Abstract
The objectives of the study were to characterize long-term neuropsychological outcomes following traumatic brain injury (TBI) sustained during early childhood, and determine whether identified neuropsychological impairments mediated the effect of TBI on long-term adaptive functioning. Participants included 16 children with severe TBI, 42 children with moderate TBI, and 72 children with orthopedic injuries (OI) sustained between ages 3 and 7 years. Children completed neuropsychological tests and caregivers completed a structured interview of child adaptive functioning at 6.9 (±1.10) years post-injury. Profile analysis and multiple mediator modeling were employed. Children with severe TBI demonstrated poorer fluid reasoning and inhibitory control than both children with moderate TBI and OI, as well as slower processing speed than the OI group. Both fluid reasoning and processing speed were significant independent mediators of the effect of severe TBI on adaptive functioning. No neuropsychological measure significantly mediated the effect of moderate TBI on adaptive functioning. Children sustaining early severe TBI demonstrate persisting neuropsychological impairments into adolescence and young adulthood. The impact of severe TBI on children's long-term adaptive functioning is mediated in part by its effects on fluid reasoning and processing speed.
Keywords: : cognitive function, neuropsychology, outcome measures, pediatric brain injury
Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability in childhood. Studies suggest that younger children (e.g., <7 years) are not only at greater risk for injuries requiring emergency treatment or hospitalization than are older children,1 but also that TBI sustained during early childhood results in poorer neuropsychological, psychosocial, and academic outcomes than injuries sustained during later childhood.2–6 Factors thought to account for these poorer outcomes include an increased susceptibility to diffuse brain insult and the detrimental effects of brain injury on subsequent development.4,7,8 Therefore, children sustaining TBI during early childhood are a special population at heightened risk for potentially lifelong impairments in neuropsychological and adaptive outcomes. Developing an understanding of how such children function in the longer term as they enter adolescence and early adulthood will help determine the very long-term public health impact of early childhood TBI, as well as assisting in predicting which individuals will go on to have both neuropsychological and adaptive functioning impairments into adulthood.
The long-term neuropsychological outcomes following early childhood TBI as children enter adolescence and early adulthood are poorly understood. In the short term, children often demonstrate deficits across neuropsychological domains, with more severe injuries and younger age at injury associated with greater impairment.4,6,9–11 Several studies have followed children with early TBI longitudinally to between 1 and 5 years post-injury, documenting variable recovery of function in mild to moderately injured children over the first 1–2 years post-injury, but limited recovery in severely injured children.4,6,9,10 Children sustaining severe injuries during early childhood show lingering long-term deficits in a range of domains, including overall intellectual functioning, verbal and nonverbal skills, learning and memory, attention and executive functions, and motor skills.4,6,9,10,12–21 Few studies, however, have characterized outcomes beyond 5 years following early TBI.
To our knowledge, the neuropsychological outcomes of only one cohort of children who sustained TBI during early childhood have previously been reported longitudinally during adolescence and early adulthood. Recruited and followed by investigators at Murdoch Children's Research Institute in Melbourne, Australia, the cohort included children who sustained TBI between 2 and 7 years of age, as well as a group of uninjured comparison children. At 10 years post-injury, overall intellectual functioning remained significantly below that of comparison children for the severely injured group,22 with scores in the low average to average range. Examination of attention and executive functions at 10 years post-injury revealed poorer selective attention, inhibition, goal setting, and processing speed, as well as poorer parent-rated attention skills,23,24 again with scores in the low average to average range. Results from this cohort suggest persisting, although often subtle, effects of early severe childhood TBI in the longer term as children enter adolescence and young adulthood. Although these findings add important information to a scant literature, they were limited by high attrition and a relatively small sample size (i.e., 40 children with TBI and 16 comparison children), warranting replication in a different and larger sample.
Even less is known regarding long-term adaptive functioning following early childhood TBI and the relation of neuropsychological skills to adaptive functioning. In their cohort, Anderson and colleagues22,25 found poorer parent-reported adaptive functioning across domains among children who sustained severe TBI in early childhood compared with control children at 10 years post-injury. Our recent article26 reports long-term adaptive functioning outcomes for the present cohort of children who sustained TBI or orthopedic injury (OI) between the ages of 3 and 7 years. Children at each level of TBI severity demonstrated significantly greater functional impairment an average of 6.9 years post-injury relative to the OI comparison children, with more severe injury associated with more significant functional impairment.
Neuropsychological impairments, especially in the domain of executive functions, are associated with deficits in adaptive functioning in both typically developing populations27–32 and persons with neurological conditions.33 Executive functions are of particular interest in relation to adaptive functioning following early TBI, because they allow individuals to engage in purposeful, goal-directed, and appropriate behavior34–38 and are particularly vulnerable to long-term impairment.34,39–41 Several studies have reported associations between neuropsychological impairments and a variety of functional outcomes within a few months to 2 years following childhood TBI. Parent ratings of executive functions are associated with lower levels of adaptive, social, and school functioning,11,42,43 lower scores on measures of memory and executive functions with poorer academic achievement,44 substandard communication skills and processing speed with difficulties in social adjustment and participation,45 and weaknesses in cognitive control with problems in adaptive and communication functioning.46 Poorer verbal learning and memory and slower speed of information processing also predict placement in special education.47 To our knowledge, the only prior report examining associations between neuropsychological impairments and long-term functional outcomes following early TBI is that of Nybo and colleagues.48,49 These investigators found that higher scores on measures of memory and executive functioning were associated with better vocational outcomes during early49 and middle48 adulthood among individuals who had sustained TBI during early childhood.
Although association studies are valuable, evidence of mediation of the effect of early childhood TBI on adaptive outcomes by specific neuropsychological skills would provide stronger support for the assertion that neuropsychological skill deficits contribute to impairments in functional outcomes. Recent studies have begun to employ mediation analyses to identify neuropsychological skills underlying functional deficits in children with TBI relative to comparison groups. These analyses have identified mediation effects of verbal working memory on math performance,50 of self-regulation skills on social and behavioral functioning,51 and of theory of mind skills on social outcomes.52 To our knowledge, no prior study has examined neuropsychological skills as mediators of the effect of childhood TBI on long-term adaptive functioning.
The first objective of this study was to characterize long-term neuropsychological outcomes following TBI sustained during early childhood. We employed a profile analysis to examine differences between and among participants who sustained TBI or OI between 3 and 7 years of age who were an average of 6.9 years post-injury. Our second aim was to employ a multiple mediator analysis to determine if neuropsychological impairments mediated the effect of TBI on long-term adaptive functioning as assessed by structured clinical interview. We hypothesized that children who had sustained early severe TBI would demonstrate persisting neuropsychological impairments at long-term follow-up, especially in the domain of executive functions, and that these neuropsychological impairments would mediate the effect of TBI on children's adaptive functioning.
Methods
Participants
The study used a prospective, longitudinal, concurrent cohort research design to examine long-term outcomes in adolescents following TBI or OI sustained during early childhood. The original cohort included children who had sustained TBI or OI between the ages of 3 and 7 years. Children and their families were recruited within 3 months post-injury, and were assessed 6, 12, and 18 months post-injury.53–56 A follow-up study 3.5 years post-injury was also conducted to assess long-term outcomes.18 The present extended follow-up study assessed long-term neuropsychological and adaptive outcomes of these children an average of 6.9 (± 1.10) years post-injury.
