Abstract
Traumatic brain injury (TBI) is a leading cause of death and disability in people younger than 45 and is a significant public health concern. In addition to primary mechanical damage to cells and tissue, TBI involves additional molecular mechanisms of injury, termed secondary injury, that continue to evolve over hours, days, weeks, and beyond. The trajectory of recovery after TBI is highly unpredictable and in many cases results in chronic cognitive and behavioral changes. Acutely after TBI, there is an unregulated release of glutamate that cannot be buffered or cleared effectively, resulting in damaging levels of glutamate in the extracellular space. This initial loss of glutamate homeostasis may initiate additional changes in glutamate regulation. The excitatory amino acid transporters (EAATs) are expressed on both neurons and glia and are the principal mechanism for maintaining extracellular glutamate levels. Diffusion of glutamate outside the synapse due to impaired uptake may lead to increased extrasynaptic glutamate signaling, secondary injury through activation of cell death pathways, and loss of fidelity and specificity of synaptic transmission. Coordination of glutamate release and uptake is critical to regulating synaptic strength, long-term potentiation and depression, and cognitive processes. In this review, we will discuss dysregulation of extracellular glutamate and glutamate uptake in the acute stage of TBI and how failure to resolve acute disruptions in glutamate homeostatic mechanisms may play a causal role in chronic cognitive symptoms after TBI.
Keywords: : EAAT, extrasynaptic, glutamate, spillover, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a leading cause of morbidity and mortality worldwide, resulting in an estimated 10 million hospitalizations or deaths per year.1 Injury is most likely during three distinct age periods: early childhood, late adolescence, and late adulthood.2 Within the United States, TBI is the primary cause of mortality in individuals younger than 45. The majority of TBIs result from automobile accidents, followed by falls and recreational injuries.2 Additionally, members of the active duty military are at elevated risk of TBI, with an estimated incidence of TBI among wounded soldiers as high as 22%.3 TBI involves both primary mechanical damage to brain tissues as well as molecular cascades that propagate injury into surrounding tissue, a phenomenon known as secondary injury.4 These metabolic effects include unregulated neurotransmitter and ion release, cell swelling, diffuse axonal injury, free radical production and oxidative stress, mitochondrial dysfunction, inhibited ATP production, inflammation, and altered gene transcription.4–9 These secondary events further exacerbate the effects of the primary injury, increasing blood–brain barrier damage, edema, ischemia, and hypoxia—ultimately augmenting the cell death process.4,8 Due to these secondary processes, TBI continues to evolve over weeks and months, making behavioral outcomes, particularly in mild injuries without focal lesions, difficult to predict.10,11
Increases in extracellular glutamate play an important role in initiating secondary injury cascades. Extracellular glutamate levels are regulated by a family of plasma membrane excitatory amino acid transporters (EAATs), localized to post-synaptic neurons and astrocytes.12 In the initial stages of TBI, extracellular glutamate levels increase and glutamate buffering and clearance is impaired.13 Loss of synaptic fidelity due to increased extrasynaptic glutamate signaling may mediate persistent cognitive and emotional symptoms after TBI. Intracellular signaling in response to injury changes localization and binding efficiency of glutamate transporters and impairs regulation of extracellular glutamate levels. Altered glutamate buffering and reuptake modulate synaptic function, leading to neuroplastic changes in learning and memory.14 Thus, if acute deficits in regulation of extracellular glutamate persist chronically, they may form the molecular basis for the long-term cognitive and emotional deficits many persons afflicted with TBI exhibit.
Animal Models of Traumatic Brain Injury
Given the high incidence of TBI, its deleterious effects, and the inability to invasively study the progression of the injury in humans, various animal models have been developed to investigate the pathophysiology of TBI. Because of their smaller size and cost effectiveness, rodents are the primary animals used to model TBI.4 However, versions of these have been adapted for use in non-rodent models, such as pig.15 These experimental models mimic various aspects of human TBI, allowing for comparison of injury type, location, region, and severity.16 Four primary injury models in rodents are commonly used: fluid percussion injury (FPI), controlled cortical impact (CCI), weight-drop impact acceleration (WDIA), and blast injury.4
Fluid percussion injury
The FPI model of injury is perhaps the most widely-used and well-characterized model of TBI.17 FPI injury is produced by striking of a pendulum to the back of a fluid reservoir, generating a wave of pressure through an opening in the skull onto the dural surface that results in temporary displacement and mechanical deformation of the brain. FPI injuries are scalable to produce mild, moderate, or severe TBI, and by adjusting the position of the craniotomy in relation to the sagittal suture, can produce midline or lateral models of injury, with lateral positioning being the most common.4 FPI produces both focal and diffuse injury, including subdural hematoma, intracranial hemorrhage, brain swelling, and axonal shearing characteristic of TBI pathology in humans.18 Additionally, FPI reliably produces the cognitive and behavioral deficits associated with TBI in humans.19 FPI is used due to its reproducibility and the ease with which injury severity can be adjusted; however, the need for a craniotomy, the cost of the FPI device, and a high mortality rate due to compromises in brainstem function are limitations of the model.20
Controlled cortical impact
The CCI model uses a pneumatic impactor device to drive a rigid rod into the surface of the exposed brain.21 The model induces deformation of the cortex around the injury site and generates widespread degradation to cortical, thalamic, and hippocampal brain regions, resulting in tissue loss, axonal shearing, and contusion.21 A primary advantage of this model is the ease with which factors such as velocity and depth of injury can be controlled.4 The extent of cortical displacement correlates with both histopathological markers of neuronal dysfunction and behavioral deficits; thus, the model can be easily adjusted to fit experimental parameters.22 CCI represents a more focal injury than other commonly-used models, which can have implications in behavioral and anatomical characterizations.20 However, the low mortality rate and reproducible pathology make CCI a useful model for biomechanical studies of TBI.4
Weight-drop impact acceleration
The WDIA model induces trauma via a free falling guided weight striking a metal disk cemented to the rodent's skull.23 A scalable injury is achieved by varying the mass of the weight and the distance that it falls.4 The impacting force generates rapid acceleration of the brain within the skull, resulting in diffuse brain injury, including petechial hemorrhage and edema in regions from the cortex to the brainstem without fracturing the skull.16 WDIA produces characteristic pathological features, including widespread and bilateral axonal and neuronal damage and extensive diffuse axonal injury, as well as similar behavioral and cognitive defects found in FPI and CCI models.4 While cost effective and useful in evoking diffuse axonal injury, this model has drawbacks due to the relative variability in injury severity.20 Despite this, the WDIA model is useful for the study of multiple concussions, an area of increasing importance in the study of sport-related injuries.
