Abstract
Recently, there has been increasing interest in outcomes after repetitive mild traumatic brain injury (rmTBI) (e.g., sports concussions). Although most of the scientific attention has focused on elite athlete populations, the sequelae of rmTBI in children and young adults have not been well studied. Prior TBI studies have suggested that developmental differences in response to injury, including differences in excitotoxicity and inflammation, could result in differences in functional and histopathological outcomes after injury. The purpose of this study is to compare outcomes in adolescent (5-week-old) versus adult (4-month-old) mice in a clinically relevant model of rmTBI. We hypothesized that functional and histopathological outcomes after rmTBI would differ in developing adolescent brains compared with mature adult brains. Male adolescent and adult (C57Bl/6) mice were subjected to a weight drop model of rmTBI (n = 10–16/group). Loss of consciousness (LOC) after each injury was measured. Functional outcomes were assessed including tests of balance (rotorod), spatial memory (Morris water maze), and impulsivity (elevated plus maze). After behavioral testing, brains were assessed for histopathological outcomes including microglial immunolabeling and N-methyl-d-aspartate (NMDA) receptor subunit expression. Injured adolescent mice had longer LOC than injured adult mice compared with their respective sham controls. Compared with sham mice, adolescent and adult mice subjected to rmTBI had impaired balance, increased impulsivity, and worse spatial memory that persisted up to 3 months after injury, and the effect of injury was worse in adolescent than in adult mice in terms of spatial memory. Three months after injury, adolescent and adult mice demonstrated increased ionized calcium binding adaptor 1 (IbA1) immunolabeling compared with sham controls. Compared with sham controls, NMDA receptor subtype 2B (NR2B) expression in the hippocampus was reduced by ∼20% in both adolescent and adult injured mice. The data suggest that injured adolescent mice may show a distinct pattern of functional deficits after injury that warrants further mechanistic studies.
Keywords: : age, pediatric brain injury, TBI
Introduction
Recently, there has been increasing scientific attention paid to the sequelae of repetitive mild traumatic brain injury (rmTBI). Clinically, rmTBI has been associated with long-term neurological impairment including memory disturbances, Parkinsonism, behavioral changes, speech irregularities, and gait abnormalities.1–5 Case series of subjects exposed to rmTBI have described gross pathological changes such as brain volume loss and histopathological features of neurodegenerative disease.6–9 However, the majority of these clinical and pathological reports have occurred in retrospective case series of adults, including professional athletes and military veterans. Developmental considerations of the effect of rmTBI have not been well studied. One recent study using magnetic resonance imaging suggested that former National Football League players exposed to American football before the age of 12 years had differences in the microstructure of the corpus callosum than those starting at a later age.10 mTBI, or concussion, is a common injury sustained by high school athletes; as many as 11.5–13.2% of sport-related concussions are recurrent injuries.11,12 A recent study demonstrated worse neurocognitive outcomes in adolescents who sustained concussion when compared with adults.13 Whether or not the pathophysiology and sequelae of adolescent rmTBI differs from that described in adults is unknown.
Pre-clinical models of rmTBI offer the opportunity to investigate age-dependent outcomes. We have previously reported an rmTBI model in adult (2–3-month-old) mice that resulted in persistent behavioral deficits in exploratory behavior, balance, and spatial memory, associated with chronic increased astrocytosis and microgliosis.14 Extending our findings to an adolescent model (5-week-old mice) of rmTBI is important to understanding whether developmental factors contribute to outcomes after rmTBI. Maturational differences and regulation of the inflammatory response and excitotoxicity after various experimental models of brain injury15,16 have previously been reported, but whether these are relevant to rmTBI is unknown. Additionally, the impact of frequency of injury on these pathophysiological variables is unknown.
Understanding whether or not outcomes after rmTBI differ between adolescent and adult mice is a vital first step in evaluating age-specific mechanistic questions, prevention strategies, and therapeutic interventions. In the present study, we compared the performance of adolescent and adult mice subjected to rmTBI on a variety of behavioral tasks assessing learning, memory, balance, behavior, and sensorimotor function. Additionally, we evaluated microgliosis and N-methyl-d-aspartate (NMDA) receptor subunit expression. For this study, we hypothesized that functional and histopathological outcomes after rmTBI would differ in developing adolescent brains compared with mature adult brains.
Methods
All experiments were approved by the Boston Children's Hospital Institutional Animal Care and Use Committee, and complied with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Adult (age 16 weeks) and adolescent (age 5 weeks) male C57BL/6 mice were obtained from the Jackson Laboratories (Bar Harbor, ME). Mice were housed in a temperature- and humidity- controlled room with a 12 h light/dark cycle, and fed at libitum.