Children were recruited from inpatient admissions between 2003 and 2006 at three Ohio children's hospitals and one Ohio general hospital, all with level 1 trauma centers. The OI group was included to provide a comparison group who had similar effects of pre-injury risk exposure and the experience of hospitalization for a traumatic injury. General eligibility criteria included age of injury between 36 and 84 months at the time of injury, and English as the primary language in the home. Children with developmental, intellectual, or neurological disorders prior to injury, or whose injuries resulted from child abuse as documented by medical record or self-report, were excluded. Children in the TBI group had a complicated mild, moderate, or severe TBI through a blunt head trauma that required overnight hospitalization. Complicated mild to moderate TBI (henceforth referred to as “moderate TBI”) was defined as a Glasgow Coma Scale57 (GCS) score of 9–12 with or without abnormal neuroimaging (moderate TBI) or a higher GCS score with abnormal neuroimaging as defined by an intracranial or parenchymal injury or depressed skull fracture (complicated mild TBI). Severe TBI was defined as one resulting in a GCS score ≤8. The GCS score assigned to the child was the lowest one recorded post-resuscitation. Eligibility in the OI group included a documented bone fracture (excluding skull fracture) that required overnight hospitalization, and the absence of any evidence of loss of consciousness or other findings suggesting the presence of a brain injury or head trauma.
Of the 217 participants eligible for the follow-up assessment, we successfully re-established contact with 163 participants (75%), of whom 17 (10%) declined to participate because they were too busy or the child was no longer interested in taking part. Seventy-two children with OI and 58 children with TBI completed the follow-up assessment. Participants included 16 of 23 children with severe TBI (69.6%) and 42 of 64 children with moderate TBI (65.6%). Participants did not differ significantly (p < 0.05) from children who did not complete follow-up with respect to injury type, sex, race, socioeconomic status (SES), or child intelligence quotient (IQ) at baseline. Within the follow-up sample, the TBI and OI groups did not differ significantly in age at injury, age at test, sex, race, median family income, or highest maternal educational attainment (see Table 1).
Table 1.
Participant Characteristics by Injury Group
| OI (n = 72) | Moderate TBI (n = 42) | Severe TBI (n = 16) | Significant differences, p < 0.05 | |
|---|---|---|---|---|
| Gender, n (% male) | ns | |||
| Male | 38 (52.8) | 25 (59.5) | 10 (62.5) | |
| Race, n (%) | ns | |||
| White | 55 (76.4) | 32 (76.2) | 10 (62.5) | |
| Non-white | 17 (23.6) | 10 (23.8) | 6 (37.5) | |
| Age at injury in years, mean (SD) | 5.10 (1.08) | 5.19 (1.21) | 5.04 (0.98) | ns |
| Age at test | 11.91 (1.08) | 12.00 (1.07) | 12.17 (1.52) | ns |
| Median family income, mean (SD)a | $59,917 (21,128) | $61,406 (24,521) | $55,957 (18,191) | ns |
| Highest maternal educational attainment, n (%) | ns | |||
| < high school | 5 (6.9) | 5 (11.9) | 3 (18.8) | |
| ≥ high school | 67 (93.1) | 37 (88.1) | 13 (81.2) | |
| Glasgow Coma Scale | – | 13.40 (2.18) | 4.00 (1.90) | Sev < Mod |
Missing median family income for one participant with severe TBI.
ns, non-significant; OI, orthopedic injury; TBI, traumatic brain injury.
Procedure
The study was approved by the institutional review boards at each participating site. Informed consent was obtained from the families before data collection. Children completed a structured neuropsychological battery for the purpose of assessing verbal skills, verbal learning, and several executive functions, including fluid reasoning, processing speed, inhibitory control, working memory, cognitive flexibility, verbal fluency, and problem-solving skills. Simultaneously, the primary caregiver of each child participated in a structured interview, which assessed adaptive functioning.
Measures
Verbal skills
The Vocabulary subtest of the Wechsler Abbreviated Scale of Intelligence58 (WASI) assessed verbal knowledge based on the child's ability to provide word definitions. The Pragmatic Judgment test from the Comprehensive Assessment of Spoken Language59 (CASL) assessed knowledge and use of pragmatic language rules by asking the child to formulate contextually appropriate verbal responses to pictured social scenarios.
Verbal learning
Verbal learning was assessed using the Verbal Paired Associates (VPA) from the Wechsler Memory Scale-Revised60 as modified for children by Gonzalez, Anderson, Wood, Mitchell, and Harvey.61 In this test, the examiner read eight word pairs (four easy word pairs and four hard word pairs) to the child and then administered three recall trials. On each recall trial, the child attempted to recall the words paired with the ones read by the examiner.
Fluid reasoning
Fluid reasoning was assessed using the WASI Matrix Reasoning subtest (WASI MR),58 which requires the child to select visual stimuli to complete a visual matrix based on their similarity with other stimuli in the matrix.
Attention, executive functions, and processing speed
Three subtests of the Test of Everyday Attention for Children62 (TEA-Ch) were used to measure different aspects of attention and executive functioning. The Walk/Don't Walk subtest assesses inhibitory control by requiring participants to mark footprints on a path in response to a “go” tone and inhibit marking in response to a “stop” tone. In Code Transmission, which assesses working memory, participants listen to a series of single digit numbers while attempting to recall the numbers immediately preceding two consecutive “5s”. The Creature Counting subtest assesses cognitive flexibility by requiring participants to count a series of creatures in either forward or backward order according to up or down cues.
The Letter Fluency condition of the Verbal Fluency test from the Delis-Kaplan Executive Function System63 (D-KEFS) was administered to measure verbal fluency. Over three trials, participants are asked to say as many words beginning with a designated letter as possible in 60 sec.
The Tower of London-Second Edition64 (TOL) was given to assess visual-motor planning and problem-solving skills. In this task, participants are asked to move beads on their tower board to match the layout of the examiner's board by making the fewest number of moves possible. Performance was measured by the Total Correct (TC) score, defined as the number of items the participant completed without making extra moves.
Processing speed was assessed by the Processing Speed Index (PSI) of the Wechsler Intelligence Scale for Children-Fourth Edition65 (WISC-IV). PSI is a composite of the two timed subtests: Coding, which requires copying of symbols that are paired with numbers, and Symbol Search, which involves visual search for geometric symbols to match target symbols.
Adaptive functioning
Adaptive functioning was assessed by the Child and Adolescent Functional Assessment Scale66 (CAFAS). The CAFAS is a structured caregiver interview designed to obtain information on functioning in eight domains: School, Home, Community, Behavior Toward Others, Moods/Emotions, Self-Harmful Behaviors, Substance Abuse, and Thinking. Functioning in each domain is rated on an ordinal scale ranging from 0 (unimpaired) to 30 (severe impairment) in 10 point increments. A total score is created by summing domain scores (range: 0–240), with scores ≤50 considered to be “unimpaired” and those >50 considered to be “impaired.” The CAFAS predicts service utilization better than either psychiatric diagnoses or standardized checklists,67,68 is used widely to assess changes in clinical outcomes for youth receiving mental health services,69 and has established validity and excellent inter-rater reliability ranging from 0.74 to 0.99.70 The CAFAS was administered by two researchers with advanced degrees in psychology or counseling who were certified as CAFAS trainers. Additional raters were trained to achieve inter-rater reliability >80%, as recommended by the creator of the CAFAS.71 Raters were unaware of the severity or nature of the child's injury. Ten percent of interviews were taped and jointly rated yielding an overall inter-rater reliability of 98.7%. The CAFAS total score was used as the adaptive functioning outcome measure.
Statistical analysis
Profile analysis
Long-term differences in neuropsychological outcomes between and among participants who sustained TBI or OI were analyzed using a profile analysis. Profile analysis is a statistical technique for examining differences among groups on a set of outcome variables.72,73 Three primary questions were addressed:
1. Level—Do some groups have higher overall mean scores when averaged across neuropsychological measures than other groups (main effect of group)?
2. Flatness—Are scores on some neuropsychological measures higher or lower than scores on other measures, averaged across groups (main effect of measure)?
3. Shape—Does the pattern of scores across neuropsychological measures differ between groups (interaction of group × measure)?
