Abstract
Mast cells are important effector cells in immunoglobulin (Ig) E-mediated allergic reactions such as asthma, atopic dermatitis and rhinitis. Vanillic acid, a natural product, has shown anti-oxidant and anti-inflammatory activities. In the present study, we investigated the anti-allergic inflammatory effects of ortho-vanillic acid (2-hydroxy-3-methoxybenzoic acid, o-VA) that was a derivative of vanillic acid isolated from Amomum xanthioides. In mouse anaphylaxis models, oral administration of o-VA (2, 10, 50 mg/kg) dose-dependently attenuated ovalbumin-induced active systemic anaphylaxis and IgE-mediated cutaneous allergic reactions such as hypothermia, histamine release, IgE production and vasodilation; administration of o-VA also suppressed the mast cell degranulator compound 48/80-induced anaphylaxis. In cultured mast cell line RBL-2H3 and isolated rat peritoneal mast cells in vitro, pretreatment with o-VA (1–100 μmol/L) dose-dependently inhibited DNP-HSA-induced degranulation of mast cells by decreasing the intracellular free calcium level, and suppressed the expression of pro-inflammatory cytokines TNF-α and IL-4. Pretreatment of RBL-2H3 cells with o-VA suppressed DNP-HSA-induced phosphorylation of Lyn, Syk, Akt, and the nuclear translocation of nuclear factor-κB. In conclusion, o-VA suppresses the mast cell-mediated allergic inflammatory response by blocking the signaling pathways downstream of high affinity IgE receptor (FcεRI) on the surface of mast cells.
Keywords: type I hypersensitivity, allergic inflammation, o-vanillic acid, histamine, pro-inflammatory cytokine, high affinity IgE receptor, RBL-2H3 cells, rat peritoneal mast cells
Introduction
Allergy is a hypersensitive response of the body's immune system caused by normally innocuous environmental substances such as pollen, dust, mites, variant proteins and chemicals1. In particular, type I hypersensitivity is a mast cell-mediated allergic reaction that results in anaphylaxis and allergic disorders such as asthma, atopic dermatitis and eczema. Activation of mast cells can be initiated by the allergen-induced cross-linking of immunoglobulin (Ig) E antibodies bound to the high affinity IgE receptor (FcεRI) expressed on the surface of mast cells, and the aggregation of FcεRI triggers an intracellular signaling cascade2,3.
Mast cells activated by the aggregation of FcεRI immediately release allergic mediators such as preformed histamine and prostaglandins. Histamine is a major factor in acute allergic reactions such as vasodilation and increases the permeability of vessels near the allergic site4. In addition, mast cells produce various cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-4 and transforming growth factor (TGF)-β, all of which play important roles in the inflammatory responses of the late-phase reaction, including the enhancement of T cell activation and B cell survival5. Therefore, suppressing histamine and pro-inflammatory cytokine release is a suitable therapeutic target for the treatment of allergic inflammation.
FcεRI cross-linking initiates mast cell activation through a complex intracellular signaling pathway. Assembly of the heterotetrameric structure of FcεRI is initiated by the phosphorylation of the immunoreceptor tyrosine-based activating motifs on the β and γ subunits by Src family kinases such as Lyn and Fyn. The phosphorylated γ subunits then serve as binding and activation sites for Syk. Phosphorylated Syk induces the activation of various downstream signaling molecules, such as linker for the activation of T cells, phospholipase Cγ and Gab2, resulting in an increase in calcium mobilization. Furthermore, the FcεRI-mediated signaling pathway also induces the activation of mitogen-activated protein kinases (MAPKs) and phosphatidylinositol-3-kinase (PI3K), Akt, and nuclear factor (NF)-κB4,6,7. As a result, these events lead to late-phase inflammatory reactions through the expression of several pro-inflammatory and chemotactic cytokines8,9.
Many anti-allergic agents such as steroids, anti-histamines and immunosuppressants are broadly used for the treatment of allergic inflammation. However, the prolonged use of steroids or non-specific immunosuppressants is known to cause a variety of side effects10. Therefore, safer and more effective drugs are needed to control allergic inflammation11. Natural products have been considered as a source of safer and effective new drugs, and many candidates derived from natural products have been approved for use as therapeutic treatments12. Previously, we demonstrated that Amomum xanthioides extract strongly inhibited mast cell-mediated allergic inflammation13. Using activity-guided fractionation, we isolated ortho-vanillic acid (2-hydroxy-3-methoxybenzoic acid, o-VA), a polyphenolic natural compound, as an active component5. o-VA is para-position derivative of vanillic acid. Vanillic acid has been shown to possess anti-oxidant and anti-inflammatory properties14,15. However, the biological and/or pharmacological activity of o-VA has not been reported. The aim of this study was to evaluate the beneficial effect of o-VA on mast cell-mediated allergic inflammation and to determine the mechanism underlying these effects.
Materials and methods
Reagents and cell culture
Dinitrophenyl-human serum albumin (DNP-HSA), anti-DNP IgE, o-phthaldialdehyde, 4-nitrophenyl N-acetyl-β-D-glucosaminide, ovalbumin (OVA), dexamethasone (Dexa) and Histodenz were purchased from Sigma-Aldrich (St Louis, MO, USA). Alum adjuvant was purchased from Thermo Scientific (Waltham, MA, USA). In this study, o-VA was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). RBL-2H3 cells and rat peritoneal mast cells (RPMCs) were grown in Dulbecco's modified Eagle's medium (DMEM) and α-minimum essential medium (Gibco, Grand Island, NY, USA), respectively, supplemented with heat-inactivated 10% fetal bovine serum and 100 units/mL penicillin G, 250 ng/mL amphotericin and 100 μg/mL streptomycin in 5% CO2 at 37 °C.
Animals
Male Sprague-Dawley (SD) rats weighing 240–280 g (10 weeks old) and male Imprinting Control Region (ICR) mice weighing 35–40 g (6 weeks old) were purchased from Dae-Han Experimental Animal Center (Daejeon, Korea). All animals had ad libitum access to standard rodent chow and filtered water during the study. The animals were housed 5 per cage in a laminar air flow room maintained at a temperature of 22±2 °C, relative humidity of 55%±5% and 12 h light:dark cycle throughout the study. The care and treatment of the animals were in accordance with the guidelines established by the Public Health Service Policy on the Humane Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Kyungpook National University.