rmTBI
Mice were randomized to either rmTBI or sham injury. The rmTBI was performed as previously described.14,17 Briefly, mice were anesthetized for 45 sec using 4% isoflurane in a 70:30 mixture of oxygen. Anesthetized mice were placed on a delicate task wiper (Kimwipe, Kimberly-Clark, Irving, TX) and the head was placed directly under a hollow guide tube. Mice were held by the tail as an impact was delivered to the dorsal aspect of the skull. The impact was delivered by dropping a 54 g metal bolt from a height of 71 cm via a guide pipe, resulting in rotational acceleration of the head through the delicate task wiper. Sham injured mice underwent anesthesia but without injury. Mice were randomized to the following groups: seven injuries in 9 days (n = 12 adult mice, n = 17 adolescent mice), four injuries in 4 days (n = 10 adult mice, n = 10 adolescent mice), seven sham exposures in 9 days (n = 11 adult mice, n = 16 adolescent mice), and four sham exposures in 4 days (n = 10 adult mice, n = 10 adolescent mice) (Fig. 1). For the seven injuries in 9 days protocol, mice were injured daily for 5 days, followed by 2 days without injury, and then by two additional daily injuries. All mice recovered in room air after injury or sham injury. Following injury or sham injury, all behavioral testing and histological and biochemical assays were conducted by investigators blinded to injury status, using color coding stored in a password-protected computer.
FIG. 1.
Study flow diagram.
Assessments of loss of consciousness, (LOC), motor function, spatial memory and impulsivity-like phenotypes
LOC, defined as the time from removal of anesthesia to spontaneous righting, was evaluated after each injury or sham injury.
Motor ability and function was assessed on days 1–3 and again at 3 months after the last injury or sham injury using a rotorod as we previously reported.14 In brief, the rotorod consists of a 4 cm diameter rotating drum, on which mice were placed. The time (sec) between placement on the rotorod and falling off of the rotorod was recorded as a measure of motor function. Rotorod testing was conducted over 3 days with 1 day of habituation followed by 2 days of testing. On testing days, mice were placed on the rod at 4 rpm for 10 sec, to acclimate to the rod speed, after which the rod was accelerated at 0.1 rpm/sec. Each mouse completed four trials on the testing days, with a minimum of 15 min rest between trials.
Spatial learning and memory were assessed using the Morris water maze (MWM) on days 6–9 following injury and again at 3 months after injury. MWM testing was conducted as previously described.14,18 Briefly, a white pool (83 cm diameter, 60 cm deep) was filled with water to 29 cm depth. Water temperature was maintained at ∼24°C, and a target platform (a round, clear, plastic platform 10 cm in diameter) was positioned 1 cm below the surface of the water. Several highly visible intra- and extra-maze cues were located in and around the pool. During hidden and visible platform trials, mice were randomized to one of four starting quadrants. Mice were placed in the tank facing the wall and given 90 sec to find the platform, mount the platform, and remain on it for 5 sec. Mice were then placed under a heat lamp to dry before their next run. Time until the mouse mounted the platform (escape latency) was measured and recorded. Mice that failed to mount the platform within the allotted time (90 sec) were guided to the platform by the experimenter and allowed 10 sec to become acquainted with its location. Each mouse was subjected to a maximum of two trials per day, each consisting of four runs, with a 45 min break between trials. For visible platform trials, a red reflector was used to mark the top of the target platform. For probe trials, mice were placed in the tank with the platform removed, and given 60 sec to explore the tank. Noldus Ethovision 9 software tracked swim speed, total distance moved, and time spent in the target quadrant where the platform had been previously located.
Impulsivity behaviors were assessed in the elevated plus maze on day 21 and again at 3 months after injury as we previously described.14 The elevated plus maze apparatus (Lafayette Instruments, Lafayette, IN) consisted of two open and two closed arms (30 × 5 cm) extended out opposite from each other from a central platform (decision zone). Mice were placed on the center platform of the maze, facing a closed arm, and allowed to explore the apparatus for 5 min. A computer-assisted video-tracking system (Noldus Ethovision) recorded the total time spent in the open center (decision zone) and the open and closed compartments. The percent of time spent in the open arms is used as a surrogate measure of impulsivity behaviors; mice with greater impulsivity spend more time in the open arms. The maze was cleaned between tests with a weak ethanol solution and dried. Data were expressed as percentage of (time in open arms)/(time in open + closed arms).
