Abstract
Traumatic brain injury (TBI) results in oxidative stress and calcium dysregulation in mitochondria. However, little work has examined perturbations of mitochondrial homeostasis in peri-injury tissue. We examined mitochondrial homeostasis after a unilateral controlled cortical impact over the sensorimotor cortex in adult male rats. There was a significant reduction in peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) messenger RNA (mRNA) at post-injury days 3 and 6 and a transient reduction in mitochondrial DNA copy number at 3 days post-injury that recovered by 6 days in the ipsi-injury striatum. In ipsilateral cortex, PGC-1α mRNA was reduced only at 6 days post-injury. Additionally, expression of mitochondrial-encoded mRNAs, cytochrome c oxidase subunit 1 and NADH dehydrogenase subunit 1, was decreased at 3 and 6 days post-injury in ipsilesional striatum and at 6 days post-injury in ipsilesional cortex. There was no observable decrease in nuclear-encoded mRNAs mitochondrial transcription factor A or NADH dehydrogenase (ubiquinone) Fe-S protein 1. We detected an acute increase in superoxide dismutase 2 mRNA expression, as well as an induction of microRNA (miR)-21 and miR-155, which have been previously demonstrated to disrupt mitochondrial homeostasis. Behaviorally, rats with TBI exhibited marked error rates in contrainjury forelimb performance on the ladder test. These findings reveal that there may be differential susceptibilities of various peri-injury brain structures to mitochondrial dysfunction and associated behavioral deficits, and that molecular pathways demonstrated to interfere with mitochondrial homeostasis and function are activated subacutely post-TBI.
Keywords: : controlled cortical impact, mitochondria, oxidative stress, traumatic brain injury
Introduction
Although traumatic brain injury (TBI) is an acute insult, it has chronic and lifelong sequelae, including functional deficits in multiple brain structures that lead to decreased ability to complete motor and memory tasks and inhibition of behavior and emotional control.1 Despite the serious burden of this disease, both on individual patients and to society at large, treatment options for TBI are largely supportive and are designed to treat the symptoms of the injury without addressing the underlying molecular pathways that contribute to or cause those long-term symptoms.
TBI can be classified into two phases: 1) the acute, immediate response attributed to mechanical damage and 2) the delayed cellular response attributed to activation of multiple pathophysiological cascades resulting in ischemia, edema, and dysfunction in neurons and other cell types.2,3 Whereas the acute immediate response is relatively limited in space to the immediate area of impact, the secondary response can be remote from the impact because of the action of cytokines, chemokines, and damage-associated molecular pathways (DAMPs), secreted immune activators that contribute to the induction of cellular injury cascades in a paracrine or endocrine fashion.4,5 Mitochondrial dysfunction is a prominent feature of the cellular injury response in this second phase of TBI and is linked to broad changes in cell and tissue structure and function, contributing to neurological deficits.6 Mitochondrial dysfunction involves increased reactive oxygen species (ROS) production, calcium dysregulation, initiation of the mitochondrial permeability transition, and, if mitochondrial injury is sufficiently severe, initiation of apoptosis, cell death, and loss.6–8
Sublethal mitochondrial dysfunction involves processes that do not necessarily induce apoptosis, but still contribute to cellular dysfunction post-TBI. This mitochondrial dysfunction can include alterations in mitochondrial homeostasis, a highly regulated balance of mitochondrial dynamics including mitochondrial biogenesis, fission and fusion, and mitophagy.9 Although increased oxidative stress, disrupted calcium homeostasis, and apoptotic signaling post-TBI have been well characterized and targeted for pharmacological intervention, less work has focused on the role of altered mitochondrial homeostasis in peri-injury cortical regions, and even less is known about the role motor cortical injuries have on interconnected regions such as the striatum. We know that integrity of the peri-injury remaining motor cortex is important for recovery of function subsequent to sensorimotor cortex (SMC) TBI, and current studies indicate that striatal health is as well.10–12 Therefore, we hypothesized that unilateral controlled cortical impact (CCI) of the SMC, a model of focal TBI, disrupts mitochondrial homeostasis in the ipsilateral striatum with marked deficits in associated behavioral changes.
Methods
Reagents
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise noted.
Animal care and use
Sixty-eight Long Evans male rats (3–4 months old) received food and water ad libitum and were kept on a 12/12 h light/dark cycle. Animals were randomly assigned to sham (n = 30) or CCI groups (n = 38). Experimenters blind to group assignment analyzed all molecular assays and behavioral tests. All work was conducted in accord with the National Institutes of Health Animal Care and Use Committee guidelines.
Controlled cortical impact
A unilateral CCI was administered over the forelimb area of the sensorimotor cortex (FL-SMC), as described previously.11,12 Briefly, rats were anesthetized with ketamine/xylazine (100 mg/10 mg/kg intraperitoneally), a 4-mm craniotomy was created, centered over the FL-SMC (0.5 mm anterior and 4 mm lateral to bregma), then a CCI was induced with a 3-mm-diameter impact tip angled 18 degrees away from the vertical (Benchmark Stereotaxic Impactor; Leica, Buffalo Grove, IL), depressing the brain at 1.7 dorsoventral, at 3.0 m/s for 300 ms. The craniotomy was then covered with gel film and dental acrylic before incision suturing. Sham animals received all procedures up to, but not including, the craniotomy, because skull removal produces behavioral and neurochemical asymmetries.13
Motor behavior assessment: ladder task
The ladder task was used to assess coordinated forelimb use, stepping accuracy, and limb placement.11,14 Animals were video recorded as they walked across a ladder (1 m long, 3 mm diameter metal pegs, spaced 1 cm apart and raised 20 cm from the table top). Three trials per test day were collected, and by slow motion playback, the number of total steps and the number of errors were recorded. Errors were counted when a rat's forelimb completely missed a rung and fell through rungs (score of 0), was placed on rung but when weight bearing the limb fell between rungs (score of 1), or slipped off rung (score of 2). Percent error was calculated as: (#0 + 1 + 2)/total steps.
