Abstract
Exposure of 3T3/A31 cells to serum-free medium, one type of apoptotic stimulus, causes a rapid increase in the sphingosine (Sph) level, which initiates a series of processes: (i) activation of caspase 3 through an enhanced “cascade” of caspases, (ii) release of the C-terminal-half kinase domain of PKCδ (PKCδ KD) by caspase 3, and (iii) activation of Sph-dependent kinase 1 (SDK1), which was previously identified as PKCδ KD. The activation of caspase 3 and release of PKCδ KD are inhibited strongly by the incubation of cells with the ceramidase inhibitor d-erythro-2-tetradecanoylamino-1-phenyl-1-propanol and, to a much lesser extent, by l-cycloserine, an inhibitor of de novo ceramide synthesis. Exogenous addition of Sph or N,N-dimethyl-Sph to U937 cells causes caspase 3 activation and release of PKCδ KD (SDK1), leading to apoptosis. The Sph-induced apoptotic process associated with activation of caspase 3 and release of PKCδ KD (SDK1) may promote the proapoptotic effect of BAD or BAX through an increase of phosphorylated 14-3-3. In addition, Sph induces apoptosis through a separate process: the blocking of “survival signal” through the Akt kinase pathway induced by α3β1-mediated cell adhesion to laminin 10/11 in extracellular matrix. We hereby propose a unified concept of Sph-dependent apoptosis based on these multiple mechanisms operating in concert.
Sphingosine (Sph; d-erythro-Sph) and its N,N-dimethyl derivative (DMS) were originally found to inhibit PKC (1–3) as counterparts of diacylglycerol, which promotes conventional PKC (α and β) (4). The current trend in the study of sphingolipids as modulators of cellular function focuses on ceramide (Cer) (for reviews, see refs. 5 and 6) and Sph-1-phosphate (7–10), based on their promoting of or inhibitory effect on signal transduction defining various cellular phenotypes.
During our initial studies on Sph-dependent protein kinases (SDKs) (11, 12), we found a kinase, termed “SDK1,” that, in the presence of Sph or DMS, phosphorylates a specific Ser at the 14-3-3 dimer interface, i.e., Ser-60, Ser-59, or Ser-58 of 14-3-3β, -η, or -ζ, respectively (13). This phosphorylation interferes with 14-3-3 dimer formation and increases phosphorylated monomer (14), thereby preventing dimer-dependent inactivation of proapoptotic BAD or BAX at the mitochondrial membrane and consequent induction of apoptosis (15–17). We recently showed that SDK1 has a molecular mass of ≈40 kDa, is released from PKCδ by caspase 3, and is the C-terminal-half kinase domain (KD) of PKCδ (PKCδ KD) (18). PKC activity of full-length PKCδ was not activated but rather was inhibited by Sph or DMS (18), similar to conventional PKCα or PKCβ (3).
Since Cer was found to be an apoptosis inducer (19), numerous studies along this line have been made (for reviews, see refs. 5, 6, and 20). On the other hand, many recent studies suggest that PKCδ activation and translocation to the mitochondrial membrane are involved in the apoptotic process; a definitive mechanism is still under debate (ref. 21 and references therein). PKCδ has been implicated as a proapoptotic kinase (22), whereas PKCα and PKCε inhibit apoptosis by phosphorylating BCL-2 and enhancing its expression (23).
Apoptotic stimulation induces a two-phase increase in the endogenous Sph level, which in turn opens up multiple mechanisms, all linked to the apoptotic process through the activation of the caspase cascade and the release of PKCδ KD. Alternatively, exogenous addition of Sph or DMS also activates caspase 3 and release of PKCδ KD, leading to apoptosis.
Separately from the above processes, Sph affects integrin-dependent cell adhesion through interaction with the extracellular matrix and blocks cell survival signaling, particularly the Akt pathway. Many cells depend on adhesion to the extracellular matrix for their continued survival, and activation of Akt through phosphorylation at Ser-473 and Thr-308 is involved in survival signaling through certain types of integrins.
Thus, multiple mechanisms act in concert to promote apoptosis. We hereby propose a unified concept of the Sph-dependent apoptotic cycle based on such mechanisms operating in vivo.
Materials and Methods
Cells and Reagents. Mouse BALB/c 3T3 clone A31 (hereafter termed “A31”) cells, human monocytic leukemia U937 cells, human promyelocytic leukemia HL60 cells, and simian virus 40-transformed human lung fibroblast VA13 cells were all obtained from the American Type Culture Collection (ATCC). Each type of cell was cultured in a different medium under different conditions, as described in the respective figure legends. Sph, DMS, Cer, other lipids, l-cycloserine (L-CS), and d-erythro-2-tetradecanoylamino-1-phenyl-1-propanol (D-MAPP) were obtained from Matreya (Pleasant Gap, PA). All other reagents were obtained from Sigma unless described otherwise.
