Abstract
Mild traumatic brain injury (mTBI) patients frequently experience emotion dysregulation symptoms, including post-traumatic stress. Although mTBI likely affects cortical activation and structure, resulting in cognitive symptoms after mTBI, early effects of mTBI on cortical emotion processing circuits have rarely been examined. To assess early mTBI effects on cortical functional and structural components of emotion processing, we assessed cortical activation to fearful faces within the first 2 weeks after motor vehicle collision (MVC) in survivors who did and did not experience mTBI. We also examined the thicknesses of cortical regions with altered activation. MVC survivors with mTBI (n = 21) had significantly less activation in left superior parietal gyrus (SPG) (−5.9, −81.8, 33.8; p = 10−3.623), left medial orbitofrontal gyrus (mOFG) (−4.7, 36.1, −19.3; p = 10−3.231), and left and right lateral orbitofrontal gyri (lOFG) (left: −16.0, 41.4, −16.6; p = 10−2.573; right: 18.7, 22.7, −17.7; p = 10−2.764) than MVC survivors without mTBI (n = 23). SPG activation in mTBI survivors within 2 weeks after MVC was negatively correlated with subsequent post-traumatic stress symptom severity at 3 months (r = −0.68, p = 0.03). Finally, the SPG region was thinner in the mTBI survivors than in the non-mTBI survivors (F = 11.07, p = 0.002). These results suggest that early differences in activation and structure in cortical emotion processing circuits in trauma survivors who sustain mTBI may contribute to the development of emotion-related symptoms.
Keywords: : human studies; MRI, TBI
Introduction
Mild traumatic brain injury (mTBI) is common worldwide, and is estimated to affect 600 of every 100,000 adults each year.1–3 Following mTBI, symptoms that include altered emotional responses, irritability, post-traumatic stress, and/or depression, may quickly develop.4–10 Although these symptoms may spontaneously remit in some patients over subsequent weeks,7,8 emotion-related symptoms remain significantly more apparent in mTBI than in non-mTBI trauma survivors at months to years with trauma that results from combat or traffic accidents.4–6,9–12 The mechanisms that link mTBI to the pathophysiology of these emotion-related symptoms remain unclear.
Functional neuroimaging studies suggest that prefrontal, cingulate, and insular cortical regions, as well as subcortical nuclei including the amygdala, are involved in emotion processing and regulation.13–15 In addition, occipital and parietal cortical regions have been linked to processing of visual emotional stimuli, and the temporal cortex has been linked to processing of auditory emotional stimuli.16–18 It is thought that mTBI may rapidly compromise some or all these circuits to cause emotion-related post-mTBI symptoms.19 However, to date, most post-mTBI functional magnetic resonance imaging (fMRI) work has focused on memory and attention,20,21 rather than on emotional responses or regulation. The findings suggest that mTBI rapidly affects cortical activation in the days to months after mTBI in ways that contribute to memory and attention deficits.20–23 A few studies in chronic mTBI patients report deficits in negative emotion processing that may contribute to post-traumatic stress disorder (PTSD) or depressive symptoms, which suggests a link between mTBI and the development of these disorders.24–26 A recent study indicates that chronic mild and moderate TBI may impair cortical processing of affective facial features, possibly contributing to impaired interpersonal skills and development of mood disorders.27 To date, studies have focused on chronic changes, and have not tested for early functional effects of mTBI. Consequently, there is little understanding of mTBI contributions to the development of early emotion processing or regulation deficits.
Advanced MRI approaches have begun to identify post-mTBI brain structural changes that were unrecognized with conventional radiological examination. Early mTBI effects on white matter (WM) integrity, microhemorrhaging,28–30 and hypo- and hyperperfusion31–33 have been reported. High resolution structural MRI (sMRI) has detected alterations in cortical thickness34–36 and volume.37,38 A few studies have simultaneously examined brain activation and structure after mTBI and suggested that structural changes might be associated with changes in activation of attention/working memory tasks.39 Although changes in the WM of emotion circuitry may explain some post-mTBI emotional symptoms,25,40–43 we recently reported that mTBI-related changes in right precuneus cortex thickness within days after motor vehicle collision (MVC) are associated with severity of acute post-traumatic stress symptoms.35 This finding raises the possibility that cortical thickness changes also contribute to post-mTBI alterations in emotion processing. However, cortical structural changes that contribute to emotional activity have not been examined.
In the current study, we assessed activation and structure of cortical regions involved in emotion processing within days after MVC in survivors who did or did not have mTBI. Survivors were asked to identify the gender of faces showing fearful expressions as contrasted with faces showing neutral expressions. These tasks activated cortical circuits that are involved in processing of emotional facial stimuli.17,18,44 These circuits include early visual areas (V1–V4), fusiform face areas, lateral fusiform and superior temporal pathways involved in processing of gender and facial expressions, and limbic frontal cortical regions involved in processing and regulating negative emotions.45,46 One goal was to examine mTBI effects on cortical processing of emotional visual stimuli. In addition, we assessed relationships between cortical activation and thickness using our previously described cross-modal analytical approach.47 Finally, we assessed post-traumatic stress symptoms at 3 months after MVC to test for potential correlations between persistent emotion-related symptoms and early cortical emotional activity after mTBI.
Methods
Participants and assessments
Forty-four adult (18–60 years old) MVC survivors were recruited from emergency departments (EDs) within 48 h of the accident. The survivors were excluded if they were: pregnant, under the influence of alcohol or recreational drugs at the time of the accident, seriously injured (Abbreviated Injury Scale score >2), or had sustained moderate to severe TBI (Glasgow Coma Scale [GCS] scores <13, or any abnormality detected with conventional computerized tomography in the ED). All survivors gave written Institutional Review Board approved informed consent. All survivors were alert and oriented upon testing. Survivors completed self-reports in the ED that included the Rivermead Post-Concussion Symptoms Questionnaire,48 as well as information about the MVC. Police traffic reports from the Ohio Department of Public Safety (https://ext.dps.state.oh.us) were used to confirm information on the MVC collected in EDs. Three months after MVC, 24 survivors completed the PTSD Checklist-Stressor Version (PCL) questionnaire with the MVC specified as the index traumatic event.49,50
mTBI diagnosis
mTBI was diagnosed according to American Congress of Rehabilitation Medicine (ACRM) criteria using ED medical records and self-reported symptoms. Using this ED information, individuals who had experienced head impact or acceleration-deceleration during MVC were considered to have sustained mTBI if they had loss of consciousness (LOC) for <30 min, post-traumatic amnesia (PTA) for <24 h, or severe neurological symptoms such as disorientation, dizziness, or headache.51 MVC survivors who did not meet any ACRM criteria served as a non-mTBI control group.