Euthanasia /tissue extraction
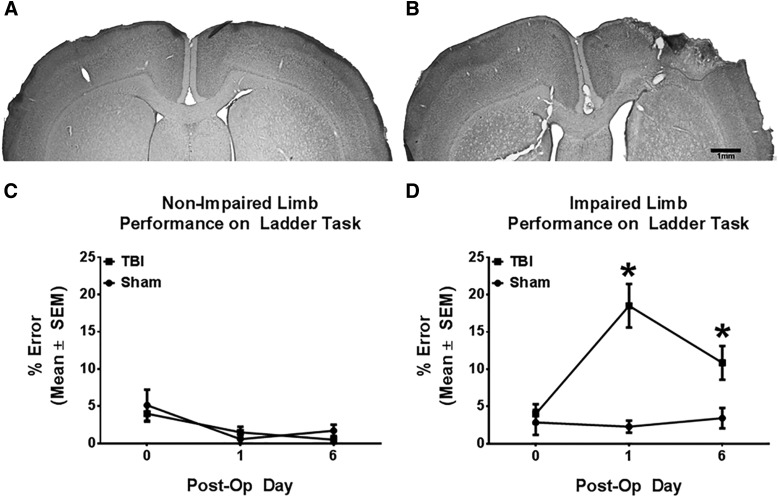
Animals were euthanized with sodium pentobarbital (∼100 mg/kg; Euthasol). Ipsi- and contrainjury frontal cortices and striatum were isolated and frozen. A subset (CCI = 2; sham = 2) of animals were intracardially perfused with 0.1 M of phosphate buffer and 0.4 M of paraformaldehyde. Coronal 50-μm sections were collected and stained with toluidine blue (Nissl stain) for representative images of injury size and placement (Fig. 1A,B). Lesion core was identified by visible mechanical damage to the motor cortex after whole-brain extraction.
FIG. 1.
Controlled cortical impact to the sensorimotor cortex causes unilateral forelimb motor deficits. Rats were subjected to either sham or controlled cortical impact treatment. Representative photomicrographs of the forelimb area of the sensorimotor cortex of typical sham (A) and controlled cortical impact (B) animals on post-operative day 6, as viewed in Nissl-stained coronal sections. Injury core is outlined. (C) After controlled cortical impact to the forelimb area of the sensorimotor cortex, nonimpaired forelimb errors were not significantly different between controlled cortical impact and sham groups. (D) Injured animals had a significant increase in impaired forelimb placing errors, compared to sham operates, at post-operative days 1 and 6. Data are reported as mean ± SEM. n ≥ 7; *p < 0.05 between sham and controlled cortical impact groups on each day. Post-Op, post-operative; SEM, standard error of the mean; TBI, traumatic brain injury.
Real-time reverse-transcriptase polymerase chain reaction: mitochondrial and antioxidant gene expression
Total RNA was extracted from cortex and striatum samples using TRIzol reagent (Invitrogen, Carlsbad, CA). Complementary DNA (cDNA) was synthesized by reverse transcription (RT) using the RevertAid First Strand cDNA kit (Thermo Fisher Scientific, Waltham, MA) with 0.5–2.0 ug of RNA. Polymerase chain reaction (PCR) products were amplified from 5 uL of cDNA template using 2× Maxima SYBR green quantitative PCR (qPCR) master mix (Thermo Fisher Scientific) and 400-nM concentrations of each primer (Integrated DNA Technologies, Inc., Coralville, IA) (Table 1). Fold changes in expression were calculated using the delta delta threshold cycle (ΔΔ-Ct) analysis method.15
Table 1.