A31 Cell Serum Starvation to Induce Apoptosis and Inhibition of Apoptosis by Treatment with Ceramidase Inhibitor and L-CS. A31 cells (5 × 105) in a culture dish (15-cm diameter) were grown in complete DMEM (see Fig. 1 legend) until confluence. For serum starvation, cells were washed three times with serum-free DMEM (with 0.1% BSA and 10 mM Hepes) and incubated in this medium for the periods indicated in the Fig. 1 legend. To observe inhibition of apoptosis, cells were incubated in serum-free DMEM containing D-MAPP plus L-CS at various concentrations (see Fig. 1 legend). The rationale for this choice of reagents is described in Results.
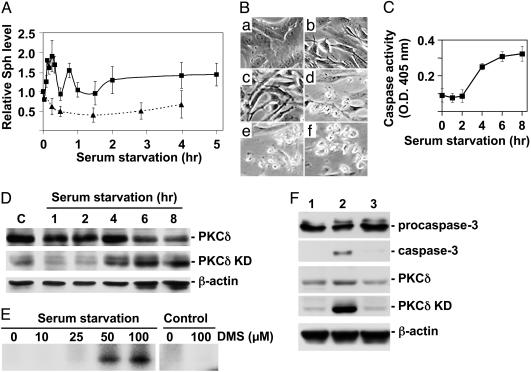
Fig. 1.
Apoptotic process of A31 cells exposed to serum-free medium. Confluent A31 cells grown in DMEM containing 10% newborn calf serum (complete DMEM) were incubated in serum-free DMEM for the indicated times, and levels of cellular Sph, caspase activity, PKCδ KD, and SDK1 activity were measured. For some experiments, to observe the effect of Sph synthesis inhibitors, A31 cells were incubated overnight in complete DMEM containing 1 mM L-CS and 100 μM D-MAPP and then for various times in serum-free DMEM with 0.5 mM L-CS and 20 μM D-MAPP. (A) Changes in the cellular Sph level in A31 cells during serum starvation (0–5 h; solid line) were determined as N-phthalyl derivative separated by HPLC with a UV monitor. Sph levels were normalized with total phospholipid levels and shown as relative value to time 0. Note the sharp increases with maxima at 15 min and 50 min and the slow increase after 2 h. The dotted line indicates Sph levels in serum-starved A31 cells treated with D-MAPP and L-CS as described above. A representative of three experiments with similar results is shown. (B) Morphology change during serum starvation. Confluent A31 cells grown in complete DMEM were incubated with this medium (a) or in serum-free DMEM for 1 h (b), 2 h (c), 4 h (d), 6 h (e), or 8 h (f). (C) Caspase activity change in serum-starved cells, determined by using chromogenic substrate Ac-Asp-Gln-Val-Asp-pNA. (D) Levels of PKCδ and PKCδ KD in serum-starved cells were determined by Western blotting. β-Actin was blotted as load control. C, control. (E) Close association of PKCδ KD release with SDK1 activity in A31 cells induced under serum starvation. A31 cells were serum-starved for 7 h or not (Control). Protein extracts from these cells were PKCδ-depleted as described in Materials and Methods and used as enzyme source. SDK1 activity was measured by using 14-3-3β as substrate. (F) Inhibitory effect of L-CS and D-MAPP on release of PKCδ KD and caspase 3 in A31 cells upon exposure to serum-free conditions. A31 cells were serum-starved (lane 2) or not (lane 1, control) or serum-starved and treated with L-CS and D-MAPP (lane 3) for 6 h as described above. Caspase 3 and PKCδ KD were determined by Western blotting. β-Actin was blotted as load control.
Apoptosis Induced with Sph and Sph Derivatives. As an example, for U937 cells, DMS or Sph was delivered in 50% ethanol to 2 × 107 cells in 40 ml of complete RPMI medium 1640 (final concentration, 20 μM), and cells were incubated at 37°C for 6 h. Cells were washed two times with PBS, 2 × 106 cells were fixed with 2% paraformaldehyde/PBS, and the apoptosis level was measured by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) (Roche), by following the manufacturer's instructions. The remaining cells were used for analysis of PKCδ fragmentation and of caspase 3 activation, by Western blotting, as described below.
Determination of Caspase Activity and Western Blot Analysis of Released Caspase 3, PKCδ, and PKCδ KD. For determination of caspase activity, serum-starved A31 cell lysates were prepared as described previously (24). Briefly, cells were extracted in 50 mM Tris (pH 7.5), 0.03% Nonidet P-40, and 1 mM DTT, kept on ice for 30 min, homogenized by five passages through a 25-gauge needle, and centrifuged at 600 × g to collect supernatant. Caspase activity was determined by using 12.4 μg of protein, following the manufacturer's protocol for caspase 3 substrate (Upstate Biotechnology, Charlottesville, VA).
Western blot analysis was performed based on protein concentration, determined by using a Micro BCA kit (Pierce Biotech, Rockford, IL). Released caspase 3 was determined as described in the protocol for anti-caspase 3 antibody from Cell Signaling Technology (Beverly, MA). For full-length PKCδ and PKCδ KD analysis, cell lysates were prepared as described previously (25). SDS/PAGE and Western blot analysis (18) were performed by using anti-PKCδ (C-17 or C-20, Santa Cruz Biotechnology), anti-caspase 3 (Cell Signaling Technology), and anti-β-actin (Sigma) antibodies.
