Abstract
US federal guidelines recommend that medical providers test all adolescents and adults for HIV infection at least once before the age of 64. The wide age range included in these guidelines may limit their utility and impact. We created an arithmetic model to estimate how HIV screening at different ages would impact the total number of years of undiagnosed HIV infection in the population and the number of persons developing clinical manifestations of HIV/AIDS. Our base case model assumed that age of infection in the screened population was the same as the estimated age of infection among all persons diagnosed with HIV in the United States in 2010. We parameterized a second model assuming age of infection was similar to the younger age distribution observed in African Americans. In the base case model, the number of years of undiagnosed HIV infection and number of persons with clinical manifestations of HIV/AIDS were both minimized by screening at age 34. If age of infection was similar to that estimated to occur among African Americans, testing at age 24 and 27 would minimize the number of years of undiagnosed infection and clinical cases, respectively. For both parameterization scenarios, testing between the ages of 21 and 38 resulted in outcomes within 10% of the model's estimated optimal age for screening. Focusing HIV screening on a narrower age range than is currently recommended may improve the impact of routine HIV screening efforts.
Keywords: : HIV, screening, mathematical model
Introduction
An estimated 13% of all HIV-infected Americans are unaware of their HIV status1 and testing these persons and linking them to effective medical care are a centerpiece of the US National HIV/AIDS Strategy.2,3 Integrating HIV testing into routine medical care is one mechanism for increasing the proportion of HIV-infected Americans who are aware of their status. In 2006, the Centers for Disease Control and Prevention (CDC) issued recommendations advocating that medical providers screen all adolescents and adults, age 13–64, for HIV infection at least once in their lifetime.4 A variant on this recommendation was subsequently endorsed by the US Preventive Services Task Force.5
Many persons at high risk for HIV test for HIV frequently,6 and CDC guidelines are meant, in large measure, to diagnose infected persons who would not be tested as part of programs designed to reach persons identified as being at high risk for infection. However, the very wide age range specified in the recommendation leaves medical providers and healthcare organizations without clear guidance as to when during a patient's adolescence or adulthood screening should occur. One-time screening at age 16 would likely result in testing most people before they might acquire HIV, while waiting to test until people are in their 60s would allow most infected persons to develop clinical manifestations of HIV/AIDS before testing.
The optimal age range for testing is unknown. We created a model to estimate the age range at which one-time HIV screening performed as part of routine medical care would ideally occur, assuming that the goal of screening is to minimize both HIV transmission and HIV/AIDS-related morbidity and mortality.
Materials and Methods
The model evaluated the impact of routine HIV testing assuming that testing consistently occurred at a specific age. We assessed three outcomes: (1) HIV test positivity; (2) the cumulative number of years of undiagnosed HIV infection in the population; and (3) the number of symptomatic HIV/AIDS cases that would occur in the population. We modeled test positivity since CDC guidelines identify test positivity of >0.1% as a threshold for defining when to undertake routine testing4 and because test positivity is widely used as a parameter in cost-effectiveness analyses.7,8 We evaluated the cumulative number of years of undiagnosed HIV infection in the population as a measure of population-level prevention impact since persons with undiagnosed infection are more likely to transmit the virus,9 and minimizing the total number of years of undiagnosed infection may be a means to avert the maximum number of HIV transmissions. Finally, we assessed the number of symptomatic HIV/AIDS cases occurring in the population as an outcome of clinical significance since HIV testing is designed to both prevent transmission and prevent deleterious health outcomes.
The model evaluated screening performed in persons aged 16–64 years using 2010 US Census data to define the size of the population at each age. We assumed that the number of persons of each age within each census-defined 5-year age grouping was equivalent.
The age at which people acquire HIV infection is a critical determinant of when routine screening would ideally occur. However, the age distribution of HIV acquisition among infected persons who are not tested though risk-based or prenatal screening—the dominant target population for routine screening—is not known. Thus, we modeled different distributions of age at time of HIV infection. In all models, we assumed that all infections occur in persons aged 16–64 years. In our base case analysis, we assumed that the age of HIV acquisition in our candidate population for screening was similar to the estimated age of acquisition of HIV observed among all persons infected in 2010. We used 2010 national CDC data to define the number of new infections occurring in persons in each age group (assuming that all infections in those under age 16 occurred in those aged 16–19 years and all those occurring in those aged >65 years occurred in persons aged 55–64 years) and calculated rates using census data to define the population at risk for infection.10 Second, because late HIV diagnosis is more common among African Americans,11 we hypothesized that the population that might benefit most from screening would be African Africans. National data suggest that the age of HIV acquisition among African Americans is younger than that of the total population of persons with HIV.10 Thus, we created a second model using the same national estimates for the total number of infections and size of the population, but assuming that the proportion of new infections occurring in each age group was similar to that estimated to occur in African Americans.