Scores on each neuropsychological measure were converted to z scores based on the mean and SD of the OI group so that all scores were of the same metric to facilitate comparison across scores. The profile analysis was conducted as a mixed repeated measures analysis of covariance (ANCOVA), with scores on each of the neuropsychological measures treated as within-subjects measures and injury group (severe TBI, moderate TBI, OI) as a between-subjects variable. Covariates included time since injury and SES (defined by averaging sample z scores for maternal education and median income).53 Follow-up post-hoc tests compared 1) groups on each neuropsychological measure to determine which measures differentiated the groups; and 2) the mean on each neuropsychological measure to the average of the remaining measures within each group to determine areas of strengths or weaknesses within each group. Post-hoc comparisons were tested at an α of p < 0.05.
Mediation analysis
First, differences in long-term adaptive functioning between groups, previously reported by Wade and coworkers,26 were confirmed using ANCOVA, controlling for time since injury and SES. These analyses were repeated here because we combined the complicated mild and moderate TBI groups, whereas our previous report examined those groups separately. Next, the neuropsychological skills that were significantly impaired in children with TBI were examined as mediators of the effect of TBI on adaptive functioning. We employed a multiple mediator model using ordinary least squares path analysis to determine the relative contributions to adaptive functioning accounted for by group and each neuropsychological measure entered in the model.74 This approach allows for the simultaneous inclusion of multiple mediators while testing the individual effects of each potential mediator after controlling for the others. In addition, predictors are permitted to be categorical and covariates can be included. Group was first entered as an independent variable, coded as two dichotomous dummy variables (severe TBI vs. OI; moderate TBI vs. OI). Neuropsychological test scores that showed significant group differences based on the profile analysis were then entered simultaneously as potential mediators of the group effects on adaptive functioning. Time since injury and SES were included as covariates. Unstandardized path coefficients (betas) were provided for each individual path of the model, scaled according to the measurement of variables in each path. The procedure provides tests of direct, indirect, and total effects (i.e., combined direct and indirect effects). To compute one tailed tests of mediation effects, we used 90% bias-corrected confidence intervals (CIs) based on 10,000 bootstrap samples. Evidence for significant mediation is provided when the effect is in the predicted direction and the CI for the indirect effect does not contain zero.
Results
Profile analysis
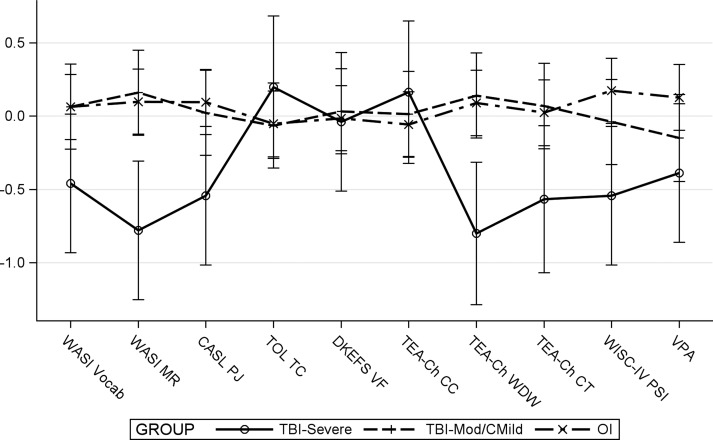
The profile analysis revealed a significant group by measure interaction (F [18, 1127] = 1.85, p = 0.017), indicating differences in the pattern of scores across neuropsychological measures between groups. When examining individual measures, the simple main effect of group was significant for WASI MR (F [2, 1127] = 6.18, p = 0.002), TEA-Ch Walk Don't Walk (WDW) (F [2, 1127] = 5.98, p = 0.003), and WISC-IV PSI (F [2, 1127] = 3.73, p = 0.024. As shown in Figure 1, children with severe TBI demonstrated poorer fluid reasoning (WASI MR) and inhibitory control (TEA-Ch WDW) relative to both children with moderate TBI (t [1127] = −3.32, p < 0.001), and OI (t [1127] = −3.30, p = 0.001), and slower processing speed (WISC-IV PSI) relative to the OI group only (t [1127] = −2.69, p = 0.007). Poorer neuropsychological performance was also associated with longer time since injury (F [1, 1127] = 10.53, p = 0.001), and lower SES (F [1, 1127] = 12.17, p < 0.001).
FIG. 1.
Results of profile analysis. Error bars are 95% confidence intervals. WASI Vocab, Wechsler Abbreviated Scale of Intelligence, Vocabulary subtest; WASI MR, Wechsler Abbreviated Scale of Intelligence, Matrix Reasoning subtest; CASL PJ, Comprehensive Assessment of Spoken Language, Pragmatic Judgment test; TOL TC, Tower of London, Second Edition, total correct score; DKEFS VF, Delis–Kaplan Executive Function System, Verbal Fluency test; TEA-Ch CC, Test of Everyday Attention for Children, Creature Counting subtest; TEA-Ch WDW, Test of Everyday Attention for Children, Walk/Don't Walk subtest; TEA-Ch CT, Test of Everyday Attention for Children, Code Transmission subtest; WISC-IV PSI, Wechsler Intelligence Scale for Children, Fourth Edition, Processing Speed Index; VPA, Verbal Paired Associates; TBI, traumatic brain injury; OI, orthopedic injury.
The simple main effect of measure was significant for the severe TBI group (F [9, 1127] = 2.68, p = 0.004), but not for the moderate TBI (F [9, 1127] = 0.48, p = 0.889), or OI (F [9, 1127] = 0.58, p = 0.817) groups, suggesting that the severe TBI group displayed significant strengths or weaknesses on certain neuropsychological measures relative to their mean performance across measures. Specifically, children with severe TBI demonstrated significant relative weaknesses in fluid reasoning (WASI MR) (t [1127] = −1.97, p = 0.049), and inhibitory control (TEA-Ch WDW) (t [1127] = −2.02, p = 0.044), and relative strengths in visual-motor planning and problem solving (TOL TC) (t [1127] = 2.72, p = 0.007), and cognitive flexibility (TEA-Ch CC) (t [1127] = 2.55, p = 0.011).
Group differences in adaptive functioning
Consistent with our previously reported findings in this cohort,26 injury groups differed significantly on the CAFAS total score (F [2, 125] = 13.18, p < 0.001). Both children with severe TBI (t [125] = −4.36, p < 0.001), and those with moderate TBI (t [125] = −3.75, p < 0.001), had poorer adaptive functioning than children with OI, whereas the severe and moderate TBI groups did not differ significantly (t [125] = −1.67, p = 0.097). Poorer adaptive functioning was also associated with lower SES (F [1, 125] = 17.01, p < 0.001), but not with time since injury (F [1, 125] = 2.01, p = 0.159).
Mediation analysis
Based on the results of the profile analysis, fluid reasoning, inhibitory control, and processing speed were examined as mediators of the effect of TBI on adaptive functioning. Both fluid reasoning and processing speed were significantly negatively correlated with the CAFAS total score (Table 2), indicating greater neuropsychological impairment associated with poorer adaptive function. Inhibitory control was not significantly associated with the CAFAS.
Table 2.