Blast injury models
In recent combat operations, explosive blast forces, such as those generated by an improvised explosive device, posed considerable risk of TBI to deployed personnel.3 Even individuals who do not experience any external injuries subsequent to an explosion can experience TBI as a result of the forces generated by the blast.3 In order to understand the mechanisms involved in the propagation of the injury from an explosive force, animal models have been developed that seek to recreate the blast injury. In these models, a shock tube and compression forces simulate non-impact blast injuries.3 Characteristic features of blast injury include cerebral edema, hyperemia, and delayed vasospasm, as well as diffuse axonal injury.4 Blast injuries also lead to behavioral and cognitive deficits similar to other models of TBI.4 The largest drawback to blast models is the difficulty in standardizing the injury procedure and in replicability of results between research groups.24
The Tripartite Synapse and Glutamate in the Healthy Brain
Glutamate release
Glutamate release, activity as a ligand, and reuptake involves the coordinated action of pre- and post-synaptic neurons, as well as astrocytes.25 Receptors, enzymes, and transporters comprise a neuron–astroglia coupled system modulating synaptic, perisynaptic, and extrasynaptic glutamate levels.26,27 In the pre-synaptic neuron, glutamine may be converted to glutamate by glutaminase and packaged by vesicular glutamate transporters (VGLUT1-3) for release into the synapse.28,29 Once released, free glutamate in the extracellular space activates post-synaptic receptors, is removed into astrocytes by glutamate transporters, or spills over into the extrasynaptic space. Glutamate in the synapse may occupy and activate ionotropic (N-methyl-D-aspartate (NMDA) receptor, α-amino-3-hydroxy-5-methyl-isoxazole propionate (AMPA), and kainate) or metabotropic glutamate receptors on both neurons and astrocytes.25,30,31
Repetitive activation of excitatory synapses increases synaptic strength, a process known as long-term potentiation (LTP).32 LTP and the related phenomenon long-term depression (LTD) exist in excitatory synapses in brain regions implicated in learning and memory.32 LTP initiation requires localized increase in intracellular calcium in the dendritic spine, typically via activation of NMDA receptors, which gate both calcium and sodium ions.32 Magnesium ions block the receptor channel at physiological concentrations in a voltage-dependent manner. Partial depolarization of the cell membrane, usually via activation of the AMPA receptor, extrudes these magnesium ions. Therefore, both pre-synaptic glutamate release and AMPA receptor-mediated post-synaptic depolarization are typically required for NMDA receptor–mediated calcium influx.33 Various receptor-associated proteins for both NMDA and AMPA can then form signaling complexes within the post-synaptic density, modulating content, morphology, and function, and ultimately initiating LTP.34,35
Neuronal and glial glutamate transporters
EAATs are the most significant means of extracellular glutamate regulation and are expressed in neurons and glia throughout the brain in a region- and cell-specific manner.36,37 EAAT1 and EAAT2 are primarily localized to astroglia, while EAAT3-4 and EAAT5 are primarily localized to neurons and the retina, respectively.36,38 EAATs mediate glutamate transport by an electrogenic exchange of 3 Na+, 1 H+, and 1 glutamate molecule into the cell and 1 K+ ion out of the cell, with the net inward movement of positive charges.38–40 The glial transporters are situated in perisynaptic processes facing the synaptic cleft.41 In the prefrontal cortex, glial transporters account for the majority of synaptic glutamate reuptake.42,43 Cortical homogenates from EAAT2-deficient mice exhibit less than 5% of uptake activity, compared with wild-type mice,44 and additional investigations show that EAAT2 accounts for more than 90% of the glutamate reuptake activity in most brain regions.45
Following EAAT-mediated uptake into astrocytes or neurons, glutamate may enter the tricarboxylic acid cycle via conversion to α-ketoglutarate, be converted to glutamine and transported back into the synapse, or be released into the extracellular space by a variety of mechanisms.46 Depending on the cell type, recovered glutamate also may contribute to lactic acid formation. Lactate production is favored in astrocytes, while lactate breakdown is favored in neurons.47 Lactate is efficiently shuttled from astrocytes to neurons and may be a preferred energy substrate in neuronal structures enveloped by astrocytic processes.47 Metabolism of glutamate in the synaptic terminals is necessary both to provide sufficient energy to sustain transmission and to replenish transmitter pools.48 Consequently, inadequate astrocytic reuptake in response to neuronal activity may both increase signaling though extrasynaptic glutamate receptors as glutamate spills out of the synapse and/or fail to sustain neuronal energy requirements and transmitter pools during periods of high demand.
Regulation of glutamate reuptake
The expression of EAATs is regulated on multiple levels, including transcription, messenger RNA (mRNA) splicing, protein synthesis, and post-translational modification.38 Receptor tyrosine kinase (RTK) signaling appears to represent a primary starting point for second messenger cascades, converging on the mitogen-activated protein kinases (MAPKs) p42 and p44, and ultimately regulating expression of EAAT2. The dual phosphorylation of p42/p44 MAPKs at threonine-202 and tyrosine-204 increases expression levels of EAAT2 in the presence of neuron-conditioned media, while inhibition of RTKs by the cell-permeable typhostin A23 blocks the induction of EAAT2.49 Growth factors can bypass the RTK-p42/44 MAPK pathway and still influence the expression of EAAT2 by directly activating transcription factors such as cAMP-responsive element modulator, cAMP responsive element-binding protein (CREB), and activating transcription factor 1 (ATF-1), although the induction of EAAT2 via these pathways is weaker than through RTK activation.49
Akt kinase also regulates the expression of EAAT2 by increasing its rate of transcription.50 Transfection with a dominant-negative AKT lentiviral vector decreased the effects of epidermal growth factor signaling on EAAT2 expression in astrocyte cultures, while constitutively active AKT increased EAAT2 expression, protein levels, and transport activity in a dose- and time-dependent manner.50 Growth factor activation of PI-3K can activate not only Akt, but also increases phosphorylation of p42/44 MAPKs, suggesting a converging network regulating EAAT2 expression.51 Thus, increased activity of a number of intracellular signaling pathways regulate EAAT2 expression, with RTK signaling cascades, p42/44 MAP kinases, and AKT protein kinase representing key mediators promoting EAAT2 expression.
Glutamate uptake also may be regulated through either sequestration of transporters into intracellular storage sites or by ubiquitin-mediated degradation of transporters. Of primary importance in selective EAAT downregulation is the activity of protein kinase C (PKC). PKC has differential effects on the EAAT subtypes; in mixed neuronal and astrocyte cultures, activation of PKC rapidly (within minutes) decreased cell-surface expression of EAAT252 and increased surface expression of the neuronal transporter EAAT3.53 These differential effects are thought to represent a switching mechanism from astrocytic to neuronal glutamate uptake. However, as astrocyte transport represents the primary uptake mechanism, the overall effect in a mixed-cell culture would be reduced reuptake and elevated extracellular levels of glutamate.53 The deletion of amino acids 475–517 on EAAT2 abolishes the effects of PKC-induced internalization, indicating a carboxyl-terminal phosphorylation site on the transporter.52 This decrease in cell-surface expression did not correspond with a reduction in total cellular levels of EAAT2, suggesting the immediate effect of phorbol ester activation of PKC involves internalization of the transporter to an intracellular sequestration site.53 Internalization can be blocked in astrocyte cultures expressing a dominant-negative variant of clathrin54 or by inhibition of the ubiquitin enzyme E1,55 demonstrating an ubiquitin-dependent, clathrin-mediated endocytic mechanism of sequestration.
In contrast to short-term activation of PKC, long-term exposure to phorbol ester was accompanied by an overall decrease in total cellular EAAT2 expression.54 Lysosomal inhibitors attenuate this decrease in EAAT2 protein, suggesting a cellular mechanism by which PKC modulates EAAT2 levels under physiological or pathological conditions.54 In summary, astrocytic and neuronal kinase signaling mechanisms may positively or negatively regulate EAAT expression and transport activity through transcriptional control or regulation of cell surface expression in a time- and concentration-dependent manner.
Extrasynaptic glutamate signaling
Removal of glutamate from the synaptic cleft involves: 1) the high affinity binding of glutamate by perisynaptic transporters (e.g., EAAT2) and 2) the transport of bound glutamate by the transporter across the plasma membrane.41,56 Once bound, glutamate may be “unbound” or released instead of transported across the plasma membrane.41,56 The relatively low rate of transport of bound glutamate relative to its binding affinity suggests that the EAATs first act as buffers for released glutamate.41 Thus, glutamate molecules may bounce from one transporter-binding site to another until transported, thereby limiting glutamate spillover from the synaptic cleft into extrasynaptic areas.
Glutamate levels in the extracellular milieu are tightly regulated, as activation of extrasynaptic glutamate receptors has potent effects. LTP and LTD can be readily induced in the adult cortex by activation of extrasynaptic GluN2B-containing NMDA receptors.57,58 While several regions have well characterized glutamate spillover between excitatory synapses, such as the cerebellum and hippocampus, there is ongoing debate regarding the extent of glutamate diffusion in other regions, including the frontal cortex, where spillover of glutamate may detrimentally lead to loss of input specificity and activation of cell death pathways.59–65 Mathematical models suggest that glutamate may diffuse and activate NMDA receptors within a radius of 0.5 μm from the release point.27 Thus, the spatial arrangement of glutamate synapses, their glutamate transporter buffering zones, and extrasynaptic glutamate receptors will determine the extent and effect of glutamate spillover.66,67
Glutamate in Acute TBI
Neurotoxicity
Elevated extracellular glutamate levels, such as through application of exogenous glutamate and related analogues into nervous tissue, are extremely toxic to cells.68,69 In humans, severe TBI results in elevated cerebrospinal fluid glutamate that may persist in some brain structures for days or perhaps weeks.70,71 Following severe TBI in rats, microdialysis determination of extracellular glutamate levels found a 9-fold increase over non-injured control rats.72 High levels of glutamate deplete ATP stores due to overstimulation and the energy expenditure involved in reuptake.68 TBI also transiently increases extracellular potassium levels by 6-fold above baseline.7 The effects of increased extracellular glutamate and potassium on ionic homeostasis and ATP expenditure are exacerbated by changes in astrocytic potassium conductance following TBI.73 Resting astrocytes are able to effectively remove glutamate from the synapse due to their high potassium conductance and a negatively charged resting membrane potential maintained in part by the inwardly rectifying potassium channel KIR4.1.74 Conditional knockout of the KIR4.1 result in cells with a diminished capacity to transport glutamate.75,76 KIR4.1 is not well studied after TBI; however, it was downregulated along with GLT-1 (EAAT2) from 4 h to at least 72 h after CCI in mice.77 The central role of potassium channels in the fidelity of glutamate transport highlights the importance of sodium/potassium gradient, as well as ATP production in recovery from TBI.