The model used data from the Concerted Action on Seroconversion to AIDS and Death in Europe (CASCADE) to define the risk of developing a CD4 count of <200 cells/mm3 over time.12 Based on those data, we assumed that 8.8% of persons develop a CD4 count <200 cells/mm3 in the first year following HIV infection and that the risk of dropping below that threshold is linear after the first year, with an additional 6.08% of persons developing a CD4 count <200 cells/mm3 each year, and all persons having a CD4 count <200 cells/mm3 16 years following infection. Our model assumed that all persons would develop symptoms leading to diagnostic testing concurrent with the decline of their CD4 to <200 cells/mm3. In reality, many people develop clinically significant symptoms before their CD4 count drops below 200 cells/mm3, while others have no symptoms until years after their CD4 count declines below that threshold.13 Cohort studies of persons infected as adults undertaken in the 1980s and 1990s found that ∼50% of persons develop symptoms of HIV infection within 7 years of infection,14 with a quarter to half developing an AIDS-defining illness in the 9–11 years following infection.14,15 The median time from infection to CD4 count <200 cells/mm3 in the model is 8 years, roughly corresponding to the median onset of illness in cohort studies.
We created the model using Excel (Microsoft Corp., Redmond, WA). Each line in the spreadsheet started with the number of persons of that age in the population, from which we estimated the number of new HIV infections occurring in persons of that age. In successive columns, we estimated the cumulative number of persons developing symptomatic HIV/AIDS in each of the next 16 years as well as the cumulative number of years of undiagnosed HIV infection among persons infected at that age. We assumed that screening occurred in the midpoint of each year and that persons contributed half a year of undiagnosed HIV in the year of their diagnosis. If screening occurred before an age group's HIV infection, we assumed those persons were not diagnosed with HIV until they developed a CD4 count <200 cells/mm3, at which point they would cease to contribute person-years of undiagnosed HIV infection to the population. For example, when modeling screening at age 20, all persons acquiring HIV after that age would be diagnosed when their CD4 count went below <200 cells/mm3. We defined the optimal age of screening as the age which resulted in the minimum total years of undiagnosed HIV infection in the population or the minimum total number of cases of symptomatic HIV/AIDS.
The following model was used to compute years of undiagnosed HIV infection and number of cases of symptomatic HIV/AIDS for any given screening age, x:
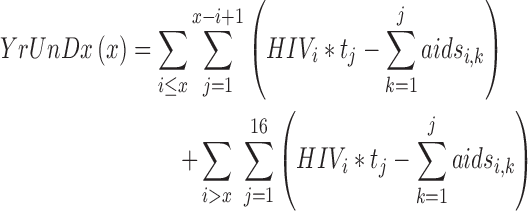 |
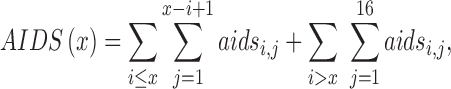 |
where
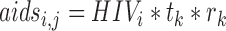 |
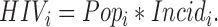 |
Popi is the population size, Incidi is HIV incidence, and wi is a weight for the ith age group, tj = 0.5 for j = 1 and 1.0 otherwise, and rk = 0.088 for k = 1 and 0.0608 otherwise.
We estimated HIV test positivity among screened persons without a prior HIV diagnosis using the following formula:
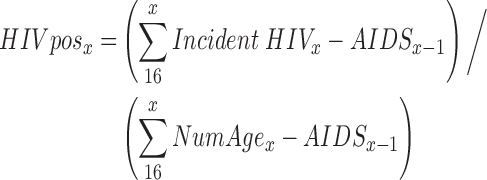 |
where incident HIVx is the number of HIV infections occurring in persons of age x, AIDSx is the number of AIDS cases occurring in persons of age x − 1, and NumAgex is the number of people in age group x, all of whom are assumed to test for HIV. Here, we subtract persons with AIDS from both the numerator and the denominator since in our model we assume those persons are tested for clinical reasons, not screening.