Pooled Within-Group Correlations Among Measures of Neuropsychological and Adaptive Functioning
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuropsychological | |||||||||||
| 1. WASI Vocab | – | ||||||||||
| 2. WASI MR | 0.40** | – | |||||||||
| 3. CASL PJ | 0.65** | 0.39** | – | ||||||||
| 4. TOL TC | 0.18* | 0.13 | 0.19* | – | |||||||
| 5. D-KEFS VF | 0.47** | 0.31** | 0.46** | 0.21* | – | ||||||
| 6. TEA-Ch CC | 0.30** | 0.21* | 0.31** | 0.13 | 0.30** | – | |||||
| 7. TEA-Ch WDW | 0.20* | 0.23* | 0.24* | 0.07 | 0.33** | 0.31** | – | ||||
| 8. TEA-Ch CT | 0.15 | 0.20* | 0.07 | 0.14 | 0.26* | 0.10* | 0.32** | – | |||
| 9. WISC-IV PSI | 0.29** | 0.21* | 0.31** | 0.24* | 0.33** | 0.19* | 0.32** | 0.24* | – | ||
| 10. VPA | 0.30** | 0.12 | 0.24* | 0.08 | 0.27* | 0.13 | 0.18* | 0.12 | 0.09 | – | |
| Adaptive | |||||||||||
| 11. CAFAS | −0.34** | −0.28* | −0.29** | −0.20 | −0.13 | −0.11 | −0.04 | −0.12 | −0.23* | −0.06 | – |
p < 0.05; **p < 0.001
CAFAS, Child and Adolescent Functional Assessment Scale; CASL PJ, Comprehensive Assessment of Spoken Language - Pragmatic Judgment; D-KEFS VF, Delis–Kaplan Executive Function System - Verbal Fluency; TEA-Ch CC, Test of Everyday Attention for Children - Creature Counting; TEA-Ch CT, Test of Everyday Attention for Children – Code Transmission; TEA-Ch WDW, Test of Everyday Attention for Children – Walk/Don't Walk; TOL TC, Tower of London – Total Correct Score; VPA, Verbal Paired Associates; WASI MR, Wechsler Abbreviated Scale of Intelligence – Matrix Reasoning; WASI Vocab, Wechsler Abbreviated Scale of Intelligence - Vocabulary; WISC-IV PSI, Wechsler Intelligence Scale for Children Fourth Edition – Processing Speed Index.
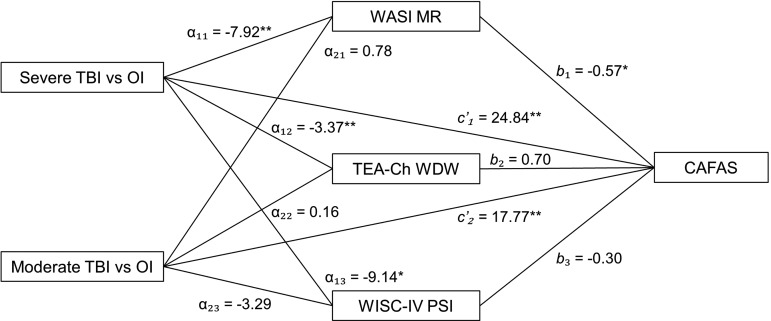
The results from the multiple mediator model are presented in Figure 2. Consistent with results of the profile analysis, the dummy variable representing the severe TBI versus OI group contrast was significant, indicating that the severe TBI group performed significantly worse than the OI group on each of the three neuropsychological mediators, whereas the dummy variable representing the moderate TBI versus OI group contrast was not significant.
FIG. 2.
Direct effect pathways of group and mediator variables. α, direct effect of group on potential mediators; b, direct effect of mediator variables on adaptive functioning; c’, direct effect of group on adaptive functioning. WASI MR, Wechsler Abbreviated Scale of Intelligence, Matrix Reasoning subtest; TEA-Ch WDW, Test of Everyday Attention for Children, Walk/Don't Walk subtest; WISC-IV PSI, Wechsler Intelligence Scale for Children, Fourth Edition, Processing Speed Index; CAFAS, Child and Adolescent Functional Assessment Scale; TBI, traumatic brain injury; OI, orthopedic injury; *p < 0.05; **p < 0.01.
Both severe TBI and moderate TBI (relative to OI) had significant direct effects on the CAFAS total score, which is again consistent with the group comparisons presented. Fluid reasoning also had a significant direct effect on adaptive functioning, whereas inhibitory control and processing speed did not.
Indirect effects (fluid reasoning, indirect effect = 4.47, lower CI = 1.00, upper CI = 11.63; and processing speed, indirect effect = 2.70, lower CI = 0.07, upper CI = 8.05), were significant independent mediators of the effect of severe TBI on adaptive functioning, whereas inhibitory control was not (indirect effect = −2.38, lower CI = −6.55, upper CI = 1.24). In contrast, no neuropsychological measure significantly mediated the effect of moderate TBI on adaptive functioning (fluid reasoning indirect effect = −0.44, lower CI = −2.64, upper CI = 0.78; inhibitory control indirect effect = 0.11, lower CI = −0.56, upper CI = 1.73; processing speed indirect effect = 0.97, lower CI = −0.10, upper CI = 4.88).
Discussion
The present study examined long-term neuropsychological outcomes following TBI sustained during early childhood and examined neuropsychological skills as mediators of the effect of TBI on long-term adaptive functioning. As expected, children with early severe TBI demonstrated persisting neuropsychological impairments in fluid reasoning, inhibitory control, and processing speed at long-term follow-up, and impairments in fluid reasoning and processing speed mediated the effect of severe TBI on children's long-term adaptive functioning.
The presence of persisting neuropsychological impairments in children who sustained early severe TBI but intact neuropsychological functioning in the moderately injured group is consistent with results from the only previously reported cohort of children sustaining early TBI that was followed into adolescence and young adulthood.22,24,34 Both investigations found that children with severe TBI had weaknesses relative to comparison groups in the domains of fluid reasoning, inhibitory control, and processing speed, with mean scores for the severe TBI group falling in the low-average to average range. These findings support the notion of particular vulnerability of the executive functions to long-term impairment.15,23 The executive functions are supported by widely distributed neural networks34,75–79 that may be particularly vulnerable to the mechanisms of TBI, including diffuse axonal injury, which is especially prevalent among children sustaining brain injuries during infancy and early childhood.80
Within the severe TBI group, children demonstrated significant relative weaknesses in fluid reasoning and inhibitory control and relative strengths in problem solving and cognitive flexibility. These findings highlight the variability of outcomes among children with severe TBI, including within specific neuropsychological domains, especially the executive functions. Whereas variability within executive functions is a common finding post-injury,23 the executive skills that are most and least affected have been inconsistent from one study to another. Some authors have suggested that skills within a rapid stage of development at the time of injury may be particularly vulnerable.15,81,82 Others have suggested that executive skills that develop prior to injury are more robust, whereas those higher-level executive functions that develop later and build upon the earlier developed skills may be more vulnerable to the effects of injury.34,83,84 The present findings do not appear to support either theory consistently. For example, both inhibitory control and cognitive flexibility are “core” executive functions with rapid developmental trajectories during the preschool years.85–89 According to the theory of vulnerability of rapidly developing skills at the time of injury, both inhibitory control and cognitive flexibility should be impaired; however, our results revealed inhibitory control to be a weakness, but cognitive flexibility to be a strength in children with severe TBI. In contrast, both fluid reasoning and planning and problem-solving skills develop later during childhood, and build upon lower-level “core” executive functions.90,91 Therefore, the theory that later developing EF skills are more vulnerable to the effects of TBI was supported by the relative weakness in fluid reasoning, but is in conflict with the relative strength in visual-motor planning and problem solving. Measurement issues inherent in comparing performance across measures normed on different samples may contribute to these discrepancies. Other sources of variability in outcomes include the specific brain regions affected, family environmental influences on recovery,53,92 and genetic differences impacting outcomes,93–95 as well as the complex interactions among these factors. Future research is needed to investigate sources of heterogeneity in neuropsychological outcomes, better understand why certain skills are more vulnerable to injury than others, and predict which children will go on to have which specific impairments.