Synaptosome preparation and immunoblot for NMDA receptors (NMDARs)
The separation of synaptic fractions was defined as previously described with slight modifications.19 Briefly, the hippocampi and cortices from injured (n = 6) and sham (n = 6) mice from adolescent and adult mice 3 months post-injury were homogenized with a tissue grinder (Thermo Fisher Scientific, MA) in buffer I (320 mM of sucrose, 10 mM of Tris, pH 7.4, 1 mM of sodium orthovanadate [Na3VO4], 5 mM of NaF, 1 mM EDTA, 1 mM of EGTA, 0.1 mM of phenylmethanesulfonylfluoride [PMSF], and protease inhibitor cocktail [Roch Life Science, IN]). The homogenates were centrifuged for 10 min at 800g at 4°C to remove uncompletely homogenized tissures and nuclei (p1). The supernatants were further spun at 10,000g for 10 min. The pellets (p2) were separated from the supernatant (s1). The p2 pellets were washed once in buffer I and resuspended in 70 μL (for hippocampus) or 140 μL (for cortex) of buffer II (10 mM of Tris, pH 7.4, 1 mM of Na3VO4, 5 mM of NaF, 1 mM EDTA, 1 mM of EGTA, 0.1 mM of PMSF and 0.5% of triton-X100). The suspensions were incubated on ice for additional 20 min with pipetting every 5 min, and then the suspensions were spun at 100,000g for an additional 1 h at 4°C. The supernants (s2, containing synaptic vesicle fraction) were transferred to new tubes. The pellets (p3, containing synaptosome fraction) were then resuspended in 50 μL (for hippocampus) or 100 μL (for cortex) of buffer I with 1% of sodium dodecyl sulfate (SDS). Protein concentrations were determined using a Bio-Rad Protein Assay kit (Bio-Rad, CA). The p3 fractions were boiled in sample buffer. Twenty μg of protein was then loaded into 4–15% TGX™ gradient gel (Bio-rad, Hercules, CA) for electrophoresis, transferred onto polyvinylidene difluoride (PVDF) membranes, and blocked with 5% of milk. Subsequently, anti-NR2B antibodies (Millipore) were applied at a 1:500 dilution and incubated overnight at 4°C. The following day, membranes were incubated with species-appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies and washed, and electrochemiluminescent substrate was applied (SuperSignal™ West Femto Maximum Sensitivity Substrate, Life Technologies). An LAS 4000 Imager (GE) was used to detect signal. Actin was used to confirm equal loading between lanes. Blots were quantified using NIH ImageJ software (http://rsb.info.nih.gov/ij). The expression levels were normalized to actin and compared with the sham group.
Immunohistochemistry
Mice (both ages) were perfused 3 months after injury, and brains were collected for histopathological analysis. Serial 20 μm coronal frozen sections from sham and injured adolescent (n = 5/group) and adult (n = 7/group) brains were cut on a cryostat (Leica) from the anterior frontal lobes through the posterior extent of the dorsal hippocampus. Every 10th section was collected sequentially and mounted on slides consistent with stereological techniques. After hydrogen peroxide treatment and incubation in a blocking solution containing 10% normal goat serum, sections were incubated overnight at 4°C in anti-ionized calcium binding adaptor 1 (IbA1) for microglia (WAKO, 1:250) antibodies. The following day, sections were washed and incubated sequentially with appropriate secondary antibodies, Vectastain ABC kit, and diaminobenzadine (DAB) (Vector), and mounted with Permount (Thermo-Fisher Scientific).
Stereological estimates
As described previously, Stereologer software (Stereology Resource Center) was used for analyzing the microglia cell immunolabeling after TBI.14 Briefly, estimates of the number of microglia were obtained in the CA1 of sham and injured mice using a thin section modification of the optical fractionator method to determine cell immunolabeling using object area fraction and region point counting.20 An investigator blinded to age and injury status quantified the total volume of the CA1, using the Calvalieri-point counting method, and the total volume of Iba1 immunolabelling. The CA1 was defined as the most dorsal peak of the hippocampus. The medial border of the CA1 was congruent with the dorsal peak of the cingulum, the lateral border was the lateral border of the molecular layer of the dentate gyrus, and the anterior margin was concomitant with the first appearance of a closed loop between the pyramidal cell layer and the dentate granule cell layer in the hippocampal midline and the posterior margin with the anterior margin of the fasciola cinereum in the mesial hippocampus. On every 10th section, the software superimposed a lattice of regularly spaced plus signs over the region of interest (ROI), and the ROI was outlined. Then, under high magnification, the number of cells within each systematically spaced unbiased sampling frame was counted. At the completion of the stereological analyses, the samples were decoded, and mean and SEM of the CA1 volumes and immunolabeling were calculated. All coefficients of error (CE) values for the stereological estimates were <10%.
White matter quanitification
An investigator blinded to age and injury status quantified white matter area using ImageJ software (Bethesda, MD) for adolescent (n = 7/group) and adult (n = 8/group) brains. After stereological estimates, sectioned brains were scanned into the computer with a ruler for scale calibration. The color and brightness of the pictures were adjusted to obtain the best visibility of the white matter through all the sections. White matter was measured on two sections from each animal: the first section closest to bregma 0.38 mm and the second section 400 μm following the first. Visible white matter from these sections was traced, and the number of pixels was calculated by the software, then converted to area. At the completion of measuring two sections per slide, the samples were decoded, and the average and SEM were calculated from each group.
Statistical analysis
Data are presented as mean ± SEM. Continuous variables were inspected for normality using the Shapiro–Wilk test and compared between injured and sham-injured mice, and between adolescent and adult mice at single time points using a Student's t test or Wilcoxon rank sum test, as approporiate. Sample size calculations were based on the t test, a moderate effect size of 0.5, power of 0.8, and an α of 0.05, estimating 10 mice/group needed for these studies. MWM and rotorod latencies were analyzed by linear regression, using clustered standard errors to account for repeated measures and time as a covariate when appropriate. The regression models were inspected with residual versus predictor plots to ensure that the residuals were normally distributed. Finally, to evaluate whether or not the effect of injury was modified by age, we created an interaction term for age and injury status, and re-ran the linear regression models with the interaction term. Statistical significance was considered p < 0.05. All analyses were performed using Stata 11.2 (StataCorp, College Station, TX).
