Abstract
Objective: In previous work, we demonstrated the development of a novel fusion protein containing stromal cell-derived growth factor-1 alpha juxtaposed to an elastin-like peptide (SDF1-ELP), which has similar bioactivity, but is more stable in elastase than SDF1. Herein, we compare the ability of a single topical application of SDF1-ELP to that of SDF1 in healing 1 × 1 cm excisional wounds in diabetic mice.
Approach: Human Leukemia-60 cells were used to demonstrate the chemotactic potential of SDF1-ELP versus SDF1 in vitro. Human umbilical vascular endothelial cells were used to demonstrate the angiogenic potential of SDF1-ELP versus SDF1 in vitro. The bioactivity of SDF1-ELP versus SDF1 after incubation in ex-vivo diabetic wound fluid was compared. The in-vivo effectiveness of SDF1-ELP versus SDF1 was compared in diabetic mice wound model by monitoring for the number of CD31+ cells in harvested wound tissues.
Results: SDF1-ELP promotes the migration of cells and induces vascularization similar to SDF1 in vitro. SDF1-ELP is more stable in wound fluids compared to SDF1. In vivo, SDF1-ELP induced a higher number of vascular endothelial cells (CD31+ cells) compared to SDF1 and other controls, suggesting increased vascularization.
Innovation: While growth factors have been shown to improve wound healing, this strategy is largely ineffective in chronic wounds. In this work, we show that SDF1-ELP is a promising agent for the treatment of chronic skin wounds.
Conclusion: The superior in vivo performance and stability of SDF1-ELP makes it a promising agent for the treatment of chronic skin wounds.
Keywords: : stromal cell-derived factor 1 (SDF1), elastin like peptides (ELP), nanoparticles, wound healing, skin

Martin L. Yarmush, MD, PhD
Introduction
The management of wound healing and subsequent scarring remains a challenge for healthcare professionals. Chronic wounds are especially difficult to treat as the wound repair process is interrupted by underlying medical conditions such as diabetes and immunosuppression, leading to a prolonged and excessive inflammatory phase,1 and persistent infections2 to wound. High levels of inflammatory cells in the chronic wound induce the production of serine proteases and matrix metalloproteinases that degrade and inactivate the components of the extra cellular matrix and growth factors needed for wound healing.3
Studies have demonstrated that topical growth factors are promising therapeutics for nonhealing wounds,4 and their effectiveness has been demonstrated in nonclinical animal models. Despite their therapeutic potential, one of the biggest challenges in the development of exogenous growth factors for clinical use is finding effective drug delivery platforms and technologies that ensure the safe and prolonged release of growth factors at the wound site during the entire skin regeneration process, and that are able to protect the growth factor from degradation by proteolytic activities in the wound. To date, the only topical growth factor that has received US Food and Drug Administration approval for wound healing is recombinant human platelet-derived growth factor (Regranex®),5 which although has been shown to be effective in treating leg ulcers, needs to be used in multiple applications due to degradation by proteinases. However, an FDA-instigated black box on the product label warns of an increased risk in cancer deaths in patients who used more than two applications of the growth factor.
One growth factor, which has been shown to enhance the closure of skin wounds, is stromal cell-derived growth factor 1 alpha (SDF1).6 SDF1 is known to promote revascularization, which is needed for reepithelialization7–9 by recruiting endothelial progenitor cells that differentiate into mature vascular endothelium.10,11 However, similar to Regranex, repeated and high doses of topical SDF1 are needed to achieve therapeutic efficacy6 in animal models, making its potential therapeutic use expensive and impractical. Translation to the clinic may face significant regulatory hurdles since the Regranex case sets a precedent on the malignancy risk associated with repeated growth factor application12; delivery strategies that minimize the number of applications may therefore be more clinically translatable.
In our previous work,13 we showed the development of an SDF1 derivative, SDF1 elastin-like peptide (SDF1-ELP) with a similar in vitro bioactivity as SDF1, but a superior in vivo efficacy. In this work, we show that while SDF1-ELP promotes the migration of cells and induces vascularization similar to SDF1 in vitro, it is more stable in wound fluid. When applied to full-thickness skin wounds in diabetic mice, wounds treated with a single topical application of SDF1-ELP exhibited more endothelial cells (CD31+ cells) compared with SDF1 and vehicle controls, suggesting increased vascularization.
Clinical Problem Addressed
The management of wound healing and subsequent scarring remains a challenge for healthcare professionals. Studies have demonstrated that topical growth factors are promising therapeutics for nonhealing wounds. However, therapeutic application of exogenous growth factors such as SDF1 (known to promote revascularization, which is needed for reepithelialization of wounds) does not work because they are also subject to proteolysis in the harsh wound environment. In this work, we show that a derivative of SDF1, SDF1-ELP, promotes the migration of cells and induces vascularization similar to SDF1 in vitro, but is more stable in wound fluid. When applied to full-thickness skin wounds in diabetic mice, wounds treated with SDF1-ELP exhibited more endothelial cells (CD31+ cells) compared with SDF1 and vehicle controls, suggesting increased vascularization. SDF1-ELP is a promising agent for the treatment of chronic skin wounds.