Both fluid reasoning and processing speed were significant independent mediators of the effect of severe TBI on adaptive functioning. None of the examined variables significantly mediated the effect of moderate TBI on adaptive functioning. These findings suggest that impairments in fluid reasoning and processing speed likely play a significant role in the emergence of poor long-term adaptive functioning following severe TBI in early childhood. Interestingly, our measures of fluid reasoning (WASI MR) and processing speed (WISC-IV PSI) were both drawn from intelligence tests. Although intelligence and executive functions are distinct constructs, aspects of intelligence, such as fluid intelligence, are highly correlated with executive functioning, and impairments in both constructs result from injury to the same brain regions.96 As a result, some authors fail to distinguish higher-level executive functions from fluid intelligence.96 In the present study, the lack of mediation in the moderate TBI group likely reflects the more limited effects of such injuries on neuropsychological skills and adaptive functioning, producing relatively weak associations between these domains. In support of this possibility, studies of typically developing children have also found weak associations between cognitive measures and adaptive functioning.97–99 In contrast, in populations with developmental disabilities and/or neurological conditions, measures of intelligence appear to be the most consistent predictors of adaptive functioning,100–105 although associations of adaptive functioning with tests of executive functions have also been reported in diverse populations.106–110 The present results suggest that impairments in certain aspects of executive functioning might be driving associations between intelligence and adaptive functioning, warranting examination of executive functions in other populations with adaptive functioning impairments.
Emerging evidence suggests that impairments in adaptive functioning during childhood and adolescence predict delayed achievement or failure to achieve milestones in adulthood, such as independent living, gainful employment, and engagement in meaningful relationships.111,112 Identifying children and adolescents with reduced adaptive functioning, and the underlying neuropsychological impairments contributing to these low levels of functioning, may allow for earlier identification of adults in most need of services. The nature of associated cognitive weaknesses also suggests possible targets of intervention, remediation, or accommodation. According to our results, children who sustain severe TBI during early childhood who have persisting impairments in fluid reasoning and processing speed are likely at high risk for poorer outcomes in adulthood. Impairments in fluid reasoning may reduce an individual's ability to problem solve and think flexibly in response to daily challenges. Similarly, reductions in processing speed may impair daily functioning across tasks such as understanding verbal instructions and meeting the demands of fast-paced employment settings. Although questions remain with regard to the generalizability of cognitive remediation in improving daily functioning,113 reducing demands upon fluid reasoning and processing speed through environmental accommodation might be effective targets for bolstering the adaptive functioning of individuals following remote severe TBI.
Several strengths of the present study highlight its unique contribution to the research literature. The prospective, longitudinal, concurrent cohort study design with 68% retention provides the largest cohort of children followed for >5 years following TBI sustained during early childhood. Additionally, the use of a structured interview to assess adaptive functioning reduced possible bias in caregiver report of functioning, and the application of the conservative and robust statistical techniques of profile analysis and multiple mediator modeling add to our confidence in the findings. However, several limitations of the study also require acknowledgement. The small size of the severe TBI group limited statistical power in subgroup analyses. Therefore, replication, especially of the mediation findings in a larger sample of severely injured children, is warranted. Our assessment of long-term neuropsychological functioning, although reasonably comprehensive, did not encompass all aspects of executive function or other cognitive domains, and did not probe learning difficulties or different types of problems in behavior and adaptive functioning. Finally, the analysis of concurrent neuropsychological and adaptive functioning precluded an examination of the temporal relations among these variables, limiting inferences of causality.
The present study provides evidence that early severe TBI results in persisting long-term impairments in fluid reasoning, inhibitory control, and processing speed into adolescence and young adulthood. Moreover, impairments in fluid reasoning and processing speed mediate the effect of early severe TBI on children's long-term adaptive functioning. Adolescents with impairments in these areas are likely at risk for poor adaptive functioning into adulthood, and the early identification of these neuropsychological skill deficits might help predict individuals at greatest risk to enable provision of earlier, targeted intervention. Future research should continue to examine the long-term effects of TBI sustained during early childhood and work toward improved prognostic tools and evidence-based interventions for addressing long-term functional impairments.
Acknowledgments
This publication was supported by an Institutional Clinical and Translational Science Award, NIH/NCRR Grant Number 1UL1RR026314. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH). Additional support provided to Dr. Wade included grant R01 HD42729 from National Institute of Child Health and Human Development (NICHD) and Trauma Research grants from the State of Ohio Emergency Medical Services.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Langlois J.A., Rutland-Brown W., and Wald M.M. (2006). The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 21, 375–378 [DOI] [PubMed] [Google Scholar]
- 2.Barnes M.A., Dennis M., and Wilkinson M. (1999). Reading after closed head injury in childhood: Effects on accuracy, fluency, and comprehension. Dev. Neuropsychol. 15, 1–24 [Google Scholar]
- 3.Ewing–Cobbs L., and Barnes M. (2002). Linguistic outcomes following traumatic brain injury in children. Semin. Pediatr. Neurol. 9, 209–217 [DOI] [PubMed] [Google Scholar]
- 4.Ewing–Cobbs L., Fletcher J.M., Levin H.S., Francis D.J., Davidson K., and Miner M.E. (1997). Longitudinal neuropsychological outcome in infants and preschoolers with traumatic brain injury. J. Int. Neuropsychol. Soc. 3, 581–591 [PubMed] [Google Scholar]
- 5.Verger K., Junque C., Jurado M.A., Tresserras P., Bartumeus F., Nogues P., and Poch J.M. (2000). Age effects on long-term neuropsychological outcome in paediatric traumatic brain injury. Brain Inj. 14, 495–503 [DOI] [PubMed] [Google Scholar]
- 6.Anderson V., Catroppa C., Morse S., Haritou F., and Rosenfeld J. (2005). Functional plasticity or vulnerability after early brain injury? Pediatrics 116, 1374–1382 [DOI] [PubMed] [Google Scholar]
- 7.Ewing–Cobbs L., Barnes M., Fletcher J.M., Levin H.S., Swank P.R., and Song J. (2004). Modeling of longitudinal academic achievement scores after pediatric traumatic brain injury. Dev. Neuropsychol. 25, 107–133 [DOI] [PubMed] [Google Scholar]
- 8.Taylor H.G., and Alden J. (1997). Age-related differences in outcomes following childhood brain insults: an introduction and overview. J. Int. Neuropsychol. Soc. 3, 555–567 [PubMed] [Google Scholar]