Primers Used for PCR
| RT-qPCR Primers | ||
|---|---|---|
| Gene | Primer sequence | |
| PGC-1α | Sense | 5′-AGGAAATCCGAGCGGAGCTGA-3′ |
| Antisense | 5′-GCAAGAAGGCGACACATCGAA-3′ | |
| ND1 | Sense | 5′-TGAATCCGAGCATCCTACC-3′ |
| Antisense | 5′-ATTCCTGCTAGGAAAATTGG-3′ | |
| COXI | Sense | 5′-CCTGAGCAGGAATAGTAGGG-3′ |
| Antisense | 5′-AGTGGTACAAGTCAGTTCCC-3′ | |
| TFAM | Sense | 5′-TGAAGTTCTTACACTGATGGC-3′ |
| Antisense | 5′-CCACGTCATCTAGTAAAGCC-3′ | |
| NDUFS1 | Sense | 5′-AGATGATTTGGGAACAACGG-3′ |
| Antisense | 5′-TAAGGCTTAGAGGTTAGGGC-3′ | |
| SOD2 | Sense | 5′-CAAGGGAGATGTTACAACTCAGG-3′ |
| Antisense | 5′-CTTAGGGCTCAGGTTTGTCCA-3′ | |
| UCP2 | Sense | 5′-GAGATACCAGAGCACTGTCG-3′ |
| Antisense | 5′-GCTCAGTACAGTTGACAATGG-3′ | |
| Tubulin | Sense | 5′-CTCTCTGTCGACTACGGAAAG-3′ |
| Antisense | 5′-TGGTGAGGATGGAATTGTAGG-3′ | |
| qPCR Primers | ||
|---|---|---|
| Gene | Primer sequence | |
| ND1 | Sense | 5′-TGAATCCGAGCATCCTACC-3′ |
| Antisense | 5′-ATTCCTGCTAGGAAAATTGG-3′ | |
| β-actin | Sense | 5′-TAAGGAACAACCCAGCATCC-3′ |
| Antisense | 5′-CAGTGAGGCCAGGATAGAGC-3′ | |
| miRNA primers | ||
|---|---|---|
| miRNA | Primer sequence | |
| miR-21 | RT | 5′-CTCAACTGGTGTCGTGGAGTCGGCAATT-3′ |
| Sense | 5′-ACACTCCAGCTGGGTAGCTTATGAGACT-3′ | |
| miR-155 | RT | 5′-CTCAACTGGTGTCGTGGAGTCGGCAATT-3′ |
| Sense | 5′-ACACTCCAGCTGGGTTAATGCTAATTGTG-3′ | |
| U6 | RT/antisense | 5′-TTCACGAATTTGCGTGTCAT-3′ |
| Sense | 5′-CGCTTCGGCAGCACATATAC-3′ | |
| Universal antisense | Antisense | 5′-TGGTGTCGTGGAGTCG-3′ |
COXI, cytochrome c oxidase subunit 1; miR/miRNA, microRNA; ND1, NADH dehydrogenase subunit 1; NDUFS1, NADH dehydrogenase (ubiquinone) Fe-S protein 1; PCR, polymerase chain reaction; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; POD, post-operative day; qPCR, quantitative PCR; RT-qPCR, reverse-transcriptase quantitative polymerase chain reaction; RT, reverse transcriptase; TBI, traumatic brain injury; TFAM, mitochondrial transcription factor A.
Mitochondrial DNA content
Relative quantity of mitochondrial DNA (mtDNA) was measured using real-time PCR. DNA was isolated using the DNEasy Blood and Tissue Kit (Qiagen, Valencia, CA), and qPCR was performed using 5 ng of cellular DNA. Expression of NADH dehydrogenase 1 (ND1), a mitochondrial gene, was measured and normalized to nuclear-encoded β-actin (Table 1). The ΔΔ-Ct analysis method was used to calculate fold changes in expression.15
Real-time reverse-transcriptase polymerase chain reaction: mitochondrial: microRNA expression
Total RNA was extracted as previously described. cDNA was synthesized by RT using the Kit iScript Advanced cDNA (Bio-Rad, Hercules, CA) with 0.5-2.0 ug of RNA and 400 nM of the specific RT primer (Table 1) for each microRNA (miRNA) using pulsed RT-PCR.16 PCR products were amplified from 5 uL of cDNA template using 22× Maxima SYBR green qPCR master mix (Thermo Fisher Scientific) and 400-nM concentrations of primers (Integrated DNA Technologies) (Table 1). Target miRNAs were amplified with a specific forward primer and a universal reverse primer. U6 was used to normalize target miRNA expression and was amplified with a specific forward primer and a reverse primer that is identical to its RT primer. Fold changes in expression were calculated using the ΔΔ-Ct analysis method.15
Statistical analysis
Data are presented as means ± standard error of the mean (SEM). Subsets of the total animal population were analyzed in batches corresponding to day of euthanasia post-injury (post-operative day [POD] 1, 3, or 6). Normality was determined using the D'Agostino and Pearson omnibus normality test. Single comparisons for normally distributed data were performed using a Student's t-test. Data not normally distributed were subjected to a Mann-Whitney U-test. Statistical outliers were identified using a Dixon's Q-test and excluded from further analysis. Molecular assays were performed with n ≥ 4 in each group. Motor performance on the ladder task was analyzed with repeated analysis of variance and Fisher's least significant difference post-hoc analysis for individual days. Single and multiple comparison data were considered statistically significantly different at p < 0.05.
Results
Controlled cortical impact to the forelimb area of the sensorimotor cortex causes unilateral forelimb motor deficits
To examine forelimb motor impairment post-TBI, forelimb placement on the ladder task was used for both sham and CCI rats. CCI produced unilateral damage centered over the forelimb FL-SMC as previously demonstrated (Fig. 1).11,14 Behaviorally, unilateral FL-SMC CCI impaired accurate forelimb placing on the ladder task compared to shams (Fig. 1C) across post-CCI testing days (group × day: F4,64 = 8.297; p ≤ 0.001). There were no significant differences between groups in errors with nonimpaired forelimb (Fig. 1D; group × day: F4,64 = 0.228; p ≥ 0.05). These results indicate that motor function is altered in this model of TBI and provide evidence that the injury sustained to the SMC by CCI is severe, specific to the site of injury, and sustained in the subacute phase after initial insult.
