Abstract
Risk factors for blunt cerebrovascular injury (BCVI) may differ between children and adults, suggesting that children at low risk for BCVI after trauma receive unnecessary computed tomography angiography (CTA) and high-dose radiation. We previously developed a score for predicting pediatric BCVI based on retrospective cohort analysis. Our objective is to externally validate this prediction score with a retrospective multi-institutional cohort. We included patients who underwent CTA for traumatic cranial injury at four pediatric Level I trauma centers. Each patient in the validation cohort was scored using the “Utah Score” and classified as high or low risk. Before analysis, we defined a misclassification rate <25% as validating the Utah Score. Six hundred forty-five patients (mean age 8.6 ± 5.4 years; 63.4% males) underwent screening for BCVI via CTA. The validation cohort was 411 patients from three sites compared with the training cohort of 234 patients. Twenty-two BCVIs (5.4%) were identified in the validation cohort. The Utah Score was significantly associated with BCVIs in the validation cohort (odds ratio 8.1 [3.3, 19.8], p < 0.001) and discriminated well in the validation cohort (area under the curve 72%). When the Utah Score was applied to the validation cohort, the sensitivity was 59%, specificity was 85%, positive predictive value was 18%, and negative predictive value was 97%. The Utah Score misclassified 16.6% of patients in the validation cohort. The Utah Score for predicting BCVI in pediatric trauma patients was validated with a low misclassification rate using a large, independent, multicenter cohort. Its implementation in the clinical setting may reduce the use of CTA in low-risk patients.
Keywords: : blunt cerebrovascular injury, computed tomography angiography, pediatrics, traumatic brain injury, Utah Score
Introduction
Previous studies have suggested that blunt cerebrovascular injury (BCVI) complicates 0.03–0.9% of pediatric traumatic brain injuries (TBIs).1–4 BCVI can lead to ischemia and neurologic morbidity, especially in the absence of prompt recognition and treatment.2,5 In adults, the use of computed tomographic angiography (CTA) of the head and neck to screen patients for BCVI3,6–9 based on well-established risk factors described by the Denver and Memphis criteria6,7,10 may be cost effective,8 but the patterns of intracranial injury in children differ from those in adults.9 In addition, the low incidence of BCVI in children has led to speculation that children with TBI are inadequately screened1; thus, more CTAs, which require high-dose radiation and are linked to greater risk of future neoplasms,11–13 are performed in children.14
We previously identified independent risk factors for BCVI specific to children and developed a clinical prediction rule to identify and screen high-risk pediatric patients.14 Our retrospective cohort study included 234 patients, 36 of whom had a BCVI. Multivariate regression analysis identified Glasgow Coma Scale (GCS) score ≤8, focal neurological deficit, fracture through the carotid canal, petrous temporal bone fracture, and hypodensity on CT consistent with ischemia as independent risk factors for BCVI.14
We created a prediction score ranging from 0 (least likely) to 11 (most likely) identifying children at high risk for BCVI (Table 1). A value ≤2 yielded a 7.9% probability of BCVI whereas a “Utah Score” of ≥3 portended a 39.3% risk (Table 2).14 The primary objective of the current study was to use an independent, multicenter sample to externally validate the Utah Score for widespread use. We also evaluated the fit of the adult Denver criteria for BCVI risk to our pediatric cohort to assess whether these criteria are appropriate for assessment of pediatric patients. We hypothesize that the Utah Score will accurately predict BCVI and perform better than the adult Denver criteria when applied to children.
Table 1.
Utah Score
| Utah Score variables | Score |
|---|---|
| Glasgow Coma Scale score ≤8 | 1 |
| Focal neurological deficit | 2 |
| Carotid canal fracture | 2 |
| Petrous temporal bone fracture | 3 |
| Cerebral infarction on computed tomography | 3 |
| 11 |
Total possible score ranges from 0 to 11.
Reproduced with permission from Ravindra, V.M., Riva-Cambrin, J., Sivakumar, W., Metzger, R.R., and Bollo, R.J. (2015). Risk factors for traumatic blunt cerebrovascular injury diagnosed by computed tomography angiography in the pediatric population: a retrospective cohort study. J. Neurosurg. Pediatr. 15, 599–606.
Table 2.
The Pediatric BCVI Prediction Score
| Score | No. of patients | Probability of BCVI* (%) |
|---|---|---|
| 0 | 92 | 6.5 |
| 1 | 56 | 10.7 |
| 2 | 30 | 6.7 |
| 3 | 37 | 32.4 |
| 4 | 9 | 44.4 |
| 5 | 7 | 57.1 |
| 6 | 2 | 50 |
| 7 | 0 | - |
| 8 | 1 | 100.0 |
| 9 | 0 | - |
| 10 | 0 | - |
| 11 | 0 | - |
The probability in the study population of having a BCVI with this score; dashes in this column denote missing data because of no patients with this score (the statistical significance of the model was p < 0.001.
In the single study cohort from Primary Children's Hospital, a patient with a score ≤2 had a 7.9% probability of blunt cerebrovascular injury and a patient with a score ≥3 had a 39.3% probability of blunt cerebrovascular injury.
Reproduced with permission from Ravindra, V.M., Riva-Cambrin, J., Sivakumar, W., Metzger, R.R., and Bollo, R.J. (2015). Risk factors for traumatic blunt cerebrovascular injury diagnosed by computed tomography angiography in the pediatric population: a retrospective cohort study. J. Neurosurg. Pediatr. 15, 599–606.
Methods
Patient population
The multicenter validation cohort comprised patients treated at three pediatric Level I trauma centers: Monroe Carell Children's Hospital (MCCH), Nashville, Tennessee; St. Louis Children's Hospital (STLCH), St. Louis, Missouri; and Texas Children's Hospital (TCH), Houston, Texas. Like the training cohort at the University of Utah/Primary Children's Hospital (PCH), the retrospective validation cohort included all pediatric patients who underwent CTA of the head or neck for suspected BCVI during the 11-year study period (January 1, 2003–December 31, 2013). Multi-institutional Institutional Review Board approval was obtained.
CT scanning across centers was uniform. The proprietary brand of CT scanners used in the study varied from institution to institution, but the overall scanning protocol and technique were similar, using either a 64- or 320-slice scanner with 1-mm slice thickness with intravenous contrast infusion with Isovue 370 or Omni 300.