Ovalbumin-induced active systemic anaphylaxis (ASA)
A total of 30 mice were divided into 6 groups (PBS only, OVA mixture only, OVA mixture and o-VA at 2, 10, or 50 mg/kg, and Dexa 10 mg/kg). Mice (n=5/group) were sensitized with the OVA mixture (100 μg of OVA and 2 mg of alum adjuvant in 200 μL of PBS) by intraperitoneal (ip) injection on d 0 and d 7. o-VA and Dexa were dissolved in saline and orally administered 3 times once every 2 d at doses of 2–50 mg/kg body weight after the second sensitization. On d 14, 200 μg of OVA was ip injected, and then the rectal temperature of the animals was measured every 10 min for 80 min. After 80 min, blood was drawn from the abdominal artery of each mouse for the measurement of serum histamine, total IgE and OVA-specific IgE levels.
IgE-mediated passive cutaneous anaphylaxis (PCA)
An IgE-dependent cutaneous reaction was elicited as described previously16. A total of 20 mice were divided into 4 groups (PBS only, DNP-HSA only, DNP-HSA and o-VA 50 mg/kg, and Dexa 10 mg/kg). To induce a PCA reaction, the skin on the ears of the mice (n=5/group) was sensitized with an intradermal injection of anti-DNP IgE (0.5 μg/site). After 48 h, each mouse received an injection of DNP-HSA (1 mg/mouse) and 4% Evans blue (1:1) mixture via the tail vein. o-VA or Dexa was dissolved in saline and orally administered at a dose of 50 mg/kg body weight 1 h before the challenge. Thirty minutes after the challenge, the mice were euthanized with CO2 and the ears were removed for measurement of the pigmented area. The amount of dye present was determined colorimetrically after extraction with 1 mL of 1 mol/L KOH and 9 mL of a mixture of acetone and phosphoric acid (5:13). The absorbance intensity was measured at 620 nm in a spectrophotometer (UV-1201; Shimadzu, Kyoto, Japan).
Compound 48/80-induced systemic anaphylaxis
A total of 60 mice were divided into 6 groups (PBS only, compound 48/80 only, compound 48/80 and o-VA at 1, 10, or 100 mg/kg, and Dexa 10 mg/kg). Mice (n=10/group) were given with an ip injection of PBS or 8 mg/kg body weight of the mast cell degranulator compound 48/80. o-VA or Dexa was dissolved in saline and orally administered at doses of 1, 10, and 100 mg/kg body weight 1 h before the injection of compound 48/80. Mortality was monitored for 1 h after the induction of anaphylactic shock.
Preparation of rat peritoneal mast cells (RPMCs)
RPMCs were isolated from SD rats as previously described17. In brief, the rats were euthanized with CO2 and injected with 40 mL of Tyrode's buffer A (137 mmol/L NaCl, 5.6 mmol/L glucose, 12 mmol/L NaHCO3, 2.7 mmol/L KCl, 0.3 mmol/L NaH2PO4, and 0.1% gelatin) into the peritoneal cavity before gentle massage of the abdomen for approximately 90 s. The peritoneal cavity was carefully opened, and the fluid containing the peritoneal cells was collected using a Pasteur pipette. The cells were collected after centrifugation at 150×g for 10 min at room temperature and then resuspended in 1 mL of Tyrode's buffer A. To separate the mast cells from the other major rat peritoneal cells, ie, macrophages and small lymphocytes, the peritoneal cells suspended in Tyrode's buffer A were layered on 2 mL of 0.235 g/mL Histodenz solution and centrifuged at 400×g for 15 min at room temperature. The cells at the buffer-Histodenz interface were discarded, and the cells in the pellet were washed and resuspended. The mast cell preparations had a purity of approximately 95% as determined by toluidine blue staining. More than 97% of the cells were viable based on trypan blue staining.
Cell viability
Cell viability was assayed using an MTT assay kit (WelGENE, Seoul, Korea). RBL-2H3 cells (3×104 cells/well in 96-well plates) were pretreated with various concentrations of o-VA for 24 h and incubated with 1 mg/mL MTT reagent at 37 °C. After 2 h, the formazan crystal by-products in the cells were dissolved with 100 μL DMSO per well. The absorbance was measured at 570 nm using a spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). Cell viability was calculated as relative absorbance compared with the control and expressed as a percentage of the control.
β-Hexosaminidase release
The release of β-hexosaminidase used as a marker of mast cell degranulation. Anti-DNP IgE (100 ng/mL)-sensitized RBL-2H3 cells (5×105 cells/well in 12-well plates) were pretreated with o-VA for 1 h after washing 3 times with PBS and then stimulated with DNP-HSA (100 ng/mL) for 4 h. After incubation, the cells were separated from the media by centrifugation at 150×g for 5 min at 4 °C, and then the supernatant (40 μL) was transferred to 96-well plates and incubated with an equal volume of substrate solution (1 mmol/L 4-nitrophenyl N-acetyl-β-D-glucosaminide in 0.1 mol/L citrate buffer, pH 4.5) for 1 h at 37 °C. The cells were lysed with 0.5% Triton X-100 before removing the supernatant to measure total β-hexosaminidase activity. The reaction was stopped by adding 200 μL stop solution (0.1 mol/L Na2CO3-NaHCO3, pH=10). The absorbance was measured at 405 nm using a spectrophotometer. β-Hexosaminidase release was calculated by dividing the content in the culture media by the combined absorbance of the culture media and cell lysate.
% Degranulation=ODCultured media/(ODCultured media+ODCell lysate)×100
Histamine release
To determine mast cell degranulation, the levels of histamine in the serum and culture medium were measured. Mouse blood was centrifuged at 400×g for 15 min at 4 °C, and the serum was collected. Anti-DNP IgE (100 ng/mL)-sensitized RBL-2H3 cells (5×105 cells/well in 12-well plates) were pretreated with o-VA for 1 h after washing 3 times with PBS, and then stimulated with DNP-HSA (100 ng/mL) for 4 h. Isolated RPMCs were seeded into 24-well plates (2×104 cells/well) with anti-DNP IgE (500 ng/mL). After 24 h, the cells were washed with PBS, pretreated with o-VA for 1 h and then stimulated with DNP-HSA (100 ng/mL) for 30 min. The cells were separated from the medium by centrifugation at 150×g for 5 min at 4 °C. For the measurement of histamine in the serum and separated medium, 0.1 mol/L HCl and 60% perchloric acid were added and then centrifuged. The supernatant was transferred to a 1.5 mL eppendorf tube, 5 mol/L NaCl, 5 mol/L NaOH and n-butanol were added, and the solution was vortexed and then centrifuged. The supernatant was shaken with 0.1 mol/L HCl and n-haptane and then centrifuged. The histamine in the aqueous layer was measured using the o-phthaldialdehyde spectrofluorometric procedure as previously described18. The fluorescence intensity was detected at emission 440 nm and excitation 380 nm using a fluorescence plate reader (Molecular Devices).
