Abstract
Environmental enrichment (EE) confers significant benefits after experimental traumatic brain injury (TBI). In contrast, the antipsychotic drug (APD) haloperidol (HAL) exerts deleterious effects on neurobehavioral and cognitive recovery. Neurorehabilitation and management of agitation, however, are integral components of the treatment strategy for patients with TBI. Hence, the goal of this study was to determine how the two therapeutic approaches interact and influence motor and cognitive recovery. Anesthetized adult male rats received a controlled cortical impact (2.8 mm tissue deformation at 4 m/sec) or sham injury and then were provided HAL (0.5 mg/kg; intraperitoneally [IP]) or vehicle (VEH; 1 mL/kg; IP) commencing 24 h after surgery and once daily for 19 days while housed in EE or standard (STD) conditions. Beam balance/walk and Morris water maze performance were assessed on post-injury days 1–5 and 14–19, respectively, followed immediately by quantification of cortical lesion volumes. The data revealed both expected and unexpected findings. It was not surprising that the TBI groups receiving EE performed significantly better than those in STD housing and that the TBI + STD + HAL group performed worse than the TBI + STD + VEH group (p < 0.05). What was surprising was that the therapeutic effects of EE were greatly reduced by concomitant administration of HAL. No differences in cortical lesion volumes were observed among the groups (p > 0.05). The potential clinical implications of these findings suggest that administering HAL to patients undergoing neurorehabilitation may be a double-edged sword because agitation must be controlled before rehabilitation can be safely initiated and executed, but its use may compromise therapeutic efficacy.
Keywords: : antipsychotic drugs, behavioral outcome, controlled cortical impact, environmental enrichment, functional recovery, learning and memory, Morris water maze, traumatic brain injury
Introduction
Approximately 5.3 million persons in the United States are currently living with significant disabilities as a result of traumatic brain injury (TBI).1 When accounting for lost employment productivity and healthcare, the cost for long-term impairments of patients with TBI is estimated to be more than $76 billion, which is a significant economic burden and establishes TBI as a pertinent national healthcare issue.2–4 Of the survivors, many continue to have long-term motor, cognitive, and psychosocial deficits for which there are limited treatments.5–7
Neurorehabilitation is one of the more promising therapeutic strategies for TBI. TBI-induced agitation and, in some instances, aggression hamper the implementation of rehabilitation, however.8 Hence, antipsychotic drugs (APDs) are commonly used in an attempt to manage agitated patients with TBI 8–13 so that rehabilitation can be safely initiated and completed.
The use of APDs, however, in treating patients with TBI has been controversial.8,14 A traditionally administered APD is the first-generation drug haloperidol (HAL). HAL, a D2-receptor antagonist, acts diffusely and indiscriminately throughout the brain affecting post-synaptic D2 receptors.15 Antagonism of D2 receptors has been negatively implicated in the recuperation of motor function and cognition after injury, because these receptors are important for both abilities. Specifically, experimental studies using cortical ablation brain injury have shown that HAL inhibits amphetamine-induced recovery of binocular depth perception and slows motor recovery on a beam walking task.16,17 Moreover, HAL hinders restoration of spatial learning after fluid percussion brain injury or controlled cortical impact (CCI) relative to vehicle-treated controls.18–22
Environmental enrichment (EE), a rodent living condition that consists of a combination of complex inanimate and social stimulation, is considered a pre-clinical model of neurorehabilitation for TBI.23,24 EE promotes improved motor performance and enhances learning and memory after experimental TBI regardless of the model of TBI, sex, and age.23–32 Hence, EE is a relevant therapeutic strategy with potential for translation into the clinical population.
While single bouts of agitation and aggression are seen in the acute hospital setting (i.e., emergency department), an increase in the number of episodes and frequency of APD administration is more likely to be observed during chronic post-injury rehabilitation. Therefore, the goals of this study were to (1) evaluate the effects of combined EE and HAL on neurobehavioral and cognitive performance after TBI, which is both relevant and warranted,8,9,11 and (2) test the hypothesis that EE would attenuate the HAL-induced deleterious effects that have been reported.18–22
Methods
Subjects
Sixty adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 300–325 g on the day of surgery were housed in standard steel-wire mesh cages and maintained in a temperature (21 ± 1°C) and light (on 7:00 AM to 7:00 PM) controlled environment with ad libitum food and water. After 1 week of acclimatization, all rats underwent a single day of beam walk training, which consisted of 3–5 trials. After training, the rats were randomly assigned to one of the following group conditions: TBI + standard (STD) + vehicle (VEH) (1.0 mL/kg; n = 10), TBI + STD + HAL (0.5 mg/kg; n = 10), TBI + EE + VEH (n = 10), TBI + EE + HAL (n = 10), Sham + STD + VEH (n = 5), Sham + STD + HAL (n = 5), Sham + EE + VEH (n = 5), or Sham + EE + HAL (n = 5). All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. Every attempt was made to limit the number of rats used and to minimize suffering.
Surgery
A CCI injury was produced as described previously.31–36 Briefly, surgical anesthesia was induced and maintained with 4% and 2% isoflurane, respectively, in 2:1 N2O:O2. After the rats were intubated, they were secured in a stereotaxic frame and ventilated mechanically. Using aseptic procedures, a midline scalp incision was made, the skin and fascia were reflected to expose the skull, and a craniectomy (6 mm in diameter) was made in the right hemisphere (encompassing bregma and lambda and between the sagittal suture and the coronal ridge) with a high-speed dental drill. The bone flap was removed, and the craniectomy was enlarged further.
Subsequently, the impacting rod was extended and the impact tip (6 mm, flat) was centered and lowered through the craniectomy until it contacted the dura mater; then the rod was retracted and the impact tip was advanced 2.8 mm farther to produce a brain injury of moderate severity (2.8 mm tissue deformation at 4 m/sec). Core body temperature was maintained at 37 ± 0.5°C with a heating blanket. Immediately after the CCI, anesthesia was discontinued, and the incision was promptly sutured. The rats were subsequently extubated and assessed for acute neurological outcome. Sham rats underwent similar surgical procedures but were not subjected to the impact.
Acute neurological evaluation
Hind limb reflexive ability was assessed immediately after the cessation of anesthesia by gently squeezing a rat's paw with forceps every 5 sec and recording the time to elicit a withdrawal response. Return of the righting reflex was determined by the time needed to turn from the supine to prone position. These tests are sensitive indicators of injury severity and duration of anesthesia.31–37
Housing conditions: Environmental manipulation
After surgery and after the effects of anesthesia abated (as evidenced by spontaneous movement in the holding cage), the rats were returned to the colony where those designated for enrichment were immediately placed in specifically designed steel-wire cages (91 × 76 × 50 cm). The EE cages consisted of three levels with ladders to ambulate from one level to another and contained various toys (e.g., balls, blocks, and tubes), nesting materials (e.g., paper towels), and ad libitum food and water.25,26 To maintain novelty, the objects were rearranged every day and changed each time the cage was cleaned, which was twice per week. Ten to 12 rats, which included HAL and vehicle-treated TBI and sham controls, were housed in the EE together to minimize variability among the groups. Rats in the STD conditions were placed in typical shoebox cages (37 × 25 × 18 cm, two rats per cage) with only food and water.