Data collection
At each center, the trauma and radiology databases were queried to identify trauma patients screened for BCVI with CTA of the head or neck. Mechanism of injury was categorized as either motor vehicle accident, pedestrian versus vehicle, >1-story fall, nonaccidental trauma, or other. Clinical information included initial GCS on neurosurgical evaluation, presence of focal neurological deficits on initial examination, and mode of treatment for TBI (medical vs. surgical). Radiological variables included presence of petrous temporal bone fracture or fracture through the carotid canal as defined in the original article.14
We abstracted any concomitant intracranial injury (epidural, subdural, or subarachnoid hemorrhage), presence of hypodensity on CT imaging consistent with ischemia, Rotterdam score15 (a 6-point score based on noncontrast CT findings that predicts 6-month mortality in moderate and severe TBI),16 and CTA radiation dose. We recorded whether dedicated cervical spine imaging was performed, and if so, the modality, cervical spine injury type, level of injury, and fracture through the foramen transversarium. We also recorded discharge disposition (home vs. other).
The primary outcome of interest, BCVI, was indicated by the presence of internal carotid artery or vertebral artery injury and grade of injury diagnosed by CTA. Each injury was classified according to the Denver grading scale for BCVI.17 Grade 1 injury is characterized by intimal irregularity with <25% narrowing, Grade 2 injury is a dissection or presence of intramural hematoma with >25% narrowing, Grade 3 injury is the presence of a pseudoaneurysm, Grade 4 injury is an occlusion, and Grade 5 injury is transection with extravasation.17
Statistical analysis
Study data were collected and managed using REDCap hosted at the University of Utah.18 All data analysis was performed in a de-identified manner at the lead site.
Data were summarized using means and standard deviations (SDs) for continuous variables and counts and frequencies for categorical variables. The demographic, clinical, and radiographic variables were compared among centers using an analysis of variance for continuous variables and χ2 for categorical variables.
The validation analysis began by scoring each patient in the validation cohort using the Utah Score (n = 411). After scoring, patients were classified as high (score ≥3) or low (score ≤2) risk. The score was split into two categories for two reasons: first, the risk of BCVI based on patient score was clearly dichotomized based on statistics alone (score of 2 = 6.7% probability of BCVI vs. score of 3 = 32.4% probability of BCVI; Table 2), and second, a dichotomized score would be most helpful in making a binary clinical decision during the initial trauma evaluation: to screen the patient or not. Patients were categorized in a 2 × 2 table assessing risk versus the presence/absence of BCVI. This comparison was analyzed using the Fisher exact test. We calculated percent misclassification, sensitivity, specificity, positive predictive value, and negative predictive value. Before the study, validation was defined as a misclassification rate of <25%.19
A logistic regression model was developed for the Utah Score risk categorization and its association with BCVI. Using this model, we calculated the area under the curve or discrimination for the dichotomized Utah Score.
In a Bayesian analysis, we applied likelihood ratios from the original model (training cohort at PCH)14 to the validation cohort to compute the post-test probability for the high- and low-risk dichotomizations for the Utah Score and compare with the actual or observed probabilities found in the validation cohort. Data were analyzed using SAS v.9.3 software.
To compare our pediatric Utah Score with previous models based on adult trauma data, we applied the Denver criteria (prediction model) to our entire cohort of pediatric patients (n = 645). The Denver criteria for screening for BCVI in adults include GCS <6, petrous temporal bone fracture, diffuse axonal injury, and LeFort Type II and III fractures for carotid artery injury and cervical spine for vertebral artery injury.10 We calculated the observed probabilities within each of the Denver strata (0–4) by scoring each child within the entire cohort (n = 645). We had not specifically collected diffuse axonal injury as a variable and used intracranial contusions as a proxy. Our observed percentages were directly compared with the predicted probabilities from Biffl and associates.10
More recent modifications to the Denver criteria suggest additional risk factors, but these were added via a descriptive nonanalytical method without presenting quantifiable risk prediction, which was included in the original article.20 Therefore, meaningful comparisons of risk prediction were only possible with the data and analysis presented in that article.
Results
Patient population characteristics
At the four centers, 645 pediatric patients were evaluated for BCVI with CTA of the head or neck (mean age 8.6 years, 63.4% male) during the 11-year study period. The validation cohort included 411 patients (mean age 8.7 years, 63.3% male) from three medical centers (TCH, MCCH, STLCH), whereas the training cohort (PCH) had 234 patients (mean age 8.3 years, 63.7% male) (Table 3).
Table 3.
Clinical and Imaging Characteristics for Patients at All Centers
| Total (n = 645) | Primary Children's Hospital (n = 234) | Monroe Carrell Children's Hospital (n = 312) | St. Louis Children's Hospital (n = 53) | Texas Children's Hospital (n = 46) | Differences between training and validation cohort (p value)* | |
|---|---|---|---|---|---|---|
| Age (years) ± SD | 8.6 ± 5.4 | 8.3 ± 4.9 | 8.6 ± 5.4 | 12.3 ± 5.2 | 6.1 ± 6.1 | <0.001 |
| Male sex (%) | 409 (63.4) | 149 (63.7) | 191 (61.2) | 40 (75.5) | 29 (63.0) | 0.14 |