Increases in extracellular glutamate in TBI also disrupt ionic homeostasis of sodium and calcium. Supra-physiological activation of glutamate receptors increases intracellular levels of sodium ions and leads to swelling of cells from osmotic pressure.78 Other pathologic effects of increased extracellular glutamate involve the actions of the neurotransmitter on ionotropic glutamate receptors that are calcium channels.7,79 Calcium is a critical signaling molecule governing myriad cellular processes and as such is tightly regulated with intracellular concentrations maintained around 100 nM.79 Prolonged increases in intracellular calcium disrupt mitochondrial production of ATP and activate proteases and kinases, including nitric oxide synthase. Increased activity of these enzymes may generate reactive oxygen species, disrupt cytoskeletal architecture, and increase transcription of genes associated with apoptosis pathways.79 Thus, the pathological results of increased extracellular glutamate are mediated in part by increases in intracellular levels of sodium and calcium, leading to cell swelling and the activation of deleterious signaling pathways, respectively.
In the absence of calcium, glutamate-mediated increases in intracellular sodium can lead to pathological cell swelling and cell death in hippocampal cultures.80 However, changes in intracellular sodium and calcium ions have disparate effects on mixed cortical cultures. In an extracellular environment with physiological concentrations of both sodium and calcium, neurons exhibited immediate morphological changes followed later by significant cell degradation and death. Removal of both ions from the extracellular environment was protective to the cells, even with prolonged glutamate exposure. However, ion substitution studies (cell cultures with extracellular environments lacking one of the two ions) suggested unique roles for each of these ions in the excitotoxic cascade. Mixed-cell cultures in a calcium-free media exhibited morphological changes, including increased swelling and granulation, but were largely spared from cell death and returned to previous size within an hour.81 Cultures in media where choline was substituted for sodium showed markedly less swelling and other morphological changes when exposed to toxic glutamate levels, but exhibited roughly the same amount of cell death as cultures with both ions at physiological levels.81 These results indicate that the acute excitotoxic event precipitated by sodium appears to be transient and largely non-toxic to cortical cells, while the glutamate induced changes in calcium ion concentrations play a key role in subsequent cell death by overwhelming the calcium regulatory mechanisms and activating downstream signaling pathways.5 Thus, in clinical TBI, it is likely that the calcium-regulated mechanisms responsible for cell death are overwhelmed and contribute to widespread neuronal cell death.
Pathophysiological activation of downstream signaling pathways depends on changes in calcium levels, calcium's route of entry into the cell, and the location of its release from intracellular storage.5 Of particular importance for glutamate mediated neurotoxicity is the ionotropic NMDA receptor. Studies examining calcium's level and route of entry into the cell found that absolute levels of intracellular free calcium roughly corresponded with neuronal survival, but survival was better predicted by the route of calcium entry and duration of calcium loading.82 Neuronal cell cultures were likely to survive excessive intracellular calcium levels when entry occurred through voltage-sensitive channels triggered by cell depolarization; however, similar levels produced by prolonged ligand-gated (glutamate) activity resulted in significantly more cell death.82 These effects seem to be restricted to glutamate's activity on NMDA receptors, as increases in calcium stimulated by glutamate from non-NMDA receptors did not have the same impact on cell survival.82 The neurotoxic effects of glutamate-evoked calcium influx are likely due to activity of the NMDA receptor signaling pathways that promote cellular degeneration.82
Transporters in acute TBI
Antisense oligonucleotide knockdown of EAATs show they are critical for maintaining glutamate below toxic levels. EAAT2 knockdown significantly increased hippocampal cell death, compared with controls, after TBI.83 Knockout of either GLAST (EAAT1) or GLT-1 in mice yields excitotoxic levels of glutamate similar to those experienced following TBI.84 These studies demonstrate the central role of astrocytic EAATs, especially EAAT2, in the maintenance of extracellular glutamate within physiological norms, and illustrate how the pathology of TBI can be exacerbated when EAAT function or expression is compromised. Studies in astrocyte cultures suggest that the half-life of EAAT2 is longer than 24 h85; thus decreases in EAAT2 expression in the hours immediately following TBI86 might not be accounted for solely by altering transcription or translation of the transporter. Along with degradation of EAAT2 by caspase-3,87 modulation by PKC could possibly represent a mechanism by which the secondary injury phase of TBI induces dysfunction in glutamate reuptake transporters and exacerbates the deleterious effects of elevated extracellular glutamate.
Astroglial injury and/or death, caspase-mediated degradation of glutamate transporters, reversal of sodium-calcium exchanger, and reversal of sodium-dependent glutamate transporters are all implicated in acute TBI pathology.87–89 CCI models demonstrate an early and consistent loss of EAAT2 protein in the ipsilateral cortex beginning 4–6 h after injury and persisting past 72 h.86 In studies utilizing lateral FPI, EAAT2 expression in the ipsilateral cortex may not change during the first 24 h; however, decreases in the ipsilateral cortex are reported at 7 days post-injury.88,90,91 Moreover, decreases may be in a specific splice variant for EAAT2, suggesting a shift in the cellular or subcellular localization of this transporter in TBI.91
These findings in animal models replicate analyses of human postmortem brain in which protein levels of the astrocytic glutamate transporters may be decreased.88,92 Compared with moderate EAAT2 staining found in control patients, extensive EAAT2 staining is present in the ipsilateral cortex of TBI cases, with survival times between 1 and 24 h post-injury, indicating an increase in EAAT2 expression in this region.92 In survival times less than 1 h or longer than 24 h, weak and sporadic EAAT2 staining patterns were observed. These data suggest dynamic regulation of EAAT2 expression after TBI. This transient increase in EAAT2 expression between 1 and 24 h may represent a compensatory reaction to the elevated extracellular glutamate levels following TBI. At longer survival times, patients exhibit a decrease in EAAT2 expression, possibly as a result of the activity of intracellular signaling pathways promoting EAAT2 degradation. It is worth noting that short-term TBI survival is correlated with a higher injury severity93; thus, these findings likely reflect the influence of both of these factors on EAAT2 expression.
While astrocytic EAATs are responsible for the majority of the removal of glutamate from the extracellular space, the neuronal EAATs also are affected by TBI. Increases in expression of EAAT4 were found in hippocampal astrocytes 3 to 7 days following lateral FPI.94 This finding is interesting in that EAAT4 is typically expressed in neurons.57 EAAT4 expression in hippocampal astrocytes may represent an endogenous neuroprotective mechanism or may be part of a phenotypic switch in reactive astrocytes.94 Other studies have found similar instances of phenotypic switching of typically “astrocytic” EAATs onto neuronal processes. For example, hypoxia results in early loss of EAAT2 in the pig hippocampus, which is followed by the aberrant induction of an EAAT2 splice variant in neuronal cell types.95 Additionally, detection of an alternately spliced variant of EAAT1 in neuronal cell cultures following hypoxia is an early and highly sensitive marker for neurons at risk of cell death, as normal expression of glutamate transporters may be negatively modulated by co-expression of these splice variants.96 Similarly, engineered expression of EAAT2 on neuronal cell types increases vulnerability to excitotoxicity in hippocampal slice cultures.97 It is plausible that glial EAAT expression in neurons may be a compensatory attempt by the brain to offset increases in extracellular glutamate following TBI; however, these efforts may ultimately harm neuronal tissue by making it more vulnerable to excitotoxicity due intracellular glutamate levels beyond the buffering or metabolic capacity of neurons.98
Extracellular glutamate concentrations may modulate the expression of glutamate transporters. Exposure of cultured cortical astrocytes to high levels of extracellular glutamate (20 mM) decreased EAAT2 expression by 25 and 40% following 24 h or 72 h exposure times, respectively.99 The decline in protein expression was not due to astrocyte death, but corresponded to increased glutamine synthetase protein. The decline in transporter expression was not attenuated by non-competitive antagonists for NMDA or AMPA receptors, supporting a receptor-independent mechanism for decreased transporter expression.99 In summary, the acute effects of TBI on expression of glutamate transporters are mediated by a number of paracrine and intracellular signaling factors influencing the function, location, and phenotype of the transporters. Long-term compromises in the tripartite glutamate system exacerbate the pathological effects of the primary injury and contribute to the persistent deficits in cognition characteristic of the secondary injury phase.
