Abstract
Traumatic brain injury (TBI) is an increasingly prevalent condition affecting soldiers, athletes, and motor vehicle accident victims. Unfortunately, it currently lacks effective therapeutic interventions. TBI is defined as a primary mechanical insult followed by a secondary cascade involving inflammation, apoptosis, release of reactive oxygen species, and excitotoxicity, all of which can cause synaptic changes, altered neuronal signaling, and, ultimately, behavioral changes. Previously we showed that preventing degradation of the endocannabinoid (EC) 2-acylglycerol (2-AG) with JZL184 after mild TBI attenuated neuroinflammation and improved recovery of neurobehavioral function during the early 24 h post-TBI period. The aim of this study was to extend the timeline of observations to 2 weeks post-injury and to investigate JZL184’s impact on synaptic transmission, which we view as a potential mechanism for TBI-induced cellular and behavioral pathology. Adult male rats underwent mild TBI (mTBI) followed by a single intraperitoneal injection of JZL184 or vehicle 30 min post-injury. JZL184 administered-TBI animals showed improved neurobehavioral recovery compared with vehicle-injected TBI animals beginning 24 h post-injury and persisting for 2 weeks. JZL184-treated animals had significantly diminished gray and white matter astrocyte activation when compared with vehicle-treated animals at day 7 post-TBI. JZL184 administration significantly attenuated the increased pGluR1S845/GluR1 and pERK 1/2/ERK and the increases in miniature excitatory postsynaptic potential (mEPSC) frequency and amplitude observed in layer 5 pyramidal neurons at 10 days post-TBI. These results suggest a neuroprotective role for ECs in ameliorating the TBI-induced neurobehavioral, neuroinflammatory, and glutamate dyshomeostasis from mTBI. Further studies elucidating the cellular mechanisms involved are warranted.
Keywords: : endocannabinoids, MAGL, neuroinflammation, TBI, 2-AG
Introduction
Traumatic brain injury (TBI) commonly affects otherwise healthy individuals such as athletes, victims of motor vehicle accidents, and soldiers, and currently lacks effective therapeutic intervention.1 Mild TBI (commonly called a concussion) is an often underreported injury because of the transient nature of clinical manifestations such as loss of consciousness.1 Recent evidence is mounting, however, that even after these transient effects dissipate, less obvious symptoms at the cellular and molecular level including inflammation, glutamate homeostasis disruptions, and excitotoxicity, blood–brain barrier disruption, and synaptic changes may persist.2
If left untreated, these cellular and molecular changes may increase the risk for a number of short-term (pain, headaches, anxiety, depression)3–9 and long-term (cognitive problems, neurodegenerative disease)10 neurobehavioral sequelae. It is for this reason that studying the mechanisms driving cellular and behavioral pathology during the acute recovery period after mild TBI and researching potential therapeutic targets is of great importance.
There are two phases of cellular pathology after TBI. There is initially a primary mechanical insult, commonly caused by acceleration-deceleration or rotational forces.11 These forces can stretch and strain neurons and microvessels resulting in diffuse axonal injury (DAI) and membrane permeability changes, triggering cellular apoptosis and glial cell activation.11 The initial mechanical injury can also destroy or disrupt normal glial cell function, which may impair blood–brain barrier integrity.11
Acute inflammation is a necessary and beneficial step after the initial mechanical insult. TBI, however, is often characterized by a secondary “sustained” inflammatory response that actually exacerbates tissue damage in a feed forward mechanism.1,12–14 Activated neuroimmune cells (microglia and astrocytes) and infiltrating peripheral immune cells release large amounts of proinflammatory cytokines triggering neuronal apoptosis, which in turn promotes increased glial proinflammatory cytokine release.1,12–14
The end result of this inflammatory sequelae is membrane permeability changes on both glial cells and neurons, potentially triggering massive release of glutamate and synaptic changes that could lead to neuronal damage or death by excitotoxicity.1,12–14 Pathologic changes in glutamate system homeostasis may be caused by post-TBI inflammation15 and can be measured by examining protein level changes after injury (such as glutamate receptor subunit phosphorylation) and by measuring changes in spontaneous excitatory synaptic transmission. In all, the common neurobehavioral pathologies associated with TBI outlined above may be ameliorated if interventions can be identified that attenuate the development of this secondary sustained inflammatory and excitotoxic cascade.
There is mounting evidence, including work from our own laboratory,16 which indicates a protective role of the endocannabinoid (EC) system in reducing inflammation and neuronal death after injury.17 ECs such as 2-arachidonoyl glycerol (2-AG) (the most bioactive and abundant EC in the brain) have been shown to have anti-inflammatory properties.18–20 In addition, 2-AG has also been shown to be effective in decreasing glutamate toxicity.17,19 Much like neurotransmitters, though, once ECs are released in response to injury, they are rapidly degraded.
The primary enzyme that breaks down 2-AG is monoacylglycerol lipase (MAGL).21,22 Previously, we showed significant improvement of behavior and neuroinflammation post-TBI in MAGL inhibitor JZL184-treated rats. Our results showed that treatment with one dose of JZL184 30 min post-TBI attenuated inflammation, blood–brain barrier permeability, and neurobehavioral and neurological severity scores for up to 24 h post-injury. Our studies showed that of the two EC degradation inhibitors, URB597 and JZL184, animals treated with JZL184 showed more consistent improvement in all outcome measures examined.
Because of these data, we sought in the current study to focus exclusively on MAGL inhibition and to extend the timeline for the studies to examine whether JZL184 treatment improved glutamate homeostasis disruptions and synaptic hyperexcitability, which we believe to be involved mechanistically in pathologic outcomes from mild TBI. We hypothesized that treatment with the EC degradation inhibitor JZL184 would attenuate behavioral, cellular, and synaptic dysfunction during the acute recovery period up to 14 days after mild TBI.
Methods
Animals
Two cohorts of adult male rats (Sprague-Dawleys for the first set of studies and Wistar rats for the second study) weighing between 250–275 g were purchased from Charles River Laboratories (Wilmington, MA) and pair-housed in a temperature and humidity controlled animal housing room with a 12 h light/dark cycle. We used Sprague-Dawley rats for neurobehavioral analysis, immunohistochemical analysis, and phosphoprotein expression analysis to stay consistent with (and extend the findings of) our previous study.16
To further explore the mechanisms involved in neuroprotection, we used Wistar rats exclusively for the electrophysiology studies. Wistar rats tend to show pronounced cortical changes after injury,23 and in parallel studies conducted by our group, they are being used to examine alcohol drinking behaviors after TBI because of their more ease in acquiring alcohol self-administration behavior. Our studies have shown similar neurobehavioral and neuroinflammatory changes following identical protocols of TBI in both strains of rats.16,24,25
Both sets of animals were exposed to identical experimental procedures. Animals had ad libitum access to water and standard rat chow, and were allowed 1 week to habituate to housing conditions before any experimental procedures. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center (LSUHSC; New Orleans, LA) and were in accord with the National Institutes of Health (NIH) guidelines.