Materials and Methods
Synthesis and characterization of SDF1-ELP
The design, development, and characterization of SDF1-ELP were previously described.13 Briefly, SDF1-ELP was made by juxtaposing human SDF1 to an ELP in a pET25B+ vector. The fusion protein was expressed in Escherichia coli and purified using a unique property conferred by the ELP, which enables it to reversibly aggregate into nanoparticles above its inverse transition temperature. Two cycles of temperature cycling and centrifugation were used to isolate SDF1-ELP. Purity and identity of SDF1-ELP were confirmed using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western Blot, respectively. Particle size was confirmed using a Zetasizer (Malvern instrument) and transmission electron microscopy. The binding and biological activity of SDF1-ELP was determined using surface plasmon resonance technology by a Biacore equipment (GE Health Care) and a calcium flux assay, respectively.
SDF1-ELP-mediated human leukemia-60 chemotaxis assay
To evaluate chemotactic responses to SDF1-ELP, we used the human leukemia-60 (HL-60) cell line, which highly expresses the SDF1 receptor CXCR4,14 in Boyden chambers. The HL-60 cells were obtained from the American Type Culture Collection (ATCC; Established cell line) and were cultured in Iscove's modified Dulbecco's medium (IMDM) (ATCC) supplemented with 20% fetal bovine serum (Gibco;Life Technologies) and 1% penicillin–streptomycin (Life Technologies). HL-60 cells were cultured in this medium until they reached a concentration of about 106 viable cells/mL. A total of 105 cells (in 100 μL) were placed on top of Transwells (Falcon™ Cell Culture Insert, Transparent Polyethylene Terephthalate Membrane 8.0 μm pore size; [Corning]). The bottom of the transwells was filled with 600 μL of SDF1-ELP, free SDF1, ELP alone, or another fusion protein, keratinocyte growth factor (KGF)-ELP.15 Plates were incubated at 37°C for 1, 2, and 4 h. The number of cells that migrated to the bottom of the well was counted using a hemocytometer (Hausser). The results were normalized to the initial 105 added cells.
Separation of chemotactic activity in monomeric and nanoparticle forms of SDF1-ELP
The SDF1-ELP fusion protein forms nanoparticles above its inverse temperature, previously determined to be about 35°C. We prepared SDF1-ELP at a concentration of 8 μM in 500 μL phosphate-buffered saline (PBS) and warmed the solution up to 40°C to initiate nanoparticle formation and pipetted it into a 1.5 mL Nanosep® and Nanosep MF centrifuge tube with a 10 nm nominal pore size (Pall Corporation). The tube was centrifuged at 5,000 g for 5 min at 40°C to separate monomers (which end up in the filtrate) from nanoparticles (which remain on top of the membrane). We reran the cell migration studies with 600 μL of the filtered SDF1-ELP monomer, or SDF1-ELP nanoparticles, made to a concentration of 250 nM in the medium as the test solutions, with a control group using unfiltered SDF1-ELP (250 nM), and additional groups using 10 nM recombinant SDF1, or plain medium.
Quantification of SDF1-ELP monomer release from nanoparticles
To investigate the release of SDF1-ELP monomers from nanoparticles over time, SDF1-ELP was prepared at a concentration of 1,000 nM in IMDM (ATCC; Established cell line) supplemented with 20% fetal bovine serum and 1% penicillin–streptomycin. The solution was incubated at 37°C to initiate nanoparticle formation. After 0, 1, 2, 3, and 6 h of incubation, SDF1-ELP monomers were separated from nanoparticles by centrifugation in Nanosep tubes as described earlier. The filtrate (50 μL) containing the SDF1-ELP monomers was pipetted onto a nitrocellulose membrane placed inside a dot blot apparatus, along with recombinant human SDF1 (Peprotech) standards between 0 and 125 nM. The nitrocellulose membrane was allowed to air dry, after which it was blocked with blotting-grade blocker (Bio-Rad Laboratories), treated with an anti-human SDF1 (Peprotech), and incubated overnight at 4°C. After thorough washing with TBST, a secondary goat anti-rabbit IgG-horseradish peroxidase antibody (Abcam) was added. Once the nitrocellulose membrane was developed, the amount of SDF-ELP monomers shown on the dot blot membrane was quantified using SDF1 standards also on the same membrane.
Mathematical modeling
COMSOL Multiphysics 5a (COMSOL, Inc.) was used to model the dynamics of release of SDF1-ELP monomers from nanoparticles and its subsequent diffusion into the top of the transwells used in the HL-60 chemotaxis assay. The model was conducted in a system composed of a 24-well plate with transwell inserts, at fixed temperature with a no-flux condition on all interfaces except the insert-bottom well interface. Media were assumed to be an incompressible Newtonian fluid with a density of 1,000 kg/m3. An incompressible Navier–Stokes model was used, as well as a diffusion–convection model, and a reaction model. Mesh optimization was performed for the simulations. A time-dependent solver was used with a time step of 10s. Diffusivity of SDF1-ELP monomers in media was estimated as 4.4 × 10−11 m2/s.16 The reaction system was modeled as first-order reaction with SDF1-ELP nanoparticles as the reactant and SDF1-ELP monomers as the product. The initial concentration of nanoparticles in the system ranged from 10 to 1,000 nM and the released concentration of monomer values were quantified at 0, 1, 2, and 4 h. The concentration of monomer in the top of the well was determined by using COMSOL's built-in surface integration and volume integration, respectively. Then, the concentration in the well was divided by initial concentration of drug in the transwell insert to obtain the percent release of the system.