Intracellular calcium
The concentration of intracellular calcium was measured using the fluorescent indicator Fluo-3/AM (Invitrogen, Carlsbad, CA, USA). Anti-DNP IgE (100 ng/mL)-sensitized RBL-2H3 cells (2×104 cells/well in 96-well plates) were preincubated with Fluo-3/AM (5 μmol/L) for 1 h at 37 °C. The cells were treated with or without o-VA for 1 h after washing 3 times with PBS and then were stimulated with DNP-HSA (100 ng/mL). The fluorescence intensity was measured using fluorescence plate reader at an excitation wavelength of 485 nm and an emission wavelength of 510 nm. The intracellular calcium level in untreated control cells was calculated as 1 relative absorbance unit.
RNA extraction and quantitative real-time polymerase chain reaction
Prior to the isolation of total cellular RNA, anti-DNP IgE (100 ng/mL)-sensitized RBL-2H3 cells (5×105 cells/well in 12-well plates) were pretreated with o-VA for 1 h and then stimulated with DNP-HSA (100 ng/mL) for 1 h. Total RNA samples were isolated using an RNAiso Plus kit (Takara Bio, Inc, Shiga, Japan) according to the manufacturer's protocol. First strand complementary DNA (cDNA) was synthesized using Maxime RT Premix (iNtRON Biotech, Sungnam, Korea). Quantitative real-time polymerase chain reaction (PCR) was carried out using a Thermal Cycler Dice TP850 (Takara Bio, Inc) according to the manufacturer's protocol. Briefly, 1.5 μL of cDNA (150 ng), 1 μL of each of the forward and reverse primers (0.4 μmol/L), 12.5 μL of SYBR Premix Ex Taq (Takara Bio, Inc), and 9 μL of dH2O were mixed together to obtain a final 25 μL reaction mixture in each reaction tube. The PCR amplification conditions were similar to the conditions in our previous research16. Relative quantification of mRNA expression was performed using TP850 software. The primer sequences used were as follows: TNF-α (F 5′-TCCCAAATGGGCTCCCTCTC-3′, R 5′-AAATGGCAAACCGGCTGACG-3′), IL-4 (F 5′-TGCACCGAGATGTTTGTACCAGA-3′, R 5′-TTGCGAAGCACCCTGGAAG-3′), and β-actin (F 5′-GAAGCTGTGCTATGTTGCCCTAGA-3′, R 5′-GTACTCCTGCTTGCTGATCCACAT-3′). The number of cycles was optimized to ensure product accumulation in the exponential range. The amplified products were separated by electrophoresis on a 1.5% agarose gel containing ethidium bromide and documented using a molecular imaging gel doc XR system (Bio-Rad, Hercules, CA, USA).
Enzyme linked immunosorbent assay (ELISA)
The assay was performed using an ELISA kit (BD Biosciences, San Diego, CA, USA) in a 96-well Nunc immuno plate according to the manufacturer's protocol. Anti-DNP IgE (100 ng/mL)-sensitized RBL-2H3 cells (5×105 cells/well in 12-well plates) were pretreated with o-VA for 1 h after washing 3 times with PBS and then were stimulated with DNP-HSA (100 ng/mL) for 6 h. In the case of OVA-specific IgE, the immune plate was coated with 20 μg of OVA instead of a capture antibody. After terminating the reaction with a substrate, the absorbance intensity was detected using a microplate reader at a wavelength of 450 nm.
Western blot
Nuclear and cytosolic proteins were extracted as previously described19. Anti-DNP IgE (100 ng/mL)-sensitized RBL-2H3 cells (1×106 cells/well in 6-well plates) were pretreated with o-VA for 1 h after washing 3 times with PBS and then stimulated with DNP-HSA (100 ng/mL) for 7 min (Lyn and Syk), 30 min (Akt) and 1 h (p65 NF-κB and IκBα). The cells were washed with PBS and resuspended in 100 mL of cell lysis buffer A (0.5% Triton X-100, 150 mmol/L NaCl, 10 mmol/L HEPES, 1 mmol/L EDTA/Na3VO4, 0.5 mmol/L PMSF/DTT, and 5 μg/mL leupeptin/aprotinin), vortexed, incubated for 5 min on ice, and centrifuged at 400×g for 5 min at 4 °C. The supernatant was collected and used as a cytosolic protein extract. The pellets were washed 3 times with 1 mL of PBS and then suspended in 25 μL of cell lysis buffer B (25% glycerol, 420 mmol/L NaCl, 20 mmol/L HEPES, 1.2 mmol/L MgCl2, 0.2 mmol/L EDTA, 1 mmol/L Na3VO4, 0.5 mmol/L PMSF/DTT, and 5 μg/mL leupeptin/aprotinin), vortexed, sonicated for 30 s, incubated for 20 min on ice, and centrifuged at 15 000×g for 15 min at 4 °C. The supernatant was collected and used as the nuclear protein extract. Equal amounts of cellular protein were electrophoresed using an 8%–12% SDS-PAGE gel and then transferred to nitrocellulose membrane. After blocking, the membrane was incubated with a primary antibody against the target and then with anti-IgG horseradish peroxidase-conjugated secondary antibody. The following antibodies were purchased from Santa Cruz Biotechnology: NF-κB (sc-109, rabbit polyclonal, 1:1000), IκBα (sc-371, rabbit polyclonal, 1:1000), actin (sc-8432, mouse monoclonal, 1:1000), lamin B (sc-6217, goat polyclonal, 1:1000). The following antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA): phospho-Lyn (#2731, Tyr507, rabbit polyclonal, 1:1000), phospho-Syk (#2711, Tyr525/526, rabbit polyclonal, 1:1000), phospho-Akt (#9271, Ser473, rabbit polyclonal, 1:1000), Lyn (#2732, rabbit polyclonal, 1:1000), Syk (#2712, rabbit polyclonal, 1:1000), Akt (#9272, rabbit polyclonal, 1:1000). Immunoreactive protein bands were visualized using a chemiluminescent substrate (Thermo Scientific).