Controlled cortical impact results in altered mitochondrial homeostasis in peri-injury cortex and striatum
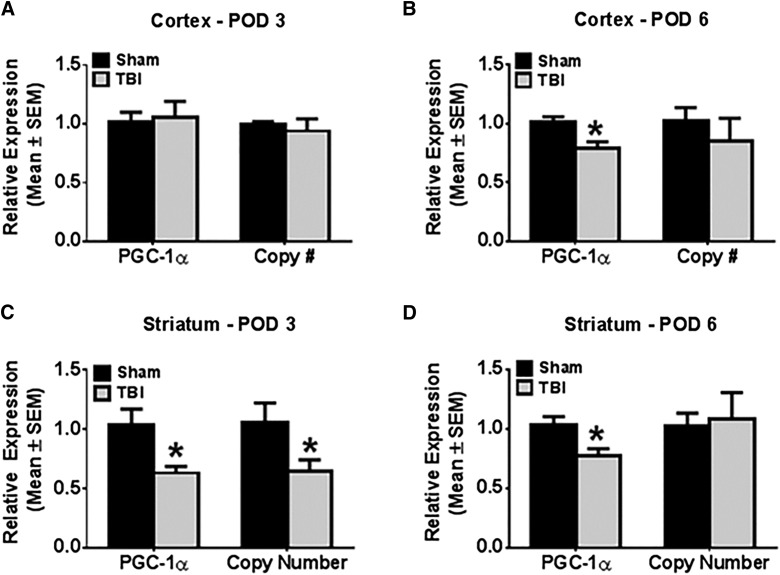
To screen for mitochondrial damage in the subacute phase post-injury, peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) messenger RNA (mRNA) expression and mtDNA copy number were measured in ipsilateral cortex and striatum at PODs 1, 3, and 6. In the ipsi-injury frontal cortex, neither PGC-1α mRNA expression nor mtDNA copy number was changed on PODs 1 or 3 compared to sham. However, on POD6, there was a 20% decrease in ipsilateral cortical PGC-1α mRNA expression (Fig. 2A,B) compared to shams (t(28) = 2.98; p < 0.05).
FIG. 2.
Controlled cortical impact results in altered mitochondrial homeostasis in tissues ipsilateral to injury. Rats were subjected to either sham or controlled cortical impact treatment. PGC-1α messenger RNA expression was determined by reverse-transcriptase polymerase chain reaction (PCR) using tubulin as a control gene. Mitochondrial DNA copy number was determined by quantitative PCR, using ND1 for the mitochondrial gene and actin for the nuclear control gene. These markers were measured in the ipsilateral cortex and striatum at post-operative days 1 (A and D), 3 (B and E), and 6 (C and F). Values reported as mean ± SEM. n > 4, *p < 0.05. PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; POD, post-operative day; SEM, standard error of the mean; TBI, traumatic brain injury.
In the ipsi-injury striatum, on POD1 there was no change in PGC-1α mRNA expression or mtDNA copy number. On POD3, both PGC-1α mRNA expression and mtDNA copy number were decreased to 60% of sham control (Fig. 2C; PGC-1α mRNA: t(12) = 3.35; p < 0.05; mtDNA copy number: t(12) = 2.33; p < 0.05). On POD6, PGC-1α mRNA expression remained suppressed to 75% of sham control expression (Fig. 2D; t(27) = 2.82, p < 0.05), but mtDNA copy number recovered to sham control levels. These results reveal a pattern of disrupted mitochondrial homeostasis in tissue ipsilateral to CCI that is initiated in the secondary phase (post-POD1) of acute injury and indicate that injury to the cortex has deleterious effects on the striatum. The differential time courses in injury between these two structures and the delay observed before detection of cortical mitochondrial dysfunction may indicate disparate susceptibilities to mitochondrial injury between different tissues of the brain.
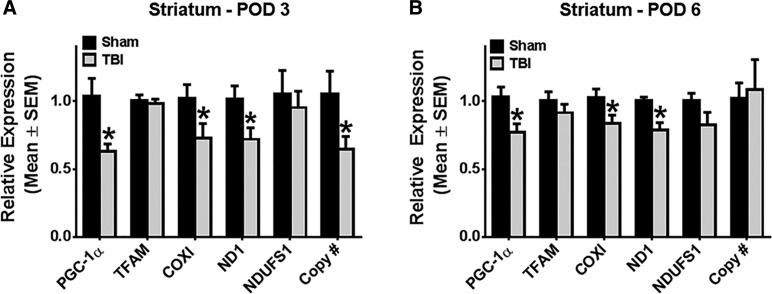
Controlled cortical impact results in decreased mitochondrial DNA transcripts in ipsilesional striatum
Because of the decrease in PGC-1α and mtDNA in the ipsi-injury striatum, we further characterized mitochondrial dysfunction using a panel of mRNAs of mitochondrial proteins, including the nuclear-encoded mitochondrial transcription factor A (TFAM) and NADH dehydrogenase (ubiquinone) Fe-S protein 1(NDUFS1) and mitochondrial-encoded cytochrome c oxidase subunit 1 (COXI) and ND1.
On POD3, mitochondrial-encoded COXI and ND1 mRNAs were decreased by 30% (COXI mRNA: t(11) = 2.87; p < 0.05; ND1 mRNA: t(10) = 2.31; p < 0.05), but no differences were observed in nuclear-encoded mRNAs (Fig. 3A). Similar to PGC-1α, both COXI and ND1 mRNA remained suppressed by 20% at POD6 (COXI mRNA: t(27) = 2.09; p < 0.05; ND1 mRNA: Mann-Whitney U = 23; nsham = 11; nCCI = 16; p < 0.05), whereas TFAM and NDUFS1 mRNA expression remained unchanged (Fig. 3B). These results reveal that the secondary phase of damage subsequent to acute CCI disrupts striatal mitochondrial homeostasis by reducing transcription of mtDNA encoded genes.
FIG. 3.