In vitro angiogenesis assay
The ability of SDF1-ELP to induce angiogenesis was measured using an in vitro vascularization assay. We cultured human umbilical vascular endothelial cells (HUVECs; Life Technologies) in a 75 cm2 tissue culture flask in Medium 200 supplemented with low serum growth supplement (Life Technologies) until they were 80% confluent. 289 μL of 10 mg/mL of Matrigel (Corning Lifesciences) was thawed on ice overnight and spread evenly over the wells of a 24-well plate. The plates were incubated for 40 min at 37°C to allow the Matrigel to gel. Trypsinized cells were transferred to a conical tube and centrifuged at 180 g for 7 min until the cells were pelleted. Cells were resuspended to a concentration of 4 × 105 cells/mL using nonsupplemented medium 200PRF (Life Technologies). SDF1-ELP (1,000) nM, SDF1 (1,000) nM, and ELP (1,000) nM were prepared in 300 μL of the cell suspension (total of 1.2 × 105 cells) and pipetted onto the Matrigel. The negative control was plain media, while the positive control was basic fibroblast growth factor (25 nM). After about 20–22 h of incubation of the plate at 37°C, the cells were removed and the plates were washed twice with HBSS. The final wash was replaced with calcein AM (Life Technologies), prepared to a concentration of 8 μg/mL in HBSS. The plates were incubated at 37°C for 30 min, after which they were washed twice with HBSS. Images were acquired at 10 × magnification using an Olympus IX81® microscope.
Stability in diabetic wound fluid
To investigate the persistence of SDF1-ELP protein nanoparticles in a wound site, 10 μM of SDF1-ELP, SDF1, or ELP were incubated for 15 days in human diabetic wound fluid or in PBS vehicle. Wound fluid from the left abdomen of a diabetic patient was used for the studies. The use of the human wound fluid was approved by the Rutgers Institutional Review Board. Initially, we investigated background bioactivity in the diabetic fluid itself, as it may mask the effect of the test proteins. We diluted the wound fluid with IMDM from 1 × (no dilution) to 1,200 × , and tested the chemotactic activity of the resulting solution as described above.
Based on our previous experience with the cell migration assay (Fig. 1a), we diluted 10 μM SDF1, which had been incubated in wound fluid for 15 days, down to 10 nM (1,000 × wound fluid dilution). Similarly, 10 μM SDF1-ELP was incubated in wound fluid for 15 days, and then diluted down to 250 nM using IMDM (40 × wound fluid dilution). We then performed the migration assay to compare the chemotactic activity of free SDF1 versus SDF1-ELP after the 15-day incubation period. As negative controls, we used 10 μM ELP preincubated for 15 days in wound fluid, also diluted down to 250 nM ELP, plain media, and plain wound fluid diluted 40 × with IMDM.
Figure 1.
(A) Chemotactic activity of SDF1-ELP toward HL-60 cells. (A) HL-60 cells were put on the top of 8 μm pore transwells. The bottom of the wells was filled with 600 μL IMDM with SDF1-ELP, free SDF1, ELP alone, or KGF-ELP at the concentrations shown. The number of migrated cells was measured after 1, 2, and 4 h at 37°C. N = 6 for each condition. SDF1-ELP migration results after 1, 2, and 4 h were statistically compared with the corresponding 10 nM SDF1 migration results (*p < 0.05, one-way ANOVA, Fisher's LSD post-test. NS, not statistically significant). (B) Chemotactic Activity of SDF-ELP Nanoparticles versus Monomeric Form. SDF1-ELP nanoparticles were separated from monomers by centrifugation through a 10 nm pore size membrane. HL-60 migration was measured using SDF1-ELP nanoparticles that remained on top of the membrane, as well as the SDF1-ELP monomers that passed through the membrane. N = 6 for each condition (*p < 0.05, one-way ANOVA, Fisher's LSD post-test). ANOVA, analysis of variance; KGF, keratinocyte growth factor. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
In vivo bioactivity of SDF-ELP versus free SDF1
Animals
All animal studies were approved by the Rutgers University Institutional Animal Care and Use Committee. Ten-week-old, genetically modified diabetic mice, BKS.Cg-Dock7m +/+ Leprdb/J, (Jackson Laboratory) were used.
Wound experiments
One day before the surgery, the hair on the back of the mice was completely removed and the shaved area was thoroughly washed with water. The next day (day of surgery), the mice were put under isoflurane anesthesia and their dorsal area sterilized for surgery with a sequential application of betadine scrub (Purdue Products) and 70% ethanol. 1 × 1 cm2 excisional wounds were created on the back of the mice using a premade template. Test solutions consisting of 1,000 nM SDF1-ELP, SDF, ELP, and plain PBS were prepared in fibrin gels as previously described13 and applied onto the wound area, after which the wounds were covered with Tegarderm™ (3M) and secured using sutures (Henry Schein).