MRI data acquisition
All participants completed MRI brain scanning within 2 weeks after MVC, except one participant who was scanned at 20 days. MVC survivors were scanned using a 3 T General Electric Signa HDX MRI scanner. Survivors were positioned in the scanner and their heads were comfortably restrained to reduce movement. Heart rate and respiration were monitored throughout the experiment. fMRI images were acquired with a T2*-weighted, Echo Planar Imaging pulse sequence (single-phase gradient echo pulse sequence, repetition time [TR] = 2000 ms, echo time [TE] = 30 ms, flip angle [FA] = 90 degrees, field of view [FOV] = 240 mm, matrix = 64 × 64, slice thickness = 3.5 mm with no gaps, 34 axial interleaved slices to cover the whole brain, with 225 phases obtained in each run). A high-resolution T1-weighted sMRI image was also obtained with our previously used three dimensional (3D) Volume Inversion Recovery Fast Spoiled Gradient Recall Echo (IR-FSPGR) protocol (TR = 7.9 ms, TE = 3 ms, inversion time [TI] = 650 ms, FOV = 25.6 × 25.6 cm, matrix = 256 × 256, slice thickness = 1 mm with no gaps, voxel dimensions = 1 × 1 × 1 mm, 164 contiguous axial slices to cover the whole brain).52 An additional T1- weighted sMRI scan for intermediate overlaying of fMRI data was obtained using a gradient recall echo (GRE) sequence (2 excitations, TR = 250 ms, TE = 3.6 ms, FA = 90 degrees, FOV = 240 mm, matrix = 64 × 64, slice thickness = 3.5 mm, 34 axial slices to cover the whole brain).
fMRI paradigm
Blood oxygenation level dependent (BOLD) activation patterns associated with implicit emotion processing and regulation were probed using a Shifted-Attention Emotion Appraisal Task (SEAT). The SEAT paradigm is composed of trials that use a gray-scaled compound picture that presents an angry, fearful, or neutral emotional face on a background of an indoor or outdoor scene (Fig. 1). The subjects are cued to judge whether: 1) the face is male or female (Male/Female); 2) the background is of an indoor or outdoor scene (Indoor/Outdoor); or 3) they like or dislike the face (Like/Dislike). These three trial types activate different neural circuits and probe activation that is respectively associated with implicit emotion induction, emotion modulation by attention, or emotion modulation by cognitive appraisal.53,54 Uncompounded pictures of either neutral faces or building scenes were used for control trials. Inter-trial intervals were randomized from 3 to 8 sec. Fourteen trials for each task and 10 control trials were organized into three runs of ∼7 min/run. The paradigms were created with E-Prime (PST, Inc., Pittsburgh, PA), and run on a Dell workstation. Stimuli were presented with a high resolution fMRI visual goggle presentation system (NordicNeuroLab). A fiberoptic button system (Psychology SoftwareTools, Inc.) was used to record responses. Time series of trials and survivor responses were logged in E-Prime.
FIG. 1.
Shifted-Attention Emotion Appraisal Task (SEAT): examples of compound pictures with different facial expressions on a background scene.
Multi-plane surface-based integrative analysis of fMRI and sMRI data
A multi-plane surface-based approach was used to test mTBI effects on cortical activation and thickness in functionally defined cortical regions (http://homepages.utoledo.edu/xwang/index.html).47 Image processing was as follows.
First, high resolution T1-weighted sMRI images were processed in FreeSurfer (version 4.5.1) (http://www.surfer.nmr.mgh.harvard.edu/fswiki)55 to create a continuous grid of vertex (1 mm2 thickness measures covering each hemisphere). Individual MRI slices were visually inspected and inaccuracies in pial and WM borders were manually corrected if necessary.
Second, fMRI data were processed using FEAT from FSL 5.0.2.2 (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). fMRI images underwent removal of the initial four volumes, skull-striping, slice timing correction, motion correction, and high-pass temporal filtering (128 sec). fMRI data were not smoothed, to ensure spatial accuracy for surface rendering. Individual level activations were calculated with fixed effect in the FMRIB's Improved Linear Model (FILM). Contrasts among different trial types were used to identify brain activation patterns associated with each trial type.
Subsequently, FLIRT/FSL was used to register each individual's activation maps with high-resolution structural images, using the low-resolution structural image as an intermediate to create initial registration parameters. Initial registration parameters were fine-tuned by rerunning all steps using FLIRT plus FreeSurfer boundary-based corrections for cortical borders. fMRI contrast images were registered to individual high-resolution structural images using optimized registration parameters and then registered to the FreeSufer atlas using the surface-based registration parameters of the individual high-resolution structural images.