Results
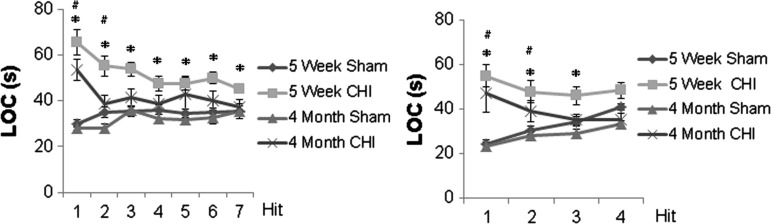
Repetitive concussive brain injury causes longer LOC in adolescent mice than in adult mice
Across all cohorts and ages, there were no convulsions after injury and all mice survived. After seven hits in 9 days, both adolescent (n = 17) and adult injured mice (n = 12) had prolonged LOC compared with their respective sham controls on day 1 and day 2 (p < 0.01); however, injured adolescent mice had persistent prolonged LOC compared with sham (p < 0.01) for all time points. There were no significant differences in LOC times between adult mice and sham on days 3–7 (Fig. 2A).
FIG. 2.
Loss of consciousness (LOC) after injury. (A) After seven injuries in 9 days, both adolescent (5 weeks old, n = 17) and adult injured mice (4 months old, n = 12) had prolonged LOC compared with their respective sham controls (16 adolescent and 12 adult, respectively) on days 1 and 2 (p < 0.01), but injured adolescent mice had persistent prolonged LOC compared with sham (p < 0.01 for all time points); there were no significant differences in adult mice on days 3–7 after injury. (B) After four injuries in 4 days, both adolescent (n = 10) and adult injured mice (n = 10) had prolonged LOC compared with their respective sham controls (10 adolescent and 10 adult, respectively) on days 1 and 2 (p < 0.05), but injured adolescent mice had persistent prolonged LOC compared with sham on day 3 (p = 0.021) as well. The data are expressed as mean ± SEM. # (for 4-month-old injured mice) and * (for 5-week-old injured mice) indicate that there is a significant difference (p < 0.05 or p < 0.01) compared to their age-matched sham littermates.
Similar results were found after four hits in 4 days: both adolescent (n = 10) and adult injured mice (n = 10) had prolonged LOC compared with their respective sham controls (n = 10 adolescent and n = 10 adult, respectively) on days 1 and 2 (p < 0.05); however, only injured adolescent mice had persistent prolonged LOC compared with sham on day 3 (p = 0.021; Fig. 2B).
Combining all injury groups, the interaction term for age and injury number was significant (p = 0.045), suggesting that the effect of injury number on LOC was different between adolescent and adult mice.
Repetitive concussive brain injury results in motor, spatial memory, and impulsivity abnormality at subacute time points after injury
One to three days after final injury, both adolescent (n = 12) and adult mice (n = 12) subjected to seven injures over 9 days had impaired performance on rotorod testing compared with their respective sham controls (n = 17 injured adolescents and n = 12 injured adults, p < 0.001 and p = 0.02 respectively, Fig. 3A). The effect of injury was not significantly different between adolescent and adult mice (p = 0.9 for interaction term).
FIG. 3.
Functional outcomes in the first month after seven injuries in 9 days. (A) Balance performance after injury. One to three days after injury (seven injuries in 9 days), both adolescent (5 weeks old n = 12) and adult injured mice (4 months old, n = 12) had impaired performance on rotorod testing compared with their respective sham controls (12 adolescent and 12 adult, p < 0.001 and p = 0.02 respectively. *p < 0.05. (B) Spatial memory. On days 13–16 after seven injuries in 9 days, both adolescent (n = 14) and adult injured mice (n = 12) had impaired performance on Morris water maze (MWM) testing compared with their respective sham controls (14 adolescent and 11 adult, p < 0.001). Adolescent sham-injured (p < 0.001 for the effect of time) and adult sham-injured mice (p < 0.001 for the effect of time) but not injured adolescent (p = 0.1) or injured adult (p = 0.7) mice demonstrated time-dependent learning. (C) Anxiety measures. On day 21 after seven injuries in 9 days, adult injured mice (n = 12) spent a similar percentage of time in open arms as sham controls (n = 11), 9.4 ± 2.1 versus 6.7 ± 1.7, p = 0.3. In contrast, injured adolescent mice (n = 14) spent a greater percentage of time in the open arms than did sham-injured controls (n = 14), 12.3 ± 1.8 versus 7.0 ± 1.2, p = 0.012. # (for 4-month- old injured mice) and * (for 5-week-old injured mice) indicate there is a significant difference (p < 0.05 or p < 0.01) compared with their age-matched sham controls. # (for 4-month-old injured mice) and * (for 5-week-old injured mice) indicate there is a significant difference (p < 0.05 or p < 0.01) compared with their age-matched sham controls. All data in these figures are expressed as mean ± SEM.