| Race (%) | <0.001 | |||||
| White | 433 (67.1) | 163 (69.7) | 224 (71.8) | 34 (64.2) | 12 (26.1) | - |
| Black | 87 (13.5) | 1 (0.4) | 57 (18.3) | 17 (32.1) | 12 (26.1) | - |
| Hispanic | 71 (11.0) | 32 (13.7) | 21 (6.7) | 0 (0) | 18 (39.1) | - |
| Asian | 7 (1.1) | 1 (0.4) | 2 (0.6) | 1 (1.9) | 3 (6.5) | - |
| Other | 46 (7.1) | 37 (15.8) | 7 (2.2) | 1 (1.9) | 1 (2.2) | - |
| Mechanism of injury (%) | <0.001 | |||||
| Motor vehicle accident | 193 (29.9) | 60 (25.6) | 109 (34.9) | 20 (37.7) | 4 (8.7) | - |
| Pedestrian vs. vehicle | 53 (8.2) | 37 (15.8) | 15 (4.8) | 0 (0) | 1 (2.2) | - |
| Fall >1 story | 28 (4.3) | 18 (7.7) | 4 (1.3) | 4 (7.5) | 2 (4.3) | - |
| Nonaccidental trauma | 25 (3.9) | 4 (1.7) | 8 (2.6) | 2 (3.8) | 11 (23.9) | - |
| Other blunt | 88 (13.6) | 44 (18.8) | 29 (9.3) | 1 (1.9) | 13 (28.3) | - |
| Penetrating | 130 (20.2) | 11 (4.7) | 109 (34.9) | 10 (18.9) | 0 (0) | - |
| Ground-level fall | 86 (13.3) | 42 (17.9) | 20 (6.4) | 11 (20.8) | 13 (28.3) | - |
| Hanging | 42 (6.5) | 18 (7.7) | 18 (5.8) | 5 (9.4) | 2 (4.3) | - |
| Death (%) | 29 (4.5) | 16 (6.8) | 10 (3.2) | 3 (5.7) | 0 (0) | 0.27 |
| Rotterdam score >3 | 40 (6.2) | 10 (4.3) | 26 (8.3) | 3 (5.7) | 1 (2.2) | 0.29 |
| Radiation dose† (mGy-cm) ± SD | mean 682.8 ± 383.4 | - | - | - | - | - |
| median 644 [393–876] | ||||||
| Any intracranial injury (%) | 278 (43.1) | 153 (65.4) | 61 (19.6) | 28 (52.8) | 36 (78.3) | <0.001 |
| Epidural | 55 (8.5) | 31 (13.2) | 14 (4.5) | 3 (5.7) | 7 (15.2) | 0.40 |
| Subdural | 130 (20.2) | 65 (27.8) | 37 (11.9) | 12 (22.6) | 16 (34.8) | 0.17 |
| Subarachnoid hemorrhage | 109 (16.9) | 54 (23.1) | 31 (9.9) | 10 (18.9) | 14 (30.4) | 0.31 |
| Contusion | 133 (20.6) | 66 (28.2) | 25 (8.0) | 20 (37.7) | 22 (47.8) | 0.02 |
| Cervical spine injury (%) | 53 (8.2) | 26 (11.1) | 23 (7.4) | 2 (3.8) | 2 (4.3) | 0.50 |
| Multiple facial fractures, frontal sinus fractures, Lefort type II or III fractures (%) | 139 (21.6) | 70 (29.9) | 55 (17.6) | 12 (22.6) | 2 (4.3) | 0.04 |
| Fracture through the foramen transversarium (%) | 14 (2.2) | 10 (4.3) | 2 (0.6) | 2 (3.8) | 0 (0) | 0.08 |
| Discharge disposition home (%) | 517 (80.2) | 165 (70.5) | 274 (87.8) | 35 (66) | 43 (93.5) | <0.001 |
SD, standard deviation.
Excludes Primary Children's Hospital patients.
Data available for 168 patients.
The training cohort had a higher incidence of motor vehicle accident and pedestrian versus vehicle than the other centers; this corresponded to the higher acuity of trauma and the higher death rate in the training cohort than in the validation cohort.10 There were 29 deaths in total (4.5%). The number of intracranial injuries varied significantly among the hospitals; two-thirds of patients (65.4%) in the training cohort had associated intracranial injury (i.e., hematomas and/or contusions), and there was a wide variation among the validation centers (19.6–78.3%) (p < 0.001).
Within the validation cohort (n = 411 patients), 22 arterial injuries (18 carotid and 4 vertebral artery) were identified (Table 4), yielding a 5.4% prevalence of BCVI. Most were low-grade injuries (Grade 1 63.4%; Grade 2, 9.1%; Grade 3, 13.6%; Grade 4, 4.6%; Grade 5, 4.6%). A total of 68.2% of patients with BCVI in the validation cohort received treatment: antiplatelet therapy (seven patients), anticoagulation (four patients), endovascular treatment (two patients), or open surgery (two patients).
Table 4.
Number (%) of Patients Presenting with Each Risk Factor in the Utah Score at Each Hospital
| Total (n = 645) | Primary Children's Hospital (n = 234) | Monroe Carrell Children's Hospital (n = 312) | St. Louis Children's Hospital (n = 53) | Texas Children's Hospital (n = 46) | Differences among validation cohort (p value)* | |
|---|---|---|---|---|---|---|
| Arterial injury (BCVI) | 58 (9.0) | 36 (15.4) | 10 (3.2) | 6 (11.3) | 6 (13.0) | 0.003 |
| Glasgow Coma Scale score ≤8 | 190 (29.4) | 107 (45.7) | 57 (18.3) | 17 (32.1) | 9 (19.6) | 0.07 |
| Focal neurological exam | 99 (15.3) | 24 (10.3) | 53 (17.0) | 7 (13.2) | 15 (32.6) | 0.002 |
| Fracture through carotid canal | 89 (13.8) | 60 (25.6) | 23 (7.4) | 6 (11.3) | 0 (0) | 0.08 |
| Petrous temporal bone fracture | 53 (8.2) | 18 (7.7) | 22 (7.1) | 9 (17.0) | 4 (8.7) | 0.06 |
| Cerebral infarction on CT | 44 (6.8) | 14 (6.0) | 23 (7.4) | 3 (5.7) | 4 (8.7) | 0.84 |
BCVI, blunt cerebrovascular injury; CT, computed tomography.
Excludes Primary Children's Hospital.
Utah Score variables
Within the validation cohort, 83 (20.2%) patients had a GCS ≤8, 75 (18.2%) patients had a focal neurological deficit, 29 (7.1%) patients had a fracture through the carotid canal, 35 (8.5%) patients had a petrous temporal bone fracture, and 30 (7.3%) patients had hypodensity on CT consistent with ischemia (Table 4). Most notably for the primary outcome of BCVI, the validation cohort had a lower prevalence of BCVI (5.4%) than the training cohort (15.4%).
Assessment of Utah Score
Each patient in the validation cohort was scored using the Utah Score and classified as high (score ≥3) or low (score ≤2) risk and by the presence or absence of BCVI (Table 5). The high-risk Utah Score was significantly associated with BCVI with an odds ratio of 8.1 [3.3, 19.8] (p < 0.001). The score discriminated well, with an area under the curve of 72%. When the Utah Score was applied to the validation cohort, we found a sensitivity of 59%, specificity of 85%, positive predictive value of 18%, and a negative predictive value of 97%.
Table 5.
Validation of the Utah Score with Data from the Validation Cohort
| + BCVI | − BCVI | ||
|---|---|---|---|
| High risk (score ≥3) | True positives | False positives | 72 |
| 13 | 59 | ||
| Low risk (score ≤2) | False negatives | True negatives | 339 |
| 9 | 330 | ||
| 22 | 389 | Total 411 |
BCVI, blunt cerebrovascular injury.
Odds ratio 8.1 (3.3, 19.8). Fisher's exact test p value <0.001.
