissolution as a function of temperature and time and have been validated against dilatometer measurements [8, 10–11].
In this study we measure the effect of pearlite decomposition on dynamic strength by performing a series of experiments where samples are rapidly heated then rapidly compressed at a strain rate of about 3000 s−1 using the pulse-heated Kolsky bar technique. Tests are conducted at temperatures roughly between A1 and A3 on 1018 and 1075 steel with ferrite-pearlite starting microstructures. The initial pearlite morphology of each steel is characterized, and tested samples are recovered and their microstructures are examined for evidence of transformation.
Experiments
Tests are conducted on commercial AISI 1018 (ASTM A108) and AISI 1075 (ASTM A684) steels acquired as cold rolled plate. Compositions, determined by spectrographic analysis (ASTM E1019 and ASTM E415) are listed in Table 1. Equilibrium thermodynamic calculations based on these compositions yield pearlite-to-austenite transformation temperatures A1 = 710 °C and A3 = 836 °C for the 1018 steel and A1 = 717 °C and A3 = 727 °C for the 1075 steel.
Table 1.
Compositions of steels investigated.
| Material | C | Mn | P | S | Si | Ni | Cr | Mo | Cu |
|---|---|---|---|---|---|---|---|---|---|
| 1018 | 0.2 | 0.6 | 0.009 | 0.006 | 0.02 | 0.08 | 0.06 | 0.02 | 0.14 |
| 1075 | 0.72 | 0.6 | 0.013 | <0.005 | 0.24 | 0.05 | 0.07 | 0.02 | 0.11 |
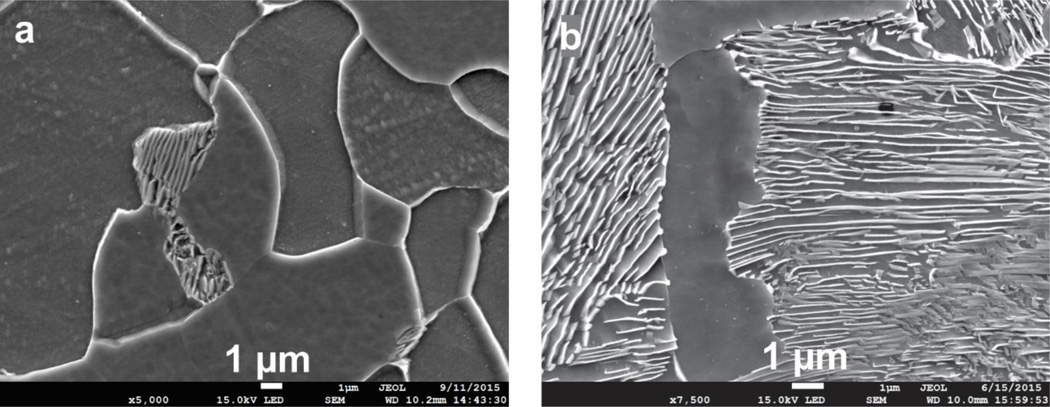
Cylindrical Kolsky test specimens measuring 2 mm thick by 4 mm diameter were cut from the plate by wire EDM such that the compression load is applied perpendicular to the rolling direction. The samples were then heat treated at 820 °C for 45 minutes then air cooled in order to form a fine, ferrite-pearlite microstructure. The initial heat treated microstructures are shown in Figure 1, which are prepared using standard metallographic polishing techniques with a 2% nital etch.
Figure 1.
Starting microstructures for 1018 steel (a) and 1075 steel (b), after heat treating. Nital etch.
The inter-lamellar spacing and average pearlite colony size are determined for the two steels examined here using a circular inspection circle placed on etched optical or SEM micrographs of the steels. The mean inter-lamellar spacing is determined by dividing the perimeter of the circle by the number of lamellae crossings:
| (1) |
Here l is the mean random inter-lamellar spacing, D is the circle diameter, and N is the number of crossings. The mean true inter-lamellar spacing is half the mean random inter-lamellar spacing [8]. Measurement of the pearlite colony size in the 1075 steel and the ferrite grain size in the 1018 steel is performed by a similar method. In these cases, the number of grain or colony crossings is noted, and the resulting average grain or colony diameter, d, is modified by the volume fraction of the phase being measured (V) [ASTM E113-12], which is determined separately by point-counting:
| (2) |
For estimating the mean pearlite colony size in the 1018 steel we use thresholded digital images and particle analysis algorithms, which work well in this material because the pearlite volume fraction is low and the individual colonies are generally well scattered through the microstructure. The average pixel area of the pearlite colonies is measured, and from this result we calculate an equivalent spherical diameter for the colonies. Measurement results for both steels are shown in Table 2. We note that the inter-lamellar spacing and the pearlite colony size in these steels is quite similar, owing to the similar heat treatments given them.