- 9.Anderson V., Catroppa C., Morse S., Haritou F., and Rosenfeld J. (2000). Recovery of intellectual ability following traumatic brain injury in childhood: impact of injury severity and age at injury. Pediatr. Neurosurg. 32, 282–290 [DOI] [PubMed] [Google Scholar]
- 10.Anderson V., Morse S.A., Catroppa C., Haritou F., and Rosenfeld J.V. (2004). Thirty Month Outcome from Early Childhood Head Injury: a prospective analysis of neurobehavioural recovery. Brain 127, 2608–2620 [DOI] [PubMed] [Google Scholar]
- 11.Ganesalingam K., Yeates K.O., Taylor H.G., Walz N.C., Stancin T., and Wade S. (2011). Executive functions and social competence in young children 6 months following traumatic brain injury. Neuropsychology 25, 466–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ewing–Cobbs L., Prasad M.R., Kramer L., Cox C.S., Jr., Baumgartner J., Fletcher S., Mendez D., Barnes M., Zhang X., and Swank P. (2006). Late intellectual and academic outcomes following traumatic brain injury sustained during early childhood. J. Neurosurg. 105, 287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catroppa C., Anderson V.A., Morse S.A., Haritou F., and Rosenfeld J.V. (2007). Children's attentional skills 5 years post-TBI. J. Pediatr. Psychol. 32, 354–369 [DOI] [PubMed] [Google Scholar]
- 14.Anderson V., Catroppa C., Morse S., Haritou F., and Rosenfeld J.V. (2009). Intellectual outcome from preschool traumatic brain injury: a 5-year prospective, longitudinal study. Pediatrics 124, e1064–1071 [DOI] [PubMed] [Google Scholar]
- 15.Ewing–Cobbs L., Prasad M.R., Landry S.H., Kramer L., and DeLeon R. (2004). Executive functions following traumatic brain injury in young children: a preliminary analysis. Dev. Neuropsychol. 26, 487–512 [DOI] [PubMed] [Google Scholar]
- 16.Anderson V., and Catroppa C. (2007). Memory outcome at 5 years post-childhood traumatic brain injury. Brain Inj. 21, 1399–1409 [DOI] [PubMed] [Google Scholar]
- 17.Crowe L.M., Anderson V., Barton S., Babl F.E., and Catroppa C. (2014). Verbal ability and language outcome following traumatic brain injury in early childhood. J. Head Trauma Rehabil. 29, 217–223 [DOI] [PubMed] [Google Scholar]
- 18.Kurowski B.G., Taylor H.G., Yeates K.O., Walz N.C., Stancin T., and Wade S.L. (2011). Caregiver ratings of long-term executive dysfunction and attention problems after early childhood traumatic brain injury: family functioning is important. PM R 3, 836–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nadebaum C., Anderson V., and Catroppa C. (2007). Executive function outcomes following traumatic brain injury in young children: a five year follow-up. Dev. Neuropsychol. 32, 703–728 [DOI] [PubMed] [Google Scholar]
- 20.Crowe L.M., Catroppa C., Babl F.E., and Anderson V. (2013). Executive function outcomes of children with traumatic brain injury sustained before three years. Child Neuropsychol. 19, 113–126 [DOI] [PubMed] [Google Scholar]
- 21.Crowe L.M., Catroppa C., Babl F.E., and Anderson V. (2012). Intellectual, behavioral, and social outcomes of accidental traumatic brain injury in early childhood. Pediatrics 129, e262–268 [DOI] [PubMed] [Google Scholar]
- 22.Anderson V., Godfrey C., Rosenfeld J.V., and Catroppa C. (2012). Predictors of cognitive function and recovery 10 years after traumatic brain injury in young children. Pediatrics 129, e254–261 [DOI] [PubMed] [Google Scholar]
- 23.Beauchamp M., Catroppa C., Godfrey C., Morse S., Rosenfeld J.V., and Anderson V. (2011). Selective changes in executive functioning ten years after severe childhood traumatic brain injury. Dev. Neuropsychol. 36, 578–595 [DOI] [PubMed] [Google Scholar]
- 24.Catroppa C., Anderson V., Godfrey C., and Rosenfeld J.V. (2011). Attentional skills 10 years post-paediatric traumatic brain injury (TBI). Brain Inj. 25, 858–869 [DOI] [PubMed] [Google Scholar]
- 25.Catroppa C., Godfrey C., Rosenfeld J.V., Hearps S.S.J.C., and Anderson V.A. (2012). Functional recovery ten years after pediatric trauamtic brain injury: outcomes and predictors. J. Neurotrauma 29, 2539–2547 [DOI] [PubMed] [Google Scholar]
- 26.Wade S.L., Zhang N., Yeates K.O., Stancin T., and Taylor H.G. (2016). Social environmental moderators of long-term functional outcomes of early childhood brain injury. J.A.M.A. Pediatr. 170, 343–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willoughby M.T., Kupersmidt J.B., and Voegler–Lee M.E. (2012). Is preschool executive function causally related to academic achievement? Child Neuropsychol. 18, 79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waber D.P., Gerber E.B., Turcios V.Y., Wagner E.R., and Forbes P.W. (2006). Executive functions and performance on high-stakes testing in children from urban schools. Dev. Neuropsychol. 29, 459–477 [DOI] [PubMed] [Google Scholar]
- 29.Molfese V.J., Molfese P.J., Molfese D.L., Rudasill K.M., Armstrong N., and Starkey G. (2010). Executive function skills of 6 to 8 year olds: brain and behavioral evidence and implications for school achievement. Contemp. Educ. Psychol. 35, 116–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell L.K., Scaduto M., van Slyke D., Niarhos F., Whitlock J.A., and Compas B.E. (2009). Executive function, coping, and behavior in survivors of childhood acute lymphocytic leukemia. J. Pediatr. Psychol. 34, 317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh K.S., Paltin I., Gioia G.A., Isquith P., Kadan–Lottick N.S., Neglia J.P., and Brouwers P. (2015). Everyday executive function in standard-risk acute lymphoblastic leukemia survivors. Child Neuropsychol. 21, 78–89 [DOI] [PubMed] [Google Scholar]
- 32.Jacobson L.A., Williford A.P., and Pianta R.C. (2011). The role of executive function in children's competent adjustment to middle school. Child Neuropsychol. 17, 255–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell L.K., Scaduto M., Van Slyke D., Niarhos F., Whitlock J.A., and Compas B.E. (2008). Executive function, coping, and behavior in survivors of childhood acute lymphocytic leukemia. J. Pediatr. Psychol. 34, 317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beauchamp M., Catroppa C., Godfrey C., Morse S., Rosenfeld J.V., and Anderson V. (2011). Selective changes in executive functioning ten years after severe childhood traumatic brain injury. Dev. Neuropsychol. 36, 578–595 [DOI] [PubMed] [Google Scholar]
- 35.Stuss D.T., and Benson D.F. (1986). The Frontal Lobes. Raven Press: New York [Google Scholar]
- 36.Shallice T. (1982). Specific impairments of planning. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 298, 199–209 [DOI] [PubMed] [Google Scholar]
- 37.Luria A.R. (1966). Higher Cortical Functions in Man. Basic Books: New York [Google Scholar]
- 38.Spreen O., Risser A.H., and Edgell D. (1985). Developmental Neuropsychology. Oxford University Press: New York [Google Scholar]
- 39.Levin H., Hanten G., Max J., Li X., Swank P., Ewing–Cobbs L., Dennis M., Menefee D.S., and Schachar R. (2007). Symptoms of attention-deficit/hyperactivity disorder following traumatic brain injury in children. J. Dev. Behav. Pediatr. 28, 108–118 [DOI] [PubMed] [Google Scholar]
- 40.Muscara F., Catroppa C., and Anderson V. (2008). The impact of injury severity on executive function 7–10 years following pediatric traumatic brain injury. Dev. Neuropsychol. 33, 623–636 [DOI] [PubMed] [Google Scholar]
- 41.Beauchamp M.H., and Anderson V. (2013). Cognitive and psychopathological sequelae of pediatric traumatic brain injury. Handb. Clin. Neurol. 112, 913–920 [DOI] [PubMed] [Google Scholar]