Drug administration
HAL (Sigma, St. Louis, MO) was prepared daily by dissolving in 1:1 dimethyl sulfoxide (DMSO)/saline, which also served as the VEH. The dose of HAL was chosen because it has been reported to be comparable to that used clinically to control psychosis38 and has been used in several brain injury studies investigating functional outcome.16–22 Treatments began 24 h after CCI or sham surgery and were provided IP once daily for 19 days. The half-life of HAL using this dose and route is reported to be 2.6 h,39 and thus it was provided after the daily behavioral assessments to circumvent sedative effects, which may confound the results. The biological effect of HAL, however, was active during the exposure to the EE because they were housed continuously.
Motor performance
Motor function was assessed using the well-established beam balance and beam walk tasks.31–37 Briefly, beam balance consisted of placing the rat on an elevated narrow beam (1.5 cm wide) and recording the time it remained on for a maximum of 60 sec. Beam walk consisted of recording the elapsed time to traverse the beam (2.5 cm wide ×100 cm long). Testing was conducted approximately 1 h before surgery (to establish a baseline measure), as well as on post-operative days 1–5, and consisted of three trials (60 sec allotted time with an intertrial interval of 30 sec) per day on each task. The average daily scores for each subject were used in the statistical analyses.
Cognitive function: Acquisition of spatial learning
Spatial learning was assessed in a Morris water maze task that has been shown to be sensitive to cognitive function after TBI.31–37,40–43 Briefly, the maze consisted of a plastic pool (180 cm diameter; 60 cm high) filled with tap water (26 ± 1°C) to a depth of 28 cm and was situated in a room with salient visual cues that remained constant throughout the study. The platform was a clear Plexiglas stand (10 cm diameter, 26 cm high) that was positioned 26 cm from the maze wall in the southwest quadrant and held constant for each rat.
Spatial learning began on post-operative day 14 and consisted of providing a block of four daily trials (4-min intertrial interval) for 5 consecutive days (14–18) to locate the platform when it was submerged 2 cm below the water surface. On day 19 the platform was made visible to the rats by raising it 2 cm above the water surface as a control procedure to determine the contributions of nonspatial factors (e.g., sensory-motor performance, motivation, and visual acuity) on cognitive performance.
For each daily block of trials, the rats were placed in the pool facing the wall at each of the four possible start locations (north, east, south, and west) in a randomized manner. Each trial lasted until the rat climbed onto the platform or until 120 sec had elapsed, whichever occurred first. Rats that failed to locate the goal within the allotted time were manually guided to it. All rats remained on the platform for 30 sec before being placed in a heated incubator between trials. The times of the four daily trials for each rat were averaged and used in the statistical analyses.
Cognitive function: Probe trial (memory retention)
One day after the final acquisition training session (i.e., day 19), all rats were given a single probe trial to measure memory retention. Briefly, the platform was removed from the pool, and the rats were placed in the maze from the location point distal to the quadrant where the platform was situated previously (i.e., “target quadrant”) and allowed to freely explore the pool for 30 sec. The percent time spent in the target quadrant was used in the statistical analysis.
Histology: quantification of cortical lesion volume
After the last behavioral assessment (i.e., post-operative day 19), the rats were anesthetized with pentobarbital (50 mg/kg IP) and then perfused transcardially with 200 mL 0.1 M phosphate buffered saline (pH 7.4) followed by 300 mL 4% paraformaldehyde. The brains were extracted, post-fixed in the perfusate for 1 week, dehydrated with alcohols, and embedded in paraffin. Coronal sections (7-μm thick) were cut at 1-mm intervals through the lesion on a microtome and mounted on microscope slides. After drying at room temperature, the sections were deparaffinized in xylenes, rehydrated, and stained with cresyl violet.
An observer blinded to experimental conditions analyzed the cortical lesion volumes (mm3) using a Nikon Eclipse 90i microscope. The area of the lesion (mm2) was first calculated by outlining the inferred area of missing cortical tissue for each section (typically 5–7) and then by summing the lesions obtained, as reported previously. 25,26,32
Data analysis
Statistical analyses were performed on data collected by observers blinded to treatment conditions using Statview 5.0.1 software. The motor and cognitive data were analyzed by repeated-measures analysis of variance (ANOVA). Acute neurological assessments and swim speed were analyzed by one-factor ANOVAs. When the overall ANOVAs revealed a significant effect, the Newman-Keuls post hoc test was used to determine specific group differences. The data are presented as the mean ± standard error of the mean (SEM) and are considered significant when corresponding p values are ≤0.05.
Results
Two rats (one from the TBI + STD + VEH group and one from the TBI + EE + HAL group) were excluded from the study because of an inability to locate the visible platform, which may be indicative of visual acuity deficits and therefore could be a potential confound given the necessity to see the cues located on the walls to acquire spatial learning. Hence, statistical analyses are based on 58 rats. There were no significant differences in any assessment among the sham control groups, regardless of treatment (VEH, HAL or housing), and thus the data were pooled into a single Sham group.
Acute neurological assessments
No significant differences were observed among the TBI groups for return of hindlimb reflex ability after a brief paw pinch (range for right: 157.2 ± 6.41 – 170.5 ± 7.4; range for left: 161.3 ± 6.3 – 174.8 ± 7.6) or righting reflex latency (range: 387.1 ± 24.2 – 440.4 ± 38.7). The lack of significant differences in post-surgical neurological assessments among the TBI groups indicates that all rats received similar levels of anesthesia and injury severity.
Motor function: Beam balance
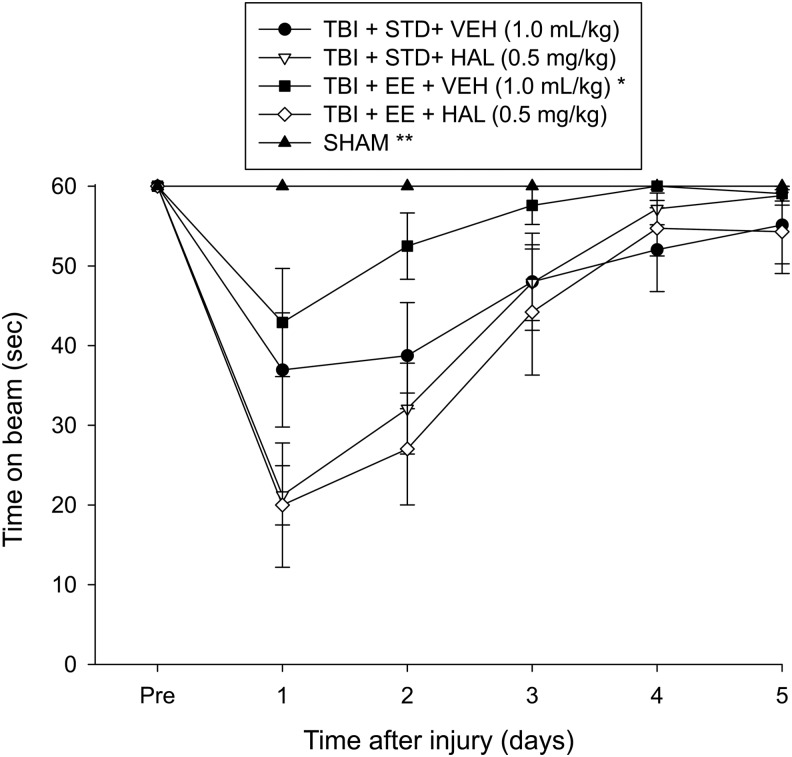
No pre-surgical differences were observed among groups because all rats were capable of balancing on the beam for the allotted 60 sec (Fig. 1). After TBI, the repeated-measures ANOVA revealed significant group (F4,53 = 10.937, p < 0.0001) and day (F5,265 = 54.379, p < 0.0001) differences, as well as a significant group × day interaction (F20,265 = 7.606, p < 0.0001). The post hoc analysis revealed that all TBI groups were significantly impaired compared with the Sham group, which was able to maintain balance for the full 60 sec (p's < 0.05). Among the TBI groups, the TBI + EE + VEH performed markedly better than the TBI + STD + VEH, TBI + STD + HAL, and TBI + EE + HAL groups (p's < 0.05). No difference was revealed between the TBI + STD + VEH and TBI + EE + HAL groups (p > 0.05). All other comparisons were statistically insignificant.