Craniotomy
Surgeries were completed as detailed previously.24 Briefly, animals were anesthetized with a mixture of ketamine (90 mg/kg) and xylazine (9 mg/kg) before craniotomy (-2 mm bregma and 3 mm lateral to bregma; over the left sensorimotor cortex). A female Luer-Loc connector was superglued over the craniotomy site and secured with dental cement (Lang Dental Manufacturing, Wheeling, IL). The female Luer-Loc was filled with sterile physiologic saline and capped.
After surgery, animals were allowed to recover for 3 days in single housed cages with ad libitum access to food and water before randomization to either Sham (craniotomy-only) or TBI groups. Based on the stereotaxic coordinates of the crianotomy center (−2 mm caudal and 3 mm lateral), the injured areas will include: body, limb, and barrel (whisker) fields on the primary somatosensory cortex, primary and secondary motor cortical areas, parietal association areas, and to a lesser degree: retrosplenial dysgranular cortex, cingulate cortex, and secondary visual cortex.26
TBI via lateral fluid percussion (LFP)
TBI was produced by LFP as described previously.24 Briefly, rats were anesthetized via inhalant isoflurane (4% induction, 3% maintenance) and placed in a stereotaxic frame (model 900; Kopf Instruments, Tujunga, CA). The Luer-Loc that was previously attached to the rat skull was connected to the LFP apparatus via pressure tubing. Dropping the mallet on the LFP apparatus from a pre-determined angle (approximately 18 degrees) produced a consistent saline pressure wave that impacted the dura (2 atm pressure for 25 msec) and resulted in a mild TBI.
This procedure results in a mild-to-moderate TBI as defined by (1) the pressure of the saline impact (1.5–2 atm), (2) the resulting righting reflex and subsequent neurological behavioral scores, and (3) resultant localized inflammation and blood brain barrier permeability (criteria by McIntosh and associates27).
Sham control animals were subject to the same procedure and connected to the LFP apparatus but did not receive an injury.
We measured apnea, respiratory rate, and righting reflex immediately after TBI. TBI caused an average apnea of 10–15 sec, a reduction in respiratory rate to approximately 70 breaths per minute (compared with 90–100 for Sham controls), and an average righting reflex of approximately 12 min. All animals were placed back into their individual home cage and continuously monitored for 2 h post-TBI with free access to food and water.
EC degradation inhibition
JZL184 is a selective inhibitor of MAGL, the enzyme responsible for 2-AG hydrolysis. MAGL inhibition after JZL184 administration is rapid (maximal inhibition achieved within 0.5 h post-treatment) and potent (>80% inhibition of 2-AG hydrolysis activity resulting in a 7–9 fold increase in brain 2-AG levels). The half-life of JZL184 is approximately 7 h.26 JZL184 (16 mg/kg) was injected intraperitoneally 30 min after TBI (TBI/JZL) in both cohorts of rats. Time-matched TBI and Sham animals received equal volumes of vehicle (0.64 mL of vehicle per kg body weight; TBI/VEH or SHAM/VEH; vehicle injections contained alcohol, emulphor, and saline (1:1:18)). Animals were studied during the 7–14 days post-TBI recovery period.
Assessment of neurobehavioral function
Neurological (neurological severity scores; NSS) and neurobehavioral (neurobehavioral scores; NBS) function were assessed at baseline (1 h before TBI) and at 2 h, 24 h, 72 h, 7 d, 14 d, 21 d, and 30 d post-TBI, as described previously.24 All animals were exposed to all tasks, trained, and evaluated before TBI using the testing parameters that were adapted from previously published methods of assessing cognition and behavior. Animals were allowed 30 min to habituate to the testing room before assessment. NSS scores range 0–25 (least to most impaired) and NBS scores range 0–12 (least to most impaired) and were based on the animal's performance on each task.
NSS evaluates motor function, sensory, reflexes, beam walking, and beam balancing. Pinna, corneal, startle, and righting reflexes were assessed, where a score of 1 indicates impaired reflex and a score of 0 indicates the reflex is intact. Animals were placed on beams of decreasing width (10, 8, 5, and 2.5 cm) to test motor coordination (allowed 60 sec to traverse each beam). In addition, beam balance was assessed where animals were placed on a 1.5-cm–wide beam and given 60 sec to balance. Failure to walk all beams and/or balance for 60 sec resulted in increased NSS total.
NBS tests sensorimotor, proprioception, exploratory behavior, and novel object recognition. Proprioception was assessed by pushing each animal laterally (lateral pulsion) on each side of its body. Each side was assessed, and failure to resist lateral pulsion on one or both sides increased the NBS total. Exploratory behavior was assessed immediately after the animal's cage top was removed—uninjured animals actively explored the top of the cage and surroundings (lower NBS score) while injured animals tended to avoid exploratory behavior (increased NBS score).
Tissue collection
After behavioral tests were completed, animals were euthanized by decapitation under light isoflurane anesthesia. Brains were rapidly dissected and snap frozen in isopentane. The site of injury was isolated using a pre-frozen standard adult rodent brain slicer matrix (Zivic Instruments, Pittsburgh, PA) and divided at midline to separate the ipsilateral (injured) from the contralateral (uninjured) region. Brain tissues were then stored at −80°C for further analyses.
Immunohistochemistry and immunofluorescence
Immunohistochemistry was performed as described previously.24,25 Briefly, a subset of animals were perfusion-fixed and brains isolated and sectioned in 40 micron slices at −20oC before permeabilization with 0.3% Triton-X 100 in phosphate-buffered saline (PBS) for 30 min. Sections were then blocked at room temperature with blocking buffer (bovine serum albumin, normal donkey serum, Triton-X 100, PBS) for 1 h.
Sections were incubated with rabbit antiglial fibrillary acid protein (GFAP; 1:200; Abcam) for 24 h at 4°C in a humidification chamber. The next day slides were washed for 15 min (3 × 5 min in fresh PBS) before secondary antibody incubation with Alexa Fluor 555 donkey antirabbit (1:200; by Life Technologies, Carlsbad, CA) for 2 h at room temperature in the dark. The slides were dried and coverslipped using mounting media with DAPI (ProLong Gold, Life Technologies Carlsbad, CA). Images were captured at 20x or 4x magnification using a 3 msec exposure time on a Nikon Eclipse TE2000-U (Nikon, Tokyo, Japan). The imaging software was NIS Elements (Version 3.22.11, Nikon, Tokyo, Japan).