Statistical analysis
Statistical analyses were performed using SAS statistical software (SAS Institute, Cary, NC, USA). Treatment effects were analyzed using analysis of variance followed by Duncan's multiple range tests. P<0.05 indicated significance.
Results
Effect of o-VA on systemic and local anaphylaxis
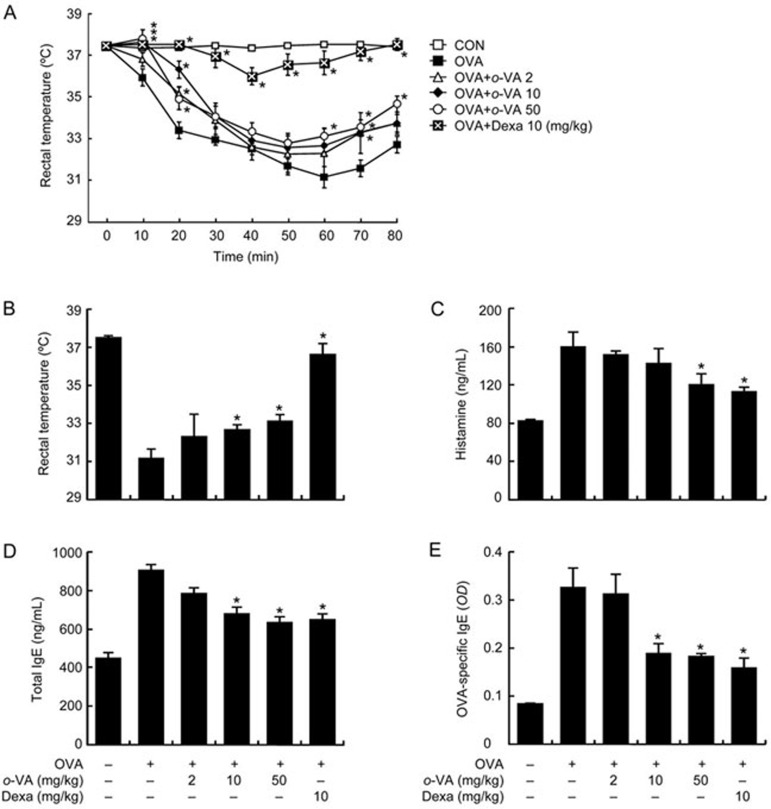
The systemic anaphylaxis model is widely used to investigate allergic responses, which are closely linked to the activation of mast cells20. Anaphylaxis was induced in mice repetitively sensitized with ovalbumin (OVA) through OVA challenge. After an ip injection of OVA, mice were observed for 80 min. The rectal temperature of the mice decreased; this effect was attenuated by the administration of o-VA, which was linked to the serum histamine level. The serum histamine level increased but was reduced by the administration of o-VA (Figure 1A–1C). In addition, total/OVA-specific IgE levels were increased after OVA challenge and reduced by o-VA (Figure 1D, 1E). IgE plays a critical role in the mast cell-mediated allergic response21. To confirm the systemic activity of o-VA, we also used a compound 48/80-induced anaphylactic shock model. Our result showed that ip injection of compound 48/80 increased the mortality rate. However, the mortality rate was reduced by oral administration of o-VA (Table 1).
Figure 1.
Effects of o-VA on ovalbumin (OVA)-induced active systemic anaphylaxis. The induction of systemic anaphylaxis and oral administration of o-VA are described in the Materials and methods section. (A) Rectal temperature was measured every 10 min for 80 min. (B) Rectal temperature of the mice at 60 min. Blood was obtained from the abdominal artery of each mouse to measure serum histamine, total IgE and OVA-specific IgE levels. (C) Histamine levels were detected using a fluorescence plate reader. (D and E) Serum total IgE and OVA-specific IgE levels were detected by ELISA. Each data point represents the mean±SEM of three independent experiments. *P<0.05. Dexa: dexamethasone.
Table 1. Effect of o-VA on compound 48/80-induced systemic anaphylaxis.
| Dose (mg/kg) | Compound 48/80 (8 mg/kg) | Mortality (%) |
|---|---|---|
| None (saline) | + | 100 |
| o-VA 1 | + | 40 |
| 10 | + | 30 |
| 100 | + | 20 |
| 100 | − | 0 |
| Dexa 10 | + | 20 |
Mice were given an intraperitoneal injection of 8 mg/kg of mast cell degranulator, compound 48/80. o-VA was orally administered at doses of 1, 10, and 100 mg/kg 1 h before the injection of compound 48/80 (n=10/group). Mortality was monitored for 1 h after induction of anaphylactic shock.
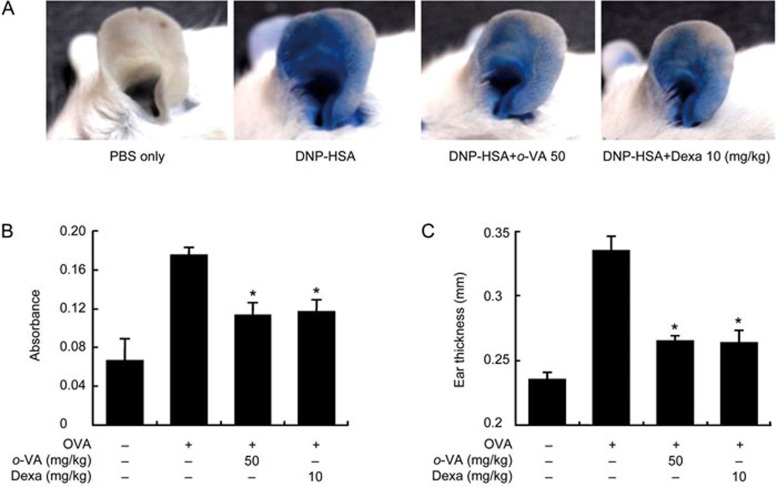
PCA is one of the most appropriate in vivo models of local allergic reaction8. After injection of 4% Evans blue mixed with antigen, the PCA reaction site indicated that vascular permeability was markedly increased, as indicated by the amount of Evans blue dye extravasating. When o-VA was orally administered to those mice, the vascular permeability of the ears was attenuated, as indicated by the extent of ear blue staining and intensity of Evans blue extraction of ears (Figure 2A, 2B). Ear thickness was also increased by antigen injection and decreased by o-VA (Figure 2C).