Next, the FreeSurfer surface-based fMRI analysis procedure (http://surfer.nmr.mgh.harvard.edu/fswiki/FsTutorial/MultiModal/) was independently performed at five planes of thickness in cortex. The five planes were at 0, 25, 50, 75, and 100% of cortical thickness from the WM surface. Activation located inside a plane within a subject was normalized to the corresponding plane in the FreeSurfer atlas. The normalized individual activation was then spatially smoothed along the surface using a two-dimensional Gaussian kernel of full width at half maximum (FWHM) of 8 mm. Group comparisons of cortical activation were performed at every vertex using a general linear model (GLM) of two sample t tests, controlling for age and gender. The maximal z scores across all five planes of each vertex were summed on the WM surface of the atlas. A cluster-wise correction for multiple comparisons within each hemisphere was performed by means of Z Monte Carlo simulations as implemented in FreeSurfer.56 Differences were considered significant after a multiple comparison correction with a cluster-wise p value <0.05 and a surface area >100 mm2.57
Finally, cortical thickness and task-related activation were extracted from functionally defined regions of interest (ROIs). Significantly active clusters in each of the five planes in the FreeSurfer template were individualized to the individual spaces. The individualized supra-threshold vertices on any plane were summed to create a surface ROI for the individual. The thickness of vertices in the individual surface ROI was extracted from the subject's thickness map, and a 3D ROI for measures of activation of the region was reconstructed. We projected the individualized supra-threshold vertices on each plane to a ribbon around the plane. The planes of the pial surface and of the WM surface were inwardly projected to 12.5% of cortical thickness. The other three planes were projected to 25% of cortical thickness centered at the plane. Each surface vertex was projected to a 1 mm3 space in the ribbon, and any 3D 1 mm3 voxel in the ribbon was included if it was hit by the projection of any of the surface vertices. The hit voxels from all ribbons were merged to create a 3D volume ROI. The 3D volume ROI was registered on a segmentation map of gray matter to remove non-cortical voxels. The task-related activations in each run were represented by the average percent change in contrast of perimeter estimates (COPE) extracted from the 3D volume ROI using FSL/featquery. The extracted COPEs were then averaged across all three runs.
Statistical analysis
SPSS-21 was used for statistical analyses. Survivor age and the time between the MVC and MRI scan were compared for mTBI and non-mTBI groups using two sample t tests. Univariate analyses of variance (ANOVA) of PCL scores and mean cortical thickness of ROIs were conducted using mTBI diagnosis as an independent variable, controlling for age and gender. Relationships between activation and PCL were examined with partial correlation analyses, controlling for age and gender. The response times and accuracy of identifying gender of fearful and neutral faces were analyzed in a repeated measure factorial design of emotion by group. Results are reported as mean ± standard deviation, with p < 0.05 considered significant.
Results
Symptoms and demographics
Twenty-one survivors met diagnostic criteria for mTBI (mTBI group), whereas the remaining 23 survivors were free of mTBI symptoms (non-mTBI group). We previously reported that the mTBI and non-mTBI groups did not significantly differ in age or the time between MVC and the MRI scan (t test, p > 0.1, Table 1).35 Gender and direction of collision were similarly distributed in both groups (Table 1). Twelve mTBI survivors and 12 non-mTBI survivors completed PCL questionnaires at 3 months after MVC, and PCL scores were not significantly different between groups (F[1, 20] = 1.40, p = 0.251, η2 = 0.07).
Table 1.
Demographic and Behavioral Information
| mTBI | non-mTBI | |
|---|---|---|
| Post-MVC days | 7.2 ± 3.1 | 9.0 ± 4.6 |
| (Range in days) | (3–14) | (2–20) |
| Number of participants | 21 | 23 |
| (Male/Female) | (9/12) | (9/14) |
| Age (years) | 34.3 ± 11.2 | 33.8 ± 11.0 |
| Frontal/rear vs. lateral/angle collision | 15 vs. 6 | 19 vs. 4 |
| PCL scores at 3 months | 30.5 ± 12.3 (n = 12) | 26.5 ± 8.0 (n = 12) |
| SEAT accuracy | ||
| Fear face in GENDER Task | 0.62 ± 0.13 | 0.64 ± 0.17 |
| Neutral face in GENDER Task | 0.65 ± 0.14 | 0.71 ± 0.15 |
| SEAT reaction time (sec) | ||
| Fear face in GENDER Task | 1.38 ± 0.27 | 1.38 ± 0.33 |
| Neutral face in GENDER Task | 1.46 ± 0.29 | 1.34 ± 0.37 |
mTBI, mild traumatic brain injury; MVC, motor vehicle collision; PCL, PTSD Checklist-Stressor Version; SEAT, Shifted-Attention Emotion Appraisal Task.
mTBI effects on cortical emotion processing
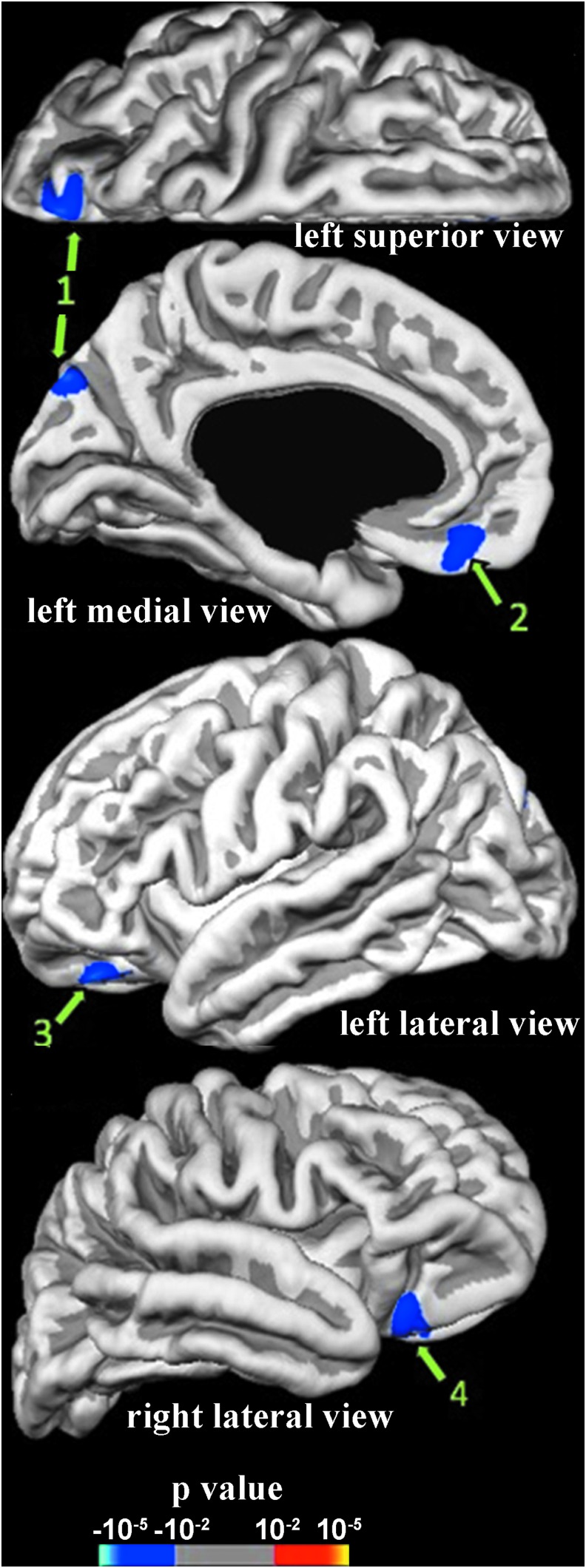
Activation associated with implicit emotional responses was revealed by contrasting the identification of the gender of fearful faces versus neutral faces. Compared with the non-mTBI group, the mTBI group had significantly less activation in response to fearful faces in clusters in the left superior parietal gyrus (SPG) and left medial orbitofrontal gyrus (mOFG), and bilaterally in the lateral orbitofrontal gyri (lOFG) (Fig. 2, Table 2).