Histological processing and analysis
After 7, 28, and 42 days postwounding, the animals were sacrificed and the wound area excised. The tissues were fixed in 10% formalin (VWR) for 24 h, and then stored at 2°C–8°C in 70% ethanol. For histology, tissues were embedded in paraffin and thin sections were stained for CD31+ cells using a primary rabbit polyclonal anti-CD31 antibody (Abcam) and also with picosirius red to visualize the morphological features of the skin.
The number of CD31+ cells in the stained sections was quantified using ImageJ (National Institutes of Health, Bethesda, MD). Values shown are averages of two different tissue sections per group, with about three different fields evaluated per section. Images of the wound sections stained with picosirius red were captured using a Stereoscope Olympus at 0.5 × magnification, and ImageJ was used to quantify the wound length not reepithelialized (defined as the distance of reepithelialization between the ends of the new epidermis growing on either side of the wound). The wound margins in the tissue sections were determined by the presence of a more organized dermis and epidermis, and the presence of hair follicles and sweat glands.
Statistical analysis
Statistical analysis was performed using KaleidaGraph software (Synergy). Data from two independent groups were analyzed using the Fisher least significant difference, after performing a one-way analysis of variance (ANOVA). A p-value of <0.05 is represented by a star (*) on the graphs, while a p-value of <0.01 is represented by two stars (**) on the graphs; both are considered statistically significant.
Results
Chemotactic activity of SDF-ELP versus Free SDF
The ability of SDF1-ELP to promote the migration of HL-60 cells, which express CXCR4,14 was evaluated in transwells. As shown in Fig. 1a, SDF1-ELP caused a dose-dependent migratory response up to 1,000 nM. A dose of 250 nM SDF1-ELP achieved approximately the same percent cell migration as 10 nM free SDF1, which was used as positive control. This concentration of free SDF1 is reported to induce a robust migratory response.17 Negative controls included vehicle consisting of plain medium with no peptide, ELP alone, or KGF-ELP, which is mitogenic for keratinocytes,15 but for which HL-60 cells do not express the receptor. Very little migration was seen in all of the negative controls used.
Chemotactic activity of SDF-ELP nanoparticles versus monomeric form
Since chemotaxis was measured at physiological temperature (37°C), which is only slightly above the SDF1-ELP inversion temperature of about 35°C, both monomeric and nanoparticle forms of SDF-ELP may be present in this assay. We therefore probed to what extent the monomers and nanoparticles contributed to the observed migration response by separating them through a 10 nm nominal pore size membrane. As a control, we also used free SDF1 on similar membranes. The HL-60 cell migration experiment was repeated using the monomers (which end up in the filtrate), nanoparticles (which remain on top of the membrane), as well as unfiltered SDF1-ELP. As shown in Fig. 1b, the chemotactic activity of the monomeric fraction was significantly greater compared with the nanoparticle fraction; however, a significant amount of migration occurred with the nanoparticle fraction as well. Thus, HL-60 migration may be contributed by both forms of SDF1-ELP, with a predominant effect of the monomeric form.
Chemotactic activity—release of SDF1-ELP monomers from nanoparticles
We measured how much SDF-ELP monomer was released out of the nanoparticles over the time course of the migration study. Dot blot quantitation of the filtrate as a function of incubation time revealed a time-dependent increase after a lag of about 1 h, as shown in Fig. 2a, b. The fraction of SDF1-ELP monomers released at the end of the incubation time of 4 h can be estimated to be about 8%. Therefore, when using a total SDF-ELP concentration of 250 nM during the HL-60 chemotaxis study, the concentration of SDF-ELP monomer can be estimated to be around 20 nM.
Figure 2.
Release of SDF1-ELP monomers from nanoparticles. (A) SDF-ELP nanoparticles were incubated in the medium at 37°C for up to 6 h. Samples retrieved at different time points were analyzed by dot blot along with free SDF1 standards. (B) The fraction of SDF-ELP monomers released at each time point was estimated by measuring the pixel intensity of the dot blot images compared with the SDF1 standards using ImageJ. N = 3
Modeling of the release and diffusion of monomers from nanoparticles in transwell migration assay
Computational fluid dynamics modeling was used to corroborate the experimental system, to determine whether the movement of SDF1-ELP monomers to the top of the transwells in the migration assay was transport or reaction rate limited. Using COMSOL, we generated a reaction-transport model and found very close prediction of the in vitro values (Table 1). Extrapolating the model out to 24 and 48 h (data not shown) indicates that the system is transport limited.
Table 1.
Release and diffusion of SDF1-ELP monomers from nanoparticles in transwell
| Initial Concentration of Nanoparticles (nM) | Expected Concentration of Monomers Released into Bottom of Transwell After 4 h (nM) | Concentration of Monomers in Transwell per Model (nM) |
|---|---|---|
| 0 | 0 | 0 |
| 10 | 0.8 | 0.79 |
| 100 | 8 | 8.1 |
| 250 | 20 | 23.24 |
| 1,000 | 80 | 81.45 |
SDF1, stromal cell-derived growth factor 1; ELP, elastin-like peptide.