The children in the validation cohort with a score of ≤2 had a 2.7% risk of BCVI, whereas patients with a score of ≥3 had an 18.1% risk of BCVI. In comparison, children in the training cohort with a score ≤2 had a 7.9% risk of BCVI and those with a score ≥3 had a 39.3% risk of BCVI.14 The Utah Score misclassified only 16.6% (59 [14.4%] false positives and 9 [2.2%] false negatives) of patients when applied to the validation cohort, which is significantly below the validation threshold of 25%.19
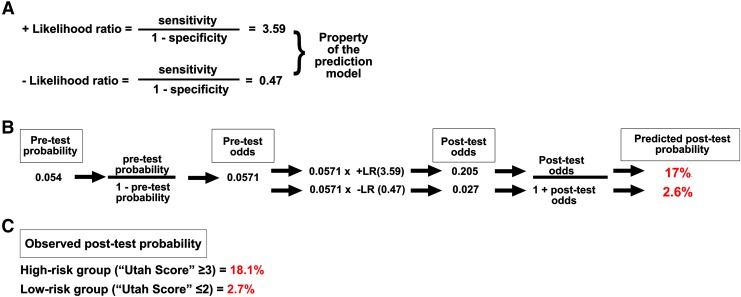
Bayesian analysis, specifically the positive likelihood ratio (3.59) and negative likelihood ratio (0.47) generated from the original Utah Score model,14 was used to determine predicted probabilities of BCVI for the high- and low-risk strata within the validation cohort. The predicted post-test probability for the high-risk strata was 17%, which was similar to the observed rate of 18.1%. The predicted post-test probability for the low-risk strata was 2.6%, which was nearly identical to the observed rate of 2.7% (Fig. 1). This high level of concordance further strengthens the external validity of the Utah Score.
FIG. 1.
(A) Bayesian analysis using original study likelihood ratios (LR). (B) Pre-test probability converted into pre-test odds and multiplied by positive and negative LR for high and low-risk groups, yielding post-test odds, then converted back to post-test probability and compared with (C) observed probability of blunt cerebrovascular injury from the validation cohort.
Application of Denver criteria to a pediatric data set
The probability of BCVI predicted by the Denver criteria in an adult patient with no risk factors was 20% compared with a 93% risk of BCVI with four risk factors. In our pediatric cohort, we found those with zero Denver criteria had a 2.9% risk of BCVI versus 25.7% with three risk factors (we had no patients with four risk factors). Although these criteria showed an escalating probability of injury with more criteria, they grossly overestimated BCVI probability in all five strata (in some cases by a factor 7) (Table 6). Therefore, the use of the Denver criteria in children would lead to overscreening with CTA and unnecessary exposure to radiation.
Table 6.
Application of the Denver Criteria to the Entire Cohort of Pediatric Trauma Patients with Blunt Cerebrovascular Injury
| Patients with carotid artery injury (n = 51) | Patients with vertebral artery injury (n = 8) | |||
|---|---|---|---|---|
| Score | Predicted by the Denver score | Observed | Predicted by the Denver score | Observed |
| 0 | 20% | 10 (2.9%) | 3% | 4 (0.7%) |
| 1 | 33–48% | 18 (10.3%) | 33% | 4 (11.4%) |
| 2 | 56–74% | 14 (15.2%) | - | - |
| 3 | 80–88% | 9 (25.7%) | - | - |
| 4 | >93% | 0 (0) | - | - |
Discussion
In this study, we externally validated the Utah Score on a cohort from three centers with a total of 411 patients. The Utah Score was significantly associated with BCVIs in the validation cohort and discriminated well with an area under the curve of 72%. The Utah Score misclassified only 16.6% of the patients at the three validation sites. For pediatric trauma patients, GCS score ≤8, focal neurological deficit, a fracture through the carotid canal, petrous temporal bone fracture, and hypodensity on noncontrast head CT are independent risk factors for BCVI. The Utah Score, which combines and weights these predictors, can be applied to help stratify patients into high- and low-risk categories to suggest whether screening with CTA should be performed (Fig. 2, Fig. 3).
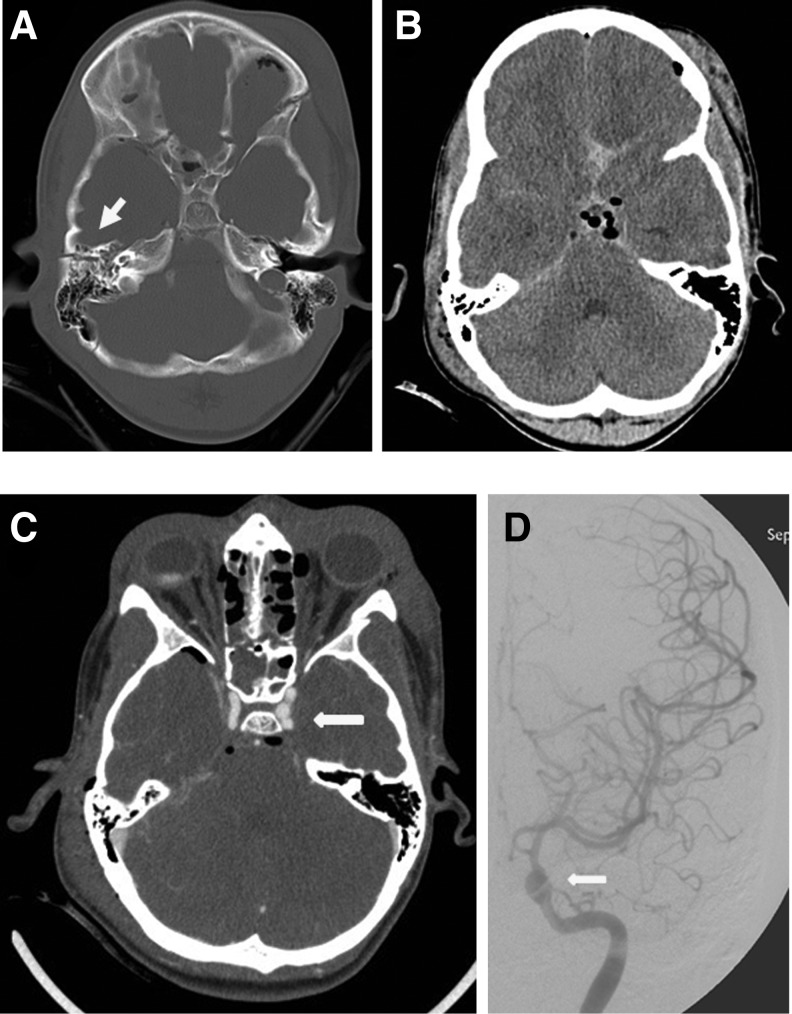
FIG. 2.