SDK1 Activity Analysis. A31 cell extract was prepared as described previously (25). Extract with 1.5 mg (320 μl) of protein was incubated with 9 μg (36 μl) of anti-N-terminal PKCδ (BD Biosciences PharMingen) and 90 μl of protein A/G agarose beads (Santa Cruz Biotechnology) overnight at 4°C. After centrifugation at 600 × g for 5 min, SDK1 activity was determined as described (18) by using 4 μl (2 μg) of supernatant and 1 μg of recombinant 14-3-3β (kindly provided by Haian Fu, Emory University, Atlanta, and purchased from Biomol, Plymouth Meeting, PA).
Sph and Phospholipid Measurement. Sph levels were determined as described previously (26, 27) with some modifications. A31 cells were washed two times with cold PBS, extracted three times by sonication with 1 ml of chloroform/methanol (C/M) (1:2, vol/vol). The extracts were combined, dried under N2, and dissolved in 1.5 ml of C/M (1:2, vol/vol). Of this extract, 300 μl was saved for phospholipid analysis. The remaining 1.2-ml extract was base-hydrolyzed (27) after the addition of 100 pmol of C20-sphinganine, and Sph was determined as an o-phthalaldehyde derivative (26). The HPLC system was composed of a Varian ProStar 230 solvent delivery module, Microsorb-MV C18 column (250 × 4.6 mm; Varian), guard C18 column (Alltech Associates), and Varian ProStar 306 fluorescence detector.
Phospholipid levels were determined on an HPTLC plate (Merck), stained with 1.3% molybdenum oxide in 4.2 M sulfuric acid, scanned, and quantified by scion image (Scion, Frederick, MD) analysis, by using phosphatidylserine as standard.
Inhibition of Integrin-Induced Survival Signal. For satisfactory determination of Sph/DMS-induced apoptosis based on inhibition of integrin-mediated survival signal, two conditions must be met: (i) cells must show a high level of integrin signaling and (ii) background signal level must be low. Therefore, we used VA13 cells showing high α3β1-induced Akt signaling, with background signal level lowered by preincubation in serum-free medium. These cells did not undergo apoptosis even when exposed to serum-free medium, in contrast to A31 cells.
When VA13 cells reached ≈95% confluence, they were cultured in serum-free MEM overnight, detached with trypsin/EDTA, added with soybean trypsin inhibitor, centrifuged, and rotary-incubated for 1.5 h at 37°C. Cells (1 × 106) were mixed with 5, 10, or 20 μM Sph, DMS, N-acetyl-Sph (C2-Cer), Sph-1-phosphate, or lysophosphatidic acid and placed in a culture dish (60-mm diameter) precoated with 1 μg/ml laminin (LN) 10/11 (Chemicon) or with 2 μg/ml fibronectin (FN) (Sigma), in PBS. For Akt phosphorylation analysis, cells were incubated for 1 h at 37°C, harvested with ice-cold PBS containing 0.5 mM sodium vanadate, centrifuged, and homogenized with RIPA buffer (150 mM NaCl/5 mM EDTA/25 mM Tris·HCl, pH 7.5/1% Triton X-100/0.5% sodium deoxycholate/0.1% SDS/5 mM pyrophosphate tetrasodium/50 mM NaF) containing aprotinin and PMSF. Ten micrograms of protein was loaded per well and analyzed by Western blotting with anti-phospho-Akt (Ser-473) (Cell Signaling Technology), anti-Akt (Cell Signaling Technology), and anti-actin (Sigma) antibodies. Apoptosis levels were measured after 15 h of incubation with Sph in LN- or FN-coated plates by using the TUNEL kit as described above.
Results
Apoptotic Stimulation of Cells Causes Two-Phase Increase of Sph Level, Followed by Activation of Caspase 3 and Release of PKCδ KD with SDK1 Activity. Exposure of A31 cells to serum-free medium caused a two-phase increase in the Sph level: a rapid, sharp increase during the first 10–50 min followed by a slow, gradual increase during the next 1–2 h (Fig. 1A). Incubation of cells with D-MAPP (which inhibits ceramidase) plus L-CS (which inhibits de novo Cer synthesis) abolished the change in the cellular Sph level induced by serum starvation (Fig. 1A, dotted line). Comparative effects of D-MAPP, L-CS, and fumonisin are described below.
Morphological changes characteristic of apoptosis were observed during a period of 2–6 h after the increase in the cellular Sph level and were essentially completed by 8 h (Fig. 1B). During this period of serum starvation, both caspase activity and PKCδ KD increased greatly, particularly after 4 h (Fig. 1 C and D), whereas full-length PKCδ decreased slightly (Fig. 1D).
Serum-starved A31 cells, which undergo apoptosis with release of PKCδ KD, displayed DMS-induced phosphorylation of 14-3-3, i.e., SDK1 activity (Fig. 1E).
Caspase 3 activation and release of PKCδ KD in A31 cells, both induced by serum-free medium, are due to the increase of Sph, because preincubation of cells with D-MAPP, with or without L-CS, completely eliminated release of caspase 3 and PKCδ KD (Fig. 1F, lane 3). The activation and release of caspase 3 by Sph are due to conversion of procaspase 3 to caspase 3 (Fig. 1F, lane 2).
