Abstract
Our and other groups have discovered that mesenchymal stem cells (MSCs) derived exosomes are a novel therapeutical modality for many diseases. In this study, we summarized a method to extract and purify hucMSCs-exosomes using ultrafiltration and gradient centrifugation in our laboratory and proved that hucMSCs-exosomes prepared according to our procedure were stable and bioactive. Results showed that exosomes derived from hucMSC were 40~100 nm and CD9 and CD81 positive. Functionally, hucMSCs-exosomes promoted cell proliferation and protected against oxidative stress-induced cell apoptosis in vitro by activation of ERK1/2 and p38. Interestingly, UV exposure abrogated the regulatory roles of exosomes under oxidative stress, indicating that hucMSCs-exosomes may regulate cell growth and apoptosis by exosomal shuttle of RNA. Furthermore, cytokine profile analysis revealed that hucMSCs-exosomes contained high dose of IL-6, IL-8, and other cytokines. The established method is practical and efficient, which provides a basis for further evaluating the potential of hucMSCs-exosomes as therapeutic agents.
1. Introduction
Exosomes are 40–100 nm extracellular membrane vesicles of endocytic origin, which were firstly discovered in the early 1980s [1–3]. Exosomes are released into the extracellular environment upon fusion of multivesicular bodies with the plasma membrane [2, 4–6]. Exosomes are secreted by most cells that have been examined so far, including mast cells, dendritic cells [7, 8], B cells [6], T cells [9], tumor cells [10, 11], and epithelial cells. In addition, exosomes have been found in many biological fluids [1, 11–16] including plasma [12], urine [13], saliva [14], and breast milk [15].
It has been shown that the exosomal protein composition depends on the cellular source of the studied exosomes. Regardless of origin, several common proteins are found in exosomes, including chaperones, cytoskeletal proteins, and tetraspanins such as CD9, CD63, and CD81 [17–20]. Furthermore, more studies have indicated that exosomes also contain a substantial amount of small molecules that can be transferred from one cell to another. Exosomes could easily communicate with target cells through specific receptor-ligand interactions and shuttle defined patterns of components such as proteins, bioactive lipids, and RNA to induce biological effects [19, 21–24]. Therefore, many investigations have been performed to demonstrate the role of exosomes in paracrine/endocrine process and genetic information exchange between different cells due to their important bioactivity in tissue microenvironment [21, 22].
Increasing studies have pointed out the potential contribution of human mesenchymal stem cells in the recovery of different types of tissue injury [25–28]. Although it has been demonstrated that MSCs mediate tissue repair through paracrine and transdifferentiation mechanisms, the details responsible for their roles are not well understood. Our recent studies showed that exosomes derived from human umbilical cord MSCs (hucMSCs) alleviate CCl4-induced liver fibrosis, enhance cutaneous wound healing, and repair cisplatin-induced acute kidney injury (AKI) [29–33]. In these studies, we established a method to extract and purify exosomes from hucMSCs with ultrafiltration and gradient centrifugation. Herein, we uncovered more detailed information about this method and other functions of hucMSC-exosome in vitro.
2. Materials and Methods
2.1. Isolation and Purification of Exosomes
HucMSCs were isolated as previously described in our work [34]. All experiment protocols were approved by the Ethics Committee of Jiangsu University. The 70%–80% of the confluent hucMSCs cultures were washed twice with phosphate-buffered saline (PBS) and then incubated in serum-free low glucose Dulbecco's modified Eagle's medium (LG-DMEM) for 48 hours. The conditioned medium was collected and centrifuged at 1,000 ×g for 20 minutes to remove cell debris, followed by centrifugation at 2,000 ×g for 20 minutes and 10,000 ×g for 20 minutes. The supernatant was collected and concentrated using 100 KDa MWCO (Millipore, USA) at 1,000 ×g for 30 minutes. The concentrated supernatant was loaded upon 5 mL of 30% sucrose/D2O cushions and then ultracentrifuged at 100,000 ×g for 60 minutes (optimal-90K, Beckman Coulter). The microvesicles-enriched fraction was harvested and diluted with PBS and then centrifuged thrice at 1,000 ×g for 30 minutes using 100 KDa MWCO. Finally, the purified exosomes were collected and subjected to filtration on 0.22 μm pore filter (Millipore, USA) and stored at −70°C.
2.2. Transmission Electron Microscopy
20 μL drops of purified exosomes were adsorbed onto copper grids, placed for 1 minute at room temperature, adsorbed onto the superfluous exosomes, and stained with 30 g/L phosphotungstic acid (pH 6.8) for 5 minutes at room temperature; the sample dried under half-watt lamp. Samples were imaged using a transmission electron microscopy (FEI Tecnai 12, Philips).
2.3. SDS-PAGE Analysis and Western Blotting
For SDS-PAGE analysis, total proteins in hucMSCs and exosomes were separated on 12% SDS-polyacrylamide gels and stained with Coomassie Blue. For Western blot assay, the proteins were electroblotted onto a nitrocellulose membrane after separating on 12% SDS-PAGE. The membrane was blocked and incubated with the primary antibodies followed by incubation with the horseradish peroxidase-coupled secondary antibody. The bands were visualized with ECL plus system from Amersham Pharmacia Biotech (Buckinghamshire, UK). The sources of primary antibodies were as follows: CD9 (Bioworld Technology, USA), CD81 (Epitomics, USA), β-actin (Bioworld Technology, USA), p-ERK1/2, T-ERK1/2, p-P38, P38 (Santa Cruz Biotechnology, USA), PCNA (Bioworld Technology, USA), and GAPDH (Shanghai KangChen Biotechnology, China).