Case example the use and application of the Utah Score for predicting blunt cerebrovascular injury (BCVI). A 10-year-old boy struck by a vehicle presented with a Glasgow Coma Scale score of 3, nonfocal neurological examination, no fracture through the carotid canal, a contralateral petrous temporal bone fracture (A, arrow), and no evidence of cerebral infarct on computed tomography (B). This patient is thus “high risk” with a Utah Score of 4, which yields a 44.4% probability of BCVI (Table 2). Computed tomographic angiography demonstrated a left internal carotid artery injury with pseudoaneurysm formation (C, arrow). This was confirmed with digital subtraction angiography (D, arrow).
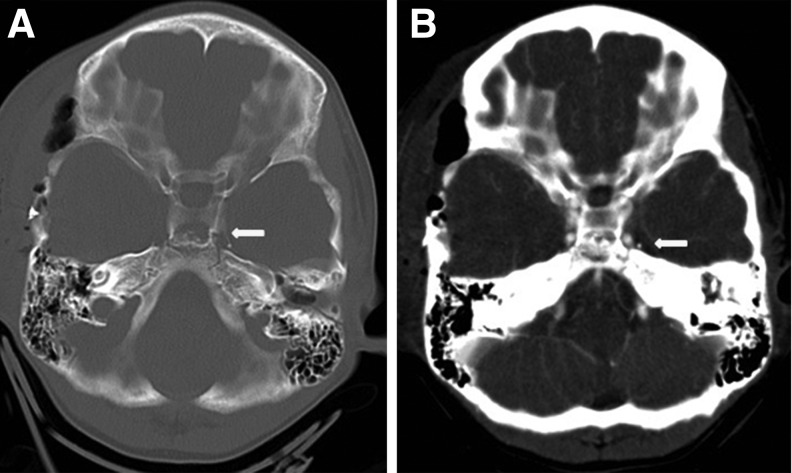
FIG. 3.
Case example demonstrating the use and application of the Utah Score for predicting cerebrovascular injury (BCVI). An 8-year-old boy involved in a motor vehicle accident who had a right temporal epidural hematoma necessitating evacuation had a Glasgow Coma Scale score of 10 and nonfocal neurological examination. He had a fracture through the carotid canal (A, arrow), no petrous temporal bone fracture, and no cerebral infarct on computed tomography. This patient is low risk with a Utah Score of 2, which yields a 6.7% probability of BCVI (Table 2). Computed tomographic angiography demonstrated no injury (B, arrow).
In this multi-institutional validation study, we found the Utah Score displayed good fit and discrimination, on par with the performance of other predictive models for other varying clinical conditions.21,22 An additional advantage of the Utah Score is its ease in use and availability to all pediatric trauma care providers.
The patient population at the four centers demonstrated heterogeneity in several variables, which strengthens the validation of the Utah Score. Specifically, the cohort from TCH demonstrated lower acuity than was seen at the other three centers. This likely reflects the fact that TCH did not become a Level I Trauma center until 2008–2009.
Although the sensitivity of the Utah Score was only 59%, the specificity was 85%. Thus, the Utah Score can be used to help rule in BCVI and suggest the need for CTA. Although it is desirable to have a high sensitivity for a screening tool such as the Utah Score, the ability to rule in disease with a high clinical suspicion to obtain further imaging can be invaluable. The positive predictive value of the score is quite low at 18%, but the negative predictive value was extremely high at 97%; thus, we can say with high certainty that patients deemed as low risk (score ≤2) have a low likelihood of having a BCVI. This is especially important to avoid missing a crucial diagnosis.
We developed the Utah Score to stratify patients to high- and low-risk groups. Among the original cohort, patients with a score ≤2 had a 7.9% risk of BCVI, whereas patients with a score ≥3 had a 39.3% risk of BCVI.14 In the validation cohort, patients with a score of ≤2 had a 2.7% risk of BCVI, whereas patients with a score of ≥3 had an 18.1% risk of BCVI. If there is strong clinical suspicion of BCVI despite a low prediction score, delayed magnetic resonance angiography may be considered as an alternative screening modality that avoids radiation in lower-risk patients.23,24 Risk modeling in adults has guided BCVI screening in practice (the Denver Criteria)10; patients with no risk factors for blunt carotid injury and for whom screening is not recommended have a 20% risk of BCVI14; comparatively, we have defined a pediatric population at much lower risk.
Whereas Kopelman and colleagues3 concluded that the risk factors for BCVI in children are similar to those in adults and recommended applying the Eastern Association for the Surgery of Trauma (EAST) guidelines6 to the pediatric trauma population, we identified GCS ≤8, focal neurological deficit, fracture through the carotid canal, petrous temporal bone fracture, and hypodensity on CT as risk factors for BCVI in children14; we suggest that these criteria be used when evaluating children for BCVI.
Similarly, we applied the original Denver criteria to the total cohort of patients (n = 645) and found that it poorly predicted BCVI in comparison with the Utah Score (Table 6). Because the Denver criteria grossly overestimated BCVI probability in all five strata, its use in children would lead to overscreening with CTA and unnecessary exposure to radiation and is not recommended. Brunetti and associates25 demonstrated that CT accounts for 32% of radiologic studies performed, but 91% of the total radiation dose; the average radiation dose per child was 17.9 mGy.
In this study, we found a mean (± SD) dose-length product of 682.8 ± 383.4 mGy-cm with a median of 644 (mGy-cm (range 393–876) among the 168 patients in the total cohort for whom the information was available. CTA of the head and neck involves 2–4 mGy of radiation exposure; thus, reducing the number of CTAs performed would significantly decrease the overall radiation exposure to children being assessed for trauma.
We suggest that use of the Utah criteria for screening for BCVI will lead to a reduction in radiation exposure by targeting CTAs for the most at-risk population. Radiation dose varies significantly with age and center, so it is difficult to say exactly how much more radiation exposure any given patient receives because a CTA was performed during the trauma evaluation. Takei and coworkers,26 however, summarized the mean dose-length products (DLP) for noncontrast head CT in children across six countries. By combining the age groups from the study, the mean radiation exposure DLP for noncontrast head CT was 543 mGy-cm.
Based on the single-center study for which the Utah Score was developed, the mean DLP for CTA was 649 mGy-cm. Considering that all patients who underwent a CTA also underwent a noncontrast head CT during the trauma evaluation, these patients received approximately 2.2 times the radiation exposure as children who did not undergo a CTA. In this setting, using the Utah criteria can reduce the increased, additional radiation exposure.