As described previously,25 two pictures were taken of three sections per brain region per animal (for a total of six uniformly sized pictures per brain region per animal), and five animals were analyzed per group. Images were taken using the same exposure time in the following regions: site of injury, contralateral cortex, ipsilateral corpus callosum, and contralateral corpus callosum. Each image was quantified, and the two quantification values per region per animal were averaged. Five animals were quantified per group and averaged together to obtain a single value for each brain region for each group. Special care was taken not to include any artifact, such as pia or tissue edges, in the images for quantification. Slide selection to determine the site of injury was made based on coordinates (-2 mm bregma) and the rat brain atlas to precisely identify white matter tracts; within each section, the area to be quantified was determined with a standard size box used for each animal and each brain region (listed above).
Quantification was completed using ImageJ software (NIH Public Domain). Briefly, RGB color images were split into single color channels, and each channel was converted to gray scale. The gray scale image was then converted to a binary image by adjusting the image threshold to highlight only the structures of interest (activated cells). The same threshold used to identify activated cells was used for every subsequent image and brain region. Once the image was in binary, analysis was completed using the ImageJ command analyze → analyze particles. Output is given as percent positive staining over total image size.
Western blot
To determine whether TBI induced sustained disruption of excitatory signaling and whether JZL184 could attenuate these changes, we examined changes in glutamate receptor subunit GluR1 and levels of extracellular signal-related kinase (ERK), a marker of neuronal activity. Individual ERK and GluR1 phosphorylation levels in the brain regional homogenates were determined as described previously.26 Regional tissue samples were obtained using 12–16 gauge punches from frozen coronal brain slices (0.5 mm thick) obtained by the use of a cryostat.
Tissue samples were homogenized by sonication in lysis buffer (320 mM sucrose, 5 mM HEPES, 1 mM EGTA, 1 mM EDTA, and 1% SDS, with protease inhibitor cocktail and phosphatase inhibitor cocktails II and III diluted 1:100; Sigma, St Louis, MO), heated at 100°C for 5 min, and stored at −80°C until the determination of protein concentration by a detergent-compatible Lowry method (Bio-Rad, Hercules, CA). Protein samples (15 μg) were subjected to SDS-polyacrylamide gel electrophoresis on 4–15% gradient acrylamide gels by using a Tris/Glycine/SDS buffer system (Bio-Rad), followed by electrophoretic transfer to polyvinylidene difluoride membranes (GE Healthcare, Piscataway, NJ).
Membranes were blocked overnight in 5% nonfat milk at 4°C and were then incubated in primary antibody recognizing the dual phosphorylated form of ERK (1:2500, 5% non-fat milk; Cell Signaling, Danvers, MA) and the protein kinase A (PKA)-phosphorylated AMPA glutamate receptor subunit pGluR1S845 (1:1000; 5% nonfat milk; Cell Signaling, Danvers, MA). Membranes were washed and labeled with species-specific peroxidase-conjugated secondary antibody (1:10 K; Bio-Rad) for 1 h at room temperature.
After chemiluminescence detection (SuperSignal West Pico; Thermo Scientific, Rockford, IL), blots were stripped for 20 min at room temperature (Restore; Thermo Scientific) and were reprobed for total protein levels of ERK (1:5000; Cell Signaling) and GluR1 (1:2500; Cell Signaling). Immunoreactivity was quantified by densitometry (ImageJ 1.45S; NIH) under linear exposure conditions. Densitized values were expressed as a percentage of the mean of control values for each gel to normalize data across blots. Individual phosphoprotein levels were normalized to individual total protein levels to generate ERK phosphorylation (pERK)/ERK and GluR1 phosphorylation (pGluR1)/GluR1 ratio values for statistical comparison.
Electrophysiology
To further validate whether TBI caused sustained changes in glutamate homeostasis disruptions and whether JZL184 could attenuate these changes, we used electrophysiology to examine spontaneous excitatory activity in the form of miniexcitatory post-synaptic currents (mEPSCs). Animals underwent the same experimental procedures described above and were sacrificed by decapitation under deep isoflurane anesthesia 10 days post-TBI. Brains were removed and sliced in an ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): 125 choline chloride, 2.5 KCl, 1.25 NaH2PO4, 2 MgSO4, 25 NaHCO3, 7 dextrose, 0.5 CaCl2, 7 MgCl2 saturated with 95% O2–5% CO2.
Coronal slices containing somatomotor cortex underlying the site of injury were then transferred to a holding chamber in a water heat bath where the holding/recording solution was kept at 36°C. The holding/recording solution consisted of (in mM): 125 NaCl, 25 NaHCO3, 1.25 NaH2PO4, 2.5 KCl, 10 dextrose, 1.25 CaCl2, 2 MgCl2, 1.3 Na-ascorbate, and 3 Na-pyruvate. After 30 min, the holding chamber was removed from the heat bath and allowed to rest at room temperature for ∼45 min before slices were used for electrophysiological recording experiments.
Whole-cell recordings were performed using borosilicate glass micropipettes (3–7 MΩ) filled with internal solution containing (in mM): 130 K-gluconate, 10 HEPES, 10 Na2-phosphocreatine, 4 MgCl2, 4 Na2-ATP, 0.4 Na-GTP, 3 ascorbic acid, 0.2 EGTA (pH 7.25, 290–295 mOsm). Recordings were obtained from pyramidal neurons because they are the primary excitatory neurons in the cortex and, because of their location, may be vulnerable to damage and altered signaling by TBI.
Postsynaptic current was measured while clamping the membrane voltage at −70 mV; mEPSCs were detected in current traces, and both frequency and amplitude were calculated to characterize glutamatergic synaptic transmission in cortical brain slices. Changes in the amplitude, kinetics, or frequency of mEPSCs were analyzed to garner information about both pre-synaptic and post-synaptic alterations29 that may occur as a result of TBI. Electrophysiological recordings were at or near the center of the craniotomy and so were all most likely in the body (shoulder, limb, and trunk) representation of primary somatosensory cortex.30
Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM). Statistical differences were determined by either one-way analysis of variance (ANOVA) or two-way ANOVA with repeated measures using GraphPad Prism 5.0 statistical software (Graphpad Software Inc., La Jolla, CA). The Tukey test for one-way ANOVA and Bonferroni test for two-way ANOVA were used for pair-wise multiple comparisons. Statistical significance was set at p < 0.05.