Figure 2.
Effect of o-VA on IgE-mediated passive cutaneous anaphylaxis. (A, B) The ear skin of mice (n=5/group) was sensitized with an intradermal injection of anti-DNP IgE (0.5 mg/site) for 48 h. o-VA was orally administered at doses of 2, 10, and 50 mg/kg body weight 1 h before the intravenous injection of a DNP-HSA and 4% Evans blue (1:1) mixture. Thirty minutes later, the thickness of both ears was measured, and the ears were collected to measure the dye pigmentation. The dye was extracted as described in the Materials and methods section and detected using a spectrophotometer. (C) Ear thickness was measured with a dial thickness gauge. Each data point represents the mean±SEM of three independent experiments. *P<0.05. Dexa: dexamethasone.
Effect of o-VA on the degranulation of mast cells
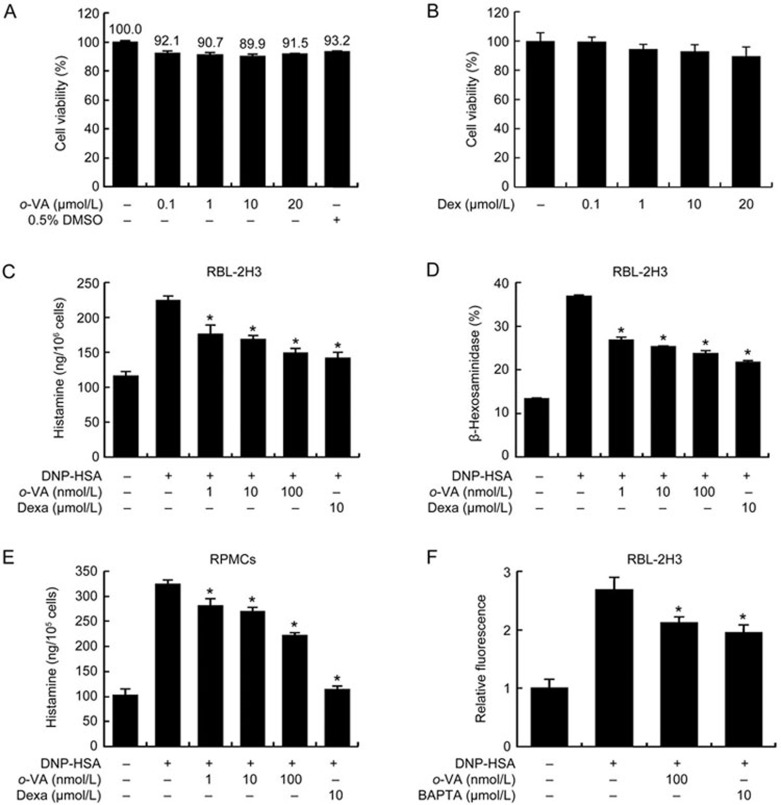
We first measured the effect of o-VA and dexamethasone (Dexa), a positive control, on the cell viability of RBL-2H3 cells using the MTT assay. RBL-2H3 cells were incubated for 24 h with various concentrations o-VA or Dexa. Pre-treatment with o-VA and Dexa at concentrations up to 20 μmol/L did not reduce the cell viability (Figure 3A, 3B). Next, we tested the ability of o-VA on the degranulation of mast cells. DNP-HSA-challenged RBL-2H3 cells released high levels of histamine and β-hexosaminidase. o-VA (1–100 nmol/L) considerably reduced the histamine and β-hexosaminidase release in DNP-HSA-challenged cells in a dose-dependent manner. Moreover, the highest dose of o-VA showed effects similar to a substantially lower (100 times) concentration of Dexa (Figure 3C, 3D). The inhibitory effect of o-VA on histamine release was confirmed in primary cultured mast cells, RPMCs (Figure 3E). To investigate the mechanisms by which o-VA reduces mast cell degranulation, we assayed intracellular calcium levels. It is known that calcium movement across the membranes of mast cells is important to histamine release. The suppression of calcium influx by anti-allergic drugs inhibits mast cell degranulation17. The inhibitory effect of o-VA on calcium influx was tested using the fluorescent indicator Fluo-3/AM. Intracellular calcium levels were elevated by DNP-HSA challenge but alleviated by o-VA pre-treatment (Figure 3F).
Figure 3.
Effects of o-VA on the degranulation of mast cells. (A, B) RBL-2H3 cells were pretreated with or without o-VA and Dexa, then incubated for 24 h. The absorbance intensity was detected using a spectrophotometer. (C, E) RBL-2H3 cells and RPMCs were pretreated with or without o-VA. Histamine levels were detected using a fluorescence plate reader. (D) β-Hexosaminidase levels were detected using a spectrophotometer. (F) RBL-2H3 cells were preincubated with Fluo-3/AM. Intracellular calcium was detected using a fluorescence plate reader. BAPTA, a calcium chelator, was used as a positive control. Each data point represents the mean±SEM of three independent experiments. *P<0.05. Dexa: dexamethasone.
Effect of o-VA on the expression and secretion of pro-inflam matory cytokines
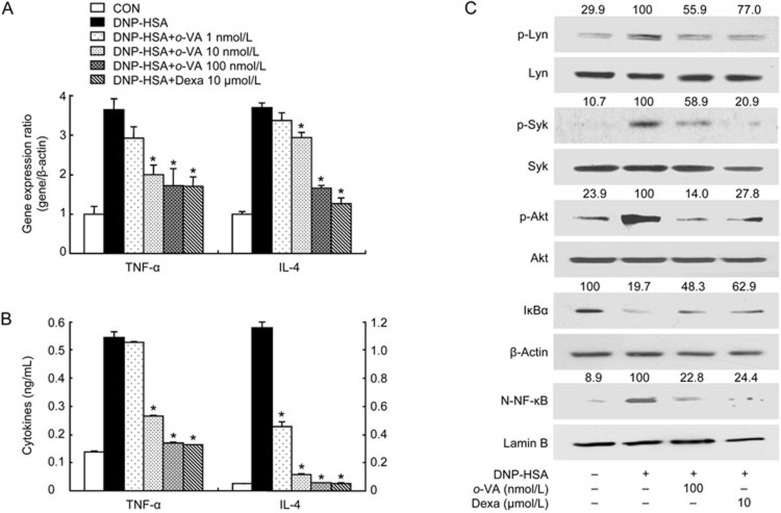
IgE molecules bind to FcεRI on the surface of mast cells. Then, antigen exposure cross-links the cell-bound IgE and secretes various pro-inflammatory cytokines, such as TNF-α and IL-44. We assessed the effect of o-VA on the expression of pro-inflammatory cytokines in RBL-2H3 cells using quantitative real-time PCR and ELISA. The gene expression of pro-inflammatory cytokines was increased after DNP-HSA challenge. However, pre-treatment with o-VA (1–100 nmol/L) suppressed the expression of pro-inflammatory cytokines. (Figure 4A). In addition, the increased secretion of TNF-α and IL-4 was dose-dependently decreased by pre-treatment with o-VA (Figure 4B).