Results
Figure 1a presents data on HIV test positivity if screening occurs at different ages using our base case assumptions. HIV test positivity is low among 16-year-olds (0.026%), but rises steeply with age until peaking at 0.28% at age 31. Positivity thereafter declines to 0.070% at age 64, reflecting the fact that most infected persons had already been diagnosed with HIV before age 64 as a result of developing symptoms. Figure 1b presents data on the cumulative number of years of undiagnosed HIV infection and number of symptomatic HIV/AIDS cases that would occur if screening happened at different ages. As shown, both the cumulative number of years of undiagnosed infection and number of symptomatic cases are highest if screening focuses on the youngest or oldest persons. Testing at age 34 minimizes both the population's number of years of undiagnosed HIV infection and the number of symptomatic cases. While this optimal age minimized adverse outcomes, small differences in the age of screening had little impact on when screening would ideally occur. For example, the age boundaries at which testing resulted in more than a 10% increase in the number of years of undiagnosed HIV infection or symptomatic cases compared with the optimal testing age (shaded gray area in Fig. 1b) were <23 and >48 years.
FIG. 1.
Estimated HIV test positivity among tested persons (a) and cumulative number of years of undiagnosed HIV infection and symptomatic HIV/AIDS cases (b) by age (base case scenario).
Figure 2 presents data from our model parameterized using the estimated age of HIV infection among African Americans in the United States in 2010. Under this assumption, compared with our base case model, test positivity among young persons is higher, peaking at age 30 at 0.30%. The optimal age of testing likewise occurs at a younger age than in our base case scenario; years of undiagnosed HIV infection are minimized by testing at age 24, and the number of symptomatic HIV/AIDS cases is minimized by testing at age 27. The curves shown in the Figure have a steeper slope than those presented in Fig. 1, indicating that the difference in outcomes between the optimal age of screening and the far ends of the age distribution is greater. For example, compared with testing at age 24, testing at age 43 is associated with 10.2% and 12.7% increase in cumulative years of undiagnosed HIV infection and symptomatic HIV/AIDS diagnoses, respectively. As in the base case, the age range in which both years of undiagnosed HIV infection and symptomatic HIV/AIDS diagnoses vary <10% from the optimal age is relatively wide. These ranges vary somewhat between our two modeled distributions of the age of infection. However, ages 21–38 are within this near optimal range under both modeled scenarios.
FIG. 2.
Estimated HIV test positivity among tested persons (a) and cumulative number of years of undiagnosed HIV infection and AIDS cases (b) by age (age distribution based on estimated incidence in African Americans).
Discussion
We used a simple model incorporating data on the age distribution of new HIV diagnoses and the rate of disease progression to estimate HIV test positivity and the optimal age for routine HIV screening. We found that HIV test positivity is highest among persons in the late 20s or early 30s and, at least in the absence of other approaches to HIV screening (i.e., testing not prompted by clinical indications), exceeds 1 per 1000 persons tested—the CDC-recommended threshold for defining populations in which testing should be routine.4 If the goal of screening all Americans at least once during their adolescence or adulthood is to minimize HIV-associated morbidity and the number of years of undiagnosed HIV infection, the optimal age for screening is likely sometime between the ages of 21 and 38. Testing within this range results in less than 10% difference in the occurrence of adverse outcomes relative to testing at the optimal ages in the scenarios we investigated.
While we believe that our findings could be helpful in refining US national guidelines to more narrowly focus routine HIV testing efforts, such a revision would require careful consideration of experiences with routine testing over the last decade and attention to how such a revision might affect different populations and operational considerations in different clinical facilities. In particular, it will be important to more carefully consider which patients' routine screening is designed to impact since many persons diagnosed with HIV infection in the United States are tested through efforts that focus on higher risk persons (e.g., men who have sex with men, persons with bacterial sexually transmitted infections, persons who inject drugs), prenatal care, or testing prompted by sex or needle sharing partner's HIV diagnosis.6,16–20 National HIV surveillance data demonstrate that late HIV diagnoses are particularly common among heterosexual men and male persons who inject drugs,21 while local studies have identified foreign birth, rural residence, and being an African American MSM as risk factors for late diagnosis.22–24 These findings highlight how existing efforts to identify persons with HIV infection are selective in their success. Decisions to change routine screening guidelines will need to consider which populations are likely to be most affected by such changes.