Results
JZL treatment attenuated TBI-induced impairments in NBS and NSS
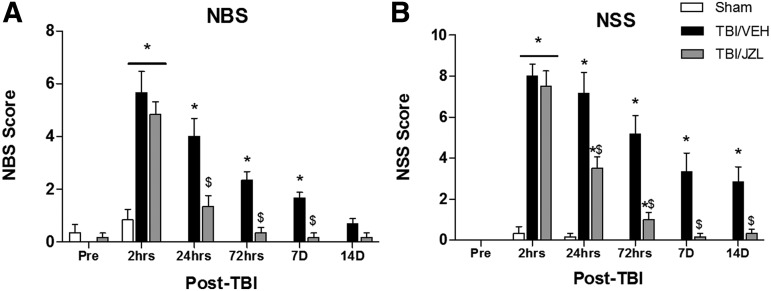
NBS and NSS for both TBI/VEH and TBI/JZL animals were greater than Sham animals 2 h post-injury (indicating greater impairment, Fig. 1). At 24 h post-TBI, however, the TBI/JZL treatment significantly attenuated NBS and NSS dysfunction (50% score reduction) compared with TBI/VEH animals. By 72h after TBI, the TBI/JZL treated animals still exhibited reduced NBS/NSS compared with TBI/VEH animals. One week post-TBI, JZL treatment still had a significant protective effect in the form of lower NBS/NSS scores compared with TBI/VEH animals. Two weeks post-TBI, NSS scores were still significantly reduced in TBI/JZL treated animals compared with TBI/VEH animals, while NBS scores were attenuated for all animals by this time point.
FIG. 1.
Neurobehavioral Scores (NBS) and Neurological Severity Scores (NSS) during the first 2 weeks post-traumatic brain injury (TBI). Higher scores indicate greater motor impairment. (A) NBS scores are attenuated with JZL treatment 7 days after TBI, and (B) NSS scores are attenuated with JZL treatment 14 days after TBI. *p < 0.05 compared with Sham, $p < 0.05 compared with TBI/VEH (vehicle).
JZL treatment attenuated astrocyte activation
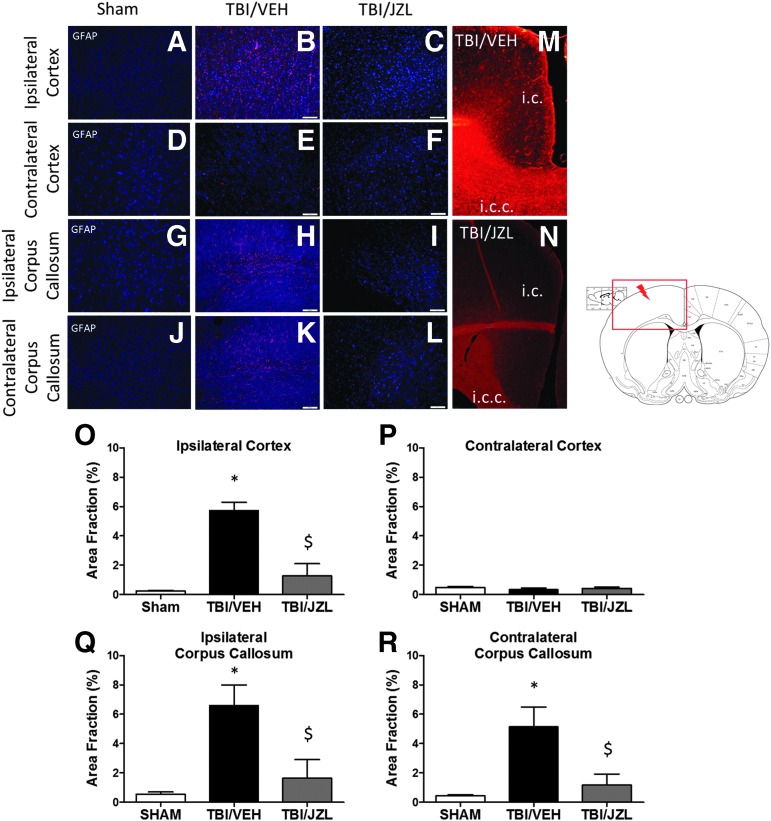
A hopeful therapeutic window after mild TBI is immediately after the primary mechanical insult before the start of the secondary injury (development of sustained neuroinflammation). While acute inflammation is important and necessary in tissue recovery, sustained inflammation may actually potentiate pathology after TBI. We wanted to examine astrocyte activation (which often accompanies inflammation and thus serves as an indirect marker of neuroinflammation) after TBI with and without the JZL administration during a critical therapeutic window (30 min post-TBI).
Astrocyte activation (as measured by GFAP immunoreactivity) was significantly increased in the ipsilateral cortex (gray matter) 1 week post-TBI and was attenuated by JZL treatment (Fig. 2). The contralateral cortex showed no significant astrocyte activation in any experimental condition. Interestingly, TBI induced white matter astrocyte activation in both the ipsilateral and contralateral corpus callosum, and this astrocyte activation was also attenuated by JZL treatment. Representative images show the diffusivity of astrocyte activation spreading from the cortex to the corpus callosum in TBI/VEH animals (Fig. 2, M) and the reduction of this astrocyte activation in TBI/JZL treated animals (Fig. 2, N).
FIG. 2.
Gray matter (cortex) and white matter (corpus callosum) astrocyte activation after traumatic brain injury (TBI). Ipsilateral (A–C) and contralateral (D–F) cortex (gray matter) astrocyte activation. Ipsilateral (G–I) and contralateral (J–L) corpus callosum (white matter) astrocyte activation. Representative images (A-L) are counterstained with DAPI. Representative images (M,N) show glial fibrillary acidic protein (GFAP) staining indicating astrocyte activation; here, JZL treatment reduces gray and white matter astrocyte activation after TBI. Quantification of IHC represented as area fraction % (O–R) where *P < 0.05 compared with Sham, $p < 0.05 compared with TBI/VEH (vehicle). i.c., ipsilateral cortex; i.c.c., ipsilateral corpus callosum. Scale bars (white) are equal to 100 μm.
JZL treatment attenuated phosphoprotein markers of increased glutamate receptor activity
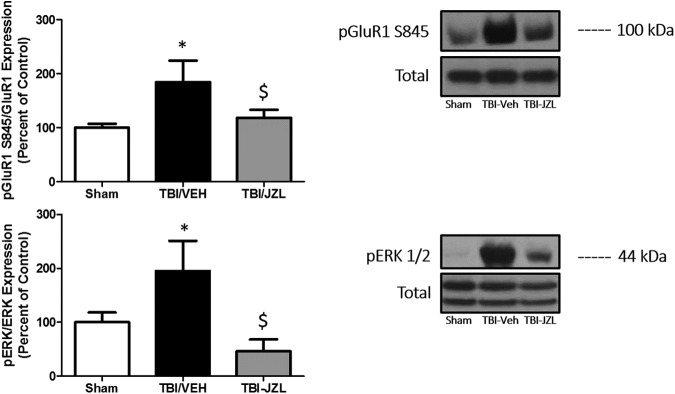
The nature of TBI lends itself to glutamate homeostasis disruptions, whether caused by stretch/strain of cortical neurons resulting in diffuse axonal injury and neuronal membrane permeability changes, or whether caused by inflammation-mediated excitotoxicity. To identify whether these glutamate homeostasis disruptions resulted in any stable phosphoprotein expression changes at glutamate synapses, we examined two protein markers associated with glutamate activity. pGluR1 S845 is an AMPA glutamate receptor subunit that, when phosphorylated, regulates opening probability of the AMPA receptor in the post-synaptic neuron. In addition, we examined ERK phosphorylation, which is a protein correlate of post-synaptic neuronal activity.