On days 13–16 after seven hits in 9 days, both adolescent (n = 14) and adult injured mice (n = 12) had impaired performance on MWM testing compared with their respective sham controls (n = 14 adolescent and n = 11 adult, p < 0.001, Fig. 3B). Adolescent sham (p < 0.001 for the effect of time) and adult sham mice demonstrated time-dependent learning (p < 0.001 for the effect of time), but injured adolescent (p = 0.1) and injured adult (p = 0.7) mice did not. The effect of injury was worse in adolescent mice than in adult mice (p = 0.03 for interaction term).
On day 21 after seven hits in 9 days, adult injured mice (n = 12) spent a similar percentage of time in open and closed arms on the elevated plus maze as their sham controls (n = 11, p = 0.3). In contrast, injured adolescent mice (n = 14) spent a greater proportion of time in the open arm than did their sham controls (n = 14, p = 0.012, Fig. 3C). The effect of injury was similar in adolescent and adult mice (p = 0.8).
Reduction in injury number diminishes neurological and cognitive deficits in adolescent and adult mice
To evaluate the effect of injury number between adolescent and adult mice, we subjected mice to a modified injury protocol of four hits in 4 days. Compared with adolescent mice who underwent the more intense injury regimen (seven hits in 9 days), injured adolescent mice on the modified regimen (n = 10) had improved performance on rotorod testing (p = 0.03) and hidden trial of the MWM (p = 0.02) but no difference in the proportion of time in the open arm of the elevated plus maze (p = 0.8, Fig. 4), although deficits in the MWM persisted when compared with sham-injured mice. Compared with the more intense seven hits in 9 days regimen, adult mice subjected to the modified four hits in 4 days regimen (n = 10) had better rotorod performance (p = 0.004), without significant difference in hidden platform MWM performance (p = 0.9) or percent of time in the open arm of the elevated plus maze (p = 0.4, Fig. 4), demonstrating persistent deficits in MWM and elevated plus performance when compared with sham controls.
FIG. 4.
Functional outcomes in the first month after four injuries in 4 days. (A) After four injuries in 4 days, there were no significant differences in rotorod performance between adolescent (5 weeks old) injured and sham mice or between adult (4 months old) injured and sham mice. (B) Both adolescent (5 weeks old) injured mice and adult (4 months old) injured mice had significant spatial memory deficits after injury compared with age-matched sham controls. (C) Elevated plus maze task. No significant differences in time spent in the open arms were observed between injured adolescent mice and sham controls (13.3 ± 2.7 vs. 6.9 ± 1.9, p = 0.07), although injured adult mice demonstrated increased time in the open arms compared with adult sham controls (12.3 ± 2.3 vs. 3.5 ± 1.1, p = 0.002). # (for 4-month-old injured mice) and * (for 5-week-old injured mice) indicate there is a significant difference (p < 0.05 or p < 0.01) compared with their age-matched sham controls. All the data are expressed as mean ± SEM.
Long-term behavioral deficits persist in both injured adolescent and adult mice
Three months after seven hits in 9 days, adolescent injured (n = 14), but not adult injured (n = 12), mice demonstrated persistent impairment in rotorod performance (p < 0.001 and p = 0.1, respectively; p < 0.001for the interaction term testing the effect of age Fig. 5A). In contrast, no deficits in rotorod performance between injured and sham mice were found 3 months after four hits in 4 days in adolescent (p = 0.8) or adult mice (p = 0.8) (data not shown). Adolescent and adult injured mice had persistently impaired MWM performance compared with sham mice 3 months after sustaining seven injuries in 9 days (p < 0.0001, Fig. 5B), as well as after the modified injury regimen of four hits in 4 days (p < 0.001, data not shown). Adult but not adolescent mice demonstrated persistent impairment in elevated plus maze performance 3 months after seven hits in 9 days (p = 0.02 for adult injured versus adult sham, Fig. 5C); however, neither adult nor adolescent injured mice had deficits in elevated plus maze performance compared with their respective shams 3 months after four hits in 4 days (p = 0.8 and p = 0.9, respectively; data not shown).
FIG. 5.
Long-term functional outcomes 3 months after seven injuries in 9 days. (A) Balance. Injured adolescent mice (n = 14), but not injured adult mice (n = 12), demonstrated persistent impairment in rotorod performance (p < 0.001 and p = 0.1, respectively). (B) Spatial memory. Adolescent and adult injured mice had persistently impaired Morris water maze (MWM) performance compared with sham mice (p < 0.0001). # (for 4-month-old CHI ) and * (for 5-week-old CHI) indicate p < 0.0001. (C) Adult, but not adolescent, mice demonstrated persistent impairment in the elevated plus maze (p = 0.02 for adult injured vs. adult sham).
Stereological estimates of microglia
Compared with their respective shams, IbA1 immunolabeling in the injured brain was significantly increased 3 months after seven hits in 9 days in both adolescent and adult mice (Fig. 6), although the effect was not different in injured adolescent mice than in injured adult mice (p = 0.3).
FIG. 6.
Representative photomicrographs of ionized calcium binding adaptor 1 (IbA1) positive cells 3 months after seven injuries in 9 days in adolescent sham (A) versus adolescent injured (B) and adult sham (C) versus injured (D) mice. IbA1 immunolabeling is significantly different between injured mice and their respective sham controls in both adolescent (n = 5/group) and adult mice (n = 7/group) (E). The data are expressed as mean ± SEM. *p < 0.05.