Controlled cortical impact results in decreased mitochondrial DNA (mtDNA)-encoded transcripts in ipsilateral striatum. Rats were subjected to either sham or controlled cortical impact treatment. PGC-1α, TFAM, COXI, ND1, and NDUFS1 messenger RNA expression were determined by reverse-transcriptase polymerase chain reaction (PCR) using tubulin as a control gene. mtDNA copy number was determined by quantitative PCR, using ND1 for the mtDNA gene and actin for the nuclear control gene. These markers were measured in the ipsilateral striatum at post-operative days 3 (A) and 6 (B). Values reported as mean ± SEM. n > 4; *p < 0.05. COXI, cytochrome c oxidase subunit 1; ND1, NADH dehydrogenase subunit 1; NDUFS1, NADH dehydrogenase (ubiquinone) Fe-S protein 1; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; POD, post-operative day; SEM, standard error of the mean; TBI, traumatic brain injury; TFAM, mitochondrial transcription factor A.
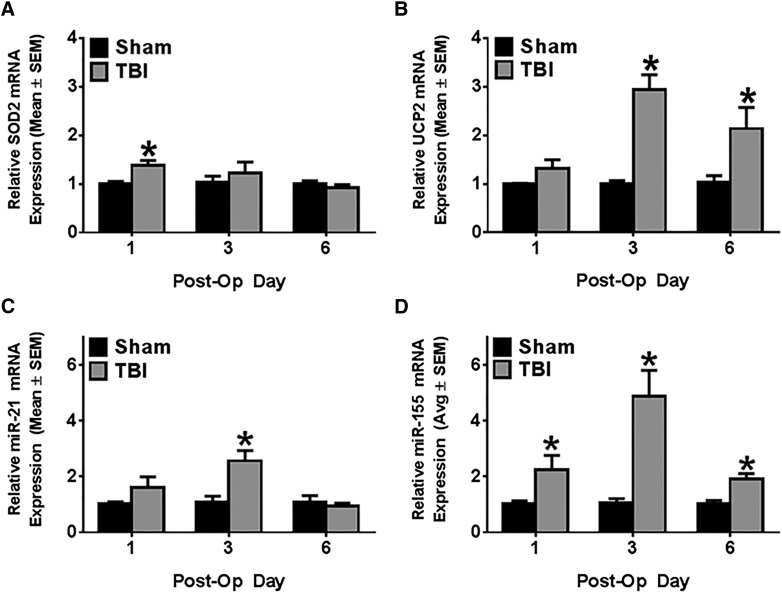
Controlled cortical impact induces antioxidant mechanisms in ipsilateral striatum
Because increased oxidative stress is a hallmark of TBI pathophysiology that disrupts mitochondrial function and propagates mitochondrial injury, we measured superoxide dismutase 2 (SOD2), a mitochondrial protein that decreases superoxide anion. In the ipsilateral striatum, SOD2 mRNA expression increased 1.4-fold over sham control at POD1 (t(13) = 3.39; p < 0.05), but returned to sham levels by POD3 (Fig. 4A). Uncoupling protein 2 (UCP2) is another protein that decreases ROS by shunting protons from the mitochondrial intermembrane space to the mitochondrial matrix, decreasing ROS.17 Ipsilateral striatal UCP2 expression remained unchanged on POD1, but increased 3-fold over sham control on POD3 (Mann-Whitney U = 0; nsham = 5; nCCI = 9; p < 0.05) and remained elevated 2-fold on POD6 (Fig. 4B; Mann-Whitney U = 1; nsham = 4; nCCI = 5; p < 0.05). These results indicate that oxidative stress is a consequence of acute CCI that begins early post-injury and persists through the first 6 days after initial insult.
FIG. 4.
Controlled cortical impact induces antioxidant mechanisms and microRNA expression in ipsilateral striatum. SOD2 and UCP2 mRNA expression were determined by reverse-transcriptase polymerase chain reaction (RT-PCR) using tubulin as a control gene. miR-21 and miR-155 mRNA expression were determined by pulsed RT-PCR using U6 as a control gene. In ipsilateral striatum, SOD2 mRNA (A), UCP2 mRNA (B), miR-21 (C), and miR-155 (D) expression were measured at post-operative days 1, 3, and 6. Values reported as mean ± SEM. n > 4; *p < 0.05. Avg, average; miR, microRNA; mRNA, messenger RNA; Post-Op, post-operative; SEM, standard error of the mean; SOD2, superoxide dismutase 2, mitochondrial; TBI, traumatic brain injury; UCP2, uncoupling protein 2.
Controlled cortical impact induces expression of microRNA-21 and microRNA-155 in ipsilateral striatum
Recent work has reported induction of several mitochondrial-associated miRNAs in the cortex and hippocampus in animal models of TBI, but little work has been done to relate them to altered expression of mitochondrial mRNAs in these models.18,19 We identified two of these miRNAs, miR-21 and miR-155, as likely mediators of striatal mitochondrial dysfunction in our CCI model. miR-21 increased 2.5-fold over sham control rats only at POD3 (Fig. 4-C; t(16) = 2.69; p < 0.05). miR-155 was induced 2-fold on POD1 (Mann-Whitney U = 14; nsham = 9; nCCI = 8; p < 0.05), increased to 5-fold on POD3 (Mann-Whitney U = 1; nsham = 6; nCCI = 11; p < 0.05), and returned to a 2-fold increase over sham control at POD6 (Fig. 4D; Mann-Whitney U = 5; nsham = 4; nCCI = 8; p < 0.05). These results implicate both miR-21 and miR-155 in the pathophysiology of striatal disruption of mitochondrial homeostasis.