At the site of injury, TBI/VEH-treated animals expressed higher levels of PKA-mediated GluR1 phosphorylation (twofold change, p < 0.05) as well as greater phosphorylation of ERK (twofold change, p < 0.05) compared with Sham animals (Fig. 3). JZL treatment significantly attenuated the heightened GluR1S845 and ERK phosphorylation. No changes in total GluR1 or ERK were observed. *p < 0.05 compared with Sham, $p < 0.05 compared with TBI/VEH analyzed via one-way ANOVA with Tukey post hoc analysis.
FIG. 3.
Phosphoprotein expression changes in the ipsilateral cortex (site of injury). Expression of phosphorylated AMPA glutamate receptor subunit (pGluR1) S845 is attenuated in JZL-treated animals compared with TBI/VEH (vehicle) animals, and post-TBI extracellular signal-related kinase (ERK) 1/2 phosphorylation is attenuated with JZL treatment. *p < 0.05 compared with Sham; $p < 0.05 compared with TBI/VEH.
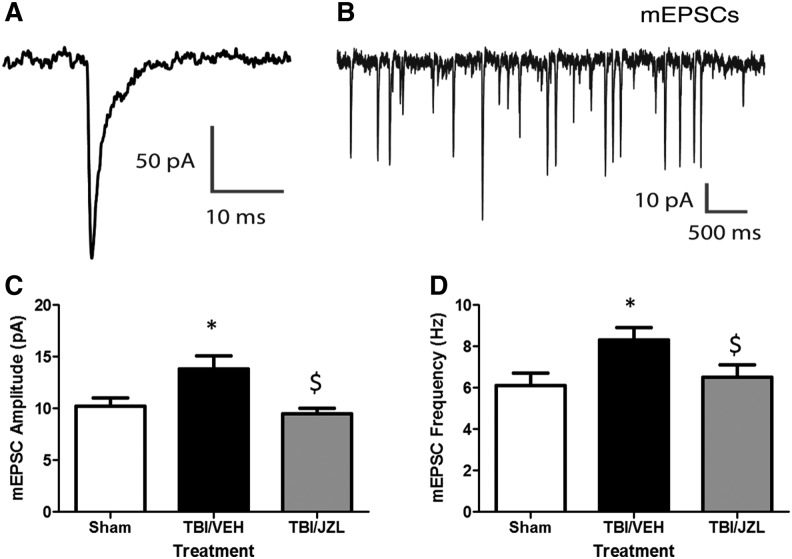
JZL treatment attenuated increases in mini excitatory post-synaptic current and amplitude at site of injury
We have shown that TBI results in phosphorylation of post-synaptic proteins associated with glutamatergic synaptic transmission at the site of injury. To investigate whether these protein changes result in measurable functional changes 10 days post-injury, we recorded mEPSCs in layer 5 cortical pyramidal neurons at the site of injury (Fig. 4A,B). Alterations in spontaneous mEPSCs may reflect changes in pre-synaptic transmitter release or post-synaptic strength.31 We found that pyramidal neurons in the cortex of animals with TBI had increased mEPSC amplitude (13.9 ± 1.3 pA) and frequency (8.3 ± 0.6 Hz) compared with sham neurons (amplitude: 10.2 ± 0.8, frequency: 6.1 ± 0.6; p = 0.025; Fig. 4C, D).
FIG. 4.
Miniexcitatory post-synaptic currents (mEPSCs) recorded from rat cortex (site of injury) via brain slice electrophysiology 10 days post-injury. A downward deflection is a depolarizing current. (A) Representative mEPSC and (B) representative trace recording. JZL treatment significantly attenuated mEPSC frequency (C) and amplitude (D) compared to traumatic brain injury/vehicle (TBI-VEH) animals. *p < 0.05 compared with Sham; $p < 0.05 compared with TBI/VEH.
Treatment with JZL resulted in mEPSC amplitudes and frequencies that are similar to sham levels (amplitude: 9.5 ± 0.5 pA, p = 0.513 vs. Sham, p = 0.005 vs. TBI; frequency: 6.5 ± 0.6 pA, p = 0.639 vs. Sham, p = 0.04 vs. TBI). Increased excitatory synaptic inputs into a cortical pyramidal neuron would act to drive its action potential output, which may then further increase synchronous neural network activity.32 Our findings suggest that both pre- and post-synaptic alterations have occurred as a result of TBI.31,33
Discussion
In the current study, we examined whether single-dose EC degradation inhibition post-TBI improved long term (up to 2 weeks) synaptic dysfunction, our proposed mechanism for TBI-induced cellular and behavioral pathology. Numerous studies implicate that exogenous EC administration may be neuroprotective,17 and previously we showed that preventing EC degradation was effective in improving short-term (up to 24 h) outcomes from mild TBI.16 For these reasons, we hypothesized in the current study that treatment with the EC degradation inhibitor JZL184 would attenuate behavioral, cellular, and synaptic dysfunction during the acute recovery period up to 14 days after mild TBI.
Our results show that JZL184 treatment improved NSS and NBS scores up to 14 days post-TBI, attenuated astrocyte reactivity at both the cortex (gray matter) and corpus callosum (white matter) 10 days post-TBI, and attenuated cortical (site of injury) expression of markers of synaptic hyperexcitability including pGluR1S845 and pERK and mEPSC frequency and amplitude in cortical neurons 10 days post-TBI. Overall, these results suggest that inhibiting the degradation of the endocannabinoid (EC) 2-AG with JZL184 is a promising therapeutic strategy that attenuates behavioral and cellular outcomes from mild TBI for up to 14 days post-injury.
The rationale for this study came from the wealth of literature indicating an important role for ECs in synaptic plasticity34–37 and neuroprotection.38–43 It is well known that TBI causes a host of cellular changes including neuroinflammation, excitotoxicity, and oxidative stress, all of which can potentially cause neuronal damage and death.2,12–14 As TBI transitions from the primary injury (mechanical insult) to the secondary injury (sustained neuroinflammation), the risk increases for long-term neurodegenerative disease, including hallmark signs of neuropathology such as beta-amyloid plaque formation, tau phosphorylation, and white matter degeneration.11 In fact, results from the current study show significant white matter astrocyte activation as well as synaptic changes indicating a hyper-excitable state after a single mild brain injury.