White matter area
Compared with their respective shams, white matter area in the injured brain was significantly decreased 3 months after seven hits in 9 days in adult mice (0.32 ± 0.01 mm2 for injured vs 0.38 ± 0.02 mm2 for sham, p = 0.02), but not in adolescent mice (0.34 ± 0.01 mm2 for injured vs 0.36 ± 0.02 mm2 for sham, p = 0.3).
NR2B receptor subunit expression
Three months after injury (seven hits in 9 days), NR2B expression in the hippocampus was reduced by ∼20% in both adolescent and adult mice compared with shams (Fig. 7) (p = 0.04 for adolescent mice and 0.03 for adult mice), although there was no difference in NR2B expression in the cortex between injured and sham adolescent or adult mice (data not shown).
FIG. 7.
N-methyl-d-aspartate receptor subtype 2B (NR2B) expression and semiquantification. Three months after injury, NR2B expression in the hippocampus was reduced in both adolescent and adult mice compared with shams. *p < 0.05.
Discussion
To our knowledge, these data represent the first comparison of outcomes after rmTBI in adolescent versus adult mice. Understanding age-dependent differences in outcomes after mTBI is important because of the large public health burden of mTBI in adolescent populations. These data extend our prior study detailing behavioral and histopathological outcomes after rmTBIs sustained by adult mice.14 Whereas most pre-clinical models of mTBI to date, including our prior studies, have focused on the pathophysiology of mTBI in adult populations, investigation of developmental considerations using less mature animals is warranted. Injury during childhood and adolescence may interrupt critical processes such as the rapid increase in gray and white matter, as well as the acquisition of functional skills such as motor dexterity, problem solving, memory, language, abstract thinking, social behavior, and emotional skills.21 Prior studies of more severe TBI have suggested age-specific differences in response to injury.22–24 We found that adolescent mice had longer LOC and age-dependent deficits in subacute MWM performance and long-term rotorod performance. It is notable that the distinct functional sequelae of rmTBI in adolescent mice were associated with a similar magnitude of gliosis as that found in adult mice, which, if progressive, could have even greater long-term significance in a young population.
Our findings may be in part explained by physics alone. Based on Newton's second law, the 5-week-old mice, exposed to the same amount of force, likely experienced greater acceleration of the head than did the older animals. In our model, exposed to the same forces, we observed that LOC in 5-week-old mice was significantly longer than in 4-month-old mice. Given the heterogeneous nature of adolescent growth patterns, we believe that this is a clinically relevant scenario in which smaller adolescents could sustain adult-magnitude injuries from their larger peers, especially in the context of collision sports.25 Although we did not find histopathological correlates of the distinct functional outcomes observed between adolescent and adult mice in this study, we studied a limited number of histopathological outcomes, and could not, therefore, assess whether age-dependent differences in outcomes are purely explained by biomechanics.
In both adolescent and adult mice, we found that deficits after injury are both dose dependent, with an increasing number of injuries associated with worse functional outcomes, and persistent, with continued functional deficits 3 months after injuries. We have previously reported the effect of increased number and frequency of injuries in adult mice, and extend these data now to adolescent populations.17 Our studies suggest distinct vulnerabilities of adolescent mice when compared with adult mice. Although we did not ascertain in this study the structural or biochemical substrate of these developmental differences, our findings suggest that the adolescent population might warrant further pre-clinical and clinical investigation to evaluate its unique vulnerability to rmTBI.
Although the pathophysiology of rmTBI is complex, one of the major pathophysiological mechanisms underlying the regional, time-dependent, and maturational vulnerability to injury is excitoxicity via the overactivation of ionotropic glutamate receptors.26 Ionotropic glutamate receptors include the α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid glutamate receptors (AMPARs), NMDARs, and kainate receptors, each of which is known to play a central role in the evolution of TBI. Glutamate-mediated neurotransmission is required for normal brain development and injury response, and glutamate receptor function is tightly regulated under normal conditions for maintenance of homeostasis.27 NMDAR subunits are developmentally regulated.27 NR2B plays a central role in learning, memory, and pain.19,28,29 Previously, it has been reported increases in cyclin dependent kinase 5 (CDK5) levels reduce synaptic NR2B levels, leading to significant impairment of learning and memory.19 We demonstrate persistently reduced synaptic NR2B expression in both adolescent and adult mice with seven closed head injuries in 9 days compared with sham animals. Specifically, synaptic NR2B protein expression is reduced at 3 months compared with sham, regardless of whether the rmTBI occurred at 5 weeks or 4 months of age. These data are consistent with our data demonstrating altered escape latency in the MWM and deficits in hippocampal dependent memory and spatial learning in both adolescent and adult injured mice. Notably, decreases in hippocampal NR2B has been reported in TBI and in the postmortem hippocampus of patients with Alzheimer's disease.30 Given its role as a critical component of molecular signaling pathways regulating calcium flux, long-term potentiation (LTP), long-term depression (LTD), synaptic growth, and plasticity,31 our findings suggest abnormal NR2B levels may be associated with loss of in-membrane stability and disruption of glutamateric homeostasis following repetitive injury. Notably, our study expands prior data indicating that NMDARs change in a regional and time-dependent fashion following closed head injury in mice for up to 3 months post-injury.26
Conclusion
In summary, we demonstrate that injured adolescent mice show a distinct pattern of functional deficits after injury when compared with adult mice. Determining whether these findings reflect differences in the biomechanics of injury, or developmental differences in the response to injury, warrants further investigation, particularly given the increasing public health burden associated with TBIs sustained by adolescents.