FIG. 1.
Mean (± standard error of the mean) time (sec) balancing on an elevated narrow beam before and after traumatic brain injury (TBI) or sham injury. *p < 0.05 vs. TBI + STD + VEH, TBI + STD + HAL, and TBI + EE + HAL. **p < 0.05 vs. all TBI groups. No other comparisons were statistically different. STD, standard; VEH, vehicle; HAL, haloperidol; EE, environmental enrichment.
Motor function: Beam walk
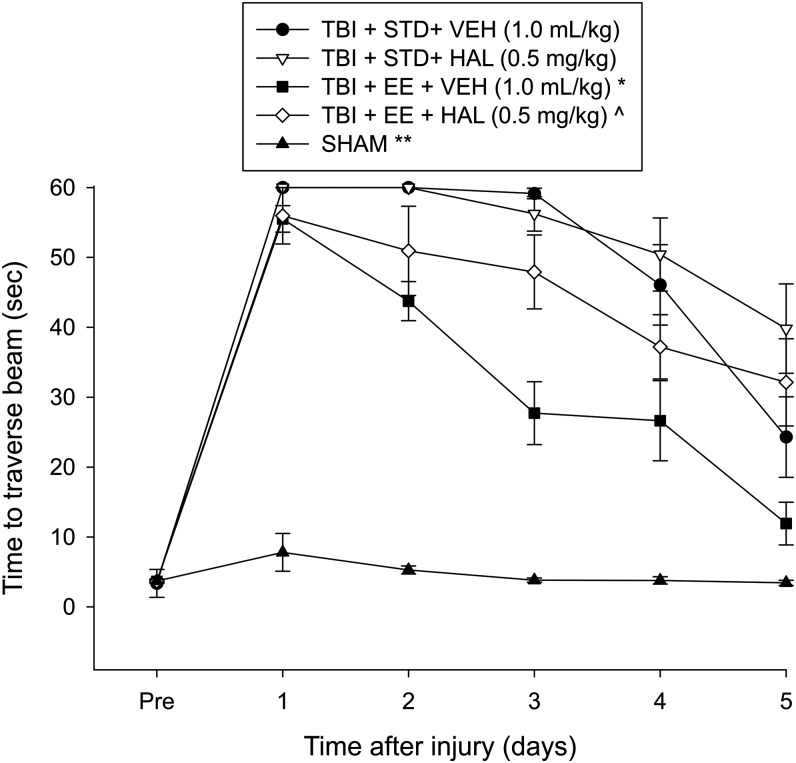
No pre-surgical differences in time to traverse the beam were revealed among groups as all rats were proficient and reached the goal box in approximately 5 sec (Fig. 2). After TBI, the repeated-measures ANOVA revealed significant group (F4,53 = 111.602, p < 0.0001) and day [F5,265 = 186.276, p < 0.0001] differences, as well as a significant group × day interaction (F20,265 = 19.402, p < 0.0001).
FIG. 2.
Mean (± standard error of the mean) time (sec) to traverse an elevated narrow beam before and after traumatic brain injury (TBI) or sham injury. *p < 0.05 vs. TBI + STD + VEH, TBI + STD + HAL, and TBI + EE + HAL. ^p < 0.05 vs. TBI + STD + HAL. **p < 0.05 vs. all TBI groups. No other comparisons were statistically different. STD, standard; VEH, vehicle; HAL, haloperidol; EE, environmental enrichment.
The post hoc analysis revealed that all TBI groups were significantly impaired relative to the Sham group (p's < 0.05). The TBI + EE + VEH group traversed the beam significantly quicker than the TBI + STD + VEH, TBI + STD + HAL, and TBI + EE + HAL groups (p < 0.05). Moreover, the TBI + EE + HAL group performed better than the TBI + STD + HAL group (p < 0.05), but did not differ from the TBI + STD + VEH group (p > 0.05). No other comparisons were statistically different.
Cognitive function: Acquisition of spatial learning
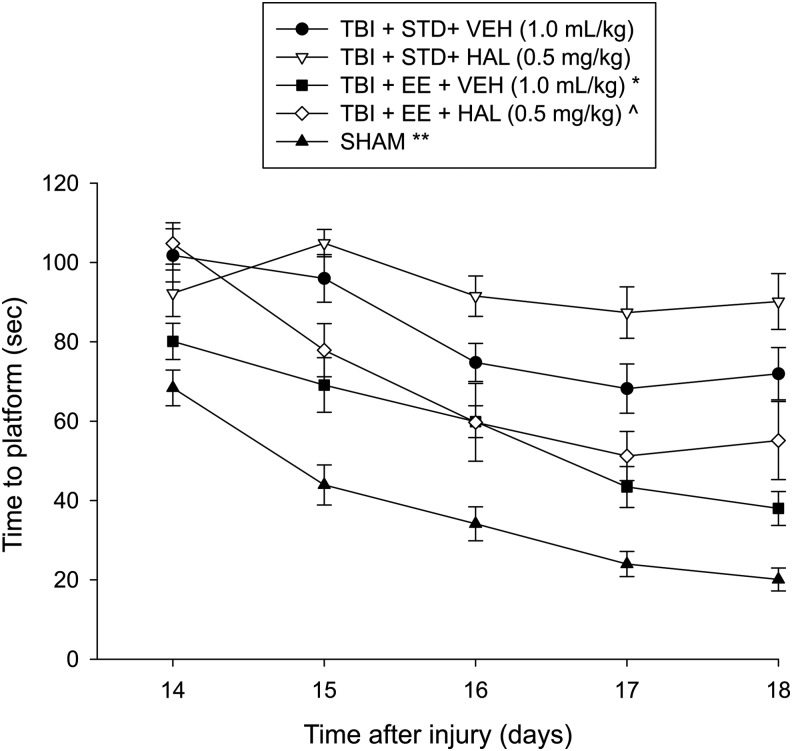
Analysis of the MWM data revealed significant group (F4,53 = 38.405, p < 0.0001) and day (F4,212 = 52.881, p < 0.0001) differences, as well as a significant group × day interaction (F16,212 = 7.606, p < 0.0001). Post hoc analyses specified that the Sham group was significantly better than all TBI groups (p's < 0.0001). The TBI + EE + VEH group was able to locate the escape platform significantly quicker over time versus the TBI + STD + VEH, TBI + STD + HAL, and TBI + EE + HAL groups (p < 0.05; Fig. 3). Moreover, the TBI + STD + VEH group performed better than the TBI + STD + HAL group (p < 0.05). Last, the TBI + EE + HAL group found the platform faster than the TBI + STD + VEH and TBI + STD + HAL groups (p's < 0.05).