FIG. 2.
Differences in activation associated with the contrast of identifying genders of fearful versus neutral faces between mild traumatic brain injury (mTBI) and non-mTBI groups within 2 weeks after motor vehicle collision (MVC). Supra-threshold regions are numbered in accord with region numbers in Table 2. mTBI group has less (blue) activation than the non-mTBI group.
Table 2.
Comparisons Between mTBI and Non-mTBI Groups in the Activation of Identification of Genders of Fearful versus Neutral Faces, and ROI Cortical Thickness
| Regiona | 1 | 2 | 3 | 4 |
| Location | Left SPG | Left mOFG | Left lOFG | Right lOFG |
| fMRI difference | mTBI < non-mTBI | mTBI < non-mTBI | mTBI < non-mTBI | mTBI < non-mTBI |
| Ppeak | 10−3.623 | 10−3.231 | 10−2.573 | 10−2.764 |
| Talarich (x,y,z) | −5.9,-81.8,33.8 | −4.7,36.1,-19.3 | −16.0,41.4,-16.6 | 18.7,22.7.-17.7 |
| Size (mm2) | 320 | 190 | 122 | 257.11 |
| ROI mean cortical thickness (mm) | mTBI<non-mTBI* | NS | NS | NS |
| mTBI | 2.141 ± 0.225 | 2.445 ± 0.275 | 2.982 ± 0.463 | 2.543 ± 0.209 |
| Non-mTBI | 2.359 ± 0.215 | 2.540 ± 0.315 | 2.973 ± 0.430 | 2.491 ± 0.238 |
| p | 0.002 | 0.404 | 0.887 | 0.404 |
| (Fb;η2) | (11.070; 0.217) | (1.032; 0.316) | (0.02; 0.001) | (0.711; 0.017) |
Regions are numbered as in Figure 2.
Contrast, error degrees of freedom (df): (1, 40).
Statistically significant at p < 0.05 level; NS = not significant.
SPG, superior parietal gyrus; mOFG, medial orbitofrontal gyrus; lOFG, lateral orbitofrontal gyrus; fMRI, functional magnetic resonance imaging; mTBI, mild traumatic brain injury; ROI, region of interest.
Early cortical emotion activation relationships to persistent emotion-related symptoms
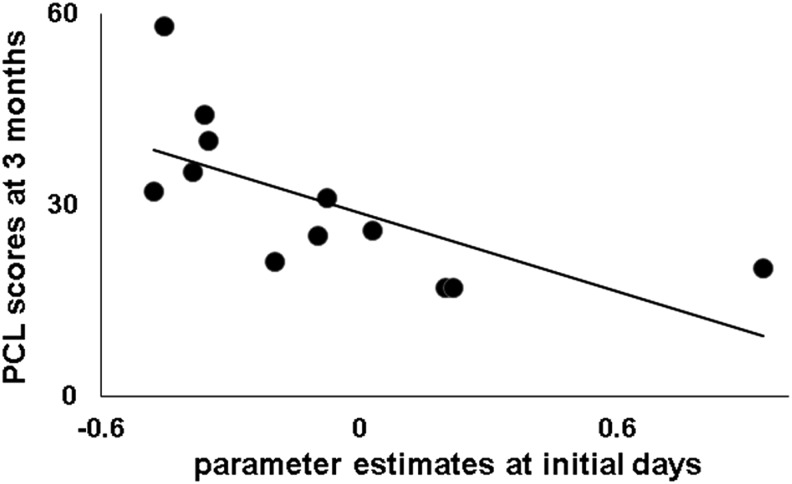
The above mentioned early decreased activation in the left SPG in the days after MVC was significantly negatively correlated with PCL scores 3 months after MVC in mTBI survivors (r = −0.68, p = 0.03, df = 8, Fig. 3). In contrast, this correlation was not significant in non-mTBI survivors (r = −0.37, p = 0.29, df = 8).
FIG. 3.
Negative correlation between left superior parietal gyrus (SPG) activation associated with identification of gender of fearful faces within 2 weeks and PTSD Checklist-Stressor Version (PCL) scores at 3 months after motor vehicle collision (MVC) in mild traumatic brain injury (mTBI) survivors.
mTBI effects on cortical thickness of functionally defined ROIs
The mean cortical thickness of the left SPG ROI with reduced activation was significantly thinner in the mTBI group than in the non-mTBI group (Table 2). Thicknesses in mOFG and lOFG ROIs were not different in the two groups.
Behavioral responses to identification of genders of emotional faces
In both groups, accuracy of identifying the gender of fearful faces was lower than on neutral face trials (F[1, 34] = 4.737, p = 0.037). The two groups did not differ in terms of accuracy (F[1, 34] = 1.05, p = 0.313, η2 = 0.03, Table 1) or response times (F[1, 34] = 0.397, p = 0.533, η2 = 0.01).