The differences we observed in the fit of the Denver criteria to adult and pediatric patients are similar to the findings from a single-center retrospective review of intracranial CT pathology in 870 adults and 336 children evaluated for blunt trauma.9 Although GCS scores were similar, pediatric patients were more likely to present with skull fracture and epidural hematoma and less likely to have a cortical contusion, subdural hemorrhage, or subarachnoid hemorrhage.8 This supports the statement that children experience trauma differently and may have different risk factors for BCVI. This may be explained by anatomical differences and divergent mechanisms of injury, both of which also underscore a different risk profile for BCVI in children compared with adults.
Jones and colleagues2 identified 45 children who had blunt BCVI; although 72% of asymptomatic injuries met adult screening criteria, more than two-thirds of patients who presented with acute neurologic findings did not meet adult screening guidelines, suggesting that screening guidelines need to be modified for children.2
Previous studies of BCVI in pediatric trauma patients have demonstrated varying rates of arterial injury that have typically been lower than the rates observed in the training and validation cohorts in this study.1–4,14 We identified a total of 22 arterial injuries in the validation cohort (n = 411) and 58 total among the total study population (n = 645). Although the majority of injuries were Grade 1 (63.4%), a large majority required treatment (68.2%), representing a true clinical finding rather than a diagnosis without consequence.
The Utah Score created from our cohorts represents a large series of patients with BCVI; it is a different representation of the prevalence of BCVI and the associated risk factors in children. In the multicenter validation cohort, we found the overall prevalence of BCVI diagnosed by CTA was 5.4% with variation among the three centers, and all centers demonstrated a higher prevalence than previous studies.
We found that, as in adult patients, petrous temporal bone fracture is an independent predictor of BCVI7,10; however, we also determined that fracture through the carotid canal, which is commonly an indication for ordering CTA on initial evaluation, is an independent risk factor in children.14 The Utah Score uses a higher GCS score cutoff to signal BCVI than is suggested in adults and highlights focal neurologic deficit and hypodensity on noncontrast CT, both of which are strongly and independently associated with BCVI.14
The difference in risk factors for BCVI between adults and children can be explained by several different factors, including response to traumatic injury, autoregulation, developmental anatomy and physiology, vessel reactivity, and cerebrovascular circulatory reserve. Our initial study and validation clearly demonstrate that BCVI in children manifests differently than in adults, and thus an adjustment in screening criteria is required. This is also supported by data from a large multicenter retrospective cohort study of 5829 children <15 years of age by Azarakhsh and associates1 that also highlights the importance of pediatric-specific risk factors and the potential usefulness of a scoring system. Only 89 of 538 patients (16.5%) who met adult criteria actually underwent screening for BCVI, and 23 BCVIs were identified.1
Interestingly, in our cohort of 644 patients, there were 53 children (8.2%) with spinal injuries—fracture dislocation, fracture, or ligamentous injury—and this finding was not associated with BCVI (p = 0.50). Although there is evidence that injuries to the cervical spine and spinal cord are less common in children than in adults,27–32 the prevalence of vertebral artery injury in the validation and training cohort was 1.24% (8/645); this is close to the incidence reported in the adult literature, which is between 0.5 and 2%.33–37 The small number of vertebral artery injuries in both the Utah cohort and the multicenter cohort, however, limits the external validity of the aforementioned finding.
Although catheter angiography is the gold standard for the diagnosis of vascular injury, CTA has become the norm for evaluation of traumatic vascular injury in both adults and children because of its speed, availability, and accuracy. Early CTA with four- and eight-slice scanners was quite poor at detecting BCVI, but the sensitivity has improved with advances in CT scanning technology. Previous data demonstrated that 16-slice scanner CTA has a sensitivity of 100% and specificity of 94% for symptomatic BCVI38; in addition, in terms of face validity, the incidence of BCVI in the studied population matched those historically that were diagnosed with conventional cerebral angiography.
Based on these data and other similar findings, Biffl and colleagues39 reversed their previous recommendation that CTA was not adequate for screening BCVI.40 Further direct comparison by Eastman and coworkers33 between CTA and digital subtraction angiography (DSA) in 162 CTAs followed by 146 cerebral angiograms demonstrated a sensitivity of 97.7%, specificity of 100%, positive predictive value of 99.3%, and negative predictive value of 99.3%. A systematic review by Roberts and associates41 examining the diagnostic accuracy of CTA angiography for BCVI found a pooled sensitivity of 66% and specificity of 97% for CTA versus DSA in the detection of BCVI.
Thus, we believe using CTA as the diagnostic tool of choice during initial contact in the trauma setting is reasonable and reflects current practice. The Denver criteria were developed using catheter angiography, but the application to CTA has been endorsed and is widely applied in the evaluation in adult trauma, although it has not been independently validated using CTA.
False positives and false negatives are issues inherent to all screening methods; while clinical prediction scores like ours may stratify patients into high-risk and low-risk groups, it is important for the clinician to assess each patient carefully, understand the limitations of the score, and integrate the tool's result into clinical decision-making.
Limitations
This multicenter validation was conducted on a retrospective cohort, which may limit the accuracy and availability of the medical record. A major limitation is the inclusion of only patients who underwent CTA for screening. In addition, we included only those patients who underwent CTA imaging as part of their trauma evaluation, so we do not know how many patients with identified risk factors did not undergo CTA for evaluation.
Patients who underwent magnetic resonance or conventional angiography for initial diagnosis were not captured, impacting the incidence of BCVI. Further, conventional angiography may have been used in patients with more severe injuries or higher suspicion of BCVI, which may bias our results toward lower-grade injuries. At all four centers examined in this study, however, CTA was the modality of choice to begin the diagnostic work-up for BCVI in the pediatric trauma population.
In addition, the original Denver criteria were designed based on catheter angiography. The Denver grading system was used as a comparison because it is an analytical method with quantifiable risk prediction using statistically supported data to screen for adult BCVI and because EAST trauma guidelines have recommended its application to the pediatric trauma population. Therefore, we thought it was critical to compare our clinical prediction score to these criteria. That comparison may be limited because the Denver grading system has not been independently validated using CTA.
Only a small number of vertebral artery injuries were identified in our multi-institutional series, limiting definitive conclusions about risk factors for vertebral injury and its relationship to cervical spine injury in children. Although most vascular injuries identified in our study cohort required treatment (68.2%), CTA may overdiagnose inconsequential injuries. This may be less of a cause for concern, however, because CTA is used as a screening test with the identification of any type of vascular injury as its objective, and more advanced, invasive imaging including catheter angiography may be used to confirm this diagnosis.