Glutamate in Chronic TBI
Glial dysfunction
Eight weeks following moderate lateral FPI, hippocampal cell loss was accompanied by increased gliosis and increased GFAP staining in the thalamus, frontal cortex, and hippocampus, indicating a persistent abnormality in astrocytes.100 Additionally, single-cell polymerase chain reaction analysis of glial acidic fibrillary protein (GFAP)–positive astrocytes found a dramatic shift in gene expression 14 days after ischemic injury in populations of reactive astrocytes.101 Reactive astrocytes have increased expression of transcripts for EAAT1, synaptosomal-associated protein 25 (SNAP25), and glutamate receptor subunits, indicating that these cells may be starting to express “neuronal” genes such as SNAP25 and some of the neuronal hyperpolarization activated cyclic nucleotide gated potassium channels (HCNs).101
Few studies have evaluated glutamate levels in chronic (>14 days post-injury) animal models of TBI. A series of studies implicated chronic dysregulation of glutamate in chronic TBI by examining late onset behavioral morbidity as indicated by increased whisker sensitivity following diffuse experimental TBI. Over 8 weeks following moderate midline FPI, rats in both injured and sham experimental conditions received manual whisker stimulation, and behavioral responses were recorded. While sham injury animals were ambivalent or soothed by whisker stimulation, animals in the injury condition demonstrated aggravated behavioral responses beginning at 1 week post-injury, which became significant at 4 weeks and persisted throughout the rest of the 8 weeks.102 A follow up study examining the molecular underpinnings for this increased whisker sensitivity supported hypersensitive glutamate circuitry as a possible causal mechanism.103 Microelectrode array studies found increased extracellular glutamate levels in the ventral posterior medial hypothalamus, compared with sham-injured animals, 4 weeks following injury.103 Imaging studies after human TBI suggest the balance of glutamate and the inhibitory transmitter γ-aminobutyric acid (GABA) may be chronically altered after TBI.71,104,105 Thus, persistent impairments in glutamate circuitry represent a possible mechanism for the chronic cognitive and emotional symptoms experienced by some patients after TBI.
In addition, several studies indirectly examine the roles of glutamate transporter expression, glutamate reuptake, and extracellular glutamate in chronic FPI using novel pharmacological therapeutics. Pharmacological blockade of sodium channels, which inhibits glutamate release, attenuated GFAP immunoreactivity 2 weeks following moderate-to-severe lateral FPI.106 Another study found treatment with riluzole, a pharmacological agent which inhibits sodium channels and decreases glutamate release, decreased cortical lesion size 2 weeks following moderate parasagittal FPI.107 Further, microtubule-associated protein 2 immunoreactivity 2 weeks after moderate lateral FPI was reduced following treatment with the glutamate receptor antagonist kynurenate, compared with untreated animals.108 These studies indirectly demonstrate the role of glutamate in chronic TBI by showing that inhibiting glutamate release or modulating glutamate receptors decreases chronic pathophysiology.
Signaling in chronic TBI
Signaling events regulating glutamate neurotransmission also are altered in chronic TBI models. Extracellular signal-regulated kinase (ERK) and CREB activation was decreased in hippocampal slices following a stimulation protocol 12 weeks after parasagittal FPI; in this same study, resting-state levels of phospho-CREB protein were decreased in the hippocampus, suggesting persistent signaling abnormalities.109 Interestingly, ERK and CREB are positive modulators of EAAT2 expression.49,51 Decreased activity in this signaling pathway is consistent with diminished EAAT expression and activity. Further, ERK and CREB are molecular markers of memory consolidation, and disruptions in these pathways downstream of glutamate neurotransmission may contribute to the chronic cognitive difficulties observed after TBI. Using electrophysiological measures, one group found increased frequency of spontaneous inhibitory post-synaptic currents in the dentate gyrus 1 month after FPI, while another found decreased capacity for LTP in mild, moderate, and severe TBI 8 weeks after injury.110,111 These data suggest a mechanism for persistent alterations in the molecular correlates of learning and memory in the chronic FPI model of TBI. There may be a threshold level of LTP that is required to initiate protein synthesis–dependent consolidation of memory112; thus, the reduced capacity for LTP found 8 weeks following TBI represents a potential mechanism for the cognitive impairment exhibited in both human patients and animal models of TBI.
Glutamate reuptake mechanisms are closely linked to the electrophysiological changes reported in chronic TBI discussed above. In the hippocampus, baseline (i.e., “normal”) LTP in the CA1 subfield is associated with increased glutamate reuptake; for example, fear conditioning increased EAAT-dependent reuptake, and blockade of EAAT3 co-localization with the NMDA receptor–signaling complex attenuated synaptic strength.14 In a similar study in ischemia, a disorder where glutamate excitotoxicity is believed to play a similar role as in TBI, transient global ischemia decreased protein expression of EAAT2 in area CA1 of the hippocampus corresponding to decreased amplitude of glutamate evoked currents from astrocytes in this area.113 Further, reverse transcription polymerase chain reaction demonstrated diminished EAAT2 mRNA in post-ischemic cells in hippocampal area CA1.113 These results suggest that disruption in EAAT2 expression and function contributes to astrocytic cell death in the hippocampus, and that glutamate reuptake is important for maintaining synaptic strength and initiating LTP in the hippocampus. Thus, impairment of glutamate reuptake could account for observed electrophysiological deficits in this region in chronic TBI.
Therapeutics addressing glutamate
Attempts to offset glutamate excitotoxicity following TBI have largely been disappointing in clinical trials.114 Numerous therapeutics agents have attempted to address the glutamate imbalance using pre-synaptic glutamate inhibiting agents, competitive and non-competitive NMDA receptor antagonists, cannabinoid agonists, nitric oxide synthase inhibitors, AMPA antagonists, serotonin agonists, and magnesium sulfates.114,115 While initially promising in phase II clinical trials, the synthetic cannabinoid receptor agonist dexanabinol failed to produce statistically relevant improvements in Glasgow Outcome Score (GOS) in phase III trials.116 The competitive NMDA receptor antagonist selfotel also was brought to phase III clinical trials with similarly disappointing results. Patients in the placebo group had statistically similar GOS scores and mortality rates to those in the drug treatment condition and the study was discontinued.117 Extracellular magnesium, which endogenously acts as a non-competitive NMDA antagonist also has been investigated. However, in moderate-to-severe TBI patients, magnesium sulfate administration resulted in no significant improvements in survival, seizure activity, or behavioral outcomes.118 Recent animal studies attempting to offset the loss of glutamate homeostasis following TBI involve the use of therapeutic drugs that upregulate glutamate transporter expression. For example, the β-lactam antibody ceftriaxone reversed the loss of EAAT2 expression in the ipsilateral frontal cortex of TBI mice 7 days post-injury and resulted in lower levels of pro-inflammatory mediators.90,119 A number of additional compounds, including riluzole, the tricyclic antidepressant amitriptyline (which upregulates EAAT expression), and the beta-carboline alkaloid harmine (which stimulates EAAT2 activity), show promise in multiple models of CNS injury and neurodegeneration.120
By focusing on neuronal protection, pharmacological intervention may be ignoring the crucial role of astrocytes in the propagation and sustainment of the disease state. We posit that there is also a need for glioprotective agents in therapeutic treatments of chronic TBI. There is still much to be learned about how traumatic injury affects EAAT expression, splice variants, trafficking, localization, activity, and turnover that could identify new therapeutic opportunities in both acute and chronic secondary injury processes. Accumulating evidence suggests that comprehensive examination of the glutamate reuptake mechanism and associated signal transduction processes may be a high yield substrate for understanding the pathophysiology of excitatory circuits in chronic TBI.