Discussion
Emotional symptoms are often reported after mTBI; however, few studies have directly assessed mTBI effects on early post-trauma emotion processing in cortical circuits. The present study reports reduced early post-mTBI activation in the left SPG and bilaterally in the orbitofrontal (OFG) in mTBI survivors during processing of fearful face visual stimuli. We also report for the first time structural thinning in the left SPG that co-localizes with reduced SPG emotion activation in mTBI survivors. Finally, the results provide evidence that supports an association between early reductions in activation in the left SPG and severity of post-traumatic stress symptoms 3 months after trauma in mTBI survivors. These findings suggest that mTBI leads to early cortical functional and structural states that contribute to later emotion-related symptoms.
Reduced early SPG activation in mTBI survivors compared with non-mTBI survivors
mTBI survivors in this study had reduced left SPG BOLD activation in a task involving viewing of fearful faces. Previous studies report that the SPG is active during working memory tasks, and that SPG activation is associated with recovery from cognitive and somatic symptoms in the weeks after a sport-related concussion.58,59 SPG activation has been linked to spatial perception and spatially directed attention.60 A bilateral intraparietal sulcus region that overlaps with the SPG cluster is active in eye gaze.61 If diminished SPG activation reflects altered processing of fearful expressions or other dynamic features of the face, it is possible that mTBI impairs this processing and contributes to impairment of interpersonal skills and mood disorders following TBI.27 Early decreased SPG activation in response to fearful faces negatively correlated with post-traumatic stress symptoms at 3 months after MVC in mTBI survivors. This suggests that early SPG emotion-related activation states may be involved in or might herald the development of post-traumatic stress symptoms. Several factors, including a history of pre-injury mental health problems, have been linked to post-traumatic stress symptoms.8 The current findings suggest that mTBI effects on emotion processing may be an additional contributor to post-traumatic stress symptoms.
Decreased SPG cortical thickness in mTBI survivors compared with non-mTBI survivors
In the mTBI group, cortical thinning occurred in the left SPG ROI where decreased activation was seen. Studies of a specific type of trauma report early alterations of cortical thickness,33,37 and studies involving diverse traumas report cortical volume changes.36 SPG is vulnerable to coup/contrecoup injury in frontal/rear axis MVCs.62,63 The present SPG thinning might reflect a structural underpinning of the reduced emotion-related activation in SPG. Co-localization of cortical thinning and lower functional activation has been reported in several diseases.64,65 Mechanistically, acute hypoperfusion caused by microvasoconstriction following mTBI may reduce cortical thickness and affect fMRI activation.66,67 From this thinking, multiple modality investigations of co-localization of cortical vascular, structural, and functional changes appear to offer promise for identifying early mechanisms by which mTBI affects cortical emotional functions and symptoms.
From our previous vertex-based, whole cortex analysis of the same subjects, we reported thickening in right precuneus and thinning in left posterior middle temporal gyrus.35 Although left SPG thickness decreases did not reach significance in these previous whole cortex analyses that used correction for multiple comparisons, decreased thickness was evident in the present analysis of mean cortical thickness extracted from a single functionally defined SPG ROI. These different findings likely reflect differences in the previous exploratory versus the present targeted ROI approaches. The observed thickness changes in right precuneus and left SPG and posterior middle temporal gyrus might also suggest that mTBI may have had different effects on the posterior left and right hemispheres. We suggest that right precuneus thickening may result from micro-edema,68,69 and that thinning in the left SPG and posterior middle temporal gyrus may result from hypoperfusion, but these possibilities require further study.
Reduced early OFG activation in MVC survivors compared with non-mTBI survivors
mTBI survivors had reduced activation in the left mOFG and bilaterally in the lOFG during emotional processing of facial expressions. However, OFG ROI thicknesses did not differ in mTBI versus non-mTBI survivors, nor was OFG ROI activation correlated with PCL symptom severity at 3 months. OFG has been implicated, for example, in integration of sensory, limbic, and prefrontal cortical inputs and in top-down inhibitory control of emotions.70–72 Abnormalities in OFG function and structure have been reported in psychiatric disorders including PTSD.73 Studies are needed to further elucidate the role of early OFG activation in post-mTBI recovery.
Limitations
This preliminary study has the following limitations. First, partly reflecting the difficulty in performing fMRI analyses during the early post-trauma stage, the sample was modest and further reduced before the 3 month follow-up was completed. Additional work is needed to confirm the present findings in larger samples. Similarly, replication in an independent sample is required to resolve the apparent inconsistency in SPG cortical thickness findings seen with our previous vertex-based whole brain analysis35 and the current ROI analysis. Second, we studied mTBI and non-mTBI survivors who had experienced MVC and had similar physical injuries. This design did not permit assessment of changes that might be present in both mTBI and non-mTBI survivors as a consequence of MVC trauma. Inclusion of trauma-free healthy controls in future studies would be useful. Third, both groups had a low level of post-traumatic stress symptoms at 3 months; therefore, our study did not test if mTBI after MVC resulted in a higher incidence of PTSD. Finally, the current report did not address possible changes in subcortical (e.g., amygdala) or archicortical structures (e.g., hippocampus).
Conclusion
The current findings demonstrate mTBI effects on BOLD activation and structure of cortical emotion circuits during the early post-mTBI period. The novel cross-modal analysis allowed examination of potential structural mechanisms that may contribute to rapid cortical functional change after mTBI. The relationship between early cortical changes and the severity of post-traumatic stress symptoms in mTBI survivors suggests potential targets for future attempts to reduce development of post-traumatic stress and other emotion-related symptoms.