FIG. 3.
Mean (± standard error of the mean) time (sec) to locate a hidden (submerged) platform in a water maze. *p < 0.05 vs. TBI + STD + VEH, TBI + STD + HAL, and TBI + EE + HAL. ^p < 0.05 vs. TBI + STD + VEH and TBI + STD + HAL. **p < 0.05 vs. all TBI groups. No other comparisons were statistically different. STD, standard; VEH, vehicle; HAL, haloperidol; EE, environmental enrichment.
No significant differences in swim speed (range = 29.5 ± 0.8 cm/sec to 32.2 ± 0.9 cm/sec) were observed among any of the TBI or Sham groups (p > 0.05). In contrast, the Sham control group located the visible platform sooner than all TBI groups, regardless of treatments (i.e., drug or housing condition). No differences in visible platform performance were revealed among the TBI groups (p > 0.05).
Cognitive function: Probe trial
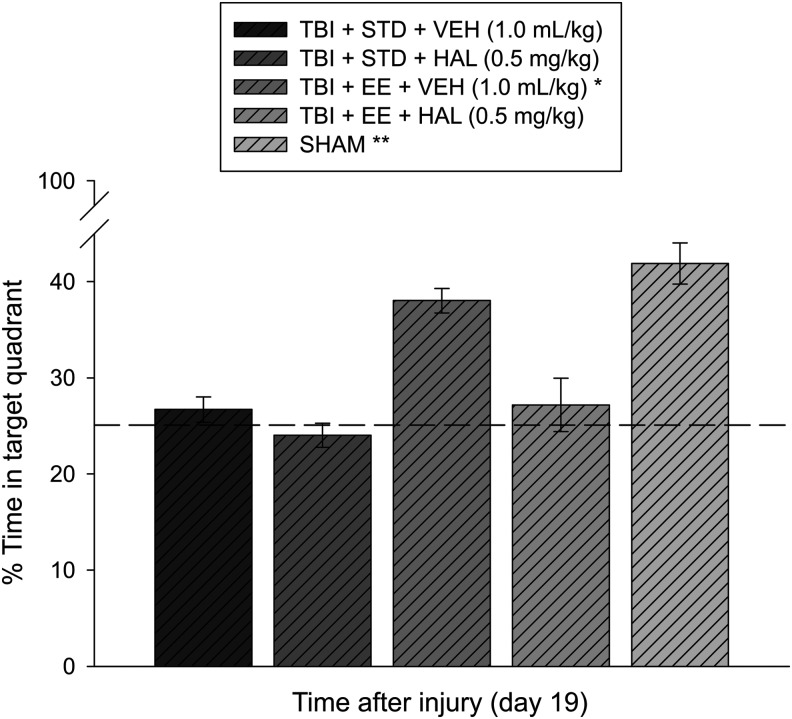
Analysis of the probe data revealed significant memory retention in the Sham and TBI + EE + VEH groups as evidenced by a greater percentage of the allotted time spent in the target quadrant (41.9 ± 3.1% and 38.0 ± 1.3%, respectively) versus the TBI + STD + VEH (26.7 ± 1.3%), TBI + STD + HAL (24.2 ± 1.3%), and TBI + EE + HAL (27.2 ± 2.8%) (p's < 0.05). No significant differences were revealed between the Sham controls and the TBI + EE + VEH group (p > 0.05) or among the other TBI comparisons (p's > 0.05; Fig. 4).
FIG. 4.
Mean (± standard error of the mean) percentage of time spent in the target quadrant (i.e., where platform was previously located) after a single probe trial 19 days after cortical impact or sham injury. *p < 0.05 vs. TBI + STD + VEH, TBI + STD + HAL, and TBI + EE + HAL. **p < 0.05 vs. TBI + STD + VEH, TBI + STD + HAL, and TBI + EE + HAL. No significant difference was revealed between the Sham and TBI + EE + VEH groups (p > 0.05). The dotted line represents performance at the chance level (25%). STD, standard; VEH, vehicle; HAL, haloperidol; EE, environmental enrichment.
Histology: Quantification of cortical lesion volume
Analysis of the cortical lesion volume data did not reveal a significant difference among the groups (F3,16 = 0.737, p = 0.54). Specifically, the lesion volumes were: TBI + STD + VEH (53.6 ± 3.3 mm3), TBI + STD + HAL (47.7 ± 4.8 mm3), TBI + EE + VEH (46.6 ± 1.6 mm3), and TBI + EE + HAL (50.0 ± 3.8 mm3).
Discussion
TBI survivors often endure motor and long-term cognitive deficits44–46 as well as disruptive behaviors such as agitation and aggression.47–50 These behavioral dysfunctions, however, require different, and likely overlapping, therapeutic interventions. EE, a pre-clinical model of neurorehabilitation has been shown to enhance motor and cognitive performance after TBI.23–30 In marked contrast, the APD HAL, which is given to manage TBI-induced agitation, impedes neurobehavioral and cognitive recovery.16–22 Nonetheless, agitation in a rehabilitation setting must be managed before therapies to alleviate motor and cognitive deficits are implemented.
Therefore, the aim of this study was to evaluate behavioral recovery in adult male rats that received a TBI via a CCI injury of moderate severity and subsequently were provided HAL or VEH daily while being housed in EE or STD conditions. It was hypothesized that EE alone would enhance functional outcome, as has been demonstrated previously.23–30 It was further hypothesized that EE provided concomitantly with HAL would attenuate the APD-induced deleterious effects that have been shown to occur after CCI or fluid percussion brain injury.19–22 As summarized below, the results from this study support the hypotheses, but also divulge an unanticipated finding.
Our results suggest that motor function assessed with the well-established beam balance and beam walk tasks, which measure gross and fine motor capabilities, respectively, was significantly impaired in all TBI groups relative to Sham controls. The TBI group receiving EE + VEH, however, performed both tasks better than the VEH and HAL-treated injured groups housed in STD conditions as well as, interestingly, the TBI group receiving HAL while housed in EE. Moreover, the brain injured EE + HAL group did not differ from the STD + HAL group on the beam balance task.
Assessment of spatial learning and memory in a MWM task revealed that the TBI groups housed in EE, regardless of whether receiving daily injections of VEH or HAL, were able to learn the location of the escape platform significantly faster than the STD-housed TBI groups. The EE + HAL group, however, was significantly impaired versus the EE + VEH group. Given that the only methodological difference between the TBI groups exposed to EE was the inclusion or exclusion of HAL, the findings indicate that EE reduced the deleterious effects of HAL, but HAL in turn attenuated the EE-mediated benefits. These findings render the combination of EE and HAL a double-edged sword.