Conclusion
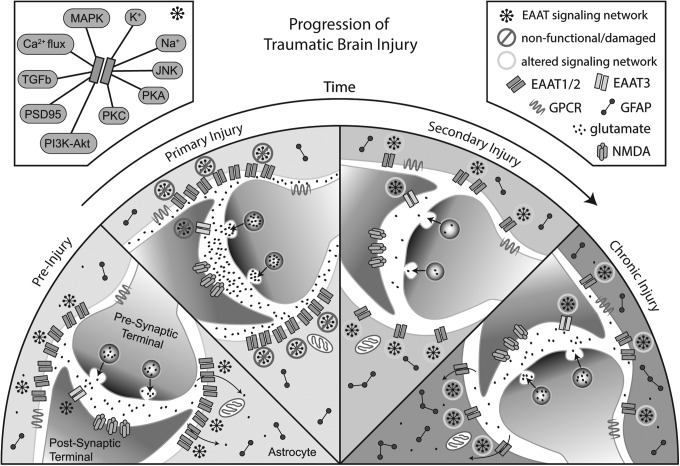
As with the proximal primary injury, alterations in glutamate homeostasis may represent a driving event in the ongoing pathophysiology of TBI. Failure to resolve the secondary injury may be due in part to changes in localization and function of glutamate transporters and their associated signaling molecules, anchoring proteins, and metabolic pathways necessary for normal glutamate transmission (Fig. 1). Given the strong link between proper glutamate reuptake and the maintenance of synaptic strength, LTP, and learning and memory, dysfunction in glutamate transport systems could play a causal role in the persistent cognitive symptoms associated with chronic TBI. Prolonged morphological and molecular changes in astroglia and astroglial glutamate uptake mechanisms may be particularly important as a single astrocyte may interconnect with thousands of synapses.121
FIG. 1.
Progression of traumatic brain injury. Schematic representation of changes in glutamate release, reuptake, and activity based on data from rodent models of traumatic brain injury. In normal brain (pre-injury), there is dense perisynaptic localization of glutamate transporters, which shapes synaptic transmission and neuroplasticity via the buffering and removal of glutamate from the synaptic cleft. During primary injury, several mechanisms may lead to diminished glutamate uptake, including changes in transporter expression, signaling pathway activation, and/or proteolytic degradation of transporter protein. We posit that transporter expression and localization is altered during secondary and chronic injury in a manner that serves to protect surviving neurons from additional injury. Finally, consolidation of neuroplastic changes during chronic injury may include alterations in glutamate release and reuptake, contributing to the cognitive defects found in chronic traumatic brain injury. MAPK, mitogen-activated protein kinase; K+, potassium; Na+, sodium; JNK, c-Jun N-terminal kinase; PKA, protein kinase A; PKC, protein kinase C; PI3K-Akt, phosphoinositide 3-kinase-protein kinase B; PSD95, post-synaptic density 95; TGFb, transforming growth factor beta; Ca2+, calcium; EAAT, excitatory amino acid transporter; GPCR, G-protein-coupled receptor; GFAP, glial fibrillary acidic protein; NMDA, N-methyl-D-aspartic.
Diminished glutamate reuptake capacity could have profound effects on cognitive and behavioral outcomes associated with chronic TBI. Inadequate management of glutamate in the synapse and glutamate spillover into extrasynaptic domains would affect initiation, amplitude, and sustainability of LTP and LTD, both of which are critically important for synaptic efficiency and cognition. Insufficient astrocytic response to neuronal activity produces electrophysiological changes consistent with glutamate spillover from the synapse.122,123 Similar mechanisms may be present in the neocortex in schizophrenia, an illness with profound cognitive impairment.33
Taken together, these mechanisms may explain the diffuse global nature of cognitive and emotional symptoms experienced following TBI; the initial injury may reset glutamate regulatory mechanisms and molecular systems associated with cognitive performance during the secondary injury phase. If not resolved, these changes consolidate, resulting in long-term alterations in glutamate homeostasis which may diminish the ability of neurons to effectively initiate or maintain synaptic plasticity (Fig. 1). This model suggests a threshold for patient symptomology, below which a patient is asymptomatic but nevertheless increasingly susceptible to developing chronic symptoms after each subsequent injury or other CNS stress due to incremental increases in damaged astroglia and associated signaling deficits.
Acknowledgments
This work was partially supported by R01NS075162 (CLF).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Langlois J.A., Rutland-Brown W., and Wald M.M. (2006). The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 21, 375–378 [DOI] [PubMed] [Google Scholar]
- 2.Bruns J., Jr. and Hauser W.A. (2003). The epidemiology of traumatic brain injury: a review. Epilepsia 44 Suppl 10, 2–10 [DOI] [PubMed] [Google Scholar]
- 3.Martin E.M., Lu W.C., Helmick K., French L., and Warden D.L. (2008). Traumatic brain injuries sustained in the Afghanistan and Iraq wars. J. Trauma Nurs. 15, 94–99 [DOI] [PubMed] [Google Scholar]
- 4.Xiong Y., Mahmood A., and Chopp M. (2013). Animal models of traumatic brain injury. Nat. Rev. Neurosci. 14, 128–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arundine M. and Tymianski M. (2004). Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell. Mol. Life Sci. 61, 657–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McIntosh T.K. (1993). Novel pharmacologic therapies in the treatment of experimental traumatic brain injury: a review. J. Neurotrauma 10, 215–261 [DOI] [PubMed] [Google Scholar]
- 7.Katayama Y., Becker D.P., Tamura T., and Hovda D.A. (1990). Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J. Neurosurg. 73, 889–900 [DOI] [PubMed] [Google Scholar]
- 8.Gaetz M. (2004). The neurophysiology of brain injury. Clin. Neurophysiol. 115, 4–18 [DOI] [PubMed] [Google Scholar]
- 9.Raghupathi R. (2004). Cell death mechanisms following traumatic brain injury. Brain Pathol. 14, 215–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyle E., Cancelliere C., Hartvigsen J., Carroll L.J., Holm L.W., and Cassidy J.D. (2014). Systematic review of prognosis after mild traumatic brain injury in the military: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch. Phys. Med. Rehabil. 95, S230–S237 [DOI] [PubMed] [Google Scholar]
- 11.Nygren-de Boussard C., Holm L.W., Cancelliere C., Godbolt A.K., Boyle E., Stalnacke B.M., Hincapie C.A., Cassidy J.D., and Borg J. (2014). Nonsurgical interventions after mild traumatic brain injury: a systematic review. Results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch. Phys. Med. Rehabil. 95, S257–S264 [DOI] [PubMed] [Google Scholar]
- 12.Danbolt N.C., Chaudhry F.A., Dehnes Y., Lehre K.P., Levy L.M., Ullensvang K., and Storm-Mathisen J. (1998). Properties and localization of glutamate transporters. Prog. Brain Res. 116, 23–43 [DOI] [PubMed] [Google Scholar]
- 13.Hinzman J.M., Thomas T.C., Quintero J.E., Gerhardt G.A., and Lifshitz J. (2012). Disruptions in the regulation of extracellular glutamate by neurons and glia in the rat striatum two days after diffuse brain injury. J. Neurotrauma 29, 1197–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levenson J., Weeber E., Selcher J.C., Kategaya L.S., Sweatt J.D., and Eskin A. (2002). Long-term potentiation and contextual fear conditioning increase neuronal glutamate uptake. Nat. Neurosci. 5, 155–161 [DOI] [PubMed] [Google Scholar]
- 15.Fievisohn E.M., Sajja V.S., Vandevord P.J., and Hardy W.N. (2014). Evaluation of impact-induced traumatic brain injury in the Gottingen Minipig using two input modes. Traffic Inj. Prev. 15 Suppl 1, S81–S87 [DOI] [PubMed] [Google Scholar]
- 16.Hallam T.M., Floyd C.L., Folkerts M.M., Lee L.L., Gong Q.Z., Lyeth B.G., Muizelaar J.P., and Berman R.F. (2004). Comparison of behavioral deficits and acute neuronal degeneration in rat lateral fluid percussion and weight-drop brain injury models. J. Neurotrauma 21, 521–539 [DOI] [PubMed] [Google Scholar]
- 17.Thompson H.J., Lifshitz J., Marklund N., Grady M.S., Graham D.I., Hovda D.A., and McIntosh T.K. (2005). Lateral fluid percussion brain injury: a 15-year review and evaluation. J. Neurotrauma 22, 42–75 [DOI] [PubMed] [Google Scholar]
- 18.Graham S.H., Chen J., and Clark R.S. (2000). Bcl-2 family gene products in cerebral ischemia and traumatic brain injury. J. Neurotrauma 17, 831–841 [DOI] [PubMed] [Google Scholar]