Table 2.
Microstructure measurements. Uncertainties are 95 % CI.
| Steel | Pearlite Volume Fraction |
Average Ferrite Grain Size d [µm] |
Average Pearlite Colony Size d [µm] |
Average Inter- Lamellar Spacing l [µm] |
|---|---|---|---|---|
| 1018 | 0.15 ± 0.03 | 8.0 ± 1.5 | 3.9 ± 0.5 | 0.18 ± 0.02 |
| 1075 | 0.77 ± 0.08 | n/a | 3.0 ± 0.2 | 0.24 ± 0.02 |
Pulse-Heated Kolsky Bar Experiments
The pulse-heated Kolsky bar technique used here is a variant of traditional Kolsky Bar or Split-Hopkinson Pressure Bar test methods [12] and is discussed in detail elsewhere [4]. We describe here only modifications to this technique that are relevant to the data presented.
The sample temperature history is controlled by modulating the heating current in order to achieve the desired radiance temperature history as indicated by a near-infrared (1200 nm central wavelength) spot pyrometer that focuses on a 1 mm square spot on one side of the specimen. Current modulation is performed via a microcontroller-based PID control loop with a loop time of 0.1 millisecond (check timing). A second, identical pyrometer measures the radiance temperature on the opposite side of the specimen to quantify the temperature uniformity during heating. The thermodynamic (true) temperature is determined from the pyrometer readings using a fine (0.005 inch diameter) k-type thermocouple spot-welded to the specimen at a point adjacent to the control pyrometer measurement spot. During heating, the thermocouple signal is ruined by electromagnetic interference. Thus the true temperature must be determined after the electromagnetic field decays to negligible levels, which occurs about 20 ms after the current is switched off. The impact wave is timed to allow the thermocouple just enough time to settle so a clean reading can be taken as close to impact as possible to minimize heat losses. From this temperature reading we obtain an effective sample emissivity, which is then used to estimate the true temperature history from the pyrometer signals. The temperature uncertainty is determined from the average difference between the two pyrometer signals.
All experiments are conducted in partial vacuum to limit surface oxidation during heating. Because the heating times are short (less than 6 seconds) and the specimens have a small diameter compared to the bars, the bars themselves remain cool beyond a very small region adjacent to the specimen. One result of this is that the elastic loading wave propagation is undisturbed by the heating process. Further, because the bars remain cool, after impact the specimen cooling rate is quite high, generally in excess of 500 °C/s. In most tests the actual cooling rate is measured by the thermocouple, which often remains intact after impact. This information is used to help interpret post-test microstructures. We finally note that the samples are subjected to a single compressive loading event by using a shortened transmission bar.
Results
Experiments are conducted over most of the equilibrium temperature range where pearlite decomposition is predicted for the steels investigated (A1 to A3 temperatures). To vary the transformation driving force, samples are held for increasingly long times for each selected test temperature. We use this technique because time control is far more precise than temperature control, since the latter is affected by small but unpredictable variations in surface emissivity and heating conditions from test to test. Typical heating profiles for short, medium and long hold times are shown in Figure 2 for a single nominal test temperature. We note these tests are nearly isothermal, particularly for the longer hold times, since the thermal rise time is usually a small fraction of the overall heating period.
Figure 2.
True temperature profiles for short, medium and long hold time experiments.
To correlate temperature and time to mechanical strength and transformation fraction, we report the average temperature during the hold period rather than the impact temperature, which can be 10 °C to 15 °C lower due to cooling of the sample that takes place as the thermocouple signal settles after the heating current is switched off (about 20 ms).