2.4. Cell Culture and Oxidative Stress Treatment
H9C2(2-1) cells were cultured in HG-DMEM with 10% fetal bovine serum (FBS, Gibco, USA). HL-7702 cells were cultured in RPMI-1640 with 20% NBS. NRK-52E cells were cultured in HG-DMEM with 10% NBS. To induce oxidative stress, H9C2(2-1), HL-7702, and NRK-52E cells were exposed to 300, 300, and 500 μM H2O2 for 24, 6, and 6 hours, respectively. H9C2(2-1), HL-7702, and NRK-52E cell lines were all bought from the Cell Bank of the Chinese Academy of Sciences, Shanghai.
2.5. Cell Viability
Cell viability was assessed by MTT assay (n = 5). 1 × 103 cells were seeded per well under normal condition and 5 × 103 cells were seeded per well under oxidative condition in 96-well plate, respectively. Then, cells were treated with different doses of hucMSCs-exosomes (100 and 800 μg/mL) for 24 and 48 hours under normal condition or pretreated with hucMSCs-exosomes (800 μg/mL) for 24 hours under oxidative condition. After incubation, the absorbance was measured at 570 nm using a microplate reader.
2.6. Cell Apoptosis
Cell apoptosis was evaluated using a terminal deoxynucleotidyl transferase mediated dUTP nick end labeling (TUNEL) assay and mitochondria membrane electric potential assay. The TUNEL assay was performed using an in situ cell death detection kit (Boster Bioengineering, Wuhan, China) according to the manufacturer's instructions. TUNEL-positive apoptotic cells were counted in 10 consecutive fields in the slides. The mitochondria membrane electric potential was performed using a JC-1 detection kit (Beyotime Institute of Biotechnology, Shanghai, China) according to the manufacturer's instructions. The fluorescent signal was observed under a fluorescence microscopy.
2.7. Immunohistochemistry
Immunohistochemical staining of proliferative cell nuclear antigen (PCNA) was performed using an SABC immunohistochemistry detection kit (Boster Bioengineering, Wuhan, China) according to the manufacturer's instructions. The cells were fixed, blocked, and incubated with the primary antibody (1 : 200) for 2 hours followed by the secondary antibody for 1 hour. PCNA-positive cells were counted in 10 consecutive fields in the slides.
2.8. UV Exposure
HucMSCs-exosomes were subjected to UV exposure (254 nm) for 1 hour at 4°C [35, 36]. The control was kept at 4°C for 1 hour without UV exposure. These two exosomes were then added to the cells at the concentration of 800 μg/mL and the cells were cultured under H2O2-induced oxidative stress.
2.9. Cytokine Array
The profile and concentration of cytokines in hucMSCs-exosomes and the conditioned medium of hucMSCs were quantified using Luminex assay.
2.10. Statistical Analysis
Data was presented as mean ± SD. Statistical variance was analyzed by ANOVA using Prism software (Graph Pad, San Diego, USA). Statistical p values less than 0.05 were considered significant.
3. Results
3.1. Characterization of HucMSCs-Exosomes
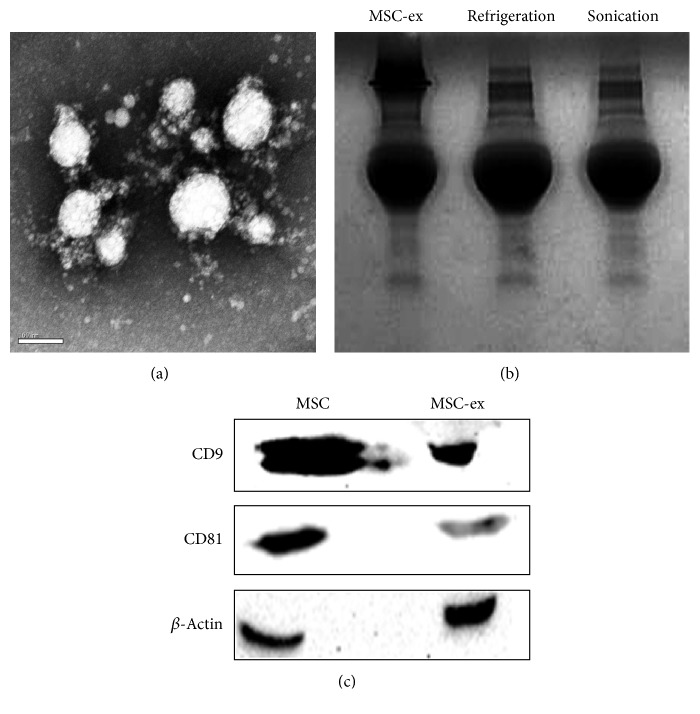
Transmission electron microscopy analysis showed a spheroid morphology of the purified exosomes, with a mean diameter of 40–100 nm (Figure 1(a)). The protein extract of exosomes was separated on 12% SDS-PAGE gel and stained with Coomassie Blue. As shown in Figure 1(b), the extract of exosomes enriched proteins with molecular weight ranging from 55 to 72 KDa and this enrichment was not affected by refrigeration or sonication. Equal amounts of protein extracts from hucMSCs and hucMSCs-exosomes were analyzed by Western blotting using antibodies specific to CD9 and CD81, which were exosomal markers. The results showed that CD9 and CD81 were constitutively expressed in hucMSCs-exosomes (Figure 1(c)). Together, these results indicate that we have successfully isolated and identified exosomes from the extracellular medium of hucMSCs.