Discussion
TBI has a complex pathophysiology, comprised of diverse inflammatory and metabolic pathways that include neurotransmitter excitotoxicity, cytokine release, calcium flux, hypoxia, and oxidative stress, all of which may contribute to mitochondrial damage.2,3,20 Many of these effects occur immediately post-injury, limiting the therapeutic window for intervention.7,8,21–24 Therefore, elucidation of the pathways that contribute to mitochondrial dysfunction during the delayed phase of injury (days post-insult) may provide additional targets with wider pharmacological treatment windows.
In our study, we report disruption of mitochondrial homeostasis as early as POD3 in the ipsi-injury striatum. In the striatum, these mitochondrial deficits included both decreases in mitochondrial content, as measured by relative mtDNA quantities and mtDNA transcripts, as well as decreases in PGC-1α mRNA. Because PGC-1a is considered the “master regulator of mitochondrial biogenesis,” decreases in its mRNA indicate that the affected cells have decreased ability to maintain mitochondrial homeostasis in response to acute injury. Additionally, whereas mtDNA copy number recovered to control levels, mtDNA transcription remained depressed through POD6. In contrast, transcripts of two nuclear-encoded mitochondrial proteins (TFAM and NDUFS1) were not affected in TBI. Whereas mechanisms of these responses and their impact on neural function will be addressed in future studies, it is possible, though completely untested, that mitochondrial dysfunction may be related to poor neural transmission in areas connected, but distant from the primary injury impact.
In the cortex, decreased PGC-1α mRNA was first detected at POD6. These results are surprising given that the cortex is the site of impact, yet the ipsilateral striatum has the greater, more-rapid disruption of mitochondrial homeostasis. The decrease in PGC-1α mRNA in ipsilateral striatum and cortex at POD6 may suggest a continued activation of DAMPs or repair mechanisms. Additionally, given that our model inflicts a severe cortical injury and the most injured cells likely undergo rapid necrosis or apoptosis and subsequent degradation by activated microglia, our methods sample the cortical cells that were functional enough to survive and therefore may not capture the true severity of mitochondrial damage in the cortex in the acute phase post-CCI.
Many studies have focused on the role of ROS in the pathophysiology of cerebral ischemic damage. Oxidative damage of proteins, lipids, and DNA causes mitochondrial damage and subsequent mitochondrial dysfunction, ultimately leading to neuronal death.25 SOD2 and UCP2 decrease these ROS and are upregulated in the setting of cerebral ischemia.25 These observations were recapitulated post-CCI. Work in other models of acute ischemic cellular injury has shown that release of ROS in acute injury results in rapid induction of SOD2.26 In ipsilesional striatum post-CCI, early increases in SOD2 mRNA were detected. On POD3, however, SOD2 mRNA returned to baseline expression, indicating that superoxide may cause acute oxidative damage, but have a lesser role in the subacute and chronic phases post-TBI.
In addition to reducing the formation of ROS in the acute injury state, it has been suggested that the proton-transporting action of UCP2 relies on translocation of membrane-bound lipids, including reactive lipid peroxides, into the intermembrane space, which has the secondary effect of sequestering these lipid peroxides from the proteins and mtDNA within mitochondrial matrix.17,27,28 In our study, UCP2 mRNA increased at POD3 and remained elevated at POD6, suggesting continued oxidative stress and mitochondrial dysfunction in the delayed injury state. Given that ROS have previously been implicated in mtDNA depletion in acute injury, the persistent presence of reactive oxidative molecules in the mitochondria post-TBI provides a potential mechanism for the observed decrease in mtDNA copy number in the ipsilateral striatum.29 Additionally, the observed decrease in SOD2 mRNA at POD3, combined with the increase in UCP2 mRNA at this same POD, may indicate a shift in the oxidative damage pathway from acute phase mediators, such as superoxide, to other reactive molecules and thus limit the efficacy of antioxidants targeting superoxide formation to use in only acute treatment of TBI.
Finally, we suggest that the observed mitochondrial dysregulation is partially attributed to induction of miRNAs. miR-21, which has been demonstrated to decrease SOD2 mRNA, was increased on POD1 and POD3 and returned to baseline at POD6.30–32 These results are consistent with previous reports that miR-21 is increased post-TBI in the cortex and could account for the rapid return of SOD2 mRNA to baseline expression after its acute increase in response to the initial bolus of ROS. Alternatively, the increase in miR-21 may decrease SOD2 prematurely and lead to persistent oxidative stress and damaged mtDNA. miR-155, which has been demonstrated to suppress PGC-1α mRNA expression, was increased in the striatum at all time points.30,33 It is maximally increased at POD3 and is correlated with maximally decreased PGC-1α mRNA expression, indicating that this miRNA may play a significant role in mitochondrial biogenesis suppression post-TBI. Further, both miR-21 and miR-155 are induced by cytokines shown previously to be increased post-TBI, including interleukin-6 and transforming growth factor β, and we suggest that mitochondrial dysregulation induced by these miRNAs in the striatum is a downstream effect of the release of inflammatory molecules at the cortical site of primary injury.19,31–36
In summary, our results suggest that the striatum, though not the primary site of impact, is highly susceptible to disruptions in mitochondrial homeostasis in the delayed phase after cortical TBI and are consistent with other reports that the striatum is more sensitive to cellular death in the setting of mtDNA depletion than either cortex or hippocampus.37 These results are further supported by a report by Sauerbeck and colleagues demonstrating that moderate CCI in combination with the environmental toxin, trichloroethylene, which results in a pathology similar to that found in Parkinson's disease, causes a significant decrease in striatal complex I–dependent oxygen consumption with a concomitant impairment of motor function; interestingly, these changes were specific to the striatum, and no decrease in oxygen consumption were observed in the substantia nigra.38 Further, in our model, mitochondrial disruptions coincide with marked motor impairment in the ladder task, which assesses both cortical and striatal injury. To our knowledge, this is the first report to suggest that miRNAs interfere with striatal mitochondrial damage response pathways after cortical injury. Additional studies are needed to probe a causative relationship between release of cytokines in the injured cortex, induction of specific miRNAs in the striatum, and disruptions in striatal antioxidant defense and mitochondrial homeostasis, which may help identify novel drug targets to mitigate secondary mitochondrial dysfunction in the striatum after cortical injuries.