Acknowledgments
The work is funded by NIH R21MH098198-01 and by a ProMedica Translational Research Stimulation Award to X.W. We thank Dr. Michael M. Dennis, Cindy Grey, Susan Yeager, Lindsey Katschke, Michelle Haunus, and the Department of Radiology at the University of Toledo for technical support; Dr. Joe Migliori Jr. for clinical support; Carol Brikmanis for editing the manuscript; and Karen Brenner of ProMedica Health System for survivor recruitment.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Cassidy J.D., Carroll L.J., Peloso P.M., Borg J., von Holst H., Holm L., Kraus J., and Coronado V.G. (2004). Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 43 Suppl, 28–60 [DOI] [PubMed] [Google Scholar]
- 2.Laker S.R. (2011). Epidemiology of concussion and mild traumatic brain injury. PM R 3, S354–358 [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (2016). Rates of TBI-related emergency department visits, hospitalizations, and deaths-United States, 2001–2010. In: Injury Prevention and Control: Traumatic Brain Injury & Concussion. CDC Center for Disease Control and Prevention; www.cdc.gov [Google Scholar]
- 4.Chossegros L., Hours M., Charnay P., Bernard M.N., Fort E., Boisson D., Sancho P.-O., Yao S.N., and Laumon B. (2011). Predictive factors of chronic post-traumatic stress disorder 6 months after a road traffic accident. Accid. Anal. Prev. 43, 471–477 [DOI] [PubMed] [Google Scholar]
- 5.Mayou R.A., Black J., and Bryant B. (2000). Unconsciousness, amnesia and psychiatric symptoms following road traffic accident injury. Br.J. Psychiatry 177, 540–545 [DOI] [PubMed] [Google Scholar]
- 6.Zhang S.R., Carroll L.J., Cassidy J.D., and Paniak C. (2009). Factors inflencing self-rated health in traffic-related mild trauma brain injury. J. Rehabil.Med. 41, 1062–1067 [DOI] [PubMed] [Google Scholar]
- 7.Cancelliere C., Hincapié C.A., Keightley M., Godbolt A.K., Côté P., Kristman V.L., Stålnacke B.-M., Carroll L.J., Hung R., Borg J., Nygren-de Boussard C., Coronado V.G., Donovan J., and Cassidy J.D. (2014). Systematic review of prognosis and return to play after sport concussion: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch. Phys. Med. Rehabil. 95, S210–S229 [DOI] [PubMed] [Google Scholar]
- 8.Carroll L.J., Cassidy J.D., Cancelliere C., Côté P., Hincapié C.A., Kristman V.L., Holm L.W., Borg J., Nygren-de Boussard C., and Hartvigsen J. (2014). Systematic review of the prognosis after mild traumatic brain injury in adults: cognitive, psychiatric, and mortality outcomes: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch. Phys. Med. Rehabil. 95, S152–S173 [DOI] [PubMed] [Google Scholar]
- 9.Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., and Castro C.A. (2008). Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 358, 453–463 [DOI] [PubMed] [Google Scholar]
- 10.Boyle E., Cancelliere C., Hartvigsen J., Carroll L.J., Holm L.W., and Cassidy J.D. (2014). Systematic review of prognosis after mild traumatic brain injury in the military: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch. Phys. Med. Rehabil. 95, S230–237 [DOI] [PubMed] [Google Scholar]
- 11.Lagarde E., Salmi L.R., Holm L.W., Contrand B., Masson F., Ribereau–Gayon R., Laborey M. and Cassidy J.D. (2014). Association of symptoms following mild traumatic brain injury with posttraumatic stress disorder vs postconcussion syndrome. JAMA Psychiatry 71, 1032–1040 [DOI] [PubMed] [Google Scholar]
- 12.Bryant R.A., Nickerson A., Creamer M., O'Donnell M., Forbes D., Galatzer–Levy I., McFarlane A.C., and Silove D. (2015). Trajectory of post-traumatic stress following traumatic injury: 6-year follow-up. Br. J. Psychiatry 206, 417–423 [DOI] [PubMed] [Google Scholar]
- 13.Frank D.W., Dewitt M., Hudgens–Haney M., Schaeffer D.J., Ball B.H., Schwarz N.F., Hussein A.A., Smart L.M., and Sabatinelli D. (2014). Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neurosci. Biobehav. Rev. 45, 202–211 [DOI] [PubMed] [Google Scholar]
- 14.Kret M.E., and Ploeger A. (2015). Emotion processing deficits: a liability spectrum providing insight into comorbidity of mental disorders. Neurosci. Biobehav. Rev. 52, 153–171 [DOI] [PubMed] [Google Scholar]
- 15.Ochsner K.N., Silvers J.A., and Buhle J.T. (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 1251, E1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neumann D., Keiski M.A., McDonald B.C., and Wang Y. (2014). Neuroimaging and facial affect processing: implications for traumatic brain injury. Brain Imaging Behav. 8, 460–473 [DOI] [PubMed] [Google Scholar]
- 17.Haxby J.V., Hoffman E.A., and Gobbini M.I. (2000). The distributed human neural system for face perception. Trends Cogn. Sci. 4, 223–233 [DOI] [PubMed] [Google Scholar]
- 18.Pallett P.M., and Meng M. (2013). Contrast negation differentiates visual pathways underlying dynamic and invariant facial processing. J. Vis. 13, 13–13 [DOI] [PubMed] [Google Scholar]
- 19.Bryant R. (2011). Post-traumatic stress disorder vs traumatic brain injury. Dialogues Clin. Neurosci. 13, 251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonald B.C., Saykin A.J., and McAllister T.W. (2012). Functional MRI of mild traumatic brain injury (mTBI): progress and perspectives from the first decade of studies. Brain Imaging Behav. 6, 193–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryer E.J., Medaglia J.D., Rostami S., and Hillary F.G. (2013). Neural recruitment after mild traumatic brain injury is task dependent: a meta-analysis. J. Int. Neuropsychol. Soc. 19, 751–762 [DOI] [PubMed] [Google Scholar]
- 22.Jantzen K.J. (2010). Functional magnetic resonance imaging of mild traumatic brain injury. J. Head Trauma Rehabil. 25, 256–266 [DOI] [PubMed] [Google Scholar]