This study externally validates the Utah Score as a clinical prediction score for BCVI in pediatric trauma patients, and we encourage its widespread implementation at other pediatric trauma centers. Nevertheless, we advise that clinical evaluation and multidisciplinary decision-making be paramount when making all diagnostic and treatment decisions.
Conclusions
We have externally validated the Utah Score for predicting BCVI in pediatric trauma patients, with a 16.6% misclassification rate. In pediatric trauma patients, fracture through the petrous temporal bone or carotid canal, focal neurological deficit, hypodensity on CT consistent with ischemia, and GCS ≤8 are independent risk factors for BCVI. The validation of these findings and the Utah Score on a large, multicenter cohort indicates that the score is appropriate for clinical use.
Physicians can utilize this easy-to-use bedside instrument to make a more informed decision about screening the at-risk pediatric population with hopes of reducing unnecessary CTA imaging for low-risk patients. Although external validation has been completed, further prospective study and tracking may strengthen these findings.
Acknowledgments
We thank Kristin Kraus, M.Sc., for editorial assistance in preparing this paper and Tracey Bach for her administrative assistance for this project.
Funding for this project was received through the Primary Children's Hospital Foundation Grant awarded to Vijay M. Ravindra in the amount of $14,750. REDcap use and management was funded by Center for Clinical and Translational Sciences grant support (8UL1TR000105 (formerly UL1RR025764) NCATS/NIH).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Azarakhsh N., Grimes S., Notrica D.M., Raines A., Garcia N.M., Tuggle D.W., Maxson R.T., Alder A.C., Recicar J., Garcia-Filion P., Greenwell C., Lawson K.A., Wan J.Y., and Eubanks J.W., 3rd, (2013). Blunt cerebrovascular injury in children: underreported or underrecognized? A multicenter ATOMAC study. J. Trauma Acute Care Surg. 75, 1006–1012 [DOI] [PubMed] [Google Scholar]
- 2.Jones T.S., Burlew C.C., Kornblith L.Z., Biffl W.L., Partrick D.A., Johnson J.L., Barnett C.C., Bensard D.D., and Moore E.E. (2012). Blunt cerebrovascular injuries in the child. Am. J. Surg. 204, 7–10 [DOI] [PubMed] [Google Scholar]
- 3.Kopelman T.R., Berardoni N.E., O'Neill P.J., Hedayati P., Vail S.J., Pieri P.G., Feiz-Erfan I., and Pressman M.A. (2011). Risk factors for blunt cerebrovascular injury in children: do they mimic those seen in adults? J. Trauma 71, 559–564 [DOI] [PubMed] [Google Scholar]
- 4.Lew S.M., Frumiento C., and Wald S.L. (1999). Pediatric blunt carotid injury: a review of the National Pediatric Trauma Registry. Pediatr. Neurosurg. 30, 239–244 [DOI] [PubMed] [Google Scholar]
- 5.Carrillo E.H., Osborne D.L., Spain D.A., Miller F.B., Senler S.O., and Richardson J.D. (1999). Blunt carotid artery injuries: difficulties with the diagnosis prior to neurologic event. J. Trauma 46, 1120–1125 [DOI] [PubMed] [Google Scholar]
- 6.Bromberg W.J., Collier B.C., Diebel L.N., Dwyer K.M., Holevar M.R., Jacobs D.G., Kurek S.J., Schreiber M.A., Shapiro M.L., and Vogel T.R. (2010). Blunt cerebrovascular injury practice management guidelines: the Eastern Association for the Surgery of Trauma. J. Trauma 68, 471–477 [DOI] [PubMed] [Google Scholar]
- 7.Miller P.R., Fabian T.C., Croce M.A., Cagiannos C., Williams J.S., Vang M., Qaisi W.G., Felker R.E., and Timmons S.D. (2002). Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes. Ann. Surg. 236, 386–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaye D., Brasel K.J., Neideen T., and Weigelt J.A. (2011). Screening for blunt cerebrovascular injuries is cost-effective. J. Trauma 70, 1051–1057 [DOI] [PubMed] [Google Scholar]
- 9.Sarkar K., Keachie K., Nguyen U., Muizelaar J.P., Zwienenberg-Lee M., and Shahlaie K. (2014). Computed tomography characteristics in pediatric versus adult traumatic brain injury. J. Neurosurg. Pediatr. 13, 307–314 [DOI] [PubMed] [Google Scholar]
- 10.Biffl W.L., Moore E.E., Offner P.J., Brega K.E., Franciose R.J., Elliott J.P., and Burch J.M. (1999). Optimizing screening for blunt cerebrovascular injuries. Am. J. Surg. 178, 517–522 [DOI] [PubMed] [Google Scholar]
- 11.Brenner D.J., and Hall E.J. (2007). Computed tomography—an increasing source of radiation exposure. N. Engl. J. Med. 357, 2277–2284 [DOI] [PubMed] [Google Scholar]
- 12.Miglioretti D.L., Johnson E., Williams A., Greenlee R.T., Weinmann S., Solberg L.I., Feigelson H.S., Roblin D., Flynn M.J., Vanneman N., and Smith-Bindman R. (2013). The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 167, 700–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearce M.S., Salotti J.A., Little M.P., McHugh K., Lee C., Kim K.P., Howe N.L., Ronckers C.M., Rajaraman P., Sir Craft A.W., Parker L., and Berrington de Gonzalez A. (2012). Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 380, 499–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravindra V.M., Riva-Cambrin J., Sivakumar W., Metzger R.R., and Bollo R.J. (2015). Risk factors for traumatic blunt cerebrovascular injury diagnosed by computed tomography angiography in the pediatric population: a retrospective cohort study. J. Neurosurg. Pediatr. 15, 599–606 [DOI] [PubMed] [Google Scholar]
- 15.Maas A.I., Hukkelhoven C.W., Marshall L.F., and Steyerberg E.W. (2005). Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery 57, 1173–1182 [DOI] [PubMed] [Google Scholar]