Figure 1.
Characterization of exosomes from hucMSCs. (a) Transmission electron microscopy analysis of extracellular vesicles secreted by hucMSCs. Scale bar: 100 nm. (b) Coomassie Blue staining. The protein extracts of hucMSCs-exosomes were separated by SDS-PAGE. (c) Western blotting analyses of exosomal markers using antibodies against CD9 and CD81.
3.2. HucMSCs-Exosomes Stimulate Cell Proliferation In Vitro
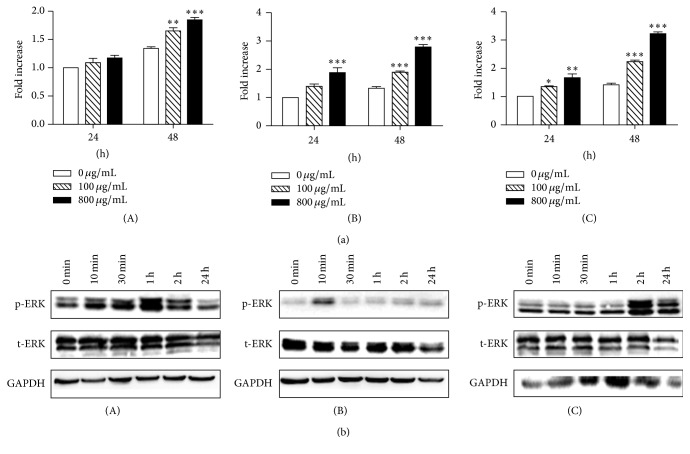
Incubation of H9C2(2-1), HL-7702, and NRK-52E cells with hucMSCs-exosomes promoted cell proliferation in a dose- and time-dependent manner compared to the control cells which were incubated with vehicle alone (conditioned medium) (Figure 2(a)). To further demonstrate the signaling pathway regulated by exosomes, we detected the levels of total and phosphorylated ERK1/2 as this pathway is tightly linked with cell growth. The results showed that exosomes treatment resulted in an increase in the phosphorylation of ERK1/2 (Figure 2(b)), suggesting that hucMSCs-exosomes may promote cell growth through upregulation of ERK1/2 phosphorylation.
Figure 2.
HucMSCs-exosomes promote cell proliferation in vitro. (a) HucMSCs-exosomes promote cell proliferation in a dose- and time-dependent manner. Results were shown as mean ± SD (n = 5). ∗ p < 0.05, ∗∗ p < 0.01, and ∗∗∗ p < 0.001 compared to control group. (b) Western blotting analysis of phosphorylated ERK1/2 in cells treated with 800 μg/mL hucMSCs-exosomes as indicated. (A) H9C2(2-1); (B) HL-7702; and (C) NRK-52E.
3.3. HucMSCs-Exosomes Protect Cell Viability In Vitro
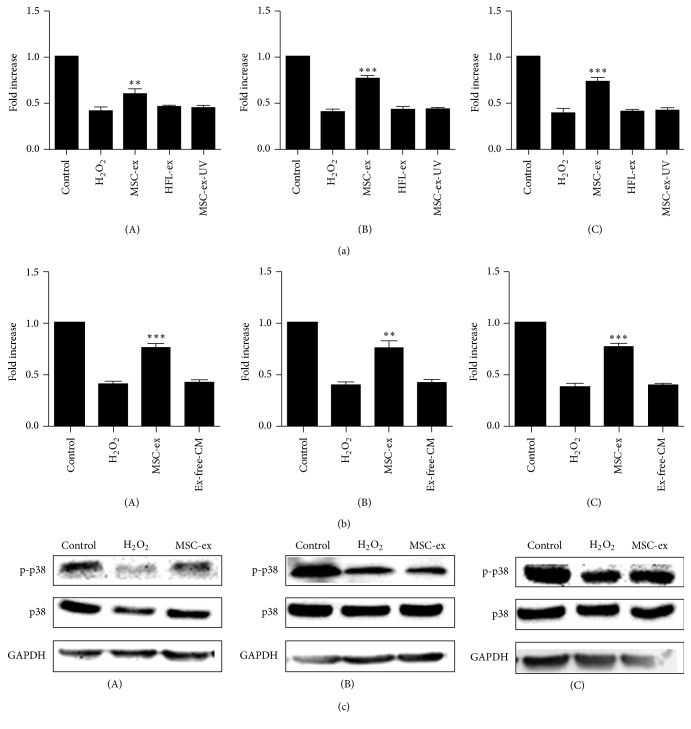
Oxidative stress induced by H2O2 leads to loss of cell viability in vitro. We further test if hucMSCs-exosomes promoted cell survival under H2O2-induced oxidative stress. Exosomes that derived from different sources were added to the cells for 24 hours, and then the cells were exposed to oxidative stress for different periods of time and the cell viability was examined by MTT assay. As shown in Figure 3(a), H2O2 decreased the viability of cells, but hucMSCs-exosomes increased the percentage of cell viability compared to H2O2 group, suggesting that exosomes' pretreatment antagonizes H2O2-induced cell death. And we also found that exosome-free conditioned medium did not show the repair role as hucMSC-exosome and could not reverse the H2O2-induced inhibition of proliferation (Figure 3(b)). We confirmed that this protective role was specific to hucMSCs-exosomes as the exosomes from human fibroblast-like cells (the Cell Bank of the Chinese Academy of Sciences, Shanghai) (HFL-exosomes) did not have the protective role. Exosomal shuttle of RNA is critical for the function of exosomes. To test if the exosomes-mediated cell protection was due to the transfer of RNA to injured cells, we exposed exosomes to UV (254 nm) for 1 hour as UV exposure inactivates RNA functions. The results showed that the exosomes that exposed to UV lost their protective effects on the cell viability (Figure 3(a)). We further demonstrated that H2O2 treatment inhibited the phosphorylation of p38 in the cells, but pretreatment with exosomes restored the level of phosphorylated p38 (Figure 3(b)).