- 42.Kurowski B.G., Wade S.L., Kirkwood M.W., Brown T.M., Stancin T., Cassedy A., and Taylor H.G. (2013). Association of parent ratings of executive function with global- and setting-specific behavioral impairment after adolescent traumatic brain injury. Arch. Phys. Med. Rehabil. 94, 543–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnett A.B., Peterson R.L., Kirkwood M.W., Taylor H.G., Stancin T., Brown T.M., and Wade S.L. (2013). Behavioral and cognitive predictors of educational outcomes in pediatric traumatic brain injury. J. Int. Neuropsychol. Soc. 19, 881–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fulton J.B., Yeates K.O., Taylor H.G., Walz N.C., and Wade S.L. (2012). Cognitive predictors of academic achievement in young children 1 year after traumatic brain injury. Neuropsychology 26, 314–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryan N.P., Anderson V., Godfrey C., Eren S., Rosema S., Taylor K., and Catroppa C. (2013). Social communication mediates the relationship between emotion perception and externalizing behaviors in young adult survivors of pediatric traumatic brain injury (TBI). Int. J. Dev. Neurosci. 31, 811–819 [DOI] [PubMed] [Google Scholar]
- 46.Levin H.S., Hanten G., and Li X. (2009). The relation of cognitive control to social outcome after paediatric TBI: Implications for intervention. Dev. Neurorehabil. 12, 320–329 [DOI] [PubMed] [Google Scholar]
- 47.Kinsella G.J., Prior M., Sawyer M., Ong B., Murtagh D., Eisenmajer R., Bryan D., Anderson V., and Klug G. (1997). Predictors and indicators of academic outcome in children 2 years following traumatic brain injury. J. Int. Neuropsychol. Soc. 3, 608–616 [PubMed] [Google Scholar]
- 48.Nybo T., Sainio M., and Müller K. (2004). Stability of vocational outcome in adulthood after moderate to severe preschool brain injury. J. Int. Neuropsychol. Soc. 10, 719–723 [DOI] [PubMed] [Google Scholar]
- 49.Nybo T., and Koskiniemi M. (1999). Cognitive indicators of vocational outcome after severe traumatic brain injury (TBI) in childhood. Brain Inj. 13, 759–766 [DOI] [PubMed] [Google Scholar]
- 50.Raghubar K.P., Barnes M.A., Prasad M., Johnson C.P., and Ewing–Cobbs L. (2013). Mathematical outcomes and working memory in children with TBI and orthopedic injury. J. Int. Neuropsychol. Soc. 19, 254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ganesalingam K., Sanson A., Anderson V., and Yeates K.O. (2007). Self-regulation as a mediator of the effects of childhood traumatic brain injury on social and behavioral functioning. J. Int. Neuropsychol. Soc. 13, 298–311 [DOI] [PubMed] [Google Scholar]
- 52.Robinson K.E., Fountain–Zaragoza S., Dennis M., Taylor H.G., Bigler E.D., Rubin K., Vannatta K., Gerhardt C.A., Stancin T., and Yeates K.O. (2014). Executive functions and theory of mind as predictors of social adjustment in childhood traumatic brain injury. J. Neurotrauma 31, 1835–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeates K.O., Taylor H.G., Walz N.C., Stancin T., and Wade S.L. (2010). The family environment as a moderator of psychosocial outcomes following traumatic brain injury in young children. Neuropsychology 24, 345–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chapman L.A., Wade S.L., Walz N.C., Taylor H.G., Stancin T., and Yeates K.O. (2010). Clinically significant behavior problems during the initial 18 months following early childhood traumatic brain injury. Rehabil. Psychol. 55, 48–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor H.G., Swartwout M.D., Yeates K.O., Walz N.C., Stancin T., and Wade S.L. (2008). Traumatic brain injury in young children: postacute effects on cognitive and school readiness skills. J. Int. Neuropsychol. Soc. 14, 734–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerrard–Morris A., Taylor H.G., Yeates K.O., Walz N.C., Stancin T., Minich N., and Wade S.L. (2010). Cognitive development after traumatic brain injury in young children. J. Int. Neuropsychol. Soc. 16, 157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teasdale G., and Jennett B. (1974). Assessment of coma and impaired consciousness. A practical scale. Lancet 2, 81–84 [DOI] [PubMed] [Google Scholar]
- 58.Wechsler D. (1999). Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation: Harcourt Brace & Company: New York [Google Scholar]
- 59.Carrow–Woolfolk E. (1999). Comprehensive Assessment of Spoken Language. American Guidance Services: San Antonio, TX [Google Scholar]
- 60.Wechsler D. (1987). Wechsler Memory Scale—Revised Manual. Psychological Corporation: San Antonio, TX [Google Scholar]
- 61.Gonzalez L.M., Anderson V.A., Wood S.J., Mitchell L.A., and Harvey A.S. (2007). The localization and lateralization of memory deficits in children with temporal lobe epilepsy. Epilepsia 48, 124–132 [DOI] [PubMed] [Google Scholar]
- 62.Manly T., Robertson I.H., Anderson V., and Nimmo–Smith I. (1999). TEA-Ch: The Test of Everyday Attention for Children Manual. Thames Valley Test Company Limited: Bury St. Edmunds [Google Scholar]
- 63.Delis D.C., Kaplan E. and Kramer J.H. (2001). Delis–Kaplan Executive Function System (D-KEFS). The Psychological Corporation: San Antonio, TX [Google Scholar]
- 64.Culbertson W.C. and Zillmer E.A. (1998). The tower of London (DX): A standardized approach to assessing executive functioning in children. Arch. Clin. Neuropsychol. 13, 285–301 [PubMed] [Google Scholar]
- 65.Wechsler D. (2004). The Wechsler Intelligence Scale for Children, 4th ed. Pearson Assessment: London [Google Scholar]
- 66.Hodges K. (1994). The Child and Adolescent Functional Assessment Scale Self-Training Manual. Eastern Michigan University, Department of Psychology: Ypsilanti, MI [Google Scholar]
- 67.Hodges K., and Wong M.M. (1997). Use of the Child and Adolescent Functional Assessment Scale to predict service utilization and cost. J. Ment. Health Adm. 24, 278–290 [DOI] [PubMed] [Google Scholar]
- 68.Achenbach T.M., and Rescorla L.A. (2001). Manual for ASEBA School-Age Forms & Profiles. University of Vermont, Research Center for Children, Youth, & Families: Burlington [Google Scholar]
- 69.Hodges K., Wong M.M., and Latessa M. (1998). Use of the Child and Adolescent Functional Assessment Scale (CAFAS) as an outcome measure in clinical settings. J. Behav. Health Serv. Res. 25, 325–336 [DOI] [PubMed] [Google Scholar]
- 70.Hodges K., and Wong M.M. (1996). Psychometric characteristics of a multidimensional measure to assess impairment: The Child and Adolescent Functional Assessment Scale. J. Child Fam. Stud. 5, 445–467 [Google Scholar]
- 71.Hodges K. (2005). CAFAS: Manual for Training Coordinators, Clinical Administrators, and Data Managers. Ann Arbor, MI: Kay Hodgess [Google Scholar]
- 72.Gioia G.A., Isquith P.K., Kenworthy L., and Barton R.M. (2002). Profiles of everyday executive function in acquired and developmental disorders. Child Neuropsychol. 8, 121–137 [DOI] [PubMed] [Google Scholar]
- 73.Harris R.J. (1985). A Primer of Multivariate Statistics, 2nd ed. Academic Press: Orlando [Google Scholar]
- 74.Preacher K.J., and Hayes A.F. (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Meth. 40, 879–891 [DOI] [PubMed] [Google Scholar]
- 75.Slomine B.S., Gerring J.P., Grados M.A., Vasa R., Brady K.D., Christensen J.R., and Denckla M.B. (2002). Performance on measures of executive function following pediatric traumatic brain injury. Brain Inj. 16, 759–772 [DOI] [PubMed] [Google Scholar]
- 76.Nowrangi M.A., Lyketsos C., and Munro C.A. (2014). Systematic review of neuroimaging correlates of executive functioning: converging evidence from different clinical populations. J. Neuropsychiatry Clin. Neurosci. 26, 114–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Collette F., Hogge M., Salmon E., and Van der Linden M. (2006). Exploration of the neural substrates of executive functioning by functional neuroimaging. Neuroscience 139, 209–221 [DOI] [PubMed] [Google Scholar]
- 78.Constantinidis C., Williams G.V., and Goldman–Rakic P.S. (2002). A role for inhibition in shaping the temporal flow of information in prefrontal cortex. Nat. Neurosci. 5, 175–180 [DOI] [PubMed] [Google Scholar]
- 79.Roberts R.J., and Pennington B.F. (1996). An interactive framework for examining prefrontal cognitive processes. Dev. Neuropsychol. 12, 105–126 [Google Scholar]