Therefore the most critical window for therapeutic intervention is the acute period immediately after the primary mechanical insult. If a therapeutic can block the transition from the primary injury to the secondary injury, long-term prognosis might improve. The EC system may play a critical role in blocking the transition to secondary injury because of its role in modulating synaptic activity and exerting neuroprotective and anti-inflammatory effects.
Synaptic plasticity occurs with changes in synaptic transmitter release and/or postsynaptic receptor binding and transmembrane current mediation. Synaptic plasticity is widely believed to be one of the most important neurochemical changes involved in learning,44–47 memory,48,49 and behavioral modification.50 The EC system plays a large role in retrograde signaling at glutamatergic synapses throughout the brain where it inhibits the release of neurotransmitter (net suppression of excitability).51,52
The primary EC involved in short-term depression of synaptic transmission is 2-AG, and this effect has been documented in numerous brain regions including the hippocampus, amygdala, cerebellum, basal ganglia, brainstem, hypothalamus, and most relevant to the present study, cerebral cortex.17,53,54 Pharmacological manipulation of the EC system to increase 2-AG, thereby preventing excitotoxicity and oxidative stress, is a promising strategy for treatment.
The EC system also has a well-known anti-inflammatory and neuroprotective effect.55 In fact, Panikashvili and colleagues55 demonstrated a neuroprotective role for the EC system in a mouse model of closed-head TBI; they demonstrated that direct 2-AG administration reduces inflammatory cytokine expression, edema formation, and blood–brain barrier permeability. While 2-AG is innately elevated in the brain after TBI, it is rapidly metabolized because of its nature as an unstable fatty acid. We hypothesized in the current study that blocking the metabolism of 2-AG to allow for strengthened EC signaling during the critical window of transition from primary to secondary injury after mild TBI would have long-lasting therapeutic benefit.
Importantly in the present study, we showed that even a single mild TBI can result in sustained neuropathology including white matter astrocyte activation and a hyperexcitable state, both of which were predictably associated with neurobehavioral dysfunction. It is well known that repetitive mild TBIs or a single severe TBI can increase the risk for developing a neurodegenerative disease now officially classified as chronic traumatic encephalopathy (CTE). Growing evidence supports the development and persistence of neuroinflammation in CTE development and, interestingly, neuroinflammation has recently been implicated in the development of other neurodegenerative diseases such as Alzheimer disease.56–59
White matter inflammation is of particular interest in brain injury because of the unique nature of how TBIs occur (stretch/strain, rotational forces, coup-contra-coup injuries) and the common occurrence of DAI (diffuse axonal injuries).60,61 In the present study, we show dramatic corpus callosum astrocyte activation up to 10 days after a single mild TBI. The corpus callosum is a highly vulnerable region to trauma that contains the axons of large neurons stretching between the two hemispheres of the brain.60,61 Interestingly, damaged axons in the corpus callosum remain damaged long after the initial injury according to analysis of post-mortem human brains, and at least one study implicates axonal damage in the white matter tract as an important source of beta-amyloid plaques in Alzheimer disease.62–65 In the present study, it was therefore particularly interesting that JZL184 administration after mild TBI not only attenuated cortical astrocyte activation but white matter tract astrocyte activation as well.
Overall, the present study showed that single mild TBI was enough to result in some of the hallmark signs of neurodegeneration including hyperexcitable signaling, white matter tract astrocyte activation, and behavioral dysfunction. We proposed that increasing the efficacy of EC signaling by inhibiting MAGL-induced EC breakdown could improve behavioral outcomes by attenuating inflammation and synaptic plasticity changes. We showed that a single dose of JZL184 30 min after mild TBI improved neurobehavioral outcomes, synaptic plasticity, and astrocyte activation up to 2 weeks after injury. These data indicate a protective role for the EC system after mild brain injury, and future work should be done exploring this translational potential of these findings.
Acknowledgments
This work was supported by NIH training (AA007577, JM, PK) and research grants AA020839 (SE), and DOD-W81XWH-11-2-0011 (PM). The authors would like to thank Dr. Nicholas Gilpin for scientific discussions during the preparation of this article and Kylie Mills for assistance with animal behavior and surgery experiments. Paige Katz was a post-doctoral fellow at LSUHSC during the completion of these studies but has since completed her fellowship.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Corrigan J.D., Selassie A.W., and Orman J.A. (2010). The epidemiology of traumatic brain injury. J. Head Trauma Rehabil. 25, 72–80 [DOI] [PubMed] [Google Scholar]
- 2.Al Nimer F., Lindblom R., Ström M., Guerreiro-Cacais A.O., Parsa R., Aeinehband S., Mathiesen T., Lidman O., and Piehl F. (2013). Strain influences on inflammatory pathway activation, cell infiltration and complement cascade after traumatic brain injury in the rat. Brain Behav. Immun. 27, 109–122 [DOI] [PubMed] [Google Scholar]
- 3.Garden N., and Sullivan K.A. (2010). An examination of the base rates of post-concussion symptoms: the influence of demographics and depression. Appl. Neuropsychol. 17, 1–7 [DOI] [PubMed] [Google Scholar]
- 4.Garden N., Sullivan K.A., and Lange R.T. (2010). The relationship between personality characteristics and postconcussion symptoms in a nonclinical sample. Neuropsychology 24, 168–175 [DOI] [PubMed] [Google Scholar]
- 5.Konrad C., Geburek A.J., Rist F., Blumenroth H., Fischer B., Husstedt I., Arolt V., Schiffbauer H., and Lohmann H. (2011). Long-term cognitive and emotional consequences of mild traumatic brain injury. Psychol. Med. 41, 1197–1211 [DOI] [PubMed] [Google Scholar]
- 6.Levin H.S., Brown S.A., Song J.X., McCauley S.R., Boake C., Contant C.F., Goodman H., and Kotrla K.J. (2001). Depression and posttraumatic stress disorder at three months after mild to moderate traumatic brain injury. J. Clin. Exp. Neuropsychol. 23, 754–769 [DOI] [PubMed] [Google Scholar]