Figure 3.
HucMSCs-exosomes protect cell viability in vitro. (a) MTT assay. H9C2(2-1), HL-7702, and NRK-52E cells were pretreated with different sources of hucMSCs-exosomes (800 μg/mL) for 24 hours, followed by exposure to H2O2 (300 μM) for 24 hours. Results were shown as mean ± SD (n = 5). ∗∗ p < 0.01 and ∗∗∗ p < 0.001 compared to H2O2 group. (b) MTT assay. H9C2(2-1), HL-7702, and NRK-52E cells were pretreated with different sources of hucMSCs-exosomes (800 μg/mL) or exosomes-free conditioned medium collected from hucMSCs (EX-free-CM) for 24 hours, followed by exposure to H2O2 (300 μM) for 24 hours. Results were shown as mean ± SD (n = 5). ∗∗ p < 0.01 and ∗∗∗ p < 0.001 compared to H2O2 group. (c) Western blotting analysis of phosphorylated p38. H9C2(2-1), HL-7702, and NRK-52E cells were pretreated with hucMSCs-exosomes (800 μg/mL) followed by exposure to H2O2 as described above.
3.4. HucMSCs-Exosomes Promote Cell Proliferation under Oxidative Stress
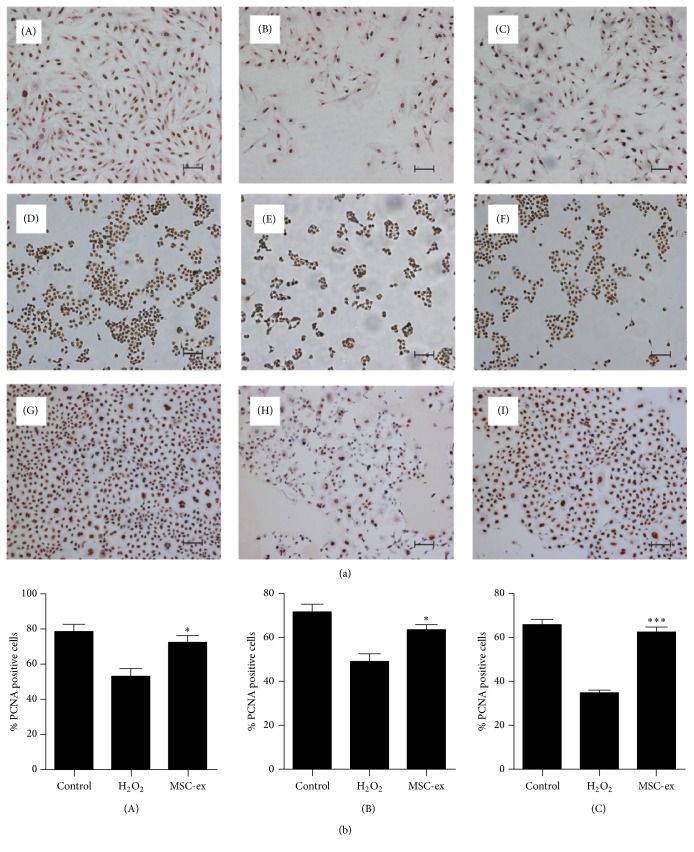
We further confirmed the protective role of hucMSCs-exosomes on cell viability by immunohistochemical staining of proliferative cell nuclear antigen (PCNA). HucMSCs-exosomes were added to the cells for 24 hours, and then the cells were exposed to oxidative stress for different periods of time. The results showed that H2O2 treatment inhibited the percentage of proliferative cells, while exosomes' treatment abrogated this effect (Figures 4(a) and 4(b)).
Figure 4.