Figure 4.
Effects of o-VA on the expression of pro-inflammatory cytokines and the activation of signaling proteins and NF-κB. RBL-2H3 cells were pretreated with or without o-VA. Extraction and analysis of mRNA/protein were performed as described in the Materials and methods section. (A) The gene expression of pro-inflammatory cytokines was determined with quantitative real-time PCR. (B) The secretion of pro-inflammatory cytokines was measured by ELISA. Each data point represents the mean±SEM of three independent experiments. (C) NF-κB translocation, IκBα degradation and the activation of signal molecules were assayed by Western blot (N-: nuclear, p-: phosphorylated). β-Actin and lamin B were used as a loading control. The band intensity was digitized and normalized to the relative ratio. The band is representative of three independent experiments. *P<0.05. Dexa: dexamethasone.
Effect of o-VA on the activation of signaling proteins in mast cells
To identify mechanisms by which o-VA inhibits pro-inflammatory cytokine production, we investigated the effect of o-VA on known intracellular signaling molecules. The aggregation of FcεRI by antigen leads to the activation of Src family protein tyrosine kinases such as Lyn and Syk. Consequently, activated Src family protein tyrosine kinases induce the phosphorylation of Akt, resulting in the translocation of NF-κB2,22. It is known that NF-κB activation is involved in the inflammatory response23. Accordingly, we measured the effect of o-VA on the activation of Lyn, Syk, Akt, IĸBα and the translocation of p65 NF-κB. Our results showed that activation of Lyn, Syk and Akt was markedly suppressed by pre-treatment of o-VA in DNP-HSA-challenged RBL-2H3 cells. In addition, o-VA hindered the DNP-HSA-induced degradation of IκBα and translocation of p65 NF-κB (Figure 4C).
Discussion
Type I hypersensitivity is induced by the release of allergic mediators and several pro-inflammatory cytokines and chemokines from activated mast cells24. After antigen exposure, mast cells are activated by the binding of antigen and the IgE-mediated cross-linking of the FcεRI complex. This event leads to the secretion of allergic mediators, which induce allergic reactions such as allergic rhinitis, atopic dermatitis, asthma and some food allergies25. Therefore, mast cells are a target for the development of drugs for allergic symptoms. In the present study, we demonstrated that o-VA suppressed mast cell-mediated allergic inflammation using in vivo and in vitro models.
Ovalbumin (OVA) is an abundant glycoprotein in egg white and one of the major allergens26. Sensitization with OVA enhances IgE production in the serum, after which re-exposure to OVA initiates an allergic response through the binding of antigen and IgE-receptor complexes on the surface of mast cells27,28. In particular, hypothermia, an allergic response to OVA challenge, is caused by increased serum histamine levels29. Therefore, the OVA-induced ASA model is an appropriate animal model for mast cell-mediated type I hypersensitivity20. The IgE-mediated PCA model is also another well-characterized animal model of allergic reaction. Local injection of anti-DNP IgE followed by an intravenous antigenic challenge induces local plasma extravasation and vascular permeability30. In both animal models, the increase in histamine release results in vasodilation, which eventually causes hypothermia and Evans blue pigmentation. In addition, the serum IgE level in the OVA-induced ASA model was increased after challenge with OVA. OVA stimulates the production of IgE by B cells, which requires the differentiation of naïve T cell into Th2 cells and is essential to mast cell activation4. It is known that increased IgE levels contribute to systemic anaphylaxis in mice20. Moreover, compound 48/80 is capable of inducing mast cell degranulation, which induces systemic anaphylactic shock8. In our results, these symptoms were reduced by the oral administration of o-VA. From these results, we suggest that o-VA suppresses the allergic reaction by inhibiting mast cell activation.
In this study, we used two types of mature mast cells to assess the anti-allergic inflammatory effect of o-VA. One was RBL-2H3 cells, one of the most popular mast cell lines used and the other was RPMCs, primary isolated mast cells from the peritoneal cavity. As previously mentioned, histamine causes immediate allergic responses, including vascular permeability, vasodilation, bronchoconstriction and hypothermia21. The mechanism underlying histamine release from mast cells is well understood. The binding of antigen to the IgE-FcεRI complex on mast cells leads to the phosphorylation of PLCγ, which catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate, resulting in the generation of inositol-1,4,5-trisphosphate (IP3) and diacylglycerol. IP3 binding to its receptor in the endoplasmic reticulum (ER) immediately releases calcium from the ER store. Finally, the extrusion of granules via fusion of the granule membrane releases histamine24,31. In our results, antigen-mediated histamine release was suppressed by o-VA treatment in both RBL-2H3 cells and RPMCs, and the intracellular calcium level was also decreased. Various reports have shown that the release of histamine is increased by intracellular calcium, an important secondary messenger5,32. Therefore, these results suggest that the inhibition of histamine release is associated with the o-VA-mediated regulation of intracellular calcium levels.
TNF-α and IL-4 have a critical biological function in allergic inflammation. In this study, we found that o-VA inhibited DNP-HSA-induced TNF-α and IL-4 expression. TNF-α promotes inflammation and adaptive immunity and stimulates immune cell maturation, migration and differentiation33. TNF-α is also involved in eosinophil survival, thereby contributing to chronic inflammation due to increased cytokine production34. IL-4 is an essential factor in the production of IgE in plasma B cells and leads to the production of allergic Th2 cells by stimulating the maturation of naïve T cells35. Thus, the suppressive effects of o-VA on the expression of TNF-α and IL-4 suggest that o-VA regulates the mast cell-mediated allergic inflammatory response.