We compared inhibitory effects of D-MAPP, L-CS, and fumonisin B1 on apoptosis and PKCδ KD release induced by serum starvation or by exogenous addition of Sph/DMS. D-MAPP had the strongest effect (80–90% inhibition). L-CS had a much smaller effect (≈10% inhibition), and fumonisin B1 had no clear effect (data not shown). Therefore, a mixture of D-MAPP and L-CS was used for inhibition experiments.
Exogenous Addition of Sph/DMS Induces PKCδ KD Release, Presumably Through Activation of Caspase 3, Leading to Apoptosis. An apoptotic process similar to that of A31 cells under the serum-free condition can be caused by exogenous addition of Sph, DMS, or C2-Cer, because these sphingolipids are readily incorporated into cells. Although the mechanism causing a high endogenous Sph level under serum-free conditions differs from the enhanced uptake of Sph or DMS after exogenous addition, both processes activate caspase 3 and induce PKCδ KD. Incubation of U937 cells with 20 μM Sph caused release of caspase 3 from procaspase 3 and increase of PKCδ KD, leading to apoptosis (Fig. 2 B and C).
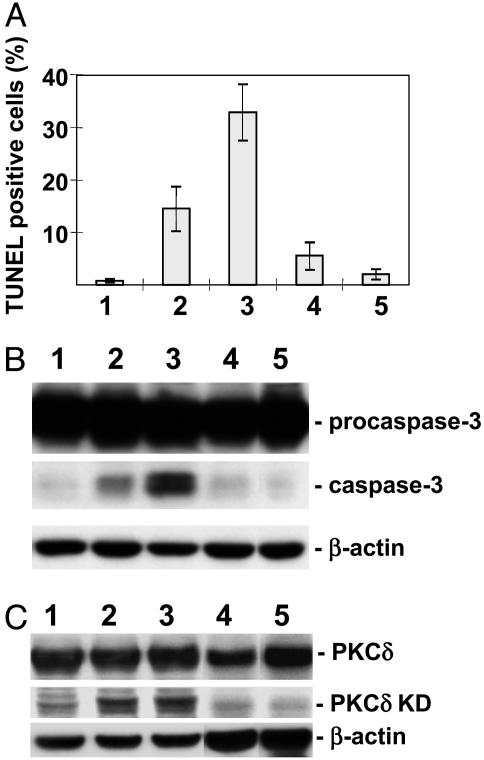
Fig. 2.
Apoptotic effect of Sph, DMS, dihydro-Sph, and C2-Cer on human monocytic leukemia U937 cells, in relation to PKCδ KD and caspase 3 expression. U937 cells grown in RPMI medium 1640 supplemented with 10% FCS were suspended at a concentration of 5 × 105 cells per ml of this medium and incubated with 20 μM Sph, DMS, dihydro-Sph, or C2-Cer. After 6 h, some of the cells were washed with PBS and fixed with 2% paraformaldehyde, and apoptosis was assessed by TUNEL (see Materials and Methods). The remaining cells were used to prepare cell lysate for detection of PKCδ KD and caspase 3 by Western blotting. β-Actin was blotted as load control. Lanes: 1, vehicle control; 2, DMS; 3, Sph; 4, dihydro-d-erythro-Sph; 5, C2-Cer. (A) Degree of apoptosis. (B) Activation of caspase 3. (C) Release of PKCδ KD.
In this apoptotic process, the effect of Sph was stronger than that of DMS. Dihydro-Sph (sphinganine) had a very weak effect, and C2-Cer had no effect (Fig. 2 A). Likewise, release of caspase 3 from procaspase 3 was strongest for Sph, followed by DMS, and dihydro-Sph and C2-Cer had no effect (Fig. 2B). A similar trend was observed for PKCδ KD release (Fig. 2C). Apoptosis of U937 or HL60 cells induced by 10 or 20 μM Sph or DMS was inhibited by 10 μM rottlerin (data not shown).
These results indicate that simultaneous activation of caspase 3 and release of PKCδ KD is an essential step for apoptosis, regardless of the apoptotic signal given (serum-free condition or exogenous Sph/DMS addition).
Inhibition of Integrin-Induced Survival Signal by Sph/DMS. Cell adhesion to LN5 or LN10/11 mediated by integrin α3β1 induces phosphatidylinositol 3-kinase (PI3K) or Akt signaling for cell survival. Similarly, α5β1-mediated adhesion to FN activates extracellular signal-regulated kinase (ERK) signaling for cell survival. The effect of Akt is stronger than that of ERK (28). VA13 cells, which express both α3β1 and α5β1, adhere well to LN10/11 and to FN, and this adhesion induces strong Akt phosphorylation. VA13 cells adhered to a LN10/11-coated plate started cell spreading (Fig. 3A1) and showed no apoptosis (Fig. 3B1). In the presence of 20 μM Sph, cell morphology became clearly apoptotic at 1 h (Fig. 3A2), whereby Akt phosphorylation (P-Akt) was greatly inhibited (Fig. 3C, lane 2 vs. lane 1), although protein levels of Akt and actin were similar in control and Sph-treated cells (Fig. 3C Middle and Bottom). Cells adhered to LN in the presence of Sph showed increasing apoptosis with time: 30–50% apoptosis after 6–10 h and >80% apoptosis after 15 h (Fig. 3B2).