In vitro endothelial tube formation
The ability of SDF1-ELP to promote angiogenesis was evaluated in a tube formation assay using HUVECs. SDF1-ELP, SDF1, and ELP were prepared to concentrations of 1,000 nM in HUVEC suspensions and pipetted onto Matrigel-coated plates. The test solutions were prepared to match the concentrations that had been previously tested in animal studies. The angiogenic activities of the test solutions were compared with bFGF, used as positive control.18 After a 22-h incubation at 37°C, followed by staining with calcein AM, fluorescence images show the multicellular structures that formed in each condition. Similar to bFGF, both SDF1-ELP and SDF1 promoted tube formation and capillary-like networks, while ELP and vehicle controls had no such effects (Fig. 3a, b).
Figure 3.
Tube formation assay. (A) Representative fluorescent images of capillary-like structures formed by HUVECs plated on matrigel (N = 3), in presence of (A) free SDF1; (B) SDF-ELP; (C) ELP alone; (D) bFGF used as positive control; and (E) plain medium, used as negative control. Scale bar: 200 μm. (B) Quantification of capillary-like structures (*p < 0.05, one-way ANOVA, Fisher's LSD post-test. NS, not significant). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
In vitro stability in human diabetic wound fluid
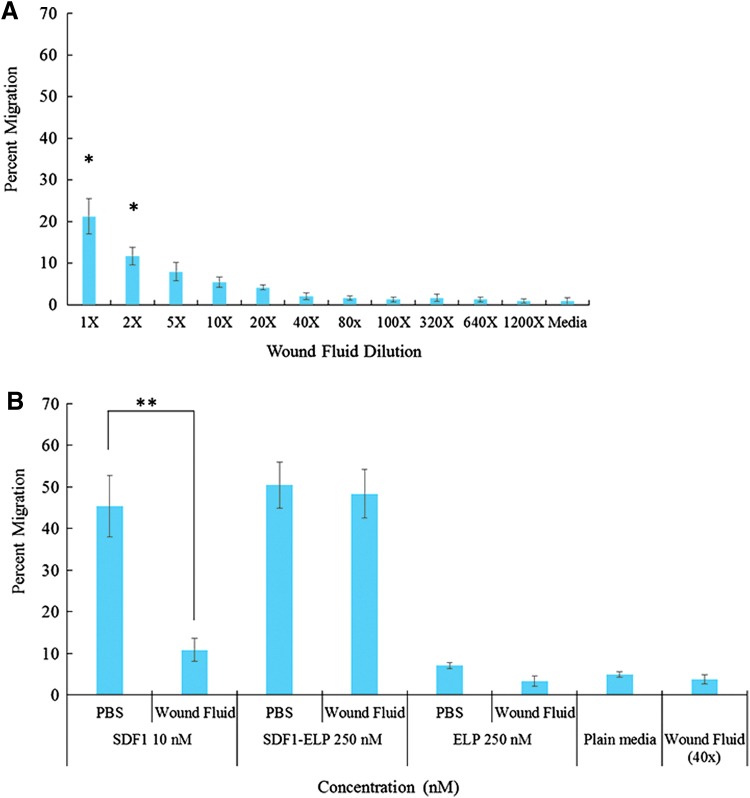
To compare the stability of SDF1-ELP versus SDF1 in a wound environment, we measured the bioactivity of each protein after prolonged incubation in human diabetic wound fluid. Because wound fluid by itself is chemotactic to HL-60 cells, we tested the chemotactic activity of different dilutions of wound fluid on HL-60 cells, and found that wound fluid diluted between a range of 40–1,200 × had a minimal chemotactic effect (Fig. 4a). After a 15-day incubation of 10 μM SDF1-ELP, SDF1, and ELP in 1 × wound fluid, we diluted the solutions to concentrations that would allow us repeating the cell migration experiment (Fig. 1), but with minimum interference from the wound fluid itself. As such, SDF1 was diluted down to 10 nM (1,000 × wound fluid dilution), whereas SDF1-ELP and ELP were diluted down to 250 nM (40 × wound fluid dilution). Additional negative controls for the experiment were wound fluid diluted 40 × , and plain media. As shown in Fig. 4b, the chemotactic activity of free SDF1 after incubation in wound fluid was significantly reduced, as compared to SDF1-ELP.
Figure 4.
Chemotactic activity of SDF-ELP versus SDF after incubation in wound fluid. (A) Wound fluid (WF) was diluted between ranges of 40 to 1,200 × with IMDM and used to perform an HL-60 cell migration assay, to understand the chemotactic effect of the wound fluid on the cells. (B) SDF1-ELP and SDF1 were incubated in wound fluid for 15 days and used to perform an HL-60 cell migration assay. N = 6 for each condition (*p < 0.05, **p < 0.01, one-way ANOVA, Fisher's LSD post-test). HL-60, human leukemia-60; IMDM, Iscove's modified Dulbecco's medium. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
SDF-ELP versus SDF1-mediated wound healing response in diabetic mice
In our previous work,13 we demonstrated the bioactivity of SDF1-ELP in excisional wounds made on diabetic mice. 1 × 1 cm2 excisional wounds created on the back of diabetic mice and treated with SDF1-ELP, SDF1, and other negative controls. Digital photographs of the wounds were captured weekly, and compared to the initial photographs using Image J (NIH). The data revealed that wounds treated with SDF1-ELP nanoparticles were 95% closed by day 21 and fully closed by day 28, while wounds treated with free SDF1, ELP alone, or vehicle took 42 days to fully close. To further substantiate these results, we also observed and measured the wound length not reepithelialized (defined as the distance of reepithelialization between the ends of the new epidermis growing from the two sides of the wound) using histological sections of wound harvested on days 7, 28, and 42 postwounding. The results indicated that wounds treated with SDF1-ELP healed significantly faster than wounds treated with SDF1 and the other controls. By day 28, wounds treated with SDF1-ELP had little to no remaining wound edge, while those treated with SDF1 and other controls were still not fully reepithelialized as expected. However, by day 42, all the wounds were fully closed (Fig. 5a, b).