- 19.McIntosh T.K., Vink R., Noble L., Yamakami I., Fernyak S., Soares H., and Faden A.L. (1989). Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience 28, 233–244 [DOI] [PubMed] [Google Scholar]
- 20.Cernak I. (2005). Animal models of head trauma. NeuroRx 2, 410–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall E.D., Sullivan P.G., Gibson T.R., Pavel K.M., Thompson B.M., and Scheff S.W. (2005). Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J. Neurotrauma 22, 252–265 [DOI] [PubMed] [Google Scholar]
- 22.Goodman J.C., Cherian L., Bryan R.M., Jr., and Robertson C.S. (1994). Lateral cortical impact injury in rats: pathologic effects of varying cortical compression and impact velocity. J. Neurotrauma 11, 587–597 [DOI] [PubMed] [Google Scholar]
- 23.Marmarou A. (2007). A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg. Focus 22, E1. [DOI] [PubMed] [Google Scholar]
- 24.Gupta R.K. and Przekwas A. (2013). Mathematical models of blast-induced TBI: current status, challenges, and prospects. Front. Neurol. 4, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salt T.E. and Eaton S.A. (1996). Functions of ionotropic and metabotropic glutamate receptors in sensory transmission in the mammalian thalamus. Prog. Neurobiol. 48, 55–72 [DOI] [PubMed] [Google Scholar]
- 26.Vernadakis A. (1996). Glia-neuron intercommunications and synaptic plasticity. Prog. Neurobiol. 49, 185–214 [DOI] [PubMed] [Google Scholar]
- 27.Rusakov D.A. and Kullmann D.M. (1998). Extrasynaptic glutamate diffusion in the hippocampus: ultrastructural constraints, uptake, and receptor activation. J. Neurosci. 18, 3158–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellocchio E.E., Reimer R.J., Fremeau R.T., Jr., and Edwards R.H. (2000). Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science 289, 957–960 [DOI] [PubMed] [Google Scholar]
- 29.Takamori S., Rhee J.S., Rosenmund C., and Jahn R. (2000). Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature 407, 189–194 [DOI] [PubMed] [Google Scholar]
- 30.Hollmann M., Boulter J., Maron C., and Heinemann S. (1994). Molecular biology of glutamate receptors. Potentiation of N-methyl-D-aspartate receptor splice variants by zinc. Renal Physiol. Biochemist. 17, 182–183 [PubMed] [Google Scholar]
- 31.Hollmann M. and Heinemann S. (1994). Cloned glutamate receptors. Annu. Rev. Neurosci. 17, 31–108 [DOI] [PubMed] [Google Scholar]
- 32.Nicoll R.A. and Malenka R.C. (1999). Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Ann. N. Y. Acad. Sci. 868, 515–525 [DOI] [PubMed] [Google Scholar]
- 33.McCullumsmith R.E., Clinton S.M., and Meador-Woodruff J.H. (2004). Schizophrenia as a disorder of neuroplasticity. Int. Rev. Neurobiol. 59, 19–45 [DOI] [PubMed] [Google Scholar]
- 34.McGee A.W. and Bredt D.S. (2003). Assembly and plasticity of the glutamatergic postsynaptic specialization. Curr. Opin. Neurobiol. 13, 111–118 [DOI] [PubMed] [Google Scholar]
- 35.Contractor A. and Heinemann S.F. (2002). Glutamate receptor trafficking in synaptic plasticity. Science's STKE 2002, re14. [DOI] [PubMed] [Google Scholar]
- 36.Arriza J.L., Kavanaugh M.P., Fairman W.A., Wu Y.N., Murdoch G.H., North R.A., and Amara S.G. (1993). Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. J. Biol. Chem. 268, 15329–15332 [PubMed] [Google Scholar]
- 37.Utsunomiya-Tate N., Endou H., and Kanai Y. (1996). Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid transporter. J. Biol. Chem. 271, 14883–14890 [DOI] [PubMed] [Google Scholar]
- 38.Danbolt N.C. (2001). Glutamate uptake. Prog. Neurobiology 65, 1–105 [DOI] [PubMed] [Google Scholar]
- 39.Zerangue N. and Kavanaugh M.P. (1996). Flux coupling in a neuronal glutamate transporter. Nature 383, 634–637 [DOI] [PubMed] [Google Scholar]
- 40.Levy L.M., Warr O., and Attwell D. (1998). Stoichiometry of the glial glutamate transporter GLT-1 expressed inducibly in a Chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. J. Neurosci. 18, 9620–9628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tzingounis A.V. and Wadiche J.I. (2007). Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat. Rev. Neuroscience 8, 935–947 [DOI] [PubMed] [Google Scholar]
- 42.Kanai Y. (1997). Substrate binding sites of glutamate transporters and structurally related neutral amino acid transporters. Jpn. J. Physiol. 47 Suppl 1, S55–S56 [PubMed] [Google Scholar]
- 43.Regan M.R., Huang Y.H., Kim Y.S., Dykes-Hoberg M.I., Jin L., Watkins A.M., Bergles D.E., and Rothstein J.D. (2007). Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J. Neurosci. 27, 6607–6619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka K., Watase K., Manabe T., Yamada K., Watanabe M., Takahashi K., Iwama H., Nishikawa T., Ichihara N., Kikuchi T., Okuyama S., Kawashima N., Hori S., Takimoto M., and Wada K. (1997). Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276, 1699–1702 [DOI] [PubMed] [Google Scholar]
- 45.Danbolt N.C., Storm-Mathisen J., and Kanner B.I. (1992). An [Na+ + K+]coupled L-glutamate transporter purified from rat brain is located in glial cell processes. Neuroscience 51, 295–310 [DOI] [PubMed] [Google Scholar]
- 46.Malarkey E.B. and Parpura V. (2008). Mechanisms of glutamate release from astrocytes. Neurochem. Int. 52, 142–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stobart J.L. and Anderson C.M. (2013). Multifunctional role of astrocytes as gatekeepers of neuronal energy supply. Front. Cell. Neurosci. 7, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tani H., Dulla C.G., Farzampour Z., Taylor-Weiner A., Huguenard J.R., and Reimer R.J. (2014). A local glutamate-glutamine cycle sustains synaptic excitatory transmitter release. Neuron 81, 888–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gegelashvili G., Dehnes Y., Danbolt N.C., and Schousboe A. (2000). The high-affinity glutamate transporters GLT1, GLAST, and EAAT4 are regulated via different signalling mechanisms. Neurochem. Int. 37, 163–170 [DOI] [PubMed] [Google Scholar]
- 50.Li L.B., Toan S.V., Zelenaia O., Watson D.J., Wolfe J.H., Rothstein J.D., and Robinson M.B. (2006). Regulation of astrocytic glutamate transporter expression by Akt: evidence for a selective transcriptional effect on the GLT-1/EAAT2 subtype. J. Neurochem. 97, 759–771 [DOI] [PubMed] [Google Scholar]
- 51.Abe K. and Saito H. (2001). Possible linkage between glutamate transporter and mitogen-activated protein kinase cascade in cultured rat cortical astrocytes. J. Neurochem. 76, 217–223 [DOI] [PubMed] [Google Scholar]
- 52.Kalandadze A., Wu Y., and Robinson M.B. (2002). Protein kinase C activation decreases cell surface expression of the GLT-1 subtype of glutamate transporter. Requirement of a carboxyl-terminal domain and partial dependence on serine 486. J. Biol. Chem. 277, 45741–45750 [DOI] [PubMed] [Google Scholar]
- 53.Gonzalez M.I. and Robinson M.B. (2004). Protein kinase C-dependent remodeling of glutamate transporter function. Mol. Interventions 4, 48–58 [DOI] [PubMed] [Google Scholar]
- 54.Susarla B.T. and Robinson M.B. (2008). Internalization and degradation of the glutamate transporter GLT-1 in response to phorbol ester. Neurochem. Int. 52, 709–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martinez-Villarreal J., Garcia Tardon N., Ibanez I., Gimenez C., and Zafra F. (2012). Cell surface turnover of the glutamate transporter GLT-1 is mediated by ubiquitination/deubiquitination. Glia 60, 1356–1365 [DOI] [PubMed] [Google Scholar]
- 56.Tong G. and Jahr C.E. (1994). Block of glutamate transporters potentiates postsynaptic excitation. Neuron 13, 1195–1203 [DOI] [PubMed] [Google Scholar]
- 57.Massie A., Cnops L., Smolders I., McCullumsmith R., Kooijman R., Kwak S., Arckens L., and Michotte Y. (2008). High-affinity Na+/K+-dependent glutamate transporter EAAT4 is expressed throughout the rat fore- and midbrain. J. Comp. Neurol. 511, 155–172 [DOI] [PubMed] [Google Scholar]