- 80.Giza C.C., Mink R.B., and Madikians A. (2007). Pediatric traumatic brain injury: not just little adults. Curr. Opin. Crit. Care 13, 143–152 [DOI] [PubMed] [Google Scholar]
- 81.Dennis M. (1988). Language and the Young Damaged Brain, Vol 7 American Psychological Association: Washington, DC [Google Scholar]
- 82.Ewing–Cobbs L., Levin H.S., Eisenberg H.M., and Fletcher J.M. (1987). Language functions following closed-head injury in children and adolescents. J. Clin. Exp. Neuropsychol. 9, 575–592 [DOI] [PubMed] [Google Scholar]
- 83.Nadebaum C., Anderson V., and Catroppa C. (2007). Executive function outcomes following traumatic brain injury in young children: a five year follow-up. Dev. Neuropsychol. 32, 703–728 [DOI] [PubMed] [Google Scholar]
- 84.Anderson V., and Catroppa C. (2005). Recovery of executive skills following paediatric traumatic brain injury (TBI): A 2 year follow-up. Brain Inj. 19, 459–470 [DOI] [PubMed] [Google Scholar]
- 85.Zelazo P.D., Reznick J.S., and Spinazzola J. (1998). Representational flexibility and response control in a multistep, multilocation search task. Dev. Psychol. 34, 203–214 [DOI] [PubMed] [Google Scholar]
- 86.Stahl L., and Pry R. (2005). Attentional flexibility and perseveration: developmental aspects in young children. Child Neuropsychol. 11, 175–189 [DOI] [PubMed] [Google Scholar]
- 87.Carlson S.M., and Moses L.J. (2001). Individual differences in inhibitory control and children's theory of mind. Child Dev. 72, 1032–1053 [DOI] [PubMed] [Google Scholar]
- 88.Clark C.A.C., Sheffield T.D., Chevalier N., Nelson J.M., Wiebe S.A., and Espy K.A. (2013). Charting early trajectories of executive control with the shape school. Dev. Psychol. 49, 1481–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Klenberg L., Korkmann M., and Lahti–Nuuttila P. (2001). Differential development of attention and executive functions in 3-to-12-year-old Finnish children. Dev. Neuropsychol. 20, 407–428 [DOI] [PubMed] [Google Scholar]
- 90.Diamond A. (2013). Executive functions. Annu. Rev. Psychol. 64, 135–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miyake A., Friedman N.P., Emerson M.J., Witzki A.H., Howerter A., and Wagner T.D. (2000). The unity and diversity of executive functions and their contributions to complex ‘frontal lobe’ tasks: a latent variable analysis. Cogn. Psychol. 41, 49–100 [DOI] [PubMed] [Google Scholar]
- 92.Wade S.L., Taylor H.G., Walz N.C., Salisbury S., Stancin T., Bernard L.A., Oberjohn K., and Yeates K.O. (2008). Parent–child interactions during the initial weeks following brain injury in young children. Rehabil. Psychol. 53, 180–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kurowski B., Martin L.J., and Wade S.L. (2012). Genetics and outcomes after traumatic brain injury (TBI): what do we know about pediatric TBI? J. Pediatr. Rehabil. Med. 5, 217–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kurowski B., Backeljauw B., Zang H., Zhang N., Martin L.J., Pilipenko V., Yeates K., Taylor H.G., and Wade S. (2015). Influence of catechol-o-methyltransferase on executive functioning longitudinally after early childhood traumatic brain injury: preliminary findings. J. Head Trauma Rehabil. [E-pub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kassam I., Gagnon F., and Cusimano M.D. (2016). Association of the APOE-ɛ4 allele with outcome of traumatic brain injury in children and youth: a meta-analysis and meta-regression. J. Neurol. Neurosurg. Psychiatry 87, 433–440 [DOI] [PubMed] [Google Scholar]
- 96.Van Aken l., Kessels R.P.C., Wingbermühle E., Van der Veld W.M., and Egger J.I.M. (2016). Fluid intelligence and executive functioning more alike than different. Acta Neuropsychiatr. 28, 31–37 [DOI] [PubMed] [Google Scholar]
- 97.Sparrow S.S., Balla D.A., and Cicchetti D.V. (1984). Vineland Adaptive Behavior Scale, 2nd ed. Pearson Assessments: Minneapolis [Google Scholar]
- 98.Sparrow S.S., Balla D.A., and Cicchetti D.V. (1984). Vineland Adaptive Behavior Scales: Interview Edition, Expanded Form Manual. American Guidance Service: Circle Pines, MN [Google Scholar]
- 99.Murray A., McKenzie K., and Murray G. (2014). To what extent does g impact on conceptual, practical and social adaptive functioning in clinically referred children? J. Intellect. Disabil. Res. 58, 777–785 [DOI] [PubMed] [Google Scholar]
- 100.Roszkowski M.J., and Bean A.G. (1980). The Adaptive Behavior Scale (ABS) and IQ: how much unshared variance is there? Psychol. Sch. 17, 452–459 [Google Scholar]
- 101.Max J.E., Koele S.L., Lindgren S.D., Robin D.A., Smith W.L., Jr., Sato Y., and Arndt S. (1998). Adaptive functioning following traumatic brain injury and orthopedic injury: a controlled study. Arch. Phys. Med. Rehabil. 79, 893–899 [DOI] [PubMed] [Google Scholar]
- 102.Schatz J., and Hamdan–Allen G. (1995). Effects of age and IQ on adaptive behavior domains for children with autism. J. Autism Dev. Disord. 25, 51–60 [DOI] [PubMed] [Google Scholar]
- 103.Carpentieri S., and Morgan S.B. (1996). Adaptive and intellectual functioning in autistic and non-autistic retarded children. J. Autism Dev. Disord. 26, 611–620 [DOI] [PubMed] [Google Scholar]
- 104.Liss M., Harel B., Fein D., Allen D., Dunn M., Feinstein C., Morris R., Waterhouse L., and Rapin I. (2001). Predictors and Correlates of Adaptive Functioning in Children with Developmental Disorders. J. Autism Dev. Disord. 31, 219–230 [DOI] [PubMed] [Google Scholar]
- 105.Loveland K.A., and Kelley M.L. (1991). Development of adaptive behavior in preschoolers with autism or Down Syndrome. Am. J. Ment. Retard. 96, 13–20 [PubMed] [Google Scholar]
- 106.Ware A.L., Crocker N., O'Brien J.W., Deweese B.N., Roesch S.C., Coles C.D., Kable J.A., May P.A., Kalberg W.O., Sowell E.R., Jones K.L., Riley E.P., Mattson S.N., and CIFASD (2012). Executive function predicts adaptive behavior in children with histories of heavy prenatal alcohol exposure and attention deficit/hyperactivity disorder. Alcoholism Clin. Exp. Res. 36, 1431–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ashford J.M., Netson K.L., Clark K.N., Merchant T.E., Santana V.m., Wu S., and Conklin H.M. (2014). Adaptive functioning of childhood brain tumor survivors following conformal radiation therapy. J. Neurooncol. 118, 193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Culhane–Shelburne K., Chapieski L., Hiscock M., and Glaze D. (2002). Executive functions in children with frontal and temporal lobe epilepsy. J. Int. Neuropsychol. Soc. 8, 623–632 [DOI] [PubMed] [Google Scholar]
- 109.Hedvall Å., Fernell E., Holm A., Åsberg Johnels J., Gillberg C., and Billstedt E. (2013). Autism, processing speed, and adaptive functioning in preschool children. ScientificWorldJournal 2013:158263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rassovsky Y., Satz P., Alfano M.S., Light R.K., Zaucha K., McArthur D.L., and Hovda D. (2006). Functional outcome in TBI II: verbal memory and information processing speed mediators. J. Clin. Exp. Neuropsychol. 28, 581–591 [DOI] [PubMed] [Google Scholar]
- 111.Davis B.E., Shurtleff D.B., Walker W.O., Seidel K.D., and Duguay S. (2006). Acquisition of autonomy skills in adolescents with myelomeningocele. Dev. Med. Child Neurol. 48, 253–258 [DOI] [PubMed] [Google Scholar]
- 112.Papazoglou A., Jacobson L.A., and Zabel T.A. (2013). More than intelligence: distinct cognitive/behavioral clusters linked to adaptive dysfunction in children. J. Int. Neuropsychol. Soc. 19, 189–197 [DOI] [PubMed] [Google Scholar]
- 113.Robinson K.E., Kaizar E., Catroppa C., Godfrey C., and Yeates K.O. (2014). Systematic review and meta-analysis of cognitive interventions for children with central nervous system disorders and neurodevelopmental disorders. J. Pediatr. Psychol. 39, 846–865 [DOI] [PubMed] [Google Scholar]