While the narrower age range we propose is potentially more complicated than the very broad age range recommended in the 2006 CDC guidelines, screening recommendations for gonorrhea and chlamydial infection; hepatitis C; and cervical, breast, and colon cancer provide guidance on the target ages or birth years for screening.25–28 Thus, there is precedent for adopting screening guidelines that are more focused than the current US HIV screening recommendations. Young adults, particularly young men, are less likely to have a usual source of medical care and to receive care than children or older adults, which may present a barrier to focusing screening on a narrower age range. On the other hand, even among men in their 20s, over 70% have a usual source of care, and many others receive at least some care through places such as urgent care clinics.29,30 Recommending HIV screening for adults in medical settings during a more narrow age range, perhaps with a suggestion to test older adults who were not screened in the recommended age range, could maximize the benefit of screening and the efficient use of HIV testing resources.
Our findings have a number of limitations. First, our model assumes that routine screening and testing of persons with clinical manifestations of HIV/AIDS are the only mechanisms for diagnosing people with HIV infection. This is clearly not the case. Many persons with identified risks for HIV infection test for HIV through programs specifically designed to serve them, many women test for HIV as part of prenatal care, and substantial numbers of persons test for HIV in response to a partner's HIV diagnosis. As a result, our model's absolute values for each outcome are overestimates, although HIV test positivity could be higher than what we estimate in some populations. Second, we do not know the true distribution of age of infection in persons who might be identified through screening integrated into routine medical care. To address this limitation, we modeled different age distributions of infection and defined an age range that optimizes routinized testing in medical care under different circumstances. Third, our model is based on 2010 estimates of age-specific HIV incidence. Insofar as historical or future incidence that varies from these estimates, our model's findings may be imprecise. Fourth, we assumed that infected persons are all diagnosed once their CD4 count drops below 200 cells/mm3. In fact, many persons develop clinically significant symptoms before their CD4 count declines below this threshold, while others remain clinically well for years with very low CD4 counts. The difficulty in estimating when infected persons might be diagnosed because of symptoms is further complicated by evidence suggesting that HIV may be evolving to progress more rapidly31 and by variance in the rate of disease progression with age.14,15 The median time to diagnosis we used is very similar to the median time to development of symptoms observed in cohort studies of hemophiliacs in the 1980s and 1990s.14,15 Thus, while our estimate of time from infection to diagnosis is imprecise, it accords with available data. Moreover, small differences in this estimate are unlikely to have a major impact on our findings. Finally, our model is static and does not include transmission effects. Persons with undiagnosed HIV are a critical source of HIV transmission,9 and decreasing the cumulative years of undiagnosed infection should decrease HIV incidence. However, we did not directly model HIV transmission. Evaluating different age-based HIV screening guidelines through the use of a dynamic model could provide additional information to inform future screening guidelines.
In summary, we found that focusing routine HIV screening on persons between the ages of 21 and 38 would likely maximize the benefits of testing as part of routine medical care. Current CDC HIV testing recommendations are now a decade old, and it may be worthwhile to refine those recommendations in light of our findings and experience gained since the guidelines were originally developed.
Acknowledgments
Research reported in this publication was supported by NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, and NIDDK of the National Institutes of Health under award number AI027757.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hall HI, An Q, Tang T, et al. Prevalence of diagnosed and undiagnosed HIV infection—United States, 2008–2012. MMWR Morb Mortal Wkly Rep 2015;64:657–662 [PMC free article] [PubMed] [Google Scholar]
- 2.White House Office on National AIDS Policy. National HIV/AIDS Strategy for the United States. Washington DC: White House Office on National AIDS Policy, 2010 [Google Scholar]
- 3.White House Office on National AIDS Policy. National HIV/AIDS Strategy for the United States: Updated to 2020; 2015
- 4.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006;55:1–17; quiz CE11-14 [PubMed] [Google Scholar]
- 5.Moyer VA. Force* USPST. Screening for HIV: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2013;159:51–60 [DOI] [PubMed] [Google Scholar]