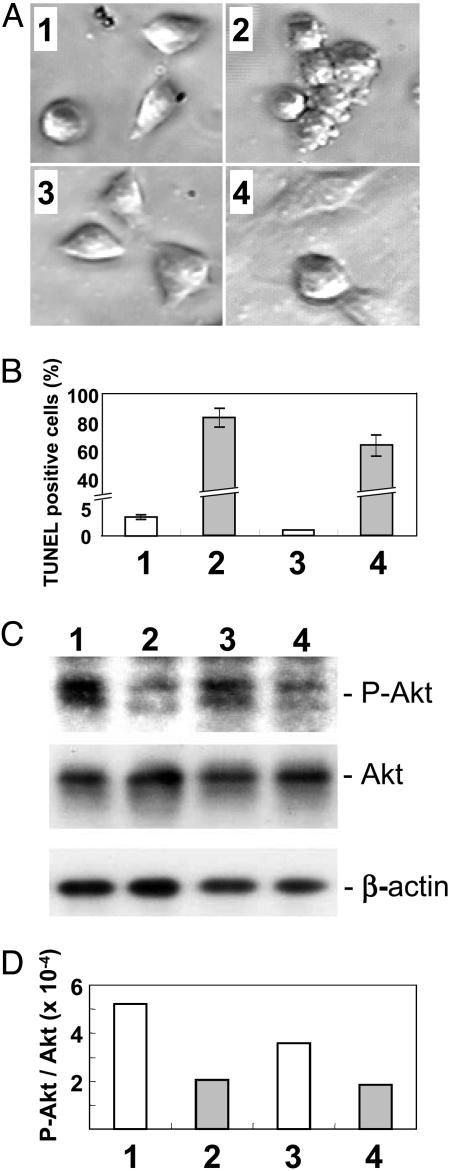
Fig. 3.
Effect of Sph on phospho-Akt (P-Akt) survival signal upon interaction of VA13 cells on LN10/11- or FN-coated plates. Simian virus 40-transformed human fibroblast VA13 cells, grown in MEM containing 10% FCS, were serum-starved, detached from tissue culture plate by trypsin/EDTA, and treated with soybean trypsin inhibitor. Cells were centrifuged, suspended in serum-free MEM (5 × 105 cells per ml), and incubated in a rotator for 1.5 h at 37°C. From this cell suspension, aliquots of 1 × 106 cells were taken, mixed with 20 μM Sph (with DMSO as control), and placed in a 6-cm dish precoated with LN10/11 (1 μg/ml PBS) or FN (2 μg/ml PBS). (A, C, and D).Cells were incubated for 1 h and subjected to determination of adhesion and signaling. For signaling assay, cells were harvested, homogenized with RIPA buffer, analyzed by Western blotting for phospho-Akt, Akt, and actin levels, and quantitated by scion image analysis. Apoptosis was quantitated by the TUNEL method at 1, 4, or 5 and 15 h; the 15-h value is shown in B.(A1) Control VA13 cells on LN-coated plates. (A2) Sph-treated VA13 cells on LN-coated plates. (A3) Control VA13 cells on FN-coated plates. (A4) Sph-treated VA13 cells on FN-coated plates. (B) Degree of apoptosis, expressed as TUNEL-positive percent of the population, in cells as in A was measured as described in Materials and Methods, at 15 h. The time course increase of apoptosis (see text) is not shown. (C) Western blot pattern of phospho-Akt, Akt, and actin of VA13 cells as in A.(D) Densitometry of phospho-Akt, Akt, or actin band by scion image analysis. The apoptosisinducing effect of DMS, C2-Cer, Sph-1-phosphate, and lysophosphatidic acid on VA13 cells adhered to LN10/11 or FN was much smaller than that of Sph (see Results).
Cells adhered to an FN-coated plate showed normal spreading (Fig. 3A3) and no apoptosis (Fig. 3B3). Cells adhered to an FN-coated plate in the presence of 20 μM Sph appeared to be nonapoptotic after 1 h of incubation (Fig. 3A4) but thereafter also showed increasing apoptosis with time, finally showing ≈60% apoptosis after 15 h (Fig. 3B4). The degree of Akt phosphorylation inhibition in the presence of Sph was lower for cells adhered to FN (Fig. 3C, lane 4 vs. lane 3) than for those adhered to LN (see above).
The degree of Sph-dependent inhibition of Akt phosphorylation was indicated by scion image analysis of densitometric pattern (Fig. 3D).
The apoptosis-inducing effect of DMS on VA13 cells adhered to LN10/11 or FN was much smaller (8.3% and 6.5% of cells were TUNEL-positive, respectively) than the effect of Sph (≈80% and ≈60%, respectively). The effects of C2-Cer, Sph-1-phosphate, and lysophosphatidic acid were even less (<5% of cells were TUNEL-positive in each case) (data not shown).