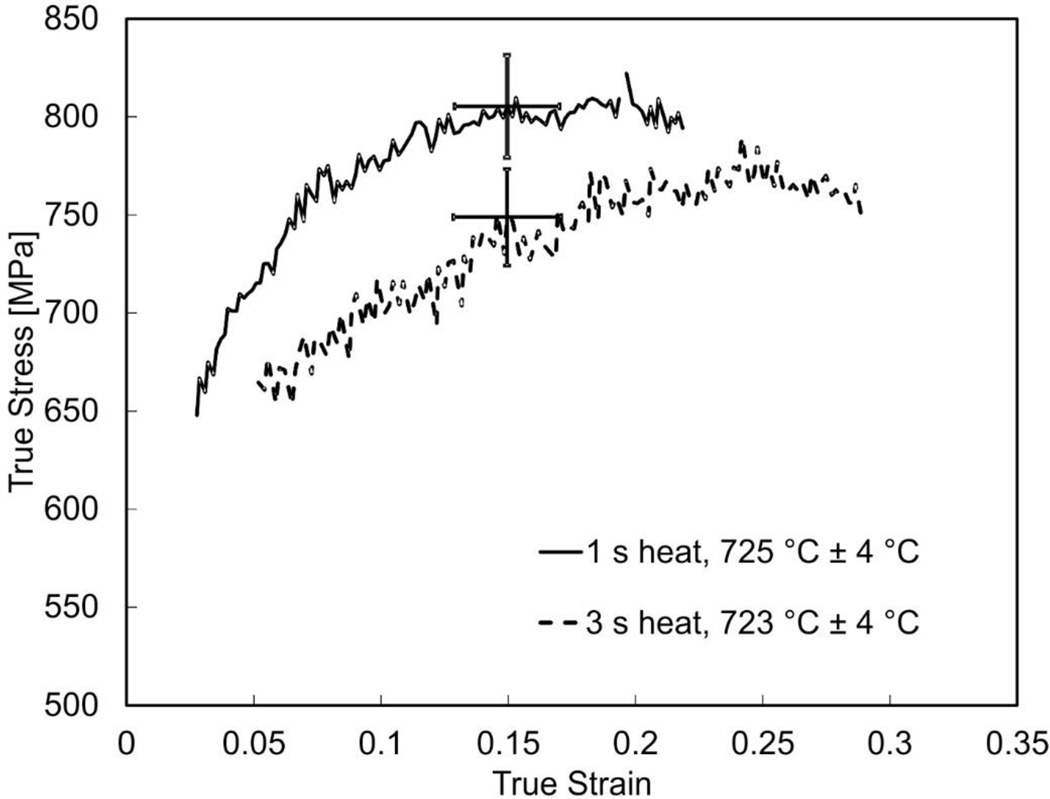
In Figure 3, dynamic stress-strain curves are shown for the 1075 steel for three increasing hold times at a true temperature of about 730 °C and at a strain rate of 3500 s−1 ± 500 s−1. At this temperature, equilibrium thermodynamics predicts almost complete transformation for this steel (A3 = 727 °C). Dynamic stress-strain curves are plotted only where good equilibrium is indicated and the strain rate has stabilized to a constant level. Thus data over the initial and final few percent strain are omitted. As Figure 3 shows, the 1 second hold time result is stronger than the 3 second result. Higher strength also correlates with a comparatively smaller total strain for the 1 second long heated test, which a result of the Kolsky bar test method used here. We believe the difference in strength observed in these two tests, conducted nominally at the same temperature and strain rate, is due to a larger amount of pearlite being dissolved in the test having the longer heating time. Microstructural evidence in support of this supposition will be given later in the paper. Finally, we note that the noise in curves presented in Figure 3 is due to the low strain gage signal levels that directly result from the need to use fairly small size specimens to achieve fast heating rates with this method. In addition, the minor variation in strain rate that occurs during these tests (± 500 s−1) or from test to test due to thermal softening is insufficient to perceptibly affect strength, which varies only logarithmically with strain rate for steels most metals for strain rates below 104 s−1 [13].
Figure 3.
Dynamic stress-strain curves for 1075 steel near 725 °C for hold times of 1 s and 3 s at a true strain rate of 3500 s−1 ± 500 s−1. Error bars are shown for a single value of true strain (0.15) and are typical of all true strains shown. Stress and strain error bars are expanded uncertainties (95% CI).