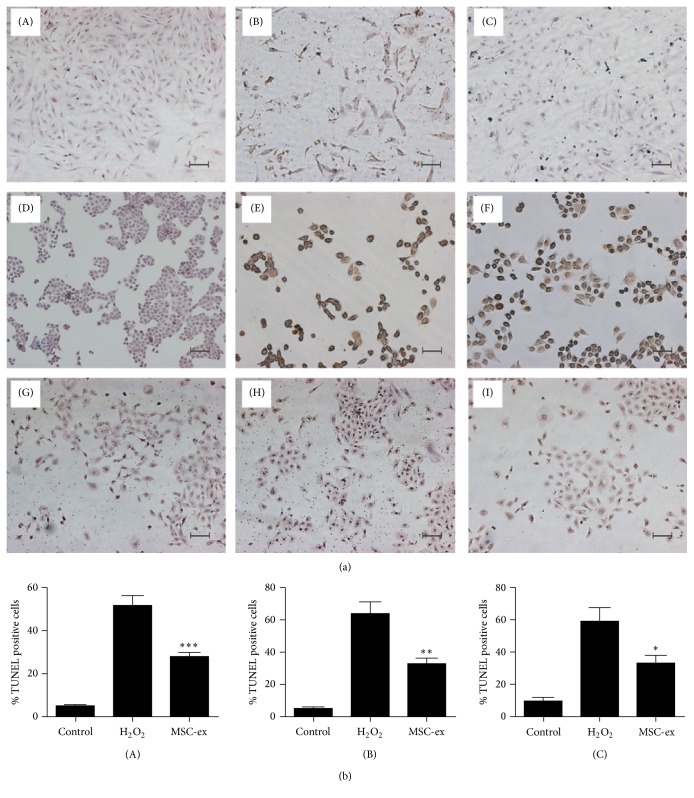
HucMSCs-exosomes promote cell proliferation under oxidative stress. (a) Immunohistochemical staining of proliferative cell nuclear antigen. H9C2(2-1), HL-7702, and NRK-52E cells were pretreated with hucMSCs-exosomes (800 μg/mL) for 24 hours, followed by exposure to H2O2 (300, 300, and 500 μM) for different time points (24, 6, and 6 hours). (A–C) H9C2(2-1); (D–F) HL-7702; and (G–I) NRK-52E. Control (A, D, and G); H2O2 (B, E, and H); and hucMSCs-exosomes (C, F, and I). Scale bar: 100 μm. (b) Quantitative analysis of the percentage of PCNA-positive cells.∗ p < 0.05 and ∗∗∗ p < 0.001 compared to H2O2 group.
3.5. HucMSCs-Exosomes Inhibit H2O2-Induced Apoptosis In Vitro
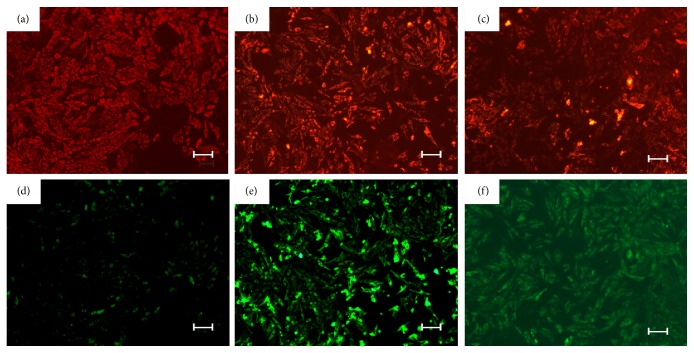
TUNEL staining showed that H2O2 treatment resulted in an increase in the percentage of apoptotic cells, but pretreatment with hucMSCs-exosomes decreased the percentage of apoptotic cells to a less extent (Figures 5(a) and 5(b)), indicating that hucMSCs-exosomes inhibited H2O2-induced apoptosis in vitro. To further demonstrate if exosomes regulated cell apoptosis pathways, we performed mitochondria membrane electric potential analysis and the results showed that H2O2 treatment increased the mitochondria membrane electric potential of H9C2(2-1), HL-7702, and NRK-52E cells (results not shown), while hucMSCs-exosomes treatment decreased the mitochondria membrane electric potential compared to that of H2O2 group (Figure 6). Collectively, hucMSCs-exosomes inhibit H2O2-induced apoptosis through regulating mitochondria-mediated cell apoptosis pathway.
Figure 5.
HucMSCs-exosomes inhibit H2O2-induced apoptosis. (a) TUNEL staining. H9C2(2-1), HL-7702, and NRK-52E cells were pretreated with hucMSCs-exosomes (800 μg/mL) for 24 hours, followed by exposure to H2O2 (300, 300, and 500 μM) for different time points (24, 6, and 6 hours). (A–C) H9C2(2-1); (D–F) HL-7702; and (G–I) NRK-52E. Control (A, D, and G); H2O2 (B, E, and H); hucMSCs-exosomes (C, F, and I). Scale bar: 100 μm. (b) Quantitative analysis of the percentage of TUNEL-positive cells. ∗ p < 0.05 compared to H2O2 group.
Figure 6.
HucMSCs-exosomes inhibit H2O2-induced mitochondria activation. H9C2(2-1) were pretreated with hucMSCs-exosomes (800 μg/mL) for 24 hours, followed by exposure to H2O2 (300 μM) for 24 hours. The cells were subjected to JC-1 staining for 15 min. The signals were observed under fluorescence microscope. Control (a, d); H2O2 (b, e); and hucMSCs-exosomes (c, f).
3.6. Cytokine Profile of HucMSCs-Exosomes
Exosomes contain certain types of cytokines which may mediate its function. We then determined the concentration of cytokines in hucMSCs-exosomes and the conditioned medium of hucMSCs by Luminex assay (n = 3). The results showed that hucMSCs-exosomes expressed a lot of cytokines including GM-CSF, IL-15, IL-6, IL-8, TNF-α, IL-1β, IL-2, and IL-10 (Table 1), in which IL-6 and IL-8 were present in high dose (>100 pg/mL).
Table 1.