Acknowledgments
The authors thank Rachel Weber for her help with behavioral assays.
This work was supported, in part, by the National Institute of Neurological Disorders and Stroke (R01 NS065866; to D.L.A.), National Institute of Diabetes and Digestive and Kidney Diseases (F30 DK091107 and T32 DK083262; to J.L.H.), and National Institute of General Medical Sciences (GM084147 [to R.G.S.] and P20GM103542 [to South Carolina COBRE in Oxidants, Redox Balance, and Stress Signaling]); National Center for Research Resources (UL1-RR029882); the Biomedical Laboratory Research and Development Program of the Department of Veterans Affairs (5I01 BX-000851; to R.G.S.); and the South Carolina Clinical and Translational Research Institute at the Medical University of South Carolina. Animal facilities were funded by the National Center for Research Resources (C06-RR015455).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Langlois J.A., Rutland-Brown W., and Wald M.M. (2006). The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 21, 375–378 [DOI] [PubMed] [Google Scholar]
- 2.William T.O.C., Aoife S., and Michael D.G. (2011). Animal models of traumatic brain injury: a critical evaluation. Pharmacol. Ther. 130, 106–113 [DOI] [PubMed] [Google Scholar]
- 3.Cheng G., Kong R.-H., Zhang L.-M., and Zhang J.-N. (2012). Mitochondria in traumatic brain injury and mitochondrial-targeted multipotential therapeutic strategies. Br. J. Pharmacol. 167, 699–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lenz A., Franklin G.A., and Cheadle W.G. (2007). Systemic inflammation after trauma. Injury 38, 1336–1345 [DOI] [PubMed] [Google Scholar]
- 5.Namas R., Ghuma A., Hermus L., Zamora R., Okonkwo D.O., Billiar T.R., and Vodovotz Y. (2009). The acute inflammatory response in trauma / hemorrhage and traumatic brain injury: current state and emerging prospects. Libyan J. Med. 4, 97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lifshitz J., Sullivan P., Hovda D., Wieloch T., and McIntosh T. (2004). Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion 4, 705–713 [DOI] [PubMed] [Google Scholar]
- 7.Niklas M., and Lars H. (2011). Animal modelling of traumatic brain injury in preclinical drug development: where do we go from here? Br. J. Pharmacol. 164, 1207–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendes Arent A., de Souza L.F., Walz R., and Dafre A.L. (2014). Perspectives on molecular biomarkers of oxidative stress and antioxidant strategies in traumatic brain injury. Biomed. Res. Int. 2014, 723060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palikaras K., and Tavernarakis N. (2014). Mitochondrial homeostasis: the interplay between mitophagy and mitochondrial biogenesis. Exp. Gerontol. 56, 182–188 [DOI] [PubMed] [Google Scholar]
- 10.Jefferson S.C., Clayton E.R., Donlan N.A., Kozlowski D.A., Jones T.A., and Adkins D.L. (2015). Cortical stimulation concurrent with skilled motor training improves forelimb function and enhances motor cortical reorganization following controlled cortical impact. Neurorehabil. Neural Repair 30, 155–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones T.A., Liput D.J., Maresh E.L., Donlan N., Parikh T.J., Marlowe D., and Kozlowski D.A. (2012). Use-dependent dendritic regrowth is limited after unilateral controlled cortical impact to the forelimb sensorimotor cortex. J. Neurotrauma 29, 1455–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adkins D.L., Ferguson L., Lance S., Pevtsov A., McDonough K., Stamschror J., Jones T.A., and Kozlowski D.A. (2015). Combining multiple types of motor rehabilitation enhances skilled forelimb use following experimental traumatic brain injury in rats. Neurorehabil. Neural Repair. 29, 989–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams F.S., Schwarting R.K. and Huston J.P. (1994). Behavioral and neurochemical asymmetries following unilateral trephination of the rat skull: is this control operation always appropriate? Physiol. Behav. 55, 947952. [DOI] [PubMed] [Google Scholar]
- 14.Adkins D.L., Ferguson L., Lance S., Pevtsov A., McDonough K., Daniels J., Ramos E., Jones T.A., and Kozlowski D.A. (2015). Combining multiple types of motor rehabilitation enhances behavioral recovery following experimental traumatic brain injury in rats. Neurorehabil Neural Repair. 29, 989–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wills L., Trager R., Beeson G., Lindsey C., Peterson Y., Beeson C., and Schnellmann R. (2012). The β2-adrenoceptor agonist formoterol stimulates mitochondrial biogenesis. J. Pharmacol. Exp. Ther. 342, 106–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varkonyi-Gasic E., Wu R., Wood M., Walton E.F., and Hellens R.P. (2007). Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods 3, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goglia F., and Skulachev V.P. (2003). A function for novel uncoupling proteins: antioxidant defense of mitochondrial matrix by translocating fatty acid peroxides from the inner to the outer membrane leaflet. FASEB J. 17, 1585–1591 [DOI] [PubMed] [Google Scholar]