The production of TNF-α and IL-4 is regulated by NF-κB, a key transcription factor that induces the expression of various genes involved in inflammatory responses23. In unstimulated cells, NF-κB is kept inactive by the binding of IκBα. After stimulation with antigen, phosphorylated IκBα is degraded, releasing NF-κB. Unbound-NF-κB translocates into the nucleus for transcriptional activity36. The nuclear translocation of NF-κB and degradation of IκBα by DNP-HSA treatment was significantly inhibited by o-VA. Our results suggest that inhibitory effects of o-VA on pro-inflammatory cytokines arise from the suppression of NF-κB nuclear translocation.
Mast cell activation is mediated by the crosslinking of FcεRI with IgE. The initiation of FcεRI signaling induces the phosphorylation of Src family kinases such as Lyn, Fyn, and Syk. After that, pLyn and pSyk phosphorylate various adaptor proteins and then induce mast cell activation. In particular, Syk kinase is an important protein in mast cell activation because it leads to the activation of various downstream signaling proteins such as PI3K, Akt, and MAPK, which cross-talks with NF-κB2,22. Therefore, the inhibition of signaling molecules might be a target for the discovery of anti-inflammatory drugs to regulate the mast cell-mediated allergic inflammatory response. A recent report showed that the allergic response is suppressed by the regulation of Syk and Akt through Lyn kinase activity37. This report supports the idea that o-VA also can act as an inhibitor for the signaling pathway responsible FcεRI crosslinking-mediated mast cell activation.
In conclusion, o-VA administration prevented systemic and cutaneous allergic reaction in animal models. o-VA reduced the degranulation of mast cells via regulating intracellular calcium levels. The expression of pro-inflammatory cytokines was suppressed by o-VA via the suppression of NF-κB, presumably through the regulation of the signaling pathway downstream of FcεRI. A recent study compared the anti-degranulation activity of vanillic acid and its derivatives in DNP-HSA-challenged RBL-2H3 cells38. It showed no effect of vanillic acid, and only 1000 μmol/L of methyl vanillate possessed a positive effect (no activity up to 300 μmol/L). This is distinctly different from our results showing a reduction in degranulation starting at 1 nmol/L of o-VA in similar conditions. However, the anti-allergic activities of o-VA in vivo are lower than those in vitro. The low bioavailability of drugs is associated with the poor aqueous solubility of drugs39. Vanillic acid does not reach the maximum serum concentration within 1 h40. This implies that the absorption and/or bioavailability of vanillic acid into the serum are low. From the differences in o-VA activity in vivo and in vitro, we speculate that o-VA may have low bioavailability similar to that observed for vanillic acid. Nevertheless, our study showed that o-VA could be a potential therapeutic candidate for mast cell-mediated allergic disorders.
Abbreviations
o-VA, o-vanillic acid; OVA, ovalbumin; PCA, passive cutaneous anaphylaxis; ASA, active systemic anaphylaxis; DNP-HSA, dinitrophenyl-human serum albumin.
Author contribution
All authors participated in the design, interpretation of the studies and analysis of the data, and review of the manuscript. Yeon-Yong KIM, In-Gyu JE, Min Jong KIM, and Byeong-Cheol KANG performed the major experiments and wrote the manuscript; Young-Ae CHOI, Moon-Chang BAEK, Byung-Heon LEE, and Jin Kyeong CHOI made substantial contributions to the conception and design of the study; Soyoung LEE, Seung-Bin YOON, and Sang-Rae LEE analyzed the data; and Tae-Yong SHIN, Dongwoo KHANG, and Sang-Hyun KIM supervised the research and co-wrote the manuscript.
Acknowledgments
This work was supported by a National Research Foundation of Korea Grant funded by the Korean Government (2014R1A5A2009242, 2012M3A9B6055416, and 2016R1A2B4008513), the KRIBB Research Initiative Program (KGM4611613), and the High Value-added Food Technology Development Program, Ministry of Agriculture, Food and Rural Affairs.
References
- Jutel M, Akdis CA. Immunological mechanisms of allergen-specific immunotherapy. Allergy 2011; 66: 725–32. [DOI] [PubMed] [Google Scholar]
- Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol 2008; 9: 1215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na H, Cho M, Chung Y. Regulation of Th2 cell immunity by dendritic cells. Immune Netw 2016; 16: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Tsai M. Ige and mast cells in allergic disease. Nat Med 2012; 18: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Je IG, Choi HG, Kim HH, Lee S, Choi JK, Kim SW, et al. Inhibitory effect of 1,2,4,5-tetramethoxybenzene on mast cell-mediated allergic inflammation through suppression of ikappab kinase complex. Toxicol Appl Pharmacol 2015; 287: 119–27. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature 2008; 454: 445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin K. The role of mast cells in allergic inflammation. Respir Med 2012; 106: 9–14. [DOI] [PubMed] [Google Scholar]
- Kim SH, Jun CD, Suk K, Choi BJ, Lim H, Park S, et al. Gallic acid inhibits histamine release and pro-inflammatory cytokine production in mast cells. Toxicol Sci 2006; 91: 123–31. [DOI] [PubMed] [Google Scholar]
- Choi JK, Oh HM, Lee S, Kwon TK, Shin TY, Rho MC, et al. Salvia plebeia suppresses atopic dermatitis-like skin lesions. Am J Chin Med 2014; 42: 967–85. [DOI] [PubMed] [Google Scholar]
- Longui CA. Glucocorticoid therapy: Minimizing side effects. J Pediatr 2007; 83: S163–77. [DOI] [PubMed] [Google Scholar]
- Bellik Y, Hammoudi SM, Abdellah F, Iguer-Ouada M, Boukraa L. Phytochemicals to prevent inflammation and allergy. Recent Pat Inflamm Allergy Drug Discov 2012; 6: 147–58. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 2016; 79: 629–61. [DOI] [PubMed] [Google Scholar]