Acknowledgments
Boston Children's Hospital Intellectual and Developmental Disabilities Research Center, Rebekah Mannix is supported by T32 HD40128-11A1, and Zhongrong Mei is supported by a Guangzhou Medical College Research Grant (1201421151).
Author Disclosure Statement
No competing financial interests exist.
William P. Meehan receives royalties from ABC-Clio Publishing for the sale of his book, Kids, Sports, and Concussion: A Guide for Coaches and Parents, and royalties from Wolters Kluwer for working as an author for UpToDate. He is under contract with ABC-Clio publishing for a future book entitled, Concussions, and with Springer International publishing for a future book entitled, Head and Neck Injuries in Young Athletes. His research is funded, in part, by a grant from the National Football League Players Association and by philanthropic support from the National Hockey League Alumni Association through the Corey C. Griffin Pro-Am Tournament.
References
- 1.Bower J.H., Maraganore D.M., Peterson B.J., McDonnell S.K., Ahlskog J.E., and Rocca W.A. (2003). Head trauma preceding PD: a case-control study. Neurology 60, 1610–1615 [DOI] [PubMed] [Google Scholar]
- 2.Critchley M. (1957). Medical aspects of boxing, particularly from a neurological standpoint. Br. Med. J. 1, 357–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grahmann H., and Ule G. (1957). Diagnosis of chronic cerebral symptoms in boxers (dementia pugilistica & traumatic encephalopathy of boxers) [in German]. Psychiatr. Neurol. (Basel) 134, 261–283 [PubMed] [Google Scholar]
- 4.Martland H. (1928). Punch drunk. J. A. M. A. 91, 1103–1107 [Google Scholar]
- 5.Plassman B.L., Havlik R.J., Steffens D.C., Helms M.J., Newman T.N., Drosdick D., Phillips C., Gau B.A., Welsh–Bohmer K.A., Burke J.R., Guralnik J.M., and Breitner J.C. (2000). Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology 55, 1158–1166 [DOI] [PubMed] [Google Scholar]
- 6.McKee A.C., Cantu R.C., Nowinski C.J., Hedley–Whyte E.T., Gavett B.E., Budson A.E., Santini V.E., Lee H.S., Kubilus C.A., and Stern R.A. (2009). Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 68, 709–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKee A.C., Stern R.A., Nowinski C.J., Stein T.D., Alvarez V.E., Daneshvar D.H., Lee H.S., Wojtowicz S.M., Hall G., Baugh C.M., Riley D.O., Kubilus C.A., Cormier K.A., Jacobs M.A., Martin B.R., Abraham C.R., Ikezu T., Reichard R.R., Wolozin B.L., Budson A.E., Goldstein L.E., Kowall N.W., and Cantu R.C. (2013). The spectrum of disease in chronic traumatic encephalopathy. Brain 136, 43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omalu B.I., DeKosky S.T., Hamilton R.L., Minster R.L., Kamboh M.I., Shakir A.M., and Wecht C.H. (2006). Chronic traumatic encephalopathy in a national football league player: part II. Neurosurgery 59, 1086–1092 [DOI] [PubMed] [Google Scholar]
- 9.Omalu B.I., DeKosky S.T., Minster R.L., Kamboh M.I., Hamilton R.L., and Wecht C.H. (2005). Chronic traumatic encephalopathy in a National Football League player. Neurosurgery 57, 128–134 [DOI] [PubMed] [Google Scholar]
- 10.Stamm J.M., Koerte I.K., Muehlmann M., Pasternak O., Bourlas A.P., Baugh C.M., Giwerc M.Y., Zhu A., Coleman M.J., Bouix S., Fritts N.G., Martin B.M., Chaisson C., McClean M.D., Lin A.P., Cantu R.C., Tripodis Y., Stern R.A., and Shenton M.E. (2015). Age at first exposure to football is associated with altered corpus callosum white matter microstructure in former professional football players. J. Neurotrauma 32, 1768–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castile L., Collins C.L., McIlvain N.M., and Comstock R.D. (2012). The epidemiology of new versus recurrent sports concussions among high school athletes, 2005–2010. Br. J. Sports Med. 46, 603–610 [DOI] [PubMed] [Google Scholar]
- 12.Marar M., McIlvain N.M., Fields S.K., and Comstock R.D. (2012). Epidemiology of concussions among United States high school athletes in 20 sports. Am. J. Sports Med. 40, 747–755 [DOI] [PubMed] [Google Scholar]
- 13.Baillargeon A., Lassonde M., Leclerc S., and Ellemberg D. (2012). Neuropsychological and neurophysiological assessment of sport concussion in children, adolescents and adults. Brain Inj. 26, 211–220 [DOI] [PubMed] [Google Scholar]
- 14.Mannix R., Berglass J., Berkner J., Moleus P., Qiu J., Andrews N., Gunner G., Berglass L., Jantzie L.L., Robinson S., and Meehan W.P., 3rd (2014). Chronic gliosis and behavioral deficits in mice following repetitive mild traumatic brain injury. J. Neurosurg. 121, 1342–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brewer G.J. (1998). Age-related toxicity to lactate, glutamate, and beta-amyloid in cultured adult neurons. Neurobiol. Aging 19, 561–568 [DOI] [PubMed] [Google Scholar]