- 18.Wang W.X., Visavadiya N.P., Pandya J.D., Nelson P.T., Sullivan P.G., and Springer J.E. (2015). Mitochondria-associated microRNAs in rat hippocampus following traumatic brain injury. Exp. Neurol. 265C, 84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lei P., Li Y., Chen X., Yang S., and Zhang J. (2009). Microarray based analysis of microRNA expression in rat cerebral cortex after traumatic brain injury. Brain Res. 1284, 191–201 [DOI] [PubMed] [Google Scholar]
- 20.Werner C., and Engelhard K. (2007). Pathophysiology of traumatic brain injury. Br. J. Anaesth. 99, 4–9 [DOI] [PubMed] [Google Scholar]
- 21.Ryan D.R., Jignesh D.P., Melanie L.M., James R.P., Joseph E.S., and Patrick G.S. (2011). Post-injury administration of the mitochondrial permeability transition pore inhibitor, NIM811, is neuroprotective and improves cognition after traumatic brain injury in rats. J. Neurotrauma 28, 1845–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lifshitz J., Friberg H., Neumar R., Raghupathi R., Welsh F., Janmey P., Saatman K., Wieloch T., Grady M., and McIntosh T. (2003). Structural and functional damage sustained by mitochondria after traumatic brain injury in the rat: evidence for differentially sensitive populations in the cortex and hippocampus. J. Cereb. Blood Flow Metab. 23, 219–231 [DOI] [PubMed] [Google Scholar]
- 23.Scheff S., and Sullivan P. (1999). Cyclosporin A significantly ameliorates cortical damage following experimental traumatic brain injury in rodents. J. Neurotrauma 16, 783–792 [DOI] [PubMed] [Google Scholar]
- 24.Sullivan P., Thompson M. and Scheff S. (1999). Cyclosporin A attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp. Neurol. 160, 226–234 [DOI] [PubMed] [Google Scholar]
- 25.Chen S.-D., Yang D.-I., Lin T.-K., Shaw F.-Z., Liou C.-W., and Chuang Y.-C. (2011). Roles of oxidative stress, apoptosis, PGC-1α and mitochondrial biogenesis in cerebral ischemia. Int. J. Mol. Sci. 12, 7199–7215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Storz P., Döppler H., and Toker A. (2005). Protein kinase D mediates mitochondrion-to-nucleus signaling and detoxification from mitochondrial reactive oxygen species. Mol. Cell. Biol. 25, 8520–8530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berardi M.J., and Chou J.J. (2014). Fatty acid flippase activity of UCP2 is essential for its proton transport in mitochondria. Cell Metab. 20, 541–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaburek M., Miyamoto S., Di Mascio P., Garlid K.D., and Jezek P. (2004). Hydroperoxy fatty acid cycling mediated by mitochondrial uncoupling protein UCP2. J. Biol. Chem. 279, 53097–53102 [DOI] [PubMed] [Google Scholar]
- 29.Furda A., Marrangoni A., Lokshin A., and Van Houten B. (2012). Oxidants and not alkylating agents induce rapid mtDNA loss and mitochondrial dysfunction. DNA Repair 11, 684–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L., Zhang J., Diao W., Wang D., Wei Y., Zhang C.-Y., and Zen K. (2014). MicroRNA-155 and MicroRNA-21 promote the expansion of functional myeloid-derived suppressor cells. J. Immunol. 192, 1034–1043 [DOI] [PubMed] [Google Scholar]
- 31.Löffler D., Brocke-Heidrich K., Pfeifer G., Stocsits C., Hackermüller J., Kretzschmar A., Burger R., Gramatzki M., Blumert C., Bauer K., Cvijic H., Ullmann A., Stadler P., and Horn F. (2007). Interleukin-6 dependent survival of multiple myeloma cells involves the Stat3-mediated induction of microRNA-21 through a highly conserved enhancer. Blood 110, 1330–1333 [DOI] [PubMed] [Google Scholar]
- 32.Zhang X., Ng W.-L., Wang P., Tian L., Werner E., Wang H., Doetsch P. and Wang Y. (2012). MicroRNA-21 modulates the levels of reactive oxygen species by targeting SOD3 and TNFα. Cancer Res. 72, 4707–4713 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 33.Chen Y., Siegel F., Kipschull S., Haas B., Fröhlich H., Meister G., and Pfeifer A. (2013). miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat. Commun. 4, 1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benarroch E. (2013). Microglia Multiple roles in surveillance, circuit shaping, and response to injury. Neurology 81, 1079–1088 [DOI] [PubMed] [Google Scholar]
- 35.Arvin B., Neville L., Barone F., and Feuerstein G. (1996). The role of inflammation and cytokines in brain injury. Neurosci. Biobehav. Rev. 20, 445–452 [DOI] [PubMed] [Google Scholar]
- 36.Lenzlinger P., Morganti-Kossmann M., Laurer H., and McIntosh T. (2001). The duality of the inflammatory response to traumatic brain injury. Mol. Neurobiol. 24, 169–181 [DOI] [PubMed] [Google Scholar]
- 37.Pickrell A.M., Fukui H., Wang X., Pinto M., and Moraes C.T. (2011). The striatum is highly susceptible to mitochondrial oxidative phosphorylation dysfunctions. J. Neurosci. 31, 9895–9904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andrew S., Randy H., Guoying B., and Patrick G.S. (2012). Traumatic brain injury and trichloroethylene exposure interact and produce functional, histological, and mitochondrial deficits. Exp. Neurol. 234, 85–94 [DOI] [PMC free article] [PubMed] [Google Scholar]