Figure 5.
Quantification of wound edge not reepithelialized. (A) Full-thickness wounds created on the back of diabetic mice were treated with SDF1-ELP, free SDF1, ELP, and plain fibrin gel (vehicle control). On days 7, 28, and 42, animals were sacrificed and their wound tissues harvested and stained with picosirius red. The entire wound was observed under a 0.5 × magnification stereoscope to observe for the wound length not reepithelialized. (B) The distance between the ends of the new epidermis growing on either side of the wound was quantified as the wound length not reepithelialized. This was done for day 7 and 28. A total of three animals per treatment group and two different tissue sections per animal were used for the analysis (**p < 0.01, one-way ANOVA, Fisher's LSD post-test. NS, not significant). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
SDF1 is known to promote revascularization, which is needed for reepithelialization of chronic wounds.7–9 SDF1-ELP was more stable during ex vivo wound fluid incubation than SDF1, under idealized in vitro conditions, and they had similar angiogenic potential. To determine whether SDF1-ELP was more effective in actual wounds, we treated excisional wounds in diabetic mice with a single dose of SDF-ELP, free SDF1, ELP alone, or plain fibrin (vehicle) control. Wound tissues were harvested after 7, 28, and 42 days postwounding and stained for CD31+ cells (Fig. 6a). The total number of CD31+ cells per field was evaluated using ImageJ.
Figure 6.
Distribution and quantification of CD31+ cells per field in wound tissues. Wound tissues were harvested after 7, 28, and 42 days postwounding and stained for CD31+ cells. Histology slides were visualized using a 10 × objective. Scale bar = 10 μm. Images shown are representative for wounds harvested on (A) Day 7: pictures of histology slides were taken at the entrance of the wound; Day 28 and 42: pictures were taken inside the wound area. Structures are labeled as follows: E, epidermis; D, dermis. (B) The number of CD31+ cells at the different time points was quantified using ImageJ. Representative images are shown. A total of three animals per treatment group were used and two different tissue sections were analyzed per group, with three (3) 10 × magnification fields evaluated per section (**p < 0.01, one-way ANOVA, Fisher's LSD post-test). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The results indicated that SDF1-ELP promoted higher numbers of CD31+ cells compared to free SDF1, ELP alone, or plain fibrin (vehicle) control (Fig. 6b).
Discussion and Conclusions
In this study, we investigated the properties of SDF1-ELP that may explain its superior in vivo performance to free SDF1 as discussed in our previous article.13 In vitro, SDF1-ELP promoted the migration of cells and induced vascularization similar to SDF1. However, when both SDF1-ELP and SDF1 were incubated in wound fluid ex vivo for an extended period of time, SDF1-ELP fusion protein nanoparticles were stable, while SDF1 lost its biological activity. When applied to an excisional wound made on the dorsal area of diabetic mice, SDF1-ELP instigated a higher amount of vascular endothelial cells compared to SDF1 and the remaining controls. Histological analysis of the harvested wound sections revealed that wounds treated with SDF1-ELP were fully reepithelialized by day 28, while the wounds treated with SDF1 and the other controls took 42 days to close.
We modeled the chemotactic potential of SDF1-ELP with HL-60 cells, which also expresses the CXCR4 receptor. We noted that a higher concentration of SDF1-ELP nanoparticles (250 nM or more) was needed to achieve the same migration effect as 10 nM of SDF1 because the migration effect mainly resulted from SDF1-ELP monomers that were released from the nanoparticles during the course of the assay. The nanoparticles acted as a depot, which slowly released monomers causing a gradient in the transwell, which instigated the movement of cells from the top to the bottom of the transwell. About 8% of SDF-ELP monomers were released from the nanoparticles during the migration assay during the 4-h migration experiment with HL-60 cells. We utilized a transport-reaction model to investigate key parameters within our monomer–nanoparticle system in the transwell migration assay and to compare the values of those parameters obtained under static in vitro environments. Our analysis demonstrates that the transwell system was transport limited.
We note that this observation is positive and attractive for in vivo applications. This is because unlike free SDF1 that is immediately released for use and could be available for rapid degradation by proteases, SDF1-ELP monomers, even if degraded by proteases, are expected to be regenerated by the depot of nanoparticles that will reside at the wound site.