- 58.Chalifoux J.R. and Carter A.G. (2011). Glutamate spillover promotes the generation of NMDA spikes. J. Neurosci. 31, 16435–16446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hardingham G.E. and Bading H. (2002). Coupling of extrasynaptic NMDA receptors to a CREB shut-off pathway is developmentally regulated. Biochim. Biophys. Acta 1600, 148–153 [DOI] [PubMed] [Google Scholar]
- 60.Hardingham G.E., Fukunaga Y., and Bading H. (2002). Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 5, 405–414 [DOI] [PubMed] [Google Scholar]
- 61.Leveille F., El Gaamouch F., Gouix E., Lecocq M., Lobner D., Nicole O., and Buisson A. (2008). Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors. FASEB J. 22, 4258–4271 [DOI] [PubMed] [Google Scholar]
- 62.Lozovaya N., Melnik S., Tsintsadze T., Grebenyuk S., Kirichok Y., and Krishtal O. (2004). Protective cap over CA1 synapses: extrasynaptic glutamate does not reach the postsynaptic density. Brain Res. 1011, 195–205 [DOI] [PubMed] [Google Scholar]
- 63.Tsvetkov E., Shin R.M., and Bolshakov V.Y. (2004). Glutamate uptake determines pathway specificity of long-term potentiation in the neural circuitry of fear conditioning. Neuron 41, 139–151 [DOI] [PubMed] [Google Scholar]
- 64.Rinholm J.E., Slettalokken G., Marcaggi P., Skare O., Storm-Mathisen J., and Bergersen L.H. (2007). Subcellular localization of the glutamate transporters GLAST and GLT at the neuromuscular junction in rodents. Neuroscience 145, 579–591 [DOI] [PubMed] [Google Scholar]
- 65.Kullmann D.M. and Asztely F. (1998). Extrasynaptic glutamate spillover in the hippocampus: evidence and implications. Trends Neurosci. 21, 8–14 [DOI] [PubMed] [Google Scholar]
- 66.Weng H.R., Chen J.H., Pan Z.Z., and Nie H. (2007). Glial glutamate transporter 1 regulates the spatial and temporal coding of glutamatergic synaptic transmission in spinal lamina II neurons. Neuroscience 149, 898–907 [DOI] [PubMed] [Google Scholar]
- 67.Sem'yanov A.V. (2005). Diffusional extrasynaptic neurotransmission via glutamate and GABA. Neurosci. Behav. Physiol. 35, 253–266 [DOI] [PubMed] [Google Scholar]
- 68.Olney J.W. (1990). Excitotoxicity: an overview. Can. Dis. Wkly. Rep. 16 Suppl 1E, 47–57 [PubMed] [Google Scholar]
- 69.Liu Z., Stafstrom C.E., Sarkisian M.R., Yang Y., Hori A., Tandon P., and Holmes G.L. (1997). Seizure-induced glutamate release in mature and immature animals: an in vivo microdialysis study. Neuroreport 8, 2019–2023 [DOI] [PubMed] [Google Scholar]
- 70.Zhang H., Zhang X., Zhang T., and Chen L. (2001). Excitatory amino acids in cerebrospinal fluid of patients with acute head injuries. Clin. Chem. 47, 1458–1462 [PubMed] [Google Scholar]
- 71.Kierans A.S., Kirov, Gonen O., Haemer G., Nisenbaum E., Babb J.S., Grossman R.I., and Lui Y.W. (2014). Myoinositol and glutamate complex neurometabolite abnormality after mild traumatic brain injury. Neurology 82, 521–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Faden A.I., Demediuk P., Panter S.S., and Vink R. (1989). The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science 244, 798–800 [DOI] [PubMed] [Google Scholar]
- 73.D'Ambrosio R., Maris D.O., Grady M.S., Winn H.R., and Janigro D. (1999). Impaired K(+) homeostasis and altered electrophysiological properties of post-traumatic hippocampal glia. J. Neurosci. 19, 8152–8162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Olsen M. (2012). Examining potassium channel function in astrocytes. Methods Mol. Biol. 814, 265–281 [DOI] [PubMed] [Google Scholar]
- 75.Djukic B., Casper K.B., Philpot B.D., Chin L.S., and McCarthy K.D. (2007). Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J. Neurosci. 27, 11354–11365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olsen M.L., Higashimori H., Campbell S.L., Hablitz J.J., and Sontheimer H. (2006). Functional expression of Kir4.1 channels in spinal cord astrocytes. Glia 53, 516–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gupta R.K. and Prasad S. (2013). Early down regulation of the glial Kir4.1 and GLT-1 expression in pericontusional cortex of the old male mice subjected to traumatic brain injury. Biogerontology 14, 531–541 [DOI] [PubMed] [Google Scholar]
- 78.Hablitz J.J. and Langmoen I.A. (1982). Excitation of hippocampal pyramidal cells by glutamate in the guinea-pig and rat. J. Physiol. 325, 317–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arundine M. and Tymianski M. (2003). Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 34, 325–337 [DOI] [PubMed] [Google Scholar]
- 80.Rothman S.M. (1985). The neurotoxicity of excitatory amino acids is produced by passive chloride influx. J. Neurosci. 5, 1483–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choi D.W. (1987). Ionic dependence of glutamate neurotoxicity. J. Neurosci. 7, 369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tymianski M., Charlton M.P., Carlen P.L., and Tator C.H. (1993). Source specificity of early calcium neurotoxicity in cultured embryonic spinal neurons. J. Neurosci. 13, 2085–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rao V.L., Dogan A., Bowen K.K., Todd K.G., and Dempsey R.J. (2001). Antisense knockdown of the glial glutamate transporter GLT-1 exacerbates hippocampal neuronal damage following traumatic injury to rat brain. Eur. J. Neurosci. 13, 119–128 [PubMed] [Google Scholar]
- 84.Rothstein J.D., Dykes-Hoberg M., Pardo C.A., Bristol L.A., Jin L., Kuncl R.W., Kanai Y., Hediger M.A., Wang Y., Schielke J.P., and Welty D.F. (1996). Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16, 675–686 [DOI] [PubMed] [Google Scholar]
- 85.Zelenaia O.A. and Robinson M.B. (2000). Degradation of glial glutamate transporter mRNAs is selectively blocked by inhibition of cellular transcription. J. Neurochem. 75, 2252–2258 [DOI] [PubMed] [Google Scholar]
- 86.Rao V.L., Baskaya M.K., Dogan A., Rothstein J.D., and Dempsey R.J. (1998). Traumatic brain injury down-regulates glial glutamate transporter (GLT-1 and GLAST) proteins in rat brain. J. Neurochem. 70, 2020–2027 [DOI] [PubMed] [Google Scholar]
- 87.Boston-Howes W., Gibb S.L., Williams E.O., Pasinelli P., Brown R.H., Jr., and Trotti D. (2006). Caspase-3 cleaves and inactivates the glutamate transporter EAAT2. J. Biol. Chem. 281, 14076–14084 [DOI] [PubMed] [Google Scholar]
- 88.van Landeghem F.K., Stover J.F., Bechmann I., Bruck W., Unterberg A., Buhrer C., and von Deimling A. (2001). Early expression of glutamate transporter proteins in ramified microglia after controlled cortical impact injury in the rat. Glia 35, 167–179 [DOI] [PubMed] [Google Scholar]
- 89.Floyd C.L., Gorin F.A., and Lyeth B.G. (2005). Mechanical strain injury increases intracellular sodium and reverses Na+/Ca2+ exchange in cortical astrocytes. Glia 51, 35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goodrich G.S., Kabakov A.Y., Hameed M.Q., Dhamne S.C., Rosenberg P.A., and Rotenberg A. (2013). Ceftriaxone treatment after traumatic brain injury restores expression of the glutamate transporter, GLT-1, reduces regional gliosis, and reduces post-traumatic seizures in the rat. J. Neurotrauma 30, 1434–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yi J.H., Pow D.V., and Hazell A.S. (2005). Early loss of the glutamate transporter splice-variant GLT-1v in rat cerebral cortex following lateral fluid-percussion injury. Glia 49, 121–133 [DOI] [PubMed] [Google Scholar]
- 92.Ikematsu K., Tsuda R., Kondo T., and Nakasono I. (2002). The expression of excitatory amino acid transporter 2 in traumatic brain injury. Forensic Sci. Int. 130, 83–89 [DOI] [PubMed] [Google Scholar]
- 93.Raj R., Skrifvars M.B., Bendel S., Selander T., Kivisaari R., Siironen J., and Reinikainen M. (2014). Predicting six-month mortality of patients with traumatic brain injury: usefulness of common intensive care severity scores. Crit. Care 18, R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yi J.H., Herrero R., Chen G., and Hazell A.S. (2007). Glutamate transporter EAAT4 is increased in hippocampal astrocytes following lateral fluid-percussion injury in the rat. Brain Res. 1154, 200–205 [DOI] [PubMed] [Google Scholar]