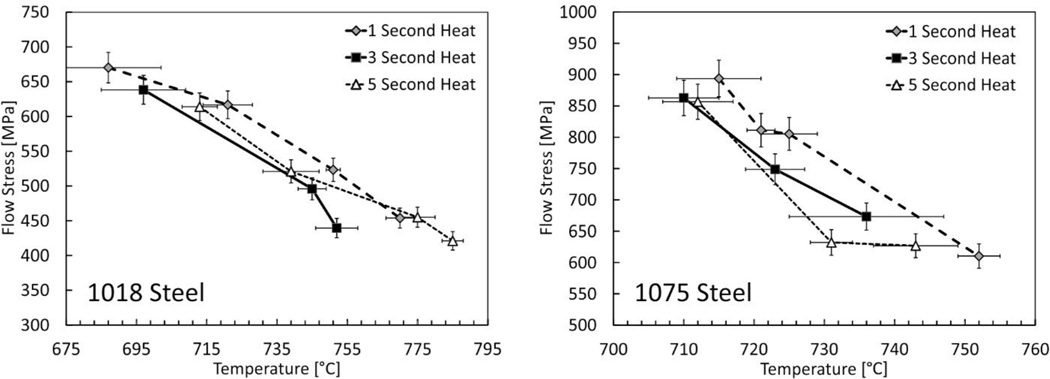
In Figure 4 we examine how heating time affects the dynamic strength through the austenite formation range. Flow stress is plotted at a single value of true strain as a function of temperature for different heating times. Lines are used to connect data points having a common heating time to guide the eye. As these plots show, both steels exhibit higher strength when heated for only 1 second compared to longer heating times over the range of temperature investigated. This transient strength effect is believed to be due to the progress of pearlite dissolution in the materials, to be confirmed by microstructural analysis of the tested specimens, which is described next for 1075 steel. Pearlite dissolution appears mostly complete after about 3 seconds in the 1018 steel since the 5 second heating time results in no significant additional weakening. For the 1075 steel there may be some further weakening, but more data are needed to draw a firm conclusion. We thus observe that the transient mechanical effects associated with the dissolving pearlite phase are present for heating times less than about 3 seconds in both steels. That the kinetics of pearlite dissolution are similar for these steels, despite the large difference in starting pearlite volume fraction, is likely due to similar inter-lamellar pearlite spacing and average pearlite colony size (Table 2) [8].
Figure 4.
Dynamic flow stress as a function of temperature and time at a true strain of 0.15. Strain rates ranged from 3500 s−1 to 4500 s−1, rising slightly as the material softens as the test temperature increases. Lines connecting data points are included as a visual guide only. Stress error bars are expanded uncertainties (95% CI), while temperature error bars represent the difference between the control and monitoring pyrometer signals.
The magnitude of the transient effect is understandably higher in the 1075 steel, which again has a larger initial pearlite volume fraction (0.77 to 0.15, Table 2). The transient strength effect, between the 1 second and 3 second heating times, is about 45 MPa for the 1018 steel and about 85 MPa for the 1075 steel. These amounts are estimated by roughly fitting straight lines to the data at each heating time and taking the average difference between the lines over the range investigated. That the transient effect is only twice as large in the 1075 steel is somewhat surprising given that it starts with five times more pearlite by volume than the 1018 steel. We also note that the overall transient strength effect associated with pearlite decomposition may be higher as these steels have likely experienced some transformation (strength loss) within the first second of heating.
For the 1075 steel the transient strength effect continues beyond the equilibrium austenite finish temperature (A3 = 727 °C), which is likely due to the rapid heating involved, which tends to increase the finish temperature above equilibrium values (Ac3 > A3) because the reaction is time-limited. While estimates of Ac3 are available for constant heating rate behavior, these estimates may not be adequate for the present experiments which do not have a constant heating rate. Predictions of austenite start and finish temperatures, under the same experimental thermal histories used here, are planned.
Conversely, for the 1018 steel, the transient effects seem to begin below A1 and apparently disappear well below the equilibrium finish temperature (A3 = 836 °C). This is to be expected if the majority of the transient strength effect associated with pearlite (cementite) dissolution are completed well before the carbon has homogenized and the microstructure becomes completely austenitic. Transformation occurring below A1 is more difficult to rationalize. One possibility is that early transformation happens due to internal thermal gradients in the specimen that are not captured by external surface measurements. Evidence of this phenomena are presented later. Regardless, further investigation is needed to confirm whether the apparent transient strength effect can be full explained by pearlite dissolution through quantitative microstructural measurements.