- 6.Cooley LA, Oster AM, Rose CE, et al. Increases in HIV testing among men who have sex with men—National HIV Behavioral Surveillance System, 20 U.S. Metropolitan Statistical Areas, 2008 and 2011. PLoS One 2014;9:e104162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States—An analysis of cost-effectiveness. N Engl J Med 2005;352:586–595 [DOI] [PubMed] [Google Scholar]
- 8.Paltiel AD, Walensky RP, Schackman BR, et al. Expanded HIV screening in the United States: Effect on clinical outcomes, HIV transmission, and costs. Ann Intern Med 2006;145:797–806 [DOI] [PubMed] [Google Scholar]
- 9.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: Implications for HIV prevention programs. J Acquir Immune Defic Syndr 2005;39:446–453 [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report 2012;17 [Google Scholar]
- 11.Centers for Disease C, Prevention. Late HIV testing −34 states, 1996–2005. MMWR Morb Mortal Wkly Rep 2009;58:661–665 [PubMed] [Google Scholar]
- 12.Lodi S, Phillips A, Touloumi G, et al. Time from human immunodeficiency virus seroconversion to reaching CD4+ cell count thresholds <200, <350, and <500 Cells/mm(3): Assessment of need following changes in treatment guidelines. Clin Infect Dis 2011;53:817–825 [DOI] [PubMed] [Google Scholar]
- 13.Hoover DR, Rinaldo C, He Y, Phair J, Fahey J, Graham NM. Long-term survival without clinical AIDS after CD4+ cell counts fall below 200 × 10(6)/l. AIDS 1995;9:145–152 [PubMed] [Google Scholar]
- 14.Lee CA, Phillips AN, Elford J, Janossy G, Griffiths P, Kernoff P. Progression of HIV disease in a haemophilic cohort followed for 11 years and the effect of treatment. BMJ 1991;303:1093–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goedert JJ, Kessler CM, Aledort LM, et al. A prospective study of human immunodeficiency virus type 1 infection and the development of AIDS in subjects with hemophilia. N Engl J Med 1989;321:1141–1148 [DOI] [PubMed] [Google Scholar]
- 16.Van Handel M, Lyons B, Oraka E, Nasrullah M, DiNenno E, Dietz P. Factors Associated with time since last HIV test among persons at high risk for HIV infection, National Survey of Family Growth, 2006–2010. AIDS Patient Care STDS 2015;29:533–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broz D, Wejnert C, Pham HT, et al. HIV infection and risk, prevention, and testing behaviors among injecting drug users—National HIV Behavioral Surveillance System, 20 U.S. cities, 2009. MMWR Surveill Summ 2014;63:1–51 [PubMed] [Google Scholar]
- 18.Ross CE, Tao G, Patton M, Hoover KW. Screening for human immunodeficiency virus and other sexually transmitted diseases among U.S. women with prenatal care. Obstet Gynecol 2015;125:1211–1216 [DOI] [PubMed] [Google Scholar]
- 19.Katz DA, Hogben M, Dooley SW, Jr, Golden MR. Increasing public health partner services for human immunodeficiency virus: Results of a second national survey. Sex Transm Dis 2010;37:469–475 [DOI] [PubMed] [Google Scholar]
- 20.Mackellar DA, Hou SI, Behel S, et al. Exposure to HIV partner counseling and referral services and notification of sexual partners among persons recently diagnosed with HIV. Sex Transm Dis 2009;36:170–177 [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Monitoring Selected National HIV Prevention and Care Objectives using HIV Surveillance Data - United States and 6 Dependent Areas, 2014. Atlanta, 2016 [Google Scholar]
- 22.Sheehan DM, Trepka MJ, Fennie KP, Prado G, Ibanez G, Maddox LM. Racial/ethnic disparities in delayed HIV diagnosis among men who have sex with men, Florida, 2000–2014. AIDS Care 2016:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardner AT, Napier R, Brown B. Risk factors for “late-to-test” HIV diagnosis in Riverside County, California. Medicine (Baltimore) 2016;95:e5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trepka MJ, Fennie KP, Sheehan DM, Lutfi K, Maddox L, Lieb S. Late HIV diagnosis: Differences by rural/urban residence, Florida, 2007–2011. AIDS Patient Care STDS 2014;28:188–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeFevre ML. Force USPST. Screening for Chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;161:902–910 [DOI] [PubMed] [Google Scholar]
- 26.Moyer VA. Force USPST. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;156:880–891, W312 [DOI] [PubMed] [Google Scholar]
- 27.Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Ward JW. Hepatitis C virus testing of persons born during 1945–1965: Recommendations from the Centers for Disease Control and Prevention. Ann Intern Med 2012;157:817–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siu AL. Force USPST. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016;164:279–296 [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Early Release of Selected Estimates Based on Data From the National Health Interview Survey, 2015; Atlanta, GA, 2016 [Google Scholar]
- 30.Blackwell DL, Lucas J. Tables of Summary Health Statistics for Adults: 2014 National Health Interview Survey (Table 1-18A); Washington, DC, 2015 [Google Scholar]
- 31.Herbeck JT, Muller V, Maust BS, et al. Is the virulence of HIV changing? A meta-analysis of trends in prognostic markers of HIV disease progression and transmission. AIDS 2012;26:193–205 [DOI] [PMC free article] [PubMed] [Google Scholar]