- 7.Moore E.L., Terryberry-Spohr L., and Hope D.A. (2006). Mild traumatic brain injury and anxiety sequelae: a review of the literature. Brain Inj. 20, 117–132 [DOI] [PubMed] [Google Scholar]
- 8.Rao V., Bertrand M., Rosenberg P., Makley M., Schretlen D.J., Brandt J., and Mielke M.M. (2010). Predictors of new-onset depression after mild traumatic brain injury. J Neuropsychiatry Clin. Neurosci. 22, 100–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanderploeg R.D., Curtiss G., Luis C.A., and Salazar A.M. (2007). Long-term morbidities following self-reported mild traumatic brain injury. J. Clin. Exp. Neuropsychol. 29 585–598 [DOI] [PubMed] [Google Scholar]
- 10.Washington P.M., Villapol S., and Burns M.P. (2015). Polypathology and dementia after brain trauma: does brain injury trigger distinct neurodegenerative diseases, or should they be classified together as traumatic encephalopathy? Exp. Neurol. 275, 381–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson V.E., Stewart W., and Smith D.H. (2013). Axonal pathology in traumatic brain injury. Exp. Neurol. 246, 35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar A., and Loane D.J. (2012). Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav. Immun. 26, 1191–1201 [DOI] [PubMed] [Google Scholar]
- 13.Loan D.J., and Faden A.I. (2010). Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol. Sci. 31, 596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McIntosh T.K., Smith D.H., Meaney D.F., Kotapka M.J., Gennarelli T.A., and Graham D.I. (1996). Neuropathological sequelae of traumatic brain injury: relationship to neurochemical and biomechanical mechanisms. Lab. Invest. 74, 315–342 [PubMed] [Google Scholar]
- 15.Prow N.A., and Irani D.N. (2008). The inflammatory cytokine, interleukin-1 beta, mediates loss of astroglial glutamate transport and drives excitotoxic motor neuron injury in the spinal cord during acute viral encephalomyelitis. J. Neurochem. 105, 1276–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz P.S., Sulzer J.K., Impastato R.A., Teng S.X., Rogers E.K., and Molina P.E. (2015). Endocannabinoid degradation inhibition improves neurobehavioral function, blood-brain barrier integrity, and neuroinflammation following mild traumatic brain injury. J. Neurotrauma 32, 297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J.Y., and Chen C. (2015). Endocannabinoids in synaptic plasticity and neuroprotection. Neuroscientist. 21, 152–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X., Zhang J., and Chen C. (2011). Endocannabinoid 2-arachidonoylglycerol protects neurons against β-amyloid insults. Neuroscience 178, 159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panikashvili D., Simeonidou C., Ben-Shabat S., Hanus L., Breuer A., Mechoulam R., and Shohami E. (2001). An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature 413, 527–531 [DOI] [PubMed] [Google Scholar]
- 20.Zhang J., and Chen C. (2008). Endocannabinoid 2-arachidonoylglycerol protects neurons by limiting COX-2 elevation. J. Biol. Chem. 283, 22601–22611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cravatt B.F., Giang D.K., Mayfield S.P., Boger D.L., Lerner R.A., and Gilula N.B. (1996). Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384, 83–87 [DOI] [PubMed] [Google Scholar]
- 22.Dinh T.P., Carpenter D., Leslie F.M., Freund T.F., Katona I., Sensi S.L., Kathuria S., and Piomelli D. (2002). Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl. Acad. Sci. U. S. A. 99, 10819–10824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuzik J., Gellért L., Oláh G., Herédi J., Kocsis K., Knapp L., Nagy D., Kincses Z.T., Kis Z., Farkas T., and Toldi J. (2013). Fundamental interstrain differences in cortical activity between Wistar and Sprague-Dawley rats during global ischemia. Neuroscience 228, 371–381 [DOI] [PubMed] [Google Scholar]
- 24.Teng S.X., and Molina P.E. (2014). Acute alcohol intoxication prolongs neuroinflammation without exacerbating neurobehavioral dysfunction following mild traumatic brain injury. J. Neurotrauma 31, 378–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayeux J.P., Teng S.X., Katz P.S., Gilpin N.W., and Molina P.E. (2015). Traumatic brain injury induces neuroinflammation and neuronal degeneration that is associated with escalated alcohol self-administration in rats. Behav. Brain Res. 279, 22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long J.Z., Li W., Booker L., Burston J.J., Kinsey S.G., Schlosburg J.E., Pavón F.J., Serrano A.M., Selley D.E., Parsons L.H., Lichtman A.H., and Cravatt B.F. (2009). Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat. Chem. Biol. 5, 37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McIntosh T.K., Noble L., Andrews B., and Faden A.I. (1987). Traumatic brain injury in the rat: characterization of a midline fluid-percussion model. Cent. Nerv. Syst. Trauma 4 119–134 [DOI] [PubMed] [Google Scholar]
- 28.Edwards S., Baynes B.B., Carmichael C.Y., Zamora-Martinez E.R., Barrus M., Koob G.F., and Gilpin N.W. (2013). Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Transl. Psychiatry 3, e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isaacson J.S., and Walmsley B. (1995). Counting quanta: direct measurements of transmitter release at a central synapse. Neuron 15, 875–884 [DOI] [PubMed] [Google Scholar]
- 30.Paxinos G., and Watson C.R. (2007). The Rat Brain in Stereotaxic Coordinates, 6th ed. Elsevier Press: Boston, MA [Google Scholar]
- 31.Zhang J., Yang Y., Li H., Cao J., and Xu L. (2005). Amplitude/frequency of spontaneous mEPSC correlates to the degree of long-term depression in the CA1 region of the hippocampal slice. Brain Res. 1050, 110–117 [DOI] [PubMed] [Google Scholar]
- 32.Kimura A., and Pavlides C. (2000). Long-term potentiation/depotentiation are accompanied by complex changes in spontaneous unit activity in the hippocampus. J. Neurophysiol. 84, 1894–1906 [DOI] [PubMed] [Google Scholar]
- 33.Oliet S.H., Malenka R.C., and Nicoll R.A. (1996). Bidirectional control of quantal size by synaptic activity in the hippocampus. Science 271, 1294–1297 [DOI] [PubMed] [Google Scholar]
- 34.Alger B.E. (2002). Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog. Neurobiol. 68, 247–286 [DOI] [PubMed] [Google Scholar]
- 35.Kano M., Ohno-Shosaku T., Hashimotodani Y., Uchigashima M., and Watanabe M. (2009). Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 89, 309–380 [DOI] [PubMed] [Google Scholar]