Exposure to EE may confer its task-specific benefits via a series of neuroplastic changes that initiate immediately on immersion into an enriched, stimulating environment, such as hippocampal neurogenesis, synaptic strengthening, and increased expression of several neurotrophic factors.51–55 Motor improvement seen in the acute recovery period (testing days 1–5), could be reliant on enhanced neurogenesis,56,57 which is thought to be primed by the increased space available for locomotion within the larger EE cage and in turn facilitate beam walking ability. A study by Sozda and colleagues26 in 2010, however, showed that the typical EE paradigm (i.e., combination of motor, sensory, and social components) provided the greatest motor benefits compared with atypical EE manipulations, each lacking one of the three components; thus, it is not just an increase in physical activity that mediates motor recovery, but most likely a complex interaction of all three EE components.
The reduction of HAL-induced detrimental effects on motor and cognitive performance after TBI by EE was not surprising given that it reliably and robustly enhances these behaviors.23–30 Moreover, EE has also been shown in non-TBI models to attenuate negative or disruptive behaviors such as dependence and voluntary consumption of morphine,58 heroin seeking,59 methamphetamine-induced withdrawal symptoms,60 and reinstatement of ethanol-induced conditioned place preference.61
The protective effects of EE may also partially result from the inoculation stress hypothesis, put forth by Crofton and colleagues,62 which posits that mild stress associated with living in a complex setting and interacting with conspecifics in a nonaggressive manner “inoculates” the rats against consequent stressors and/or drugs of abuse. This theory could certainly be extrapolated to explain the EE-related attenuation of HAL-induced detrimental effects observed in the current study.
Despite its robust effects after TBI, EE is usually provided alone or in combination with pharmacotherapies that are capable of exerting benefit on their own, but not with those that produce deleterious effects. Nevertheless, while unexpected, the attenuation of EE's efficacy by concomitant administration of HAL is a sensible result given that HAL has consistently been shown to impair recovery acutely and chronically after TBI.19–22 Indeed, the current data show that in STD-housed rats, HAL once again significantly impaired the acquisition of spatial learning relative to the VEH-treated group. These findings indicate that the robust negative effects of HAL on behavioral outcome cannot be entirely overturned by EE, despite no difference in cortical lesion size among the groups.
Previous studies further characterized the inability of HAL to provide beneficial effects or protect against stress-related detrimental effects, respectively. Chronic HAL treatment impaired working memory in a cross-maze task63 whereas extended administration (up to 320 days) impeded various performance parameters in the acquisition and performance of a two–radial-arm maze task and a five-choice serial reaction-time task.64 Also, Klitenick and colleagues65 in 1996 hypothesized that chronic HAL administration in rats would attenuate acute stress-induced (i.e., tail pinch) dopamine (DA) activation, but surprisingly found that HAL failed to impair the ability of tail pinch stress to increase DA release in the nucleus accumbens.
In more recent studies, Park and coworkers66 in 2013 showed that dendritic outgrowth in rat hippocampal neurons was induced by olanzapine (OLZ) and aripiprazole (ARIP), whereas HAL did not confer such benefits. Further, unlike the atypical APDs OLZ and ARIP, HAL administration (1 mg/kg, IP for 3 weeks) did not attenuate chronic immobilization stress-induced decreases in the expression of synapse-associated proteins (e.g., GSK-3β phosphorylation, β-catenin) in the frontal cortex.67
HAL administration, which acts as a high-affinity D2 post-synaptic receptor blocker, is also known to produce a plethora of cellular and anatomical changes in the central nervous system, such as enhanced DA turnover in the rat striatum,68 which could result in excessive production of damaging free radicals, such as hydrogen peroxide, superoxide radical, and hydroxyl radical, through oxidation processes and reacting with iron or copper ions,69 as well as tyrosine hydroxylase (TH) depletion in the substantia nigra.70 Both the central DA blockade and TH depletion effects persisted for at least 1 month after drug withdrawal.70,71 In addition, APDs are thought to induce cellular dysfunction through loss of antioxidant enzymes and increased lipid peroxidation,72 as well as oxidative stress, mitochondrial dysfunction, and even cell death.73–75
Conversely, some of these detrimental effects may be successfully overcome by EE exposure. In a study employing transient global cerebral ischemic injury in rats, Briones and associates76 in 2011 demonstrated that EE housing post-insult significantly decreased oxidative damage and neuronal degeneration in the hippocampus,76 likely through the modulation of glutamate activation, which is pivotal to both excitotoxicity and neural plasticity in TBI models. Moreover, synaptic glutamate receptor activation may also boost the antioxidant defenses of neurons by detoxifying peroxide and reactive oxygen species through the thioredoxin–peroxiredoxin system.77 Additional support for the premise that the deleterious effect of HAL on behavioral outcome after TBI is mediated, at least in part, by antagonizing D2 receptors comes from several independent studies showing that D2 receptor agonists confer significant beneficial effects after TBI.78–83
Conclusion
The data support the hypothesis that EE facilitates both motor and cognitive recovery after injury, even in the presence of the APD HAL, a treatment implicated in harmful effects on neurobehavioral outcome when provided alone. HAL-treated rats housed in EE exhibited functional benefits relative to HAL-treated rats housed in STD conditions, and thereby demonstrate EE's ability to attenuate the deleterious effects of the APD. The results, however, also demonstrated that HAL administration diminished the efficacy of EE.
These findings are important with regard to the translation of EE as a multimodal model of clinical neurorehabilitation. For patients receiving the clinical correlate of EE, the concurrent, routine administration of typical APDs such as HAL to subdue episodes of agitation could possibly hinder the individual's potential for a greater level of recovery. Alternatively, this also means that patients who require APDs should have more rigorous rehabilitation goals to enhance the potency of the treatment and elicit the greatest benefit possible. To expand these findings into a more clinically relevant paradigm, currently ongoing and future studies will provide a more thorough understanding of the effect of a combination of APDs with delayed and/or abbreviated daily periods of EE on functional recovery after experimental TBI, which may help to guide translational application.