Discussion
A number of recent studies on the mechanism of apoptotic signaling indicate that Cer and PKCδ cooperate to initiate apoptosis, particularly cytochrome c release at the mitochondrial membrane. A definitive mechanism has not been established (21). Cer may also activate PKCδ and translocate it to the Golgi network as an initial step in the apoptotic process (29).
We previously observed Sph/DMS-dependent activation of PKCδ and release of KD therefrom by caspase 3. These processes appear to occur simultaneously, and the PKCδ KD released was identified as the previously known SDK1, which phosphorylates Ser of 14-3-3 at the dimer interface. The processes are considered as an initial step leading to apoptosis, in which Cer or Sph-1-phosphate is not involved. Other target molecule(s) of PKCδ KD may exist that have not been characterized.
Sph has been suggested to be a “second messenger” because its low level greatly increases upon stimulation of cells. For example, platelet-derived growth factor and insulin-like growth factor cause activation of ceramidase that converts Cer to Sph and fatty acid (30, 31). Similarly, treatment of HL60 cells with phorbol ester increases the Sph level 3-fold, whereby the cells differentiate into macrophages. Treatment of the same cells with exogenous Sph causes apoptosis (32).
Our results indicate that Sph/DMS modulate functions of multiple signaling molecules, i.e., PKCδ, caspase 3, PKCδ KD (SDK1), α3β1 integrin, and BCL-2, presumably in concert. A key initial mechanism is enhanced Sph level upon exposure of cells to apoptotic stimuli. Importantly, in these cells, PKCδ activation, caspase 3 activation, KD release, and SDK1 activity occurred simultaneously, were affected by enhanced Sph/DMS level, and were abolished by D-MAPP as major effector, with slight enhancement by L-CS. Fumonisin, the inhibitor of Cer synthesis, had no inhibitory effect on this process.
With a different approach, intracellular Sph level was enhanced by exogenous addition of Sph/DMS-activated caspase 3, resulting in the release of PKCδ KD, previously identified as SDK1.
Either of these approaches (serum depletion or exogenous Sph/DMS addition) involves common processes, i.e., activation of caspase 3 and release of PKCδ KD, that initiate apoptosis in the presence of Sph/DMS, thereby activating 14-3-3 phosphorylation. Phosphorylation of 14-3-3 promotes the association of proapoptotic BAD/BAX with and consequent inhibition of antiapoptotic BCL-2 or BCL-X, leading to apoptosis. Significantly, Sph has a “double effect” in this overall process: it activates caspase 3 to release PKCδ KD, and it catalyzes PKCδ KD to phosphorylate 14-3-3. It is unknown whether PKCδ KD is involved in other phosphorylation processes or whether PKCδ is activated by Sph/DMS in such processes.
The apoptotic effect of Sph/DMS described above is based on the direct interaction of Sph/DMS with key molecules involved in the apoptotic process. In contrast, our previous studies indicate that Sph/DMS inhibits BCL-2 mRNA expression (33). The inhibitory effect of DMS on BCL-2 mRNA expression in HL60 cells was confirmed by our recent data (unpublished work).
A finding of the present study is the strong inhibitory effect of Sph on Akt kinase, the survival signal associated with α3β1-mediated cell adhesion to LN. Notably, only Sph (not DMS, C2-Cer, Sph-1-phosphate, or lysophosphatidic acid) had such an inhibitory effect and induced strong apoptosis. Sph also inhibited Akt signaling induced by α5β1-mediated adhesion to FN, to a lesser extent.
Adherent cells showed less susceptibility to apoptosis than nonadherent cells; this may be due in part to the presence of a strong survival signal associated with integrin-dependent cell adhesion in adherent cells. The concept of Sph-induced apoptosis through inhibition of integrin-mediated survival signal, particularly Akt, is compatible with the process of anoikis, i.e., programmed death of cells detached from their “home ground” (34). Taking all these findings and concepts into consideration, we propose a Sph-dependent apoptotic cycle as shown in Fig. 4 and described in its legend.
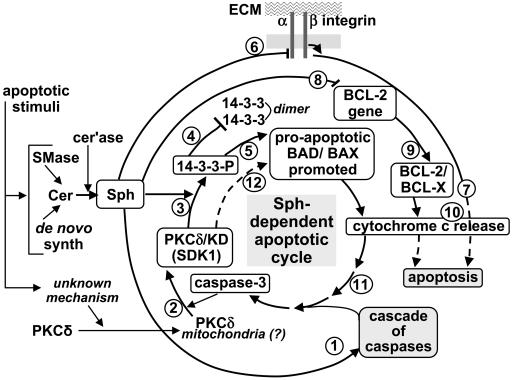
Fig. 4.