- 23.Eierud C., Craddock R.C., Fletcher S., Aulakh M., King–Casas B., Kuehl D., and LaConte S.M. (2014). Neuroimaging after mild traumatic brain injury: Review and meta-analysis. Neuroimage Clin 4, 283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maki–Marttunen V., Kuusinen V., Brause M., Perakyla J., Polvivaara M., dos Santos Ribeiro R., Ohman J., and Hartikainen K.M. (2015). Enhanced attention capture by emotional stimuli in mild traumatic brain injury. J. Neurotrauma 32, 272–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthews S.C., Strigo I.A., Simmons A.N., O'Connell R.M., Reinhardt L.E., and Moseley S.A. (2011). A multimodal imaging study in U.S. veterans of Operations Iraqi and Enduring Freedom with and without major depression after blast-related concussion. Neuroimage 54 Suppl 1, S69–75 [DOI] [PubMed] [Google Scholar]
- 26.Shu I.W., Onton J.A., Prabhakar N., O'Connell R.M., Simmons A.N., and Matthews S.C. (2014). Combat veterans with PTSD after mild TBI exhibit greater ERPs from posterior-medial cortical areas while appraising facial features. J. Affect. Disord. 155, 234–240 [DOI] [PubMed] [Google Scholar]
- 27.Radice–Neumann D., Zupan B., Babbage D.R., and Willer B. (2007). Overview of impaired facial affect recognition in persons with traumatic brain injury. Brain Inj. 21, 807–816 [DOI] [PubMed] [Google Scholar]
- 28.Benson R.R., Gattu R., Sewick B., Kou Z., Zakariah N., Cavanaugh J.M., and Haacke E.M. (2012). Detection of hemorrhagic and axonal pathology in mild traumatic brain injury using advanced MRI: implications for neurorehabilitation. NeuroRehabilitation 31, 261–279 [DOI] [PubMed] [Google Scholar]
- 29.Wang X., Wei X.E., Li M.H., Li W.B., Zhou Y.J., Zhang B., and Li Y.H. (2014). Microbleeds on susceptibility-weighted MRI in depressive and non-depressive patients after mild traumatic brain injury. Neurol. Sci. 35, 1533–1539 [DOI] [PubMed] [Google Scholar]
- 30.Huang Y.L., Kuo Y.S., Tseng Y.C., Chen D.Y., Chiu W.T., and Chen C.J. (2015). Susceptibility-weighted MRI in mild traumatic brain injury. Neurology 84, 580–585 [DOI] [PubMed] [Google Scholar]
- 31.Ge Y., Patel M.B., Chen Q., Grossman E.J., Zhang K., Miles L., Babb J.S., Reaume J., and Grossman R.I. (2009). Assessment of thalamic perfusion in patients with mild traumatic brain injury by true FISP arterial spin labelling MR imaging at 3T. Brain Inj. 23, 666–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maugans T.A., Farley C., Altaye M., Leach J., and Cecil K.M. (2012). Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics 129, 28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doshi H., Wiseman N., Liu J., Wang W., Welch R.D., O'Neil B.J., Zuk C., Wang X., Mika V., Szaflarski J.P., Haacke E.M., and Kou Z. (2015). Cerebral hemodynamic changes of mild traumatic brain injury at the acute stage. PLoS One 10, e0118061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tate D.F., York G.E., Reid M.W., Cooper D.B., Jones L., Robin D.A., Kennedy J.E., and Lewis J. (2014). Preliminary findings of cortical thickness abnormalities in blast injured service members and their relationship to clinical findings. Brain Imaging Behav. 8, 102–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X., Xie H., Cotton A.S., Tamburrino M.B., Brickman K.R., Lewis T.J., McLean S.A., and Liberzon I. (2015). Early cortical thickness change after mild traumatic brain injury following motor vehicle collision. J. Neurotrauma 32, 455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ling J.M., Klimaj S., Toulouse T., and Mayer A.R. (2013). A prospective study of gray matter abnormalities in mild traumatic brain injury. Neurology 81, 2121–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Depue B.E., Olson–Madden J.H., Smolker H.R., Rajamani M., Brenner L.A., and Banich M.T. (2014). Reduced amygdala volume is associated with deficits in inhibitory control: a voxel- and surface-based morphometric analysis of comorbid PTSD/mild TBI. Biomed. Res. Int. Article ID 691505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y., Kierans A., Kenul D., Ge Y., Rath J., Reaume J., Grossman R.I., and Lui Y.W. (2013). Mild traumatic brain injury: longitudinal regional brain volume changes. Radiology 267, 880–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keightley M.L., Chen J.K., and Ptito A. (2012). Examining the neural impact of pediatric concussion: a scoping review of multimodal and integrative approaches using functional and structural MRI techniques. Curr. Opin. Pediatr. 24, 709–716 [DOI] [PubMed] [Google Scholar]
- 40.Niogi S.N., Mukherjee P., Ghajar J., Johnson C., Kolster R.A., Sarkar R., Lee H., Meeker M., Zimmerman R.D., Manley G.T., and McCandliss B.D. (2008). Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am. J. Neuroradiol. 29, 967–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayer A.R., Ling J., Mannell M.V., Gasparovic C., Phillips J.P., Doezema D., Reichard R., and Yeo R.A. (2010). A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology 74, 643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geary E.K., Kraus M.F., Pliskin N.H., and Little D.M. (2010). Verbal learning differences in chronic mild traumatic brain injury. J. Int. Neuropsychol. Soc. 16, 506–516 [DOI] [PubMed] [Google Scholar]
- 43.Singh M., Jeong J., Hwang D., Sungkarat W., and Gruen P. (2010). Novel diffusion tensor imaging methodology to detect and quantify injured regions and affected brain pathways in traumatic brain injury. Magn. Reson. Imaging 28, 22–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruce V. and Young A. (1986). Understanding face recognition. Br. J. Psychol. 77, 305–327 [DOI] [PubMed] [Google Scholar]
- 45.Vuilleumier P. (2005). How brains beware: neural mechanisms of emotional attention. Trends Cogn. Sci. 9, 585–594 [DOI] [PubMed] [Google Scholar]