- 16.Liesemer K., Riva-Cambrin J., Bennett K.S., Bratton S.L., Tran H., Metzger R.R., and Bennett T.D. (2014). Use of Rotterdam CT scores for mortality risk stratification in children with traumatic brain injury. Pediatr. Crit. Care. Med. 15, 554–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biffl W.L., Moore E.E., Offner P.J., Brega K.E., Franciose R.J., and Burch J.M. (1999). Blunt carotid arterial injuries: implications of a new grading scale. J. Trauma 47, 845–853 [DOI] [PubMed] [Google Scholar]
- 18.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., and Conde J.G. (2009). Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42, 377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riva-Cambrin J., Detsky A.S., Lamberti-Pasculli M., Sargent M.A., Armstrong D., Moineddin R., Cochrane D.D., and Drake J.M. (2009). Predicting postresection hydrocephalus in pediatric patients with posterior fossa tumors. J. Neurosurg. Pediatr. 3, 378–385 [DOI] [PubMed] [Google Scholar]
- 20.Burlew C.C., Biffl W.L., Moore E.E., Barnett C.C., Johnson J.L., and Bensard D.D. (2012). Blunt cerebrovascular injuries: redefining screening criteria in the era of noninvasive diagnosis. J. Trauma Acute Care Surg. 72, 330–337 [DOI] [PubMed] [Google Scholar]
- 21.Burroughs A.K., Sabin C.A., Rolles K., Delvart V., Karam V., Buckels J., O'Grady J.G., Castaing D., Klempnauer J., Jamieson N., Neuhaus P., Lerut J., de Ville de Goyet J., Pollard S., Salizzoni M., Rogiers X., Muhlbacher F., Garcia Valdecasas J.C., Broelsch C., Jaeck D., Berenguer J., Gonzalez E.M., and Adam R. (2006). 3-month and 12-month mortality after first liver transplant in adults in Europe: predictive models for outcome. Lancet 367, 225–232 [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni A.V., Drake J.M., Mallucci C.L., Sgouros S., Roth J., and Constantini S. (2009). Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. J. Pediatr. 155, 254–259.e1. [DOI] [PubMed] [Google Scholar]
- 23.Bok A.P., and Peter J.C. (1996). Carotid and vertebral artery occlusion after blunt cervical injury: the role of MR angiography in early diagnosis. J. Trauma 40, 968–972 [DOI] [PubMed] [Google Scholar]
- 24.Wang A.C., Charters M.A., Thawani J.P., Than K.D., Sullivan S.E., and Graziano G.P. (2012). Evaluating the use and utility of noninvasive angiography in diagnosing traumatic blunt cerebrovascular injury. J. Trauma Acute Care Surg. 72, 1601–1610 [DOI] [PubMed] [Google Scholar]
- 25.Brunetti M.A., Mahesh M., Nabaweesi R., Locke P., Ziegfield S., and Brown R. (2011). Diagnostic rediation exposure in pediatric trauma patients. J. Trauma 70, E24–E28 [DOI] [PubMed] [Google Scholar]
- 26.Takei Y., Miyazaki O., Matsubara K., Shimada Y., Muramatsu Y., Akahane K., Fujii K., Suzuki S., and Koshida K. (2016). Nationwide survey of radiation exposure during pediatric computed tomography examinations and proposal of age-based diagnostic reference levels for Japan. Pediatr. Radiol. 46 280–285 [DOI] [PubMed] [Google Scholar]
- 27.Burke D.C. (1974). Traumatic spinal paralysis in children. Paraplegia 11, 268–276 [DOI] [PubMed] [Google Scholar]
- 28.Melzak J. (1969). Paraplegia among children. Lancet 2, 45–48 [DOI] [PubMed] [Google Scholar]
- 29.Dula D.J. (1979). Trauma to the cervical spine. JACEP 8, 504–507 [DOI] [PubMed] [Google Scholar]
- 30.Hubbard D.D. (1974). Injuries of the spine in children and adolescents. Clin. Orthop. Relat. Res. 56–65 [PubMed] [Google Scholar]
- 31.Kalfas I., Wilberger J., Goldberg A., and Prostko E.R. (1988). Magnetic resonance imaging in acute spinal cord trauma. Neurosurgery 23, 295–299 [DOI] [PubMed] [Google Scholar]
- 32.Rang M. (1974). Children's Fractures. JB Lippincott: Philadelphia [Google Scholar]
- 33.Eastman A.L., Chason D.P., Perez C.L., McAnulty A.L., and Minei J.P. (2006). Computed tomographic angiography for the diagnosis of blunt cervical vascular injury: is it ready for primetime? J. Trauma 60, 925–929 [DOI] [PubMed] [Google Scholar]
- 34.Fassett D.R., Dailey A.T., and Vaccaro A.R. (2008). Vertebral artery injuries associated with cervical spine injuries: a review of the literature. J. Spinal Disord. Tech. 21, 252–258 [DOI] [PubMed] [Google Scholar]
- 35.Cothren C.C., and Moore E.E. (2005). Blunt cerebrovascular injuries. Clinics (Sao Paulo) 60, 489–496 [DOI] [PubMed] [Google Scholar]
- 36.Biffl W.L., Cothren C.C., Moore E.E., Kozar R., Cocanour C., Davis J.W., McIntyre R.C., Jr., West M.A., and Moore F.A. (2009). Western Trauma Association critical decisions in trauma: screening for and treatment of blunt cerebrovascular injuries. J. Trauma 67, 1150–1153 [DOI] [PubMed] [Google Scholar]
- 37.Berne J.D., and Norwood S.H. (2009). Blunt vertebral artery injuries in the era of computed tomographic angiographic screening: incidence and outcomes from 8,292 patients. J. Trauma 67, 1333–1338 [DOI] [PubMed] [Google Scholar]
- 38.Berne J.D., Norwood S.H., McAuley C.E., and Villareal D.H. (2004). Helical computed tomographic angiography: an excellent screening test for blunt cerebrovascular injury. J. Trauma 57, 11–19 [DOI] [PubMed] [Google Scholar]
- 39.Biffl W.L., Moore E.E., and Mestek M. (1999). Patients with blunt carotid and vertebral artery injuries. J. Trauma 47, 438–439 [DOI] [PubMed] [Google Scholar]
- 40.Biffl W.L., Egglin T., Benedetto B., Gibbs F., and Cioffi W.G. (2006). Sixteen-slice computed tomographic angiography is a reliable noninvasive screening test for clinically significant blunt cerebrovascular injuries. J. Trauma 60, 745–752 [DOI] [PubMed] [Google Scholar]
- 41.Roberts D.J., Chaubey V.P., Zygun D.A., Lorenzetti D., Faris P.D., Ball C.G., Kirkpatrick A.W., and James M.T. (2013). Diagnostic accuracy of computed tomographic angiography for blunt cerebrovascular injury detection in trauma patients: a systematic review and meta-analysis. Ann. Surg. 257, 621–632 [DOI] [PubMed] [Google Scholar]