Microstructural Analysis
To confirm the hypothesis that the transient strength effect observed in the Kolsky bar data is due to pearlite dissolving in the microstructure, samples recovered from single-impact tests were examined by optical microscopy. For both steels the cooling rates achieved in the tests exceed 500 °C/s. For 1075 steel this cooling rate is sufficiently high to ensure any austenite that formed during heating or impact will transform to martensite. For the 1018 steel, the cooling rate is not necessarily sufficient to guarantee martensite. We also expect other quenched phases may exist in both microstructures as the austenite reaction is interrupted and thus we may be quenching partially-transformed material. We will now describe observations of quenched 1075 samples with this in mind. Characterization of the quenched 1018 structure was incomplete at the time this article was prepared.
Figure 5 shows three optical micrographs of a 1075 steel sample heated for 3 seconds at with a test temperature of 723 °C ± 4 °C. The optical micrograph (Figure 5a) was prepared with La Pera’s etch [14], which was applied for 60 seconds. The etch attacks cementite-ferrite boundaries while leaving martensite only lightly attacked. The main goal of our microstructural analysis is to examine the extent of transformation of pearlite to austenite with increasing temperature and/or holding time. We do this by examining the tested samples for regions where the microstructure departs significantly from the starting microstructure. Because we are quenching unstable austenite in regions that have transformed, the identification of the final phases can be difficult since the carbon distribution is not uniform and is changing rapidly on impact. Instead, we simply try to distinguish untransformed pearlite or ferrite from quenched phases, which is more straightforward. Figure 5a is obtained at low magnification (50 ×) montage to show the macroscopic transformation structure, which consists of a central disk of mostly transformed material surrounded by a region with gradually less transformation to the edge of the specimen. This points to radial thermal gradients existing within the specimen which are undetectable to the surface temperature sensors employed in the experiment. Reasons for this thermal gradient are under investigation. While undesirable, this feature is helpful in quickly assessing, on a qualitative basis, the relative amount of transformation present in samples heated for different lengths of time, and how this correlates to measured strength, as will be discussed later. High magnification SEM images are also shown in Figure 5b and Figure 5c to reveal the predominant microstructural features in the center and at the edges of the tested specimen. Near the edge we find a mixture of martensite, retained ferrite and pearlite or perhaps bainite existing with a wide range of scales. In the center we find mostly martensite with some scattered very fine phases that are likely either quenched partially-dissolved pearlite, or bainite. While no retained ferrite is shown in the SEM image from the sample center, we expect it exists throughout the microstructure as it is the last phase to dissolve in the transformation.
Figure 5.
Optical micrographs of a 1075 experiment at a temperature of 723 °C ± 4 °C and a 3 second heating time. Figure 5a: 50× montage optical micrograph of sample prepared with La Pera’s etch showing the macroscopic transformation structure. Figure 5b: SEM image (nital etch) near the edge showing small amount of transformation three identified phases: retained ferrite (F), martensite (M) and pearlite/bainite (P/B). Figure 5c: SEM image (nital etch) showing the heavily transformed central region consisting of a matrix of M with scattered P/B. Retained ferrite is expected in the center but it is not captured in this particular micrograph.
Figure 6 compares low magnification montage images of 1075 steel specimens, one tested at 725 °C ± 4 °C for 1 second heating time (Figure 6a) and the other tested at 723 °C ± 4 °C with a 3 second heating time (Figure 6b). The two samples shown here correspond to the stress-strain curves shown earlier in Figure 3. We clearly see a larger area of austenite formation, which consists of the lightly attacked central martensite region, for the test conducted with the longer hold time. This correlates well with the softer response of the specimen heated for the longer time period. Thus we conclude that the primary transient strength effect we observed in these steels between A1 and A3 is caused by the varying amount of dissolved pearlite at impact.
Figure 6.
Low magnification (50×) montage optical micrographs (La Pera’s etch) showing how the size of the central transformed region, indicated roughly with the dashed circles, increases with heating time. Figure 6a: 1 second heat, impact temperature = 725 °C ± 4 °C. Figure 6b: 3 second heat, impact temperature = 723 °C ± 4 °C. Stress-strain curves for these two tests were shown previously in Figure 3.