Identification of cytokine from hucMSCs-exosomes and hucMSCs using Luminex.
| Cytokine name | HucMSCs-exosomes (pg/mL) | HucMSCs (pg/mL) |
|---|---|---|
| GM-CSF | 1.75 | 8.94 |
| IL-15 | 4.02 | 0.51 |
| IL-17 | <0 | <0 |
| IL-6 | 123.57 | 649.96 |
| IL-8 | 285.26 | 5024.88 |
| TNF-α | 0.02 | 0.11 |
| IL-β | 0.05 | 0.31 |
| IL-2 | 0.06 | 0.21 |
| EGF | <0 | 3.54 |
| IL-10 | 0.74 | 0.73 |
| VEGF | <0 | 13.65 |
4. Discussion
In this study, we have isolated and identified exosomes from human umbilical cord MSCs in terms of their biophysical and biological properties: (1) floating at a cushion of 30% sucrose that is a specific attribute of exosomes; (2) nanometer-sized distribution of exosomes (40–100 nm); and (3) exosomal protein expression (CD9 and CD81) [17–20]. Our results confirmed that the microvesicles we isolated from the extracellular medium of hucMSCs were exosomes. The method we established is a practical and efficient procedure to isolate and purify exosomes from hucMSCs.
The exact functions of exosomes are not yet fully understood, although exosomes harvested from different cells have been shown to mediate a multitude of biological effects, including antigen presentation, induction of apoptosis, and promotion of cancer cell growth [6, 37–42]. It seems that the exosomes from different cells have various functions. In the current study, we demonstrated that exosomes derived from hucMSCs had stimulatory effect on cell proliferation and protective role against H2O2-induced cell death [43], suggesting that the exosomes from hucMSCs prepared using our procedure are bioactive and have supportive role in the growth of cells such as cardiomyocytes, hepatocytes, and renal cells.
The work from different laboratories has indicated that the release of exosomes is a mechanism by which cells transfer material and signals to other cells. Exosomes are round membrane vesicles. The nucleic acid and proteins in the exosomes are protected by the membrane structure. Exosomes have a particular composition reflecting their origin and can transfer not only membrane components but also nucleic acid between different cells. UV exposure of exosomes abrogated their effects on preventing oxidative stress-induced cell death, suggesting that the RNA shuttled by exosomes is one of the critical effectors of their biological effects. We also verify that the exosomes contain several types of cytokines, in which IL-6 and IL-8 are the major ones. Further studies are required to define the physiological role of these cytokines in hucMSCs-exosomes.
The advantage of using exosomes in regenerative medicine rather than the stem cells themselves is avoidance of possible long-term pathologic differentiation of engrafted cells. Compared to cell-based therapies, non-cell-based therapies are generally easier to manufacture and safer as they are nonviable and do not elicit immune rejection. Therefore, the isolation and identification of exosomes from hucMSCs provide a novel approach to treat diseases such as myocardial ischemia/reperfusion injury.
In addition to their tissue regenerative capacity, MSCs and their exosome also display immune-modulatory properties [44, 45]. MSCs constitutively express low levels of major histocompatibility complex-I molecules and do not express costimulatory molecules such as CD80, CD86, or CD40, thus lacking immunogenicity [46]. Based on these properties, MSCs are being used in the treatment of autoimmune diseases and graft-versus-host disease. The earliest indications of the immunosuppressive nature of MSC were derived from studies with human, baboon, and murine MSC demonstrating that MSCs were able to suppress T-lymphocyte activation and proliferation in vitro [46–49]. MSCs inhibit immunoglobulin production and arrest B-lymphocytes in the G0/G1 phase of the cell cycle [50]. In conclusion, MSCs possess a remarkably diverse array of immunosuppressive characteristics [51]. MSCs-exosomes might play a different role in immune-modulating activities and mechanisms. Rahman et al. reported that abnormal or excess exosomes released by these MSC-like precursor cells in islets may trigger tissue-specific autoimmunity in the NOD mouse strain [52]. MSC-derived extracellular vesicles also prevent postischemic immunosuppression [45]. These two studies hold the review that MSC-exosome can enhance the activation of immunity. However, other groups believed that MSC-exosome inhibited the inflammatory response to induce tissue regeneration. MSCs-derived exosome suppressed the secretion of proinflammatory factors TNF-α and IL-1β but increased the concentration of anti-inflammatory factor TGF-β [53]. Zhang et al. considered that MSC-secreted exosome has the potential to attenuate an activated immune system through the induction of anti-inflammatory cytokines and Tregs [54]. The aforementioned studies show that immune regulation may also be an important mechanism of exosome in tissue regeneration.
In conclusion, in this study, we have established a practical and efficient method to isolate and identify exosomes from human umbilical cord stem cells and demonstrated the role of hucMSCs-exosomes in stimulating cell proliferation and protecting against oxidative stress-induced apoptosis. Our work provides a basis for further evaluating the potential of hucMSCs-exosomes as therapeutic agents.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grants nos. 31340040, 81272481, 81270214, 81572075, 81670549, and 81670502), Jiangsu Province for Outstanding Sci-Tech Innovation Team in Colleges and Universities (Grant SJK2013-10), Jiangsu Province's Outstanding Medical Academic Leader and Sci-Tech Innovation Team Program (Grant no. LJ201117), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Competing Interests
The authors declare that they have no competing interests.