Multiple Sph-induced mechanisms leading to apoptosis. Apoptotic stimulation causes (i) a higher Cer level, resulting from higher sphingomyelinase (SMase) activity or its de novo synthesis, followed by release of Sph by ceramidase (cer'ase) and (ii) an unknown mechanism causing translocation of PKCδ and its possible accumulation in mitochondrial membrane. These two events are considered to trigger multiple Sph-induced channels for apoptosis processes 1–12 as described below. (Process 1) Sph activates a “cascade of caspases” leading to caspase 3 activation. (Process 2) Activated caspase-3 cleaves PKCδ to produce PKCδ KD (SDK1). (Process 3) Sph activates SDK1 to phosphorylate 14-3-3 (→ 14-3-3-P). (Processes 4 and 5) 14-3-3 phosphate (14-3-3-P) inhibits 14-3-3 dimer formation (process 4), which in turn inhibits binding of 14-3-3 to proapoptotic BAD/BAX, promoting their proapoptotic effect (process 5). (Processes 6 and 7) Sph inhibits integrin-dependent survival signal, e.g., Akt (process 6), based on interaction of integrin α3β1 with extracellular matrix (ECM) components (e.g., LN10/11). Inhibition of survival signal leads to apoptosis (process 7). (Processes 8 and 9) Sph inhibits BCL-2 gene expression (process 8), thereby inhibiting antiapoptotic BCL-2/BCL-X (process 9). (Processes 10 and 11) Processes 5, 7, and 9 cause release of cytochrome c (process 10), which contributes to caspase-3 activation (process 11). (Process 12) Activated caspase 3 releases PKCδ KD (SDK1), promoting a “vicious cycle” of Sph-induced apoptosis. The overall process is based on enhanced Sph level and translocation of PKCδ. The higher the Sph level, the greater the effect of the cycle through released caspase 3 and released PKCδ KD. Susceptibility of adherent tumor cells to Sph-induced apoptosis is less than that of nonadherent cells, because adherent cells require inhibition of integrin-dependent survival signal by Sph (process 6). Which Sph-induced mechanism is dominant may vary depending on type of cell.
Which pathway is predominant depends on cell type, particularly in tumor cells. The apoptotic process seen under serum-starvation conditions is pronounced in adherent cells such as A31, whereas Sph/DMS-induced apoptosis due to PKCδ KD release is pronounced in promyelocytic leukemia HL60 and monocytic leukemia U937 cells. Suppression of the mRNA level of BCL-2 by Sph/DMS is most clearly observed in HL60 cells. The successful clinical trial of DMS for treatment of myelocytic leukemia (35) is likely attributable to multiple factors susceptible to DMS, as discussed in this paper.
Author contributions: E.S., K.H., and M.S.T. performed research; E.S. analyzed the data; S.H. wrote the paper; K.H. and S.H. designed the research; and E.S., K.H., and M.S.T. contributed new reagents/analytical tools.
Abbreviations: Sph, sphingosine; DMS, N,N-dimethyl-Sph; Cer, ceramide; C2-Cer, N-acetyl-Sph; SDK, Sph-dependent protein kinase; KD, kinase domain; L-CS, l-cycloserine; D-MAPP, d-erythro-2-tetradecanoylamino-1-phenyl-1-propanol; LN, laminin; FN, fibronectin; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling.
References
- 1.Hannun, Y. A. & Bell, R. M. (1989) Science 243, 500–507. [DOI] [PubMed] [Google Scholar]
- 2.Merrill, A. H. J., Nimkar, S., Menaldino, D., Hannun, Y. A., Loomis, C. R., Bell, R. M., Tyagi, S. R., Lambeth, J. D., Stevens, V. L., Hunter, R. & Liotta, D. C. (1989) Biochemistry 28, 3138–3145. [DOI] [PubMed] [Google Scholar]
- 3.Igarashi, Y., Hakomori, S., Toyokuni, T., Dean, B., Fujita, S., Sugimoto, M., Ogawa, T., El-Ghendy, K. & Racker, E. (1989) Biochemistry 28, 6796–6800. [DOI] [PubMed] [Google Scholar]
- 4.Nishizuka, Y. (1984) Nature 308, 693–698. [DOI] [PubMed] [Google Scholar]
- 5.Kolesnick, R. N. & Kronke, M. (1998) Annu. Rev. Physiol. 60, 643–665. [DOI] [PubMed] [Google Scholar]
- 6.Pettus, B. J., Chalfant, C. E. & Hannun, Y. A. (2002) Biochim. Biophys. Acta 1585, 114–125. [DOI] [PubMed] [Google Scholar]
- 7.Sano, T., Baker, D., Virag, T., Wada, A., Yatomi, Y., Kobayashi, T., Igarashi, Y. & Tigyi, G. (2002) J. Biol. Chem. 277, 21197–21206. [DOI] [PubMed] [Google Scholar]