Other features of note in this figure are bands of higher amounts of transformation in the right micrograph that are associated with microsegregation of alloying elements on solidification and subsequent rolling of the original material [15], and the appearance of small, localized and approximately spherical regions of higher transformation within the dashed circle in the left sample, which could be individual centers of nucleation and growth of the austenite.
Moving forward, we plan a complete characterization of both 1018 and 1075 steels including measurements of transformation volume fraction for correlation with the dynamic strength results presented here. In addition, experiments will be performed where specimens will be heated without impact to determine whether the dynamic plastic deformation that occurs with impact significantly changes the amount of transformation observed. Parallel studies on the transformation kinetics of the materials investigated here using dilatometry are ongoing.
Summary
Pearlite decomposition causes a transient strength effect in both 1018 and 1075 steel that is complete within about three seconds of heating. The kinetics of pearlite dissolution appear similar for these steels, despite large difference in starting pearlite volume fraction, owing to similarity in inter-lamellar pearlite spacing and average pearlite colony size. The 1075 steel experiences about twice the strength drop as the 1018 due to higher starting pearlite content. Pearlite dissolution occurs in these experiments with a characteristic macrostructure: it begins in the center and grows outward toward the specimen edges with increased heating time. This is most likely due to radial temperature gradients within the specimen likely due to convective and/or radiative surface cooling. Regardless, a clear connection exists between the time-dependent volume of pearlite dissolved and dynamic strength. Thus it is likely that steels undergoing manufacturing processes that involve rapid plastic deformation at austenite formation temperatures will not be predicted well with strength models that ignore pearlite dissolution kinetics.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the support of the NIST Mechanical Performance Group and James Warren, NIST Technical Program Director for Materials Genomics. We also acknowledge the efforts of several NIST staff, including Mrs. Sandy Claggett for extensive assistance with metallography, Mrs. Maureen Williams for SEM imaging, and Chris Amigo for instrument fabrication. We also acknowledge the valuable assistance of Mr. Eran Vax and Mr. Eli Marcus of the Nuclear Research Center, Negev, Israel, for many improvements to the electrical heating control system.
References
- 1. www.mgi.gov. [Google Scholar]
- 2.Özel T, Karpat Y. Mater. Manuf. Process. 2007;22:659. [Google Scholar]
- 3.Burns TJ, Mates SP, Rhorer RL, Whitenton EP, Basak D. J. Mech. Phys. Solids. 2015;86:220. doi: 10.1016/j.jmps.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mates SP, Rhorer R, Whitenton E, Burns T, Basak D. Exp. Mech. 2008;48:799. doi: 10.1016/j.jmps.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han S, Melkote SN, Haluska MS, Watkins TR. Mat. Sci. Eng. A-Struct. 2008;488:195. [Google Scholar]
- 6.Esin VA, Denand B, Le Bihan Qu, Dehmas M, Teixeira J, Geandier G, Denis S, Sourmail T, Aeby-Gautier E. Acta Mat. 2014;80:118. [Google Scholar]
- 7.Datta DP, Gokhale AM. Metall. Trans. A. 1981;12A:443. [Google Scholar]
- 8.Caballero FG, Capdevila C, Garcia de Andres C. Mater. Sci. Tech. Ser. 2001;17:686. [Google Scholar]
- 9.Olivera FLG, Andrade MS, Cota AB. Mater. Charact. 2007;58:256. [Google Scholar]
- 10.Dykhuizen RC, Robino CV, Knorovsky GA. Metall. Mater. Trans. B. 1999;30B:107. [Google Scholar]
- 11.Gaudi-Fugarolas D, Bhadeshia HKDH. J. Mater. Sci. 2003;38:1195. [Google Scholar]
- 12.Gray GT., III . Metals Handbook. 10th. Vol. 8. Materials Park, Ohio: American Society of Metals; 2000. pp. 462–476. [Google Scholar]
- 13.Meyers MA. Dynamic Behavior of Materials. New York, NY: John Wiley & Sons, Inc.; 1994. p. 328. [Google Scholar]
- 14.LePera FS. Metallography. 1979;12:263. [Google Scholar]
- 15.Majka TF, Matlock DK, Krauss G. Metall. Mater. Trans. A. 2002;33A:1627. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.