- Kim SH, Lee S, Kim IK, Kwon TK, Moon JY, Park WH, et al. Suppression of mast cell-mediated allergic reaction by amomum xanthiodes. Food Chem Toxicol 2007; 45: 2138–44. [DOI] [PubMed] [Google Scholar]
- Itoh A, Isoda K, Kondoh M, Kawase M, Kobayashi M, Tamesada M, et al. Hepatoprotective effect of syringic acid and vanillic acid on concanavalin a-induced liver injury. Biol Pharm Bull 2009; 32: 1215–9. [DOI] [PubMed] [Google Scholar]
- Kumar S, Prahalathan P, Raja B. Antihypertensive and antioxidant potential of vanillic acid, a phenolic compound in L-NAME-induced hypertensive rats: A dose-dependence study. Redox Rep 2011; 16: 208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae Y, Lee S, Kim SH. Chrysin suppresses mast cell-mediated allergic inflammation: Involvement of calcium, caspase-1 and nuclear factor-kappab. Toxicol Appl Pharmacol 2011; 254: 56–64. [DOI] [PubMed] [Google Scholar]
- Kim HH, Park SB, Lee S, Kwon TK, Shin TY, Park PH, et al. Inhibitory effect of putranjivain a on allergic inflammation through suppression of mast cell activation. Toxicol Appl Pharmacol 2014; 274: 455–61. [DOI] [PubMed] [Google Scholar]
- Singh TS, Lee S, Kim HH, Choi JK, Kim SH. Perfluorooctanoic acid induces mast cell-mediated allergic inflammation by the release of histamine and inflammatory mediators. Toxicol Lett 2012; 210: 64–70. [DOI] [PubMed] [Google Scholar]
- Choi JK, Oh HM, Lee S, Park JW, Khang D, Lee SW, et al. Oleanolic acid acetate inhibits atopic dermatitis and allergic contact dermatitis in a murine model. Toxicol Appl Pharmacol 2013; 269: 72–80. [DOI] [PubMed] [Google Scholar]
- Finkelman FD, Rothenberg ME, Brandt EB, Morris SC, Strait RT. Molecular mechanisms of anaphylaxis: Lessons from studies with murine models. J Allergy Clin Immunol 2005; 115: 449–57. [DOI] [PubMed] [Google Scholar]
- Kawakami T, Kashiwakura J, Kawakami Y. Histamine-releasing factor and immunoglobulins in asthma and allergy. Allergy Asthma Immunol Res 2014; 6: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KC, Huang DY, Huang DW, Tzeng SJ, Lin WW. Inhibition of AMPK through lyn-syk-akt enhances fcepsilonri signal pathways for allergic response. J Mol Med 2016; 94: 183–94. [DOI] [PubMed] [Google Scholar]
- Caamano J, Hunter CA. Nf-kappab family of transcription factors: Central regulators of innate and adaptive immune functions. Clin Microbiol Rev 2002; 15: 414–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol 2005; 23: 749–86. [DOI] [PubMed] [Google Scholar]
- Galli SJ. Mast cells and basophils. Curr Opin Hematol 2000; 7: 32–9. [DOI] [PubMed] [Google Scholar]
- Pablos-Tanarro A, Lopez-Exposito I, Lozano-Ojalvo D, Lopez-Fandino R, Molina E. Antibody production, anaphylactic signs, and T-cell responses induced by oral sensitization with ovalbumin in BALB/c and C3H/HeOuJ mice. Allergy Asthma Immunol Res 2016; 8: 239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang J, Zhao R, Jin J, Yu Y, Li W, et al. Topical application of IL-28a attenuates allergic conjunctivitis in an ovalbumin-induced mouse model. Invest Ophthalmol Vis Sci 2016; 57: 604–10. [DOI] [PubMed] [Google Scholar]
- Lim HB, Kim SH. Inhallation of e-cigarette cartridge solution aggravates allergen-induced airway inflammation and hyper-responsiveness in mice. Toxicol Res 2014; 30: 13–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Je IG, Kim DS, Kim SW, Lee S, Lee HS, Park EK, et al. Tyrosol suppresses allergic inflammation by inhibiting the activation of phosphoinositide 3-kinase in mast cells. PLoS One 2015; 10: e0129829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Ng DS, Chan TK, Guan SP, Ho WE, Koh AH, et al. Anti-allergic action of anti-malarial drug artesunate in experimental mast cell-mediated anaphylactic models. Allergy 2013; 68: 195–203. [DOI] [PubMed] [Google Scholar]
- Guo Z, Turner C, Castle D. Relocation of the t-snare snap-23 from lamellipodia-like cell surface projections regulates compound exocytosis in mast cells. Cell 1998; 94: 537–48. [DOI] [PubMed] [Google Scholar]
- Eisenhut M, Wallace H. Ion channels in inflammation. Pflugers Arch 2011; 461: 401–21. [DOI] [PubMed] [Google Scholar]
- Hart PH. Regulation of the inflammatory response in asthma by mast cell products. Immunol Cell Biol 2001; 79: 149–53. [DOI] [PubMed] [Google Scholar]
- Walczak H. TNF and ubiquitin at the crossroads of gene activation, cell death, inflammation, and cancer. Immunol Rev 2011; 244: 9–28. [DOI] [PubMed] [Google Scholar]
- Chen L, Grabowski KA, Xin JP, Coleman J, Huang Z, Espiritu B, et al. IL-4 induces differentiation and expansion of Th2 cytokine-producing eosinophils. J Immunol 2004; 172: 2059–66. [DOI] [PubMed] [Google Scholar]
- Pasparakis M. Role of NF-kappaB in epithelial biology. Immunol Rev 2012; 246: 346–58. [DOI] [PubMed] [Google Scholar]
- Lee SH, Shin HJ, Kim DY, Shim DW, Kim TJ, Ye SK, et al. Streptochlorin suppresses allergic dermatitis and mast cell activation via regulation of Lyn/Fyn and Syk signaling pathways in cellular and mouse models. PLoS One 2013; 8: e74194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimata N, Ito H, Tai A. Structure–activity relationships of vanillic acid ester analogs in inhibitory effect of antigen-mediated degranulation in rat basophilic leukemia RBL-2H3 cells. Bioorg Med Chem Lett 2016; 26: 3533–6. [DOI] [PubMed] [Google Scholar]
- Savjani KT, Gajjar AK, Savjani JK. Drug solubility: Importance and enhancement techniques. ISRN Pharm 2012; 2012: 195727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ferrars RM, Czank C, Zhang Q, Botting NP, Kroon PA, Cassidy A, et al. The pharmacokinetics of anthocyanins and their metabolites in humans. Br J Pharmacol 2014; 171: 3268–82. [DOI] [PMC free article] [PubMed] [Google Scholar]