- 8.Kihara, A., Ikeda, M., Kariya, Y., Lee, E.-Y., Lee, Y.-M. & Igarashi, Y. (2003) J. Biol. Chem. 278, 14578–14585. [DOI] [PubMed] [Google Scholar]
- 9.Spiegel, S., English, D. & Milstien, S. (2002) Trends Cell Biol. 12, 236–242. [DOI] [PubMed] [Google Scholar]
- 10.Maceyka, M., Payne, S. G., Milstien, S. & Spiegel, S. (2002) Biochim. Biophys. Acta 1585, 193–201. [DOI] [PubMed] [Google Scholar]
- 11.Megidish, T., White, T., Takio, K., Titani, K., Igarashi, Y. & Hakomori, S. (1995) Biochem. Biophys. Res. Commun. 216, 739–747. [DOI] [PubMed] [Google Scholar]
- 12.Megidish, T., Takio, K., Titani, K., Iwabuchi, K., Hamaguchi, A., Igarashi, Y. & Hakomori, S. (1999) Biochemistry 38, 3369–3378. [DOI] [PubMed] [Google Scholar]
- 13.Megidish, T., Cooper, J., Zhang, L., Fu, H. & Hakomori, S. (1998) J. Biol. Chem. 273, 21834–21845. [DOI] [PubMed] [Google Scholar]
- 14.Woodcock, J. M., Murphy, J., Stomski, F. C., Berndt, M. C. & Lopez, A. F. (2003) J. Biol. Chem. 278, 36323–36327. [DOI] [PubMed] [Google Scholar]
- 15.Deckwerth, T. L., Elliott, J. L., Knudson, C. M., Johnson, E. M. J., Snider, W. D. & Korsmeyer, S. J. (1996) Neuron 17, 401–411. [DOI] [PubMed] [Google Scholar]
- 16.Xiang, H., Kinoshita, Y., Knudson, C. M., Korsmeyer, S. J., Schwartzkroin, P. A. & Morrison, R. S. (1998) J. Neurosci. 18, 1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scorrano, L. & Korsmeyer, S. J. (2003) Biochem. Biophys. Res. Commun. 304, 437–444. [DOI] [PubMed] [Google Scholar]
- 18.Hamaguchi, A., Suzuki, E., Murayama, K., Fujimura, T., Hikita, T., Iwabuchi, K., Handa, K., Withers, D. A., Masters, S. C., Fu, H. & Hakomori, S. (2003) J. Biol. Chem. 278, 41557–41565. [DOI] [PubMed] [Google Scholar]
- 19.Obeid, L. M., Linardic, C. M., Karolak, L. A. & Hannun, Y. A. (1993) Science 259, 1769–1771. [DOI] [PubMed] [Google Scholar]
- 20.Kolesnick, R. N. (2002) J. Clin. Invest. 110, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grant, S. & Spiegel, S. (2002) J. Clin. Invest. 109, 717–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blass, M., Kronfeld, I., Kazimirsky, G., Blumberg, P. M. & Brodie, C. (2002) Mol. Cell. Biol. 22, 182–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruvolo, P. P., Deng, X., Carr, B. K. & May, W. S. (1998) J. Biol. Chem. 273, 25436–25442. [DOI] [PubMed] [Google Scholar]
- 24.Wright, S. C., Schellenberger, U., Wang, H., Kinder, D. H., Talhouk, J. W. & Larrick, J. W. (1997) J. Exp. Med. 186, 1107–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kayali, A. G., Austin, D. A. & Webster, N. J. (2002) Endocrinology 143, 3884–3896. [DOI] [PubMed] [Google Scholar]
- 26.Wilson, E., Wang, E., Mullins, R. E., Uhlinger, D. J., Liotta, D. C., Lambeth, J. D. & Merrill, A. H. J. (1988) J. Biol. Chem. 263, 9304–9309. [PubMed] [Google Scholar]
- 27.Riley, R. T., Norred, W. P., Wang, E. & Merrill, A. H. (1999) Nat. Toxins 7, 407–414. [DOI] [PubMed] [Google Scholar]
- 28.Gu, J., Fujibayashi, A., Yamada, K. M. & Sekiguchi, K. (2002) J. Biol. Chem. 277, 19922–19928. [DOI] [PubMed] [Google Scholar]
- 29.Kajimoto, T., Shirai, Y., Sakai, N., Yamamoto, T., Matsuzaki, H., Kikkawa, U. & Saito, N. (2004) J. Biol. Chem. 279, 12668–12676. [DOI] [PubMed] [Google Scholar]
- 30.Spiegel, S. & Milstien, S. (1995) J. Membrane Biol. 146, 225–237. [DOI] [PubMed] [Google Scholar]
- 31.Coroneos, E., Martinez, M., McKenna, S. & Kester, M. (1995) J. Biol. Chem. 270, 23305–23309. [DOI] [PubMed] [Google Scholar]
- 32.Ohta, H., Sweeney, E. A., Masamune, A., Yatomi, Y., Hakomori, S. & Igarashi, Y. (1995) Cancer Res. 55, 691–697. [PubMed] [Google Scholar]
- 33.Sakakura, C., Sweeney, E. A., Shirahama, T., Hakomori, S. & Igarashi, Y. (1996) FEBS Lett. 379, 177–180. [DOI] [PubMed] [Google Scholar]
- 34.Liotta, L. A. & Kohn, E. (2004) Nature 430, 973–974. [DOI] [PubMed] [Google Scholar]
- 35.Jendiroba, D. B., Klostergaard, J., Keyhani, A., Pagliaro, L. & Freireich, E. J. (2002) Leuk. Res. 26, 301–310. [DOI] [PubMed] [Google Scholar]