Several investigators have reported the ability of SDF1 to not only attract endothelial progenitor cells but also to induce vascularization in endothelial cells. For example, Strasser et al.19 and Chen et al.20 reported angiogenesis induced by SDF1 in HUVECs, while Mirshahi et al.21 reported that SDF1 induced vascularization in human microvasculature endothelial cells. We observed that SDF1-ELP (and SDF1) induced vascularization using HUVEC cells similar to what had been previously reported.
However, despite the similar in vitro bioactivity of SDF1-ELP to SDF1, we observed SDF1-ELP to be more stable in ex vivo wound fluid when compared to SDF1. SDF1-ELP was stable after prolonged incubation in wound fluid, while free SDF1 was biologically inactive. This is similar to Olekson et al.'s22 report that SDF-1 was rendered biologically inactive, when incubated in wound fluid ex vivo.
As such, we used our animal studies to investigate if the better stability profile of SDF1-ELP in wound fluid will imply that it will enable a higher amount of endothelial cells in the wound area. As expected, SDF1-ELP promoted a higher amount of vascular endothelial cells throughout the wound healing timeline, compared to SDF1 and the remaining controls. This was corroborated by histological analysis, which indicated that wounds treated with SDF1-ELP were fully reepithelialized by day 28, while the wounds treated with SDF1 and the other controls were still open.
We therefore conclude that the stability of SDF1-ELP allows it to be retained in the chronic wound to promote a higher amount of revascularization, which leads a higher rate of wound healing compared to SDF1 and other controls. SDF1-ELP is a promising agent for the treatment of chronic skin wounds.
Innovation
The fusion of proteins to ELPs for purification purposes was reported in 1999.23 Due to their interesting biological properties, ELPs have been used in several biotechnology and pharmaceutical applications, including targeted delivery of therapeutic drugs24 and prolonging the half-life of drugs in vivo.25 The fusion of ELPs to KGF was successfully completed by Koria et al.15 However, the fusion of SDF1 to an ELP for use in chronic wounds is innovative. While this research has focused on chronic wounds, SDF1-ELP could be exploited in other injury types where SDF1 is needed to recruit stem cells to promote local tissue regeneration.
Abbreviations and Acronyms
- ANOVA
analysis of variance
- ATCC
American type culture collection
- bFGF
fibroblast growth factor
- Calcein AM
acetomethoxy derivate of calcein
- CD31
cluster of differentiation 31
- CXCR4
chemokine (C-X-C Motif) Receptor 4
- ELP
elastin-like peptide
- FDA
Food and Drug Administration
- GE
healthcare general electric healthcare
- HL-60
human leukemia-60 cells
- HUVEC
human umbilical vascular endothelial cells
- IMDM
Iscove's modified Dulbecco's medium
- KGF
keratinocyte growth factor
- NIH
National Institute of Health
- PBS
phosphate-buffered saline
- SDF1
stromal cell-derived growth factor 1
Acknowledgments and Funding Sources
This work was partially supported by the Shriners Hospitals for Children and the New Jersey Commission for Spinal Cord Research. A.Y. was supported by a National Institutes of Health-funded biotechnology training fellowship, and a Schlumberger Faculty for the Future fellowship. We would like to thank the Digital Imaging and Histology Core at the Rutgers-New Jersey Medical School Cancer Center for their histological staining services.
Author Disclosure and Ghostwriting
Authors have neither financial interests nor conflicts of interest to disclose. No ghostwriters were used in the writing of the article.
About the Authors
Agnes Yeboah, PhD, received her BSc in Chemical Engineering from Columbia University and her MSc and PhD in Chemical Engineering from Rutgers University. Tim Maguire, PhD, is a faculty at the Department of Biomedical Engineering at Rutgers University. He obtained his B.Sc. and PhD in Chemical Engineering from Rutgers University. Rene Schloss, PhD, is a faculty at the Department of Biomedical Engineering at Rutgers University. She obtained her PhD in Applied Immunology from Harvard University. Francois Berthiaume, PhD, is a faculty at the Department of Biomedical Engineering at Rutgers University. He obtained his PhD in Chemical Engineering from the Pennsylvania State University. Martin L. Yarmush, MD, PhD, is a faculty at the Department of Biomedical Engineering at Rutgers University and the Center for Engineering in Medicine, Massachusetts General Hospital, and Shriners Burns Hospital, Harvard Medical School. He obtained his PhD in Biophysical Chemistry from the Rockefeller University, his PhD studies in Chemical Engineering from the Massachusetts Institute of Technology, and his MD in Medicine from Yale University.
Key Findings.
• SDF1-ELP promotes the migration of cells and induces vascularization similar to SDF1 in vitro.
• SDF1-ELP is more stable in wound fluids compared to SDF1.
• SDF1-ELP induced a higher number of vascular endothelial cells compared to SDF1 and the remaining controls in vivo.