Acknowledgments
This work was supported, in part, by the National Institutes of Health grants R01NS060005, R01HD069620, HD069620-S1, R01NS084967 (AEK) and the University of Pittsburgh Physicians /UPMC Academic Foundation (COB).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Thurman D.J., Alverson C., Dunn K.A., Guerrero J., and Sniezek J.E. (1999). Traumatic brain injury in the United States: a public health perspective. J. Head Trauma Rehabil. 14, 602–615 [DOI] [PubMed] [Google Scholar]
- 2.Coronado V.G., McGuire L.C., Sarmiento K., Bell J., Lionbarger M.R., Jones C.D., Geller A.I., Khoury N., and Xu L. (2012). Trends in traumatic brain injury in the U.S. and the public health response: 1995–2009. J. Safety Res. 43, 299–307 [DOI] [PubMed] [Google Scholar]
- 3.Faul M., Xu L., Wald M.M., and Coronado V.G. (2010). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, Ga [Google Scholar]
- 4.Langlois J.A., Rutland-Brown W., and Thomas K.E. (2004). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, Ga [Google Scholar]
- 5.Selassie A.W., Zaloshnja E., Langlois J.A., Miller T., Jones P., and Steiner C. (2008). Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J. Head Trauma Rehabil. 23, 123–131 [DOI] [PubMed] [Google Scholar]
- 6.Millis S.R., Rosenthal M., Novack T.A., Sherer M., Nick T.G., Kreutzer J.S., High W.M., Jr., and Ricker J.H. (2001). Long-term neuropsychological outcome after traumatic brain injury. J. Head Trauma Rehabil. 16, 343–355 [DOI] [PubMed] [Google Scholar]
- 7.Maas A.I., Stocchetti N., and Bullock R. (2008). Moderate and severe traumatic brain injury in adults. Lancet Neurol. 7, 728–741 [DOI] [PubMed] [Google Scholar]
- 8.Chew E., and Zafonte R.D. (2009). Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J. Rehabil. Res. Dev. 46, 851–879 [DOI] [PubMed] [Google Scholar]
- 9.Lombard L.A., and Zafonte R.D. (2005). Agitation after traumatic brain injury: considerations and treatment options. Am. J. Phys. Med. Rehabil. 84, 797–812 [DOI] [PubMed] [Google Scholar]
- 10.McNett M., Sarver W., and Wilczewski P. (2012). The prevalence, treatment and outcomes of agitation among patients with brain injury admitted to acute care units. Brain Inj. 26, 1155–1162 [DOI] [PubMed] [Google Scholar]
- 11.Elovic E.P., Jasey N.N., and Eisenberg M.E. (2008). The use of atypical antipsychotics after traumatic brain injury. J. Head Trauma Rehabil. 23, 132–135 [DOI] [PubMed] [Google Scholar]
- 12.Rao V., Koliatsos V., Ahmed F., Lyketsosm C., and Kortte K. (2015). Neuropsychiatric disturbances associated with traumatic brain injury: a practical approach to evaluation and management. Semin. Neurol. 35, 64–82 [DOI] [PubMed] [Google Scholar]
- 13.Stanislav S.W. (1997). Cognitive effects of antipsychotic agents in persons with traumatic brain injury. Brain Inj. 11, 335–341 [DOI] [PubMed] [Google Scholar]
- 14.Saoût V., Gambart G., Leguay D., Ferrapie A.L., Launay C., and Richard I. (2011). Aggressive behavior after traumatic brain injury. Ann. Phys. Rehabil. Med. 54, 259–269 [DOI] [PubMed] [Google Scholar]
- 15.Roemer R.A., Richelson E., Shagass C., and Leventhal L. (1996). A method to estimate in vivo D2 receptor occupancy by antipsychotic drugs. J. Psychiatry Neurosci. 21, 325–333 [PMC free article] [PubMed] [Google Scholar]
- 16.Hovda D.A., and Feeney D.M. (1985). Haloperidol blocks amphetamine induced recovery of binocular depth perception after bilateral visual cortex ablation in cat. Proc. West Pharmacol. Soc. 28, 209–211 [PubMed] [Google Scholar]
- 17.Feeney D.M., Gonzalez A., and Law W.A. (1982). Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science 217, 855–857 [DOI] [PubMed] [Google Scholar]
- 18.Wilson M.S., Gibson C.J., and Hamm R.J. (2003). Haloperidol, but not olanzapine, impairs cognitive performance after traumatic brain injury in rats. Am. J. Phys. Med. Rehabil. 82, 871–879 [DOI] [PubMed] [Google Scholar]
- 19.Kline A.E., Massucci J.L., Zafonte R.D., Dixon C.E., DeFeo J.R., and Rogers E.H. (2007). Differential effects of single versus multiple administrations of haloperidol and risperidone on functional outcome after experimental brain trauma. Crit. Care Med. 35, 919–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman A.N., Cheng J.P., Zafonte R.D., and Kline A.E. (2008). Administration of haloperidol and risperidone after neurobehavioral testing hinders the recovery of traumatic brain injury-induced deficits. Life Sci. 83, 602–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kline A.E., Hoffman A.N., Cheng J.P., Zafonte R.D., and Massucci J.L. (2008). Chronic administration of antipsychotics impede behavioral recovery after experimental traumatic brain injury. Neurosci. Lett. 448, 263–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phelps T.I., Bondi C.O., Ahmed R.H., Olugbade Y.T., and Kline A.E. (2015). Divergent long-term consequences of chronic treatment with haloperidol, risperidone, and bromocriptine on traumatic brain injury-induced cognitive deficits. J. Neurotrauma 32, 590–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bondi C.O., Klitsch K.C., Leary J.B., and Kline A.E. (2014). Environmental enrichment as a viable neurorehabilitation strategy for experimental traumatic brain injury. J. Neurotrauma 31, 873–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bondi C.O., Semple B.D., Noble-Haeusslein L.J., Osier N.D., Carlson S.W., Dixon C.E., Giza C.C., and Kline A.E. (2015). Found in translation: Understanding the biology and behavior of experimental traumatic brain injury. Neurosci Biobehav Rev. 58, 123–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kline A.E., Wagner A.K., Westergom B.P., Malena R.R., Zafonte R.D., Olsen A.S., Sozda C.N., Luthra P., Panda M., Cheng J.P., and Aslam H.A. (2007). Acute treatment with the 5-HT1A receptor agonist 8-OH-DPAT and chronic environmental enrichment confer neurobehavioral benefit after experimental brain trauma. Behav. Brain Res. 177, 186–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sozda C.N., Hoffman A.N., Olsen A.S., Cheng J.P., Zafonte R.D., and Kline A.E. (2010) Empirical comparison of typical and atypical environmental enrichment paradigms on functional and histological outcome after experimental traumatic brain injury. J. Neurotrauma 27, 1047–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamm R.J., Temple M.D., O'Dell D.M., Pike B.R., and Lyeth B.G. (1996). Exposure to environmental complexity promotes recovery of cognitive function after traumatic brain injury. J. Neurotrauma 13, 41–47 [DOI] [PubMed] [Google Scholar]
- 28.Passineau M.J., Green E.J., and Dietrich W.D. (2001). Therapeutic effects of environmental enrichment on cognitive function and tissue integrity following severe traumatic brain injury in rats. Exp. Neurol. 168, 373–384 [DOI] [PubMed] [Google Scholar]