- 36.Wilson R.I., and Nicoll R.A. (2002). Endocannabinoid signaling in the brain. Science 296, 678–682 [DOI] [PubMed] [Google Scholar]
- 37.Xu J.Y., Chen R., Zhang J., and Chen C. (2010). Endocannabinoids differentially modulate synaptic plasticity in rat hippocampal CA1 pyramidal neurons. PLoS One 5, e10306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bisogno T., and Di Marzo V. (2010). Cannabinoid receptors and endocannabinoids: role in neuroinflammatory and neurodegenerative disorders. CNS Neurol. Disord. Drug Targets 9, 564–573 [DOI] [PubMed] [Google Scholar]
- 39.Du H., Chen X., Zhang J., and Chen C. (2011). Inhibition of COX-2 expression by endocannabinoid 2-arachidonoylglycerol is mediated via PPAR-γ. Br. J. Pharmacol. 163, 1533–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eljaschewitsch E., Witting A., Mawrin C., Lee T., Schmidt P.M., Wolf S., Hoertnagl H., Raine C.S., Schneider-Stock R., Nitsch R., and Ullrich O. (2006). The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron 49, 67–79 [DOI] [PubMed] [Google Scholar]
- 41.Sarne Y., and Mechoulam R. (2005). Cannabinoids: between neuroprotection and neurotoxicity. Curr. Drug Targets CNS Neurol. Disord. 4, 677–684 [DOI] [PubMed] [Google Scholar]
- 42.Stella N. (2009). Endocannabinoid signaling in microglial cells. Neuropharmacology. 56, Suppl 1, 244–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Stelt M., and Di Marzo V. (2005). Cannabinoid receptors and their role in neuroprotection. Neuromolecular Med. 7, 37–50 [DOI] [PubMed] [Google Scholar]
- 44.Bliss T.V., and Collingridge G.L. (1993). A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39 [DOI] [PubMed] [Google Scholar]
- 45.Barnes C.A. (1995). Involvement of LTP in memory: are we “searching under the street light”? Neuron 15, 751–754 [DOI] [PubMed] [Google Scholar]
- 46.Jeffery K.J. (1997). LTP and spatial learning—where to next? Hippocampus 7, 95–110 [DOI] [PubMed] [Google Scholar]
- 47.Shors T.J., and Matzel L.D. (1997). Long-term potentiation: what's learning got to do with it? Behav. Brain Sci. 20, 597–655 [DOI] [PubMed] [Google Scholar]
- 48.Abraham W.C., Mason-Parker S.E., and Logan B. (1996). Low-frequency stimulation does not readily cause long-term depression or depotentiation in the dentate gyrus of awake rats. Brain Res. 722, 217–221 [DOI] [PubMed] [Google Scholar]
- 49.Abraham W.C., and Bear M.F. (1996). Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 19, 126–130 [DOI] [PubMed] [Google Scholar]
- 50.Hawkins R.D., and Kandel E.R. (1984). Is there a cell-biological alphabet for simple forms of learning? Psychol. Rev. 91, 375–391 [PubMed] [Google Scholar]
- 51.Diana M.A., and Marty A. (2004). Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE). Br. J. Pharmacol. 142, 9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson R.I., and Nicoll R.A. (2001). Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410, 588–592 [DOI] [PubMed] [Google Scholar]
- 53.Kreitzer A.C., and Regehr W.G. (2001). Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J. Neurosci. 21, RC174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohno-Shosaku T., Tsubokawa H., Mizushima I., Yoneda N., Zimmer A., and Kano M. (2002). Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J. Neurosci. 22, 3864–3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Panikashvili D., Shein N.A., Mechoulam R., Trembovler V., Kohen R., Alexandrovich A., and Shohami E. (2006). The endocannabinoid 2-AG protects the blood-brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines. Neurobiol. Dis. 22, 257–264 [DOI] [PubMed] [Google Scholar]
- 56.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., Cooper N.R., Eikelenboom P., Emmerling M., Fiebich B.L., Finch C.E., Frautschy S., Griffin W.S., Hampel H., Hull M., Landreth G., Lue L., Mrak R., Mackenzie I.R., McGeer P.L., O'Banion M.K., Pachter J., Pasinetti G., Plata-Salaman C., Rogers J., Rydel R., Shen Y., Streit W., Strohmeyer R., Tooyoma I., Van Muiswinkel F.L., Veerhuis R., Walker D., Webster S., Wegrzyniak B., Wenk G., and Wyss-Coray T. (2000). Inflammation and Alzheimer's disease. Neurobiol. Aging 21, 383–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshiyama Y., Higuchi M., Zhang B., Huang S.M., Iwata N., Saido T.C., Maeda J., Suhara T., Trojanowski J.Q., and Lee V.M. (2007). Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53, 337–351 [DOI] [PubMed] [Google Scholar]
- 58.Eikelenboom P., van Exel E., Hoozemans J.J., Veerhuis R., Rozemuller A.J., and van Gool W.A. (2010). Neuroinflammation—an early event in both the history and pathogenesis of Alzheimer's disease. Neurodegener. Dis. 7, 38–41 [DOI] [PubMed] [Google Scholar]
- 59.Brettschneider J., Libon D.J., Toledo J.B., Xie S.X., McCluskey L., Elman L., Geser F., Lee V.M., Grossman M., and Trojanowski J.Q. (2012). Microglial activation and TDP-43 pathology correlate with executive dysfunction in amyotrophic lateral sclerosis. Acta Neuropathol. 123, 395–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adams J.H., Graham D.I., Murray L.S., and Scott G. (1982). Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann. Neurol. 12, 557–563 [DOI] [PubMed] [Google Scholar]
- 61.Geddes J.F., Vowles G.H., Beer T.W., and Ellison D.W. (1997). The diagnosis of diffuse axonal injury: implications for forensic practice. Neuropathol. Appl. Neurobiol. 23, 339–347 [PubMed] [Google Scholar]
- 62.Smith D.H., Chen X.H., Nonaka M., Trojanowski J.Q., Lee V.M., Saatman K.E., Leoni M.J., Xu B.N., Wolf J.A., and Meaney D.F. (1999). Accumulation of amyloid beta and tau and the formation of neurofilament inclusions following diffuse brain injury in the pig. J. Neuropathol. Exp. Neurol. 58, 982–992 [DOI] [PubMed] [Google Scholar]
- 63.Stone J.R., Okonkwo D.O., Singleton R.H., Mutlu L.K., Helm G.A., and Povlishock J.T. (2002). Caspase-3-mediated cleavage of amyloid precursor protein and formation of amyloid Beta peptide in traumatic axonal injury. J. Neurotrauma 19, 601–614 [DOI] [PubMed] [Google Scholar]
- 64.Johnson V.E., Stewart W., and Smith D.H. (2010). Traumatic brain injury and amyloid-β pathology: a link to Alzheimer's disease? Nat. Rev. Neurosci. 11, 361–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tran H.T., LaFerla F.M., Holtzman D.M., and Brody D.L. (2011). Controlled cortical impact traumatic brain injury in 3xTg-AD mice causes acute intra-axonal amyloid-β accumulation and independently accelerates the development of tau abnormalities. J. Neurosci. 31, 9513–9525 [DOI] [PMC free article] [PubMed] [Google Scholar]