- 16.Claus C.P., Tsuru–Aoyagi K., Adwanikar H., Walker B., Manvelyan H., Whetstone W., and Noble–Haeusslein L.J. (2010). Age is a determinant of leukocyte infiltration and loss of cortical volume after traumatic brain injury. Dev. Neurosci. 32, 454–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meehan W.P., 3rd, Zhang J., Mannix R., and Whalen M.J. (2012). Increasing recovery time between injuries improves cognitive outcome after repetitive mild concussive brain injuries in mice. Neurosurgery 71, 885–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris R. (1984). Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 11, 47–60 [DOI] [PubMed] [Google Scholar]
- 19.Zhang X., Xin X., Dong Y., Zhang Y., Yu B., Mao J., and Xie Z. (2013). Surgical incision-induced nociception causes cognitive impairment and reduction in synaptic NMDA receptor 2B in mice. J. Neurosci. 33, 17,737–17,748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jantzie L.L., Corbett C.J., Berglass J., Firl D.J., Flores J., Mannix R., and Robinson S. (2014). Complex pattern of interaction between in utero hypoxia–ischemia and intra-amniotic inflammation disrupts brain development and motor function. J. Neuroinflammation 11, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahl R.E. (2004). Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann. N. Y. Acad. Sci. 1021, 1–22 [DOI] [PubMed] [Google Scholar]
- 22.Cernak I., Chang T., Ahmed F.A., Cruz M.I., Vink R., Stoica B., and Faden A.I. (2010). Pathophysiological response to experimental diffuse brain trauma differs as a function of developmental age. Dev. Neurosci. 32, 442–453 [DOI] [PubMed] [Google Scholar]
- 23.Mannix R.C., Zhang J., Park J., Lee C., and Whalen M.J. (2011). Detrimental effect of genetic inhibition of B-site APP-cleaving enzyme 1 on functional outcome after controlled cortical impact in young adult mice. J. Neurotrauma 28, 1855–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mannix R.C., Zhang J., Park J., Zhang X., Bilal K., Walker K., Tanzi R.E., Tesco G., and Whalen M.J. (2011). Age-dependent effect of apolipoprotein E4 on functional outcome after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 31, 351–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kriz P.K., Stein C., Kent J., Ruggieri D., Dolan E., O'Brien M., and Meehan W.P., 3rd (2016). Physical maturity and concussion symptom duration among adolescent ice hockey players. J. Pediatr. 171, 234–239.e2 [DOI] [PubMed] [Google Scholar]
- 26.Biegon A., Fry P.A., Paden C.M., Alexandrovich A., Tsenter J., and Shohami E. (2004). Dynamic changes in N-methyl-d-aspartate receptors after closed head injury in mice: Implications for treatment of neurological and cognitive deficits. Proc. Natl. Acad. Sci. U. S. A. 101, 5117–5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jantzie L.L., Talos D.M., Jackson M.C., Park H.K., Graham D.A., Lechpammer M., Folkerth R.D., Volpe J.J., and Jensen F.E. (2015). Developmental expression of N-methyl-d-aspartate (NMDA) receptor subunits in human white and gray matter: potential mechanism of increased vulnerability in the immature brain. Cereb. Cortex 25, 482–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plattner F., Hernandez A., Kistler T.M., Pozo K., Zhong P., Yuen E.Y., Tan C., Hawasli A.H., Cooke S.F., Nishi A., Guo A., Wiederhold T., Yan Z., and Bibb J.A. (2014). Memory enhancement by targeting Cdk5 regulation of NR2B. Neuron 81, 1070–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferreira J.S., Schmidt J., Rio P., Aguas R., Rooyakkers A., Li K.W., Smit A.B., Craig A.M., and Carvalho A.L. (2015). GluN2B-containing NMDA receptors regulate AMPA receptor traffic through anchoring of the synaptic proteasome. J. Neurosci. 35, 8462–8479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bi H., and Sze C.I. (2002). N-methyl-D-aspartate receptor subunit NR2A and NR2B messenger RNA levels are altered in the hippocampus and entorhinal cortex in Alzheimer's disease. J. Neurol. Sci. 200, 11–18 [DOI] [PubMed] [Google Scholar]
- 31.Muller T., Albrecht D., and Gebhardt C. (2009). Both NR2A and NR2B subunits of the NMDA receptor are critical for long-term potentiation and long-term depression in the lateral amygdala of horizontal slices of adult mice. Learn. Mem. 16, 395–405 [DOI] [PubMed] [Google Scholar]