- 29.Hicks R.R., Zhang L., Atkinson A., Stevenon M., Veneracion M., and Seroogy K.B. (2002). Environmental enrichment attenuates cognitive deficits, but does not alter neurotrophin gene expression in the hippocampus following lateral fluid percussion brain injury. Neuroscience 112, 631–637 [DOI] [PubMed] [Google Scholar]
- 30.Briones T.L., Woods J., and Rogozinska M. (2013). Decreased neuroinflammation and increased brain energy homeostasis following environmental enrichment after mild traumatic brain injury is associated with improvement in cognitive function. Acta Neuropathol. Commun. 1, 57. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Monaco C.M., Gebhardt K.M., Chelbowski S.M., Shaw K.E., Cheng J.P., Henchir J.J., Zupa M.F., and Kline A.E. (2014). A combined therapeutic regimen of buspirone and environmental enrichment is more efficacious than either alone in enhancing spatial learning in brain-injured pediatric rats. J. Neurotrauma 31, 1934–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monaco C.M., Mattiola V.V., Folweiler K.A., Tay J.K., Yelleswarapu N.K., Curatolo L.M., Matter A.M., Cheng J.P., and Kline A.E. (2013). Environmental enrichment promotes robust functional and histological benefits in female rats after controlled cortical impact injury. Exp. Neurol. 247, 410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixon C.E., Clifton G.L., Lighthall J.W., Yaghmai A.A., and Hayes R.L. (1991). A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods 39, 253–262 [DOI] [PubMed] [Google Scholar]
- 34.Kline A.E., McAloon R.L., Henderson K.A., Bansal U.K., Ganti B.M., Ahmed R.H., Gibbs R.B., and Sozda C.N. (2010). Evaluation of a combined therapeutic regimen of 8-OH-DPAT and environmental enrichment after experimental traumatic brain injury. J. Neurotrauma 27, 2021–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Witt B.W., Ehrenberg K.M., McAloon R.L., Panos A.H., Shaw K.E., Raghavan P.V., Skidmore E.R., and Kline A.E. (2011). Abbreviated environmental enrichment enhances neurobehavioral recovery comparably to continuous exposure after traumatic brain injury. Neurorehabil. Neural Repair 25, 343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matter A.M., Folweiler K.A., Curatolo L.M., and Kline A.E. (2011). Temporal effects of environmental enrichment-mediated functional improvement after experimental traumatic brain injury in rats. Neurorehabil. Neural Repair 25, 558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bondi C.O., Cheng J.P., Tennant H.M., Monaco C.M., and Kline A.E. (2014). Old dog, new tricks: the attentional set-shifting test as a novel cognitive behavioral task after controlled cortical impact injury. J. Neurotrauma 31, 926–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosengarten H., and Quartermain D. (2002). The effect of chronic treatment with typical and atypical antipsychotics on working memory and jaw movements in three-and eighteen-month-old rats. Prog. Neuropsychopharmacol. Bio. Psychiatry 26, 1047–1054 [DOI] [PubMed] [Google Scholar]
- 39.Kapetanovic I.M., Sweeney D.J., and Rapoport S.I. (1982). Age effects on haloperidol pharmacokinetics in male, Fischer-344 rats. J. Pharmacol. Exp. Ther. 221, 434–438 [PubMed] [Google Scholar]
- 40.Kline A.E., Massucci J.L., Marion D.W., and Dixon C.E. (2002). Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J. Neurotrauma 19, 415–425 [DOI] [PubMed] [Google Scholar]
- 41.Kline A.E., Massucci J.L., Ma X., Zafonte R.D., and Dixon C.E. (2004). Bromocriptine reduces lipid peroxidation and enhances spatial learning and hippocampal neuron survival in a rodent model of focal brain trauma. J. Neurotrauma 21, 1712–1722 [DOI] [PubMed] [Google Scholar]
- 42.Hamm R.J., Dixon C.E., Gbadebo D.M., Singha A.K., Jenkins L.W., Lyeth B.G., and Hayes R.L. (1992). Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J. Neurotrauma 9, 11–20 [DOI] [PubMed] [Google Scholar]
- 43.Scheff S.W., Baldwin S.A., Brown R.W., and Kraemer P.J. (1997). Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J. Neurotrauma 14, 615–627 [DOI] [PubMed] [Google Scholar]
- 44.Mazaux J.M., Masson F., Levin H.S., Alaoui P., Maurette P., and Barat M. (1997). Long-term neuropsychological outcome and loss of social autonomy after traumatic brain injury. Arch. Phys. Med. Rehabil. 78, 1316–1320 [DOI] [PubMed] [Google Scholar]
- 45.Cristofori I., and Levin H.S. (2015). Traumatic brain injury and cognition. Handb. Clin. Neurol. 128, 579–611 [DOI] [PubMed] [Google Scholar]
- 46.Rabinowitz A.R., and Levin H.S. (2014). Cognitive sequelae of traumatic brain injury. Psychiatr. Clin. North Am. 37, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levin H.S., and Grossman R.G. (1978). Behavioral sequelae of closed head injury. A quantitative study. Arch. Neurol. 35, 720–727 [DOI] [PubMed] [Google Scholar]
- 48.Wolf A.P., Gleckman A.D., Cifu D.X., and Ginsburg P.C. (1996). The prevalence of agitation and brain injury in skilled nursing facilities: a survey. Brain Inj. 10, 241–245 [DOI] [PubMed] [Google Scholar]
- 49.Nott M.T., Chapparo C., Heard R., and Baguley I.J. (2010). Patterns of agitated behaviour during acute brain injury rehabilitation. Brain Inj. 24, 1214–1221 [DOI] [PubMed] [Google Scholar]
- 50.Ciurli P., Formisano R., Bivona U., Cantagallo A., and Angelelli P. (2011). Neuropsychiatric disorders in persons with severe traumatic brain injury: prevalence, phenomenology, and relationship with demographic, clinical, and functional features. J. Head Trauma Rehabil. 26, 116–126 [DOI] [PubMed] [Google Scholar]
- 51.Diamond M.C., Krech D., and Rosenzweig M.R. (1964). The effects of an enriched environment on the histology of the rat cerebral cortex. J. Comp. Neurol. 123, 111–120 [DOI] [PubMed] [Google Scholar]
- 52.Diamond M.C., Law F., Rhodes H., Lindner B., Rosenzweig M.R., Krech D., and Bennett E.L. (1966). Increases in cortical depth and glia numbers in rats subjected to enriched environment. J. Comp. Neurol. 128, 117–126 [DOI] [PubMed] [Google Scholar]
- 53.Diamond M.C., Ingham C.A., Johnson R.E., Bennett E.L., and Rosenzweig M.R. (1976). Effects of environment on morphology of rat cerebral cortex and hippocampus. J. Neurobiol. 7, 75–85 [DOI] [PubMed] [Google Scholar]
- 54.van Praag, H., Kempermann G., and Gage F.H. (2000). Neural consequences of environmental enrichment. Nat. Rev. Neurosci. 1, 191–198 [DOI] [PubMed] [Google Scholar]
- 55.Will B., Galani R., Kelche C., and Rosenzweig M.R. (2004). Recovery from brain injury in animals: relative efficacy of environmental enrichment, physical exercise or formal training (1990–2002). Prog. Neurobiol. 72, 167–182 [DOI] [PubMed] [Google Scholar]
- 56.Paylor R., Morrison S.K., Rudy J.W., Waltrip L.T., and Wehner J.M. (1992). Brief exposure to an enriched environment improves performance on the Morris water task and increases hippocampal cytosolic protein kinase C activity in young rats. Behav. Brain Res. 52, 49–59 [DOI] [PubMed] [Google Scholar]