Authors' Contributions
Bin Zhang, Li Shen, and Hui Shi contributed equally to this work.
References
- 1.Johnstone R. M., Adam M., Hammond J. R., Orr L., Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) The Journal of Biological Chemistry. 1987;262(19):9412–9420. [PubMed] [Google Scholar]
- 2.Pan B.-T., Teng K., Wu C., Adam M., Johnstone R. M. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. Journal of Cell Biology. 1985;101(3):942–948. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan B.-T., Johnstone R. M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 4.Chaput N., Taïeb J., Schartz N. E. C., André F., Angevin E., Zitvogel L. Exosome-based immunotherapy. Cancer Immunology, Immunotherapy. 2004;53(3):234–239. doi: 10.1007/s00262-003-0472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Théry C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nature Reviews Immunology. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 6.Raposo G., Nijman H. W., Stoorvogel W., et al. B lymphocytes secrete antigen-presenting vesicles. The Journal of Experimental Medicine. 1996;183(3):1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zitvogel L., Regnault A., Lozier A., et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nature Medicine. 1998;4(5):594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 8.Théry C., Regnault A., Garin J., et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. The Journal of Cell Biology. 1999;147(3):599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanchard N., Lankar D., Faure F., et al. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/ζ complex. Journal of Immunology. 2002;168(7):3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- 10.Wolfers J., Lozier A., Raposo G., et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nature Medicine. 2001;7(3):297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 11.Andre F., Schartz N. E. C., Movassagh M., et al. Malignant effusions and immunogenic tumour-derived exosomes. The Lancet. 2002;360(9329):295–305. doi: 10.1016/s0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 12.Caby M.-P., Lankar D., Vincendeau-Scherrer C., Raposo G., Bonnerot C. Exosomal-like vesicles are present in human blood plasma. International Immunology. 2005;17(7):879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 13.Pisitkun T., Shen R.-F., Knepper M. A. Identification and proteomic profiling of exosomes in human urine. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(36):13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palanisamy V., Sharma S., Deshpande A., Zhou H., Gimzewski J., Wong D. T. Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS ONE. 2010;5(1, article e8577) doi: 10.1371/journal.pone.0008577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Admyre C., Johansson S. M., Qazi K. R., et al. Exosomes with immune modulatory features are present in human breast milk. The Journal of Immunology. 2007;179(3):1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 16.Admyre C., Grunewald J., Thyberg J., et al. Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. The European Respiratory Journal. 2003;22(4):578–583. doi: 10.1183/09031936.03.00041703. [DOI] [PubMed] [Google Scholar]
- 17.Olver C., Vidal M. Proteomic analysis of secreted exosomes. Sub-Cellular Biochemistry. 2007;43:99–131. doi: 10.1007/978-1-4020-5943-8_7. [DOI] [PubMed] [Google Scholar]
- 18.Keller S., Sanderson M. P., Stoeck A., Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunology Letters. 2006;107(2):102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J. J., Lötvall J. O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 20.Stoorvogel W., Kleijmeer M. J., Geuze H. J., Raposo G. The biogenesis and functions of exosomes. Traffic. 2002;3(5):321–330. doi: 10.1034/j.1600-0854.2002.30502.x. [DOI] [PubMed] [Google Scholar]
- 21.Simons M., Raposo G. Exosomes—vesicular carriers for intercellular communication. Current Opinion in Cell Biology. 2009;21(4):575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Pap E., Pállinger É., Pásztói M., Falus A. Highlights of a new type of intercellular communication: microvesicle-based information transfer. Inflammation Research. 2009;58(1):1–8. doi: 10.1007/s00011-008-8210-7. [DOI] [PubMed] [Google Scholar]
- 23.Chen T. S., Lai R. C., Lee M. M., Choo A. B. H., Lee C. N., Lim S. K. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Research. 2009;38(1):215–224. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koh W., Sheng C. T., Tan B., et al. Analysis of deep sequencing microRNA expression profile from human embryonic stem cells derived mesenchymal stem cells reveals possible role of let-7 microRNA family in downstream targeting of hepatic nuclear factor 4 alpha. BMC Genomics. 2010;11(supplement 1, article S6) doi: 10.1186/1471-2164-11-s1-s6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun L., Wang D., Liang J., et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis & Rheumatism. 2010;62(8):2467–2475. doi: 10.1002/art.27548. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y., Qian H., Zhu W., et al. Hepatocyte growth factor modification promotes the amelioration effects of human umbilical cord mesenchymal stem cells on rat acute kidney injury. Stem Cells and Development. 2011;20(1):103–113. doi: 10.1089/scd.2009.0495. [DOI] [PubMed] [Google Scholar]
- 27.Cao H., Qian H., Xu W., et al. Mesenchymal stem cells derived from human umbilical cord ameliorate ischemia/reperfusion-induced acute renal failure in rats. Biotechnology Letters. 2010;32(5):725–732. doi: 10.1007/s10529-010-0207-y. [DOI] [PubMed] [Google Scholar]