- 46.Lieberman M.D., Inagaki T.K., Tabibnia G., and Crockett M.J. (2011). Subjective responses to emotional stimuli during labeling, reappraisal, and distraction. Emotion 11, 468–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X., Garfinkel S.N., King A.P., Angstadt M., Dennis M.J., Xie H., Welsh R.C., Tamburrino M.B., and Liberzon I. (2010). A multiple-plane approach to measure the structural properties of functionally active regions in the human cortex. Neuroimage 49, 3075–3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.King N.S., Crawford S., Wenden F.J., Moss N.E., and Wade D.T. (1995). The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J. Neurol. 242, 587–592 [DOI] [PubMed] [Google Scholar]
- 49.McDonald S.D., and Calhoun P.S. (2010). The diagnostic accuracy of the PTSD Checklist: a critical review. Clin. Psychol. Rev. 30, 976–987 [DOI] [PubMed] [Google Scholar]
- 50.Wilkins K.C., Lang A.J., and Norman S.B. (2011). Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress. Anxiety 28, 596–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.America Congres o Rehabilitation Medicine (1993). Defination of mild traumatic brain injury. J. Head Trauma Rehabil. 8, 86–87 [Google Scholar]
- 52.Wang X., Gerken M., Dennis M., Mooney R., Kane J., Khuder S., Xie H., Bauer W., Apkarian A.V., and Wall J. (2010). Profiles of precentral and postcentral cortical mean thicknesses in individual subjects over acute and subacute time-scales. Cereb. Cortex 20, 1513–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sripada R.K., Marx C.E., King A.P., Rampton J.C., Ho S.S., and Liberzon I. (2013). Allopregnanolone elevations following pregnenolone administration are associated with enhanced activation of emotion regulation neurocircuits. Biol Psychiatry 73, 1045–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liberzon I., Ma S.T., Okada G., Shaun Ho S., Swain J.E., and Evans G.W. (2015). Childhood poverty and recruitment of adult emotion regulatory neurocircuitry. Soc. Cogn. Affect. Neurosci. 10, 1596–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fischl B. (2012). FreeSurfer. Neuroimage 62, 774–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hagler D.J., Saygin A.P., and Sereno M.I. (2006). Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage 33, 1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cruz–Orive L.M., Gelsvartas J., and Roberts N. (2014). Sampling theory and automated simulations for vertical sections, applied to human brain. J. Microsc. 253, 119–150 [DOI] [PubMed] [Google Scholar]
- 58.Pardini J.E., Pardini D.A., Becker J.T., Dunfee K.L., Eddy W.F., Lovell M.R., and Welling J.S. (2010). Postconcussive symptoms are associated with compensatory cortical recruitment during a working memory task. Neurosurgery 67, 1020–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lovell M.R., Pardini J.E., Welling J., Collins M.W., Bakal J., Lazar N., Roush R., Eddy W.F., and Becker J.T. (2007). Functional brain abnormalities are related to clinical recovery and time to return-to-play in athletes. Neurosurgery 61, 352–360 [DOI] [PubMed] [Google Scholar]
- 60.Glover S. (2004). Separate visual representations in the planning and control of action. Behav. Brain Sci. 27, 3–78 [DOI] [PubMed] [Google Scholar]
- 61.Hoffman E.A., and Haxby J.V. (2000). Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nat. Neurosci. 3, 80–84 [DOI] [PubMed] [Google Scholar]
- 62.Lillie E.M., Urban J.E., Lynch S.K., Whitlow C.T., and Stitzel J.D. (2013). Evaluation of the extent and distribution of diffuse axonal injury from real world motor vehicle crashes – biomed 2013. Biomed. Sci. Instrum. 49, 297–304 [PubMed] [Google Scholar]
- 63.Urban J.E., Whitlow C.T., Edgerton C.A., Powers A.K., Maldjian J.A., and Stitzel J.D. (2012). Motor Vehicle crash-related subdural hematoma from real-world head impact data. J. Neurotrauma 29, 2774–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Remy F., Mirrashed F., Campbell B., and Richter W. (2005). Verbal episodic memory impairment in Alzheimer's disease: a combined structural and functional MRI study. Neuroimage 25, 253–266 [DOI] [PubMed] [Google Scholar]
- 65.Rasser P.E., Johnston P., Lagopoulos J., Ward P.B., Schall U., Thienel R., Bender S., Toga A.W., and Thompson P.M. (2005). Functional MRI BOLD response to Tower of London performance of first-episode schizophrenia patients using cortical pattern matching. Neuroimage 26, 941–951 [DOI] [PubMed] [Google Scholar]
- 66.Tuor U.I., Hudzik T.J., Malisza K., Sydserff S., Kozlowski P., and Del Bigio M.R. (2001). Long-term deficits following cerebral hypoxia-ischemia in four-week-old rats: correspondence between behavioral, histological, and magnetic resonance imaging assessments. Exp. Neurol. 167, 272–281 [DOI] [PubMed] [Google Scholar]
- 67.Abu–Judeh H.H., Parker R., Aleksic S., Singh M.L., Naddaf S., Atay S., Kumar M., Omar W., El-Zeftawy H., Luo J.Q., and Abdel-Dayem H.M. (2000). SPECT brain perfusion findings in mild or moderate traumatic brain injury. Nucl. Med. Rev. Cent. East. Eur. 3, 5–11 [PubMed] [Google Scholar]
- 68.Caner B., Hou J., Altay O., Fuj M., and Zhang J.H. (2012). Transition of research focus from vasospasm to early brain injury after subarachnoid hemorrhage. J. Neurochem. 123, 12–21 [DOI] [PubMed] [Google Scholar]
- 69.Bederson J.B., Levy A.L., Ding W.H., Kahn R., DiPerna C.A., Jenkins A.L., 3rd, and Vallabhajosyula P. (1998). Acute vasoconstriction after subarachnoid hemorrhage. Neurosurgery 42, 352–360 [DOI] [PubMed] [Google Scholar]
- 70.Kringelbach M. (2004). The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog. Neurobiol. 72, 341–372 [DOI] [PubMed] [Google Scholar]
- 71.Rolls E.T., and Grabenhorst F. (2008). The orbitofrontal cortex and beyond: From affect to decision-making. Prog. Neurobiol. 86, 216–244 [DOI] [PubMed] [Google Scholar]
- 72.Vuilleumier P., and Pourtois G. (2007). Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia 45, 174–194 [DOI] [PubMed] [Google Scholar]
- 73.Jackowski A.P., Araujo Filho G.M., Almeida A.G., Araujo C.M., Reis M., Nery F., Batista I.R., Silva I., and Lacerda A.L. (2012). The involvement of the orbitofrontal cortex in psychiatric disorders: an update of neuroimaging findings. Rev. Bras. Psiquiatr. 34, 207–212 [DOI] [PubMed] [Google Scholar]