- 57.Kempermann G., Kuhn H.G., and Gage F.H. (1997). More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495 [DOI] [PubMed] [Google Scholar]
- 58.Hammami-Abrand Abadi, A., Miladi-Gorji H., and Bigdeli I. (2016). Effect of environmental enrichment on physical and psychological dependence signs and voluntary morphine consumption in morphine-dependent and morphine-withdrawn rats. Behav. Pharmacol. 2–3, 270–278 [DOI] [PubMed] [Google Scholar]
- 59.Peck J.A., Galaj E., Eshak S., Newman K.L., and Ranaldi R. (2015). Environmental enrichment induces early heroin abstinence in an animal conflict model. Pharmacol. Biochem. Behav. 138, 20–25 [DOI] [PubMed] [Google Scholar]
- 60.Hajheidari S., Miladi-Gorji H., and Bigdeli I. (2015). Effects of environmental enrichment during induction of methamphetamine dependence on the behavioral withdrawal symptoms in rats. Neurosci. Lett. 605, 39–43 [DOI] [PubMed] [Google Scholar]
- 61.Li X., Meng L., Huang K., Wang H., and Li D. (2015). Environmental enrichment blocks reinstatement of ethanol-induced conditioned place preference in mice. Neurosci. Lett. 599, 92–96 [DOI] [PubMed] [Google Scholar]
- 62.Crofton E.J., Zhang Y., and Green T.A. (2015). Inoculation stress hypothesis of environmental enrichment. Neurosci. Biobehav. Rev. 49, 19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karl T., Duffy L., O'Brien E., Matsumoto I., and Dedova I. (2006). Behavioural effects of chronic haloperidol and risperidone treatment in rats. Behav. Brain Res. 171, 286–294 [DOI] [PubMed] [Google Scholar]
- 64.Hutchings E.J., Waller J.L., and Terry A.V., Jr. (2013). Differential long-term effects of haloperidol and risperidone on the acquisition and performance of tasks of spatial working and short-term memory and sustained attention in rats. J. Pharmacol. Exp. Ther. 347, 547–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klitenick M.A., Taber M.T., and Fibiger H.C. (1996). Effects of chronic haloperidol on stress- and stimulation-induced increases in dopamine release: tests of the depolarization block hypothesis. Neuropsychopharmacology 15, 424–428 [DOI] [PubMed] [Google Scholar]
- 66.Park S.W., Lee C.H., Cho H.Y., Seo M.K., Lee J.G., Lee B.J., Seol W., Kee B.S., and Kim Y.H. (2013). Effects of antipsychotic drugs on the expression of synaptic proteins and dendritic outgrowth in hippocampal neuronal cultures. Synapse 67, 224–234 [DOI] [PubMed] [Google Scholar]
- 67.Seo M.K, Lee C.H., Cho H.Y., You Y.S., Lee B.J., Lee J.G., Park S.W., and Kim Y.H. (2015). Effects of antipsychotic drugs on the expression of synapse-associated proteins in the frontal cortex of rats subjected to immobilization stress. Psychiatry Res. 229, 968–974 [DOI] [PubMed] [Google Scholar]
- 68.Benes F.M., Paskevich P.A., Davidson J., and Domesick V.B. (1985). The effects of haloperidol on synaptic patterns in the rat striatum. Brain Res. 329, 265–273 [DOI] [PubMed] [Google Scholar]
- 69.Naidu P.S., Singh A., and Kulkarni S.K. (2002). Carvedilol attenuates neuroleptic-induced orofacial dyskinesia: possible antioxidant mechanisms. Br. J. Pharmacol. 136, 193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Besret L., Caldwell M.A., Torres E.M., and Dunnett S.B. (2000). Antioxidant strategy to counteract the side effects of antipsychotic therapy: an in vivo study in rats. Eur. J. Pharmacol. 408, 35–39 [DOI] [PubMed] [Google Scholar]
- 71.Campbell A., Baldessarini R.J., Teicher M.H., and Kula N.S. (1985). Prolonged antidopaminergic actions of single doses of butyrophenones in the rat. Psychopharmacology (Berl) 87, 161–166 [DOI] [PubMed] [Google Scholar]
- 72.Pillai A., Parikh V., Terry A.V., Jr., and Mahadik S.P. (2007). Long-term antipsychotic treatments and crossover studies in rats: differential effects of typical and atypical agents on the expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J. Psychiatr. Res. 41, 372–386 [DOI] [PubMed] [Google Scholar]
- 73.Ukai W., Ozawa H., Tateno M., Hashimoto E., and Saito T. (2004). Neurotoxic potential of haloperidol in comparison with risperidone: implication of Akt-mediated signal changes by haloperidol. J. Neural Transm. 111, 667–681 [DOI] [PubMed] [Google Scholar]
- 74.Cadet J.L., and Kahler L.A. (1994). Free radical mechanisms in schizophrenia and tardive dyskinesia. Neurosci. Biobehav. Rev. 18, 457–467 [DOI] [PubMed] [Google Scholar]
- 75.Gil-Ad I., Weizman A., Melamed E., and Offen D. (2000). Haloperidol-induced neurotoxicity—possible implications for tardive dyskinesia. J. Neural Transm. 107, 479–490 [DOI] [PubMed] [Google Scholar]
- 76.Briones T.L., Rogozinska M., and Woods J. (2011). Modulation of ischemia-induced NMDAR1 activation by environmental enrichment decreases oxidative damage. J. Neurotrauma 28, 2485–2492 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Papadia S., Soriano F.X., Léveille F., Martel MA., Dakin K.A., Hansen H.H., Kaindl A., Sifringer M., Fowler J., Stefovska V., McKenzie G., Craigon M., Corriveau R., Ghazal P., Horsburgh K., Yankner B.A., Wyllie D.J.A., Ikonomidou C., and Hardingham G.E. (2008). Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat. Neurosci. 11, 476–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dixon C.E., Kraus M.F., Kline A.E., Ma X., Yan H.Q., Griffith R.G., Wolfson B.M., and Marion D.W. (1999). Amantadine improves water maze performance without affecting motor behavior following traumatic brain injury in rats. Restor. Neurol. Neurosci. 14, 285–294 [PubMed] [Google Scholar]
- 79.Kline A.E., Massucci J.L., Ma X., Zafonte R.D., and Dixon C.E. (2004). Bromocriptine reduces lipid peroxidation and enhances spatial learning and hippocampal neuron survival in a rodent model of focal brain trauma. J. Neurotrauma 21, 1712–1722 [DOI] [PubMed] [Google Scholar]
- 80.Kline A.E., Yan H.Q., Bao J., Marion D.W., and Dixon C.E. (2000). Chronic methylphenidate treatment enhances water maze performance following traumatic brain injury in rats. Neurosci. Lett. 280, 163–166 [DOI] [PubMed] [Google Scholar]
- 81.Kline A.E., Massucci J.L., Marion D.W., and Dixon C.E. (2002). Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J. Neurotrauma 19, 415–425 [DOI] [PubMed] [Google Scholar]
- 82.Zhu J., Hamm R.J., Reeves T.M., Povlishock J.T., and Phillips L.L. (2000). Postinjury administration of L-deprenyl improves cognitive function and enhances neuroplasticity after traumatic brain injury. Exp. Neurol. 166, 136–152 [DOI] [PubMed] [Google Scholar]
- 83.Leary L.B., Bondi C.O., LaPorte M.J., Carlson L.C., Cheng J.P., and Kline A.E. (2016). The therapeutic efficacy of environmental enrichment and methylphenidate alone and in combination after controlled cortical impact injury. J. Neurotrauma. Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]