- 95.Pow D.V., Naidoo T., Lingwood B.E., Healy G.N., Williams S.M., Sullivan R.K., O'Driscoll S., and Colditz P.B. (2004). Loss of glial glutamate transporters and induction of neuronal expression of GLT-1B in the hypoxic neonatal pig brain. Brain Res. Dev. Brain Res. 153, 1–11 [DOI] [PubMed] [Google Scholar]
- 96.Sullivan S.M., Macnab L.T., Bjorkman S.T., Colditz P.B., and Pow D.V. (2007). GLAST1b, the exon-9 skipping form of the glutamate-aspartate transporter EAAT1 is a sensitive marker of neuronal dysfunction in the hypoxic brain. Neuroscience 149, 434–445 [DOI] [PubMed] [Google Scholar]
- 97.Selkirk J.V., Stiefel T.H., Stone I.M., Naeve G.S., Foster A.C., and Poulsen D.J. (2005). Over-expression of the human EAAT2 glutamate transporter within neurons of mouse organotypic hippocampal slice cultures leads to increased vulnerability of CA1 pyramidal cells. Eur. J. Neurosci. 21, 2291–2296 [DOI] [PubMed] [Google Scholar]
- 98.Lin C.L., Bristol L.A., Jin L., Dykes-Hoberg M., Crawford T., Clawson L., and Rothstein J.D. (1998). Aberrant RNA processing in a neurodegenerative disease: the cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron 20, 589–602 [DOI] [PubMed] [Google Scholar]
- 99.Lehmann C., Bette S., and Engele J. (2009). High extracellular glutamate modulates expression of glutamate transporters and glutamine synthetase in cultured astrocytes. Brain Res. 1297, 1–8 [DOI] [PubMed] [Google Scholar]
- 100.Bramlett H.M., Dietrich W.D., Green E.J., and Busto R. (1997). Chronic histopathological consequences of fluid-percussion brain injury in rats: effects of post-traumatic hypothermia. Acta Neuropathol. 93, 190–199 [DOI] [PubMed] [Google Scholar]
- 101.Rusnakova V., Honsa P., Dzamba D., Stahlberg A., Kubista M., and Anderova M. (2013). Heterogeneity of astrocytes: from development to injury—single cell gene expression. PloS One 8, e69734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McNamara K.C., Lisembee A.M., and Lifshitz J. (2010). The whisker nuisance task identifies a late-onset, persistent sensory sensitivity in diffuse brain-injured rats. J. Neurotrauma 27, 695–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thomas T.C., Hinzman J.M., Gerhardt G.A., and Lifshitz J. (2012). Hypersensitive glutamate signaling correlates with the development of late-onset behavioral morbidity in diffuse brain-injured circuitry. J. Neurotrauma 29, 187–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chamard E., Lassonde M., Henry L., Tremblay J., Boulanger Y., De Beaumont L., and Theoret H. (2013). Neurometabolic and microstructural alterations following a sports-related concussion in female athletes. Brain Inj. 27, 1038–1046 [DOI] [PubMed] [Google Scholar]
- 105.Tremblay S., Beaule V., Proulx S., de Beaumont L., Marjanska M., Doyon J., Pascual-Leone A., Lassonde M., and Theoret H. (2013). Relationship between transcranial magnetic stimulation measures of intracortical inhibition and spectroscopy measures of GABA and glutamate+glutamine. J. Neurophysiol. 109, 1343–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sun F.Y. and Faden A.I. (1995). Pretreatment with antisense oligodeoxynucleotides directed against the NMDA-R1 receptor enhances survival and behavioral recovery following traumatic brain injury in rats. Brain Res. 693, 163–168 [DOI] [PubMed] [Google Scholar]
- 107.Zhang C., Raghupathi R., Saatman K.E., Smith D.H., Stutzmann J.M., Wahl F., and McIntosh T.K. (1998). Riluzole attenuates cortical lesion size, but not hippocampal neuronal loss, following traumatic brain injury in the rat. J. Neurosci. Res. 52, 342–349 [DOI] [PubMed] [Google Scholar]
- 108.Hicks R.R., Smith D.H., and McIntosh T.K. (1995). Temporal response and effects of excitatory amino acid antagonism on microtubule-associated protein 2 immunoreactivity following experimental brain injury in rats. Brain Res. 678, 151–160 [DOI] [PubMed] [Google Scholar]
- 109.Atkins C.M., Falo M.C., Alonso O.F., Bramlett H.M., and Dietrich W.D. (2009). Deficits in ERK and CREB activation in the hippocampus after traumatic brain injury. Neurosci. Lett. 459, 52–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Santhakumar V., Ratzliff A.D., Jeng J., Toth Z., and Soltesz I. (2001). Long-term hyperexcitability in the hippocampus after experimental head trauma. Ann. Neurol. 50, 708–717 [DOI] [PubMed] [Google Scholar]
- 111.Sanders M.J., Sick T.J., Perez-Pinzon M.A., Dietrich W.D., and Green E.J. (2000). Chronic failure in the maintenance of long-term potentiation following fluid percussion injury in the rat. Brain Res. 861, 69–76 [DOI] [PubMed] [Google Scholar]
- 112.Nguyen P.V. and Kandel E.R. (1996). A macromolecular synthesis-dependent late phase of long-term potentiation requiring cAMP in the medial perforant pathway of rat hippocampal slices. J. Neurosci. 16, 3189–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yeh T.H., Hwang H.M., Chen J.J., Wu T., Li A.H., and Wang H.L. (2005). Glutamate transporter function of rat hippocampal astrocytes is impaired following the global ischemia. Neurobiol. Dis. 18, 476–483 [DOI] [PubMed] [Google Scholar]
- 114.McConeghy K.W., Hatton J., Hughes L., and Cook A.M. (2012). A review of neuroprotection pharmacology and therapies in patients with acute traumatic brain injury. CNS Drugs 26, 613–636 [DOI] [PubMed] [Google Scholar]
- 115.Maas A.I. (2001). Neuroprotective agents in traumatic brain injury. Expert Opin. Investig. Drugs 10, 753–767 [DOI] [PubMed] [Google Scholar]
- 116.Maas A.I., Murray G., Henney H., 3rd, Kassem N., Legrand V., Mangelus M., Muizelaar J.P., Stocchetti N., andKnoller N., Pharmos TBI investigators. (2006). Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomised, placebo-controlled, clinical trial. Lancet. Neurol. 5, 38–45 [DOI] [PubMed] [Google Scholar]
- 117.Morris G.F., Bullock R., Marshall S.B., Marmarou A., Maas A., and Marshall L.F. (1999). Failure of the competitive N-methyl-D-aspartate antagonist Selfotel (CGS 19755) in the treatment of severe head injury: results of two phase III clinical trials. The Selfotel Investigators. J. Neurosurg. 91, 737–743 [DOI] [PubMed] [Google Scholar]
- 118.Temkin N.R., Anderson G.D., Winn H.R., Ellenbogen R.G., Britz G.W., Schuster J., Lucas T., Newell D.W., Mansfield P.N., Machamer J.E., Barber J., and Dikmen S.S. (2007). Magnesium sulfate for neuroprotection after traumatic brain injury: a randomised controlled trial. Lancet. Neurol. 6, 29–38 [DOI] [PubMed] [Google Scholar]
- 119.Wei J., Pan X., Pei Z., Wang W., Qiu W., Shi Z., and Xiao G. (2012). The beta-lactam antibiotic, ceftriaxone, provides neuroprotective potential via anti-excitotoxicity and anti-inflammation response in a rat model of traumatic brain injury. J. Trauma Acute Care Surg. 73, 654–660 [DOI] [PubMed] [Google Scholar]
- 120.Fontana A.C. (2015). Current approaches to enhance glutamate transporter function and expression. J. Neurochem. 134, 982–1007 [DOI] [PubMed] [Google Scholar]
- 121.Parpura V. and Verkhratsky A. (2012). The astrocyte excitability brief: from receptors to gliotransmission. Neurochem. Int. 61, 610–621 [DOI] [PubMed] [Google Scholar]
- 122.Tanaka M., Shih P.Y., Gomi H., Yoshida T., Nakai J., Ando R., Furuichi T., Mikoshiba K., Semyanov A., and Itohara S. (2013). Astrocytic Ca2+ signals are required for the functional integrity of tripartite synapses. Mol. Brain. 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shen H.W., Scofield M.D., Boger H., Hensley M., and Kalivas P.W. (2014). Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. J. Neurosci. 34, 5649–5657 [DOI] [PMC free article] [PubMed] [Google Scholar]