• SDF1-ELP is sustained longer at the wound site, allowing it to promote a higher amount of revascularization, which leads to a higher rate of wound healing compared to SDF1 and other controls. SDF1-ELP is a promising agent for the treatment of chronic skin wounds
References
- 1.Tellechea A, Leal E, Veves A, Carvalho E. Inflammatory and angiogenic abnormalities in diabetic wound healing: role of neuropeptides and therapeutic perspectives. Open Circ Vasc J 2010;3:43–55 [Google Scholar]
- 2.Edwards R, Harding K. Bacteria and wound healing. Curr Opin Infect Dis 2004;17:91–96 [DOI] [PubMed] [Google Scholar]
- 3.Eming SA, Kreig T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 2007;127:514–525 [DOI] [PubMed] [Google Scholar]
- 4.Grazul-Bilska A. Wound healing: the role of growth factors. Drugs Today 2003;39:787–800 [DOI] [PubMed] [Google Scholar]
- 5.Murphy PS, Evans GRD. Advances in wound healing: a review of current wound healing products. Plastic Surgery International 2012;2012:190436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarkar A, Tatildede S, Scherer SS, Orgill DP, Berthiaume F. Combination of stromal cell derived factor-1 and collagen-glycosaminoglycan scaffold delays contraction and accelerates reepithilization of dermal wounds in wild-type mice. Wound Repair Regen 2011;19:71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalka C, Masuda H, Takahashi T, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A 2000;97:3422–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majka SM, Jackson KA, Kienstra KA, Majesky MW, Goodell MA, Hirschi KK. Distinct progenitor populations in skelatal muscle are bone marrow derived and exhibit different cell fates during vascular regeneration. J Clin Invest 2003;111:71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 1999;5:434–438 [DOI] [PubMed] [Google Scholar]
- 10.Xu X, Zhu FQ, Zhang M, et al. Stromal cell-derived factor-1 enhances wound healing through recruiting bone marrow-derived mesenchymal stem cells to the wound area and promoting neovascularization. Cells Tissues Organs 2013;197:103–113 [DOI] [PubMed] [Google Scholar]
- 11.George AL, Bangalore-Prakash P, Rajoria S, et al. Endothelial progenitor cell biology in disease and tissue regeneration. J Hematol Oncol 2011;4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res 2010;16:2927–2931 [DOI] [PubMed] [Google Scholar]
- 13.Yeboah A, Cohen RI, Faulknor R, Schloss R, Yarmush ML, Berthiaume F. The development and characterization of SDF1α-elastin-like-peptide nanoparticles for wound healing. J Control Release 2016;232:238–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogani C, Ponziani V, Guglielmelli P, et al. Hypermethylation of CXCR4 promoter in CD34+ cells from patients with primary myelofibrosis. Stem Cells 2008;26:1920–1930 [DOI] [PubMed] [Google Scholar]
- 15.Koria P, Yagi H, Kitagawa Y, et al. Self-assembling elastin-like peptides growth factor chimeric nanoparticles for the treatment of chronic wounds. Proc Natl Acad Sci U S A 2011;108:1034–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arrio-Dupont M, Cribier S, Foucault G, Devaux PF, d'Albis A. Diffusion of fluorescently labeled macromolecules in cultured muscle cells. Biophys J 1996;70:2327–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aiuti A, Webb IJ, Bleulm C, Springer T. The chemokine SDF-1 is a chemoattractant for human CD34 hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34 progenitors to peripheral blood. J Exp Med 1997;185:111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bastaki M, Nelli EE, Dell'Era P, et al. Basic fibroblast growth factor-induced angiogenic phenotype in mouse endothelium. A study of aortic and microvascular endothelial cell lines. Arteriosclerosis, thrombosis, and vascular biology 1997;17:454–464 [DOI] [PubMed] [Google Scholar]
- 19.Strasser GA, Kaminker JS, Tessier-Lavigne M. Microarray analysis of retinal endothelial tip cells identifies CXCR4 as a mediator of tip cell morphology and branching. Blood 2010;115:5102–5110 [DOI] [PubMed] [Google Scholar]
- 20.Chen T, Bai H, Shao Y, et al. Stromal cell-derived factor-1/CXCR4 signaling modifies the capillary-like organization of human embryonic stem cell-derived endothelium in vitro. Stem cells 2007;25:392–401 [DOI] [PubMed] [Google Scholar]
- 21.Mirshahi F, Pourtau J, Li H, et al. SDF-1 activity on microvascular endothelial cells: consequences on angiogenesis in in vitro and in vivo models. Thromb Res 2000;99:587–594 [DOI] [PubMed] [Google Scholar]
- 22.Olekson MA, Faulknor R, Bandekar A, Sempkowski M, Hsia HC, Berthiaume F. SDF-1 liposomes promote sustained cell proliferation in mouse diabetic wounds. Wound Repair Regen 2015;23:711–723 [DOI] [PubMed] [Google Scholar]
- 23.Meyer DE, Chilkoti A. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat Biotechnol 1999;17:1112–1115 [DOI] [PubMed] [Google Scholar]
- 24.McDaniel JR, Callahan DJ, Chilkoti A. Drug delivery to solid tumors by elastin-like polypeptides. Adv Drug Deliv Rev 2010;62:1456–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amiram M, Luginbuhl KM, Li X, Feinglos MN, Chilkoti A. A depot-forming glucagon-like peptide-1 fusion protein reduces blood glucose for five days with a single injection. J Control Release 2013;172:144–151 [DOI] [PMC free article] [PubMed] [Google Scholar]