- 28.Yan Y., Xu W., Qian H., et al. Mesenchymal stem cells from human umbilical cords ameliorate mouse hepatic injury in vivo. Liver International. 2009;29(3):356–365. doi: 10.1111/j.1478-3231.2008.01855.x. [DOI] [PubMed] [Google Scholar]
- 29.Li T., Yan Y., Wang B., et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells and Development. 2013;22(6):845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang B., Wang M., Gong A., et al. HucMSc-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33(7):2158–2168. doi: 10.1002/stem.1771. [DOI] [PubMed] [Google Scholar]
- 31.Zhang B., Wu X., Zhang X., et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Translational Medicine. 2015;4(5):513–522. doi: 10.5966/sctm.2014-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y., Xu H., Xu W., et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro . Stem Cell Research & Therapy. 2013;4(2, article 34) doi: 10.1186/scrt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y., Sun X., Cao W., et al. Exosomes derived from human umbilical cord mesenchymal stem cells relieve acute myocardial ischemic injury. Stem Cells International. 2015;2015:12. doi: 10.1155/2015/761643.761643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiao C., Xu W., Zhu W., et al. Human mesenchymal stem cells isolated from the umbilical cord. Cell Biology International. 2008;32(1):8–15. doi: 10.1016/j.cellbi.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Ponta H., Pfennig-Yeh M.-L., Wagner E. F., Schweiger M., Herrlich P. Radiation sensitivity of messenger RNA. Molecular & General Genetics. 1979;175(1):13–17. doi: 10.1007/bf00267850. [DOI] [PubMed] [Google Scholar]
- 36.Wurtmann E. J., Wolin S. L. RNA under attack: cellular handling of RNA damage. Critical Reviews in Biochemistry and Molecular Biology. 2009;44(1):34–49. doi: 10.1080/10409230802594043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camussi G., Deregibus M. C., Tetta C. Paracrine/endocrine mechanism of stem cells on kidney repair: role of microvesicle-mediated transfer of genetic information. Current Opinion in Nephrology and Hypertension. 2010;19(1):7–12. doi: 10.1097/mnh.0b013e328332fb6f. [DOI] [PubMed] [Google Scholar]
- 38.Eldh M., Ekström K., Valadi H., et al. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS ONE. 2010;5(12) doi: 10.1371/journal.pone.0015353.e15353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu W., Huang L., Li Y., et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Letters. 2012;315(1):28–37. doi: 10.1016/j.canlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Admyre C., Johansson S. M., Paulie S., Gabrielsson S. Direct exosome stimulation of peripheral human T cells detected by ELISPOT. European Journal of Immunology. 2006;36(7):1772–1781. doi: 10.1002/eji.200535615. [DOI] [PubMed] [Google Scholar]
- 41.Karlsson M., Lundin S., Dahlgren U., Kahu H., Pettersson I., Telemo E. ‘Tolerosomes’ are produced by intestinal epithelial cells. European Journal of Immunology. 2001;31(10):2892–2900. doi: 10.1002/1521-4141(2001010)31:10<2892::aid-immu2892>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 42.Clayton A., Tabi Z. Exosomes and the MICA-NKG2D system in cancer. Blood Cells, Molecules, & Diseases. 2005;34(3):206–213. doi: 10.1016/j.bcmd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Takeda M., Shirato I., Kobayashi M., Endou H. Hydrogen peroxide induces necrosis, apoptosis, oncosis and apoptotic oncosis of mouse terminal proximal straight tubule cells. Nephron. 1999;81(2):234–238. doi: 10.1159/000045282. [DOI] [PubMed] [Google Scholar]
- 44.Maria Spaggiari G., Moretta L. Cellular and molecular interactions of mesenchymal stem cells in innate immunity. Immunology and Cell Biology. 2013;91(1):27–31. doi: 10.1038/icb.2012.62. [DOI] [PubMed] [Google Scholar]
- 45.Doeppner T. R., Herz J., Görgens A., et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Translational Medicine. 2015;4(10):1131–1143. doi: 10.5966/sctm.2015-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herrero C., Perez-Simon J. A. Immunomodulatory effect of mesenchymal stem cells. Brazilian Journal of Medical and Biological Research. 2010;43(5):425–430. doi: 10.1590/s0100-879x2010007500033. [DOI] [PubMed] [Google Scholar]
- 47.Bartholomew A., Sturgeon C., Siatskas M., et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Experimental Hematology. 2002;30(1):42–48. doi: 10.1016/S0301-472X(01)00769-X. [DOI] [PubMed] [Google Scholar]
- 48.Di Nicola M., Carlo-Stella C., Magni M., et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 49.Glennie S., Soeiro I., Dyson P. J., Lam E. W.-F., Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105(7):2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 50.Corcione A., Benvenuto F., Ferretti E., et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 51.English K., Mahon B. P. Allogeneic mesenchymal stem cells: agents of immune modulation. Journal of Cellular Biochemistry. 2011;112(8):1963–1968. doi: 10.1002/jcb.23119. [DOI] [PubMed] [Google Scholar]
- 52.Rahman M. J., Regn D., Bashratyan R., Dai Y. D. Exosomes released by islet-derived mesenchymal stem cells trigger autoimmune responses in NOD mice. Diabetes. 2014;63(3):1008–1020. doi: 10.2337/db13-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen W., Huang Y., Han J., et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunologic Research. 2016;64(4):831–840. doi: 10.1007/s12026-016-8798-6. [DOI] [PubMed] [Google Scholar]
- 54.Zhang B., Yin Y., Lai R. C., Tan S. S., Choo A. B. H., Lim S. K. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells and Development. 2014;23(11):1233–1244. doi: 10.1089/scd.2013.0479. [DOI] [PubMed] [Google Scholar]