Abstract
In addition to antigen-specific stimulation of T cell receptor (TCR) by a peptide-MHC complex, the functional outcome of TCR engagement is regulated by antigen-independent costimulatory signals. Costimulatory signals are provided by an array of interactions involving activating and inhibitory receptors expressed on T cells and their cognate ligands on antigen presenting cells. T cell immunoglobulin and ITIM domain (TIGIT), a recently identified immune receptor expressed on T and NK cells, upon interaction with either of its two ligands, nectin-2 or poliovirus receptor (PVR), inhibits activation of T and NK cells. Here we report the crystal structure of the human TIGIT ectodomain, which exhibits the classic two-layer β-sandwich topology observed in other immunoglobulin super family (IgSF) members. Biophysical studies indicate that TIGIT is monomeric in solution but can form a dimer at high concentrations, consistent with the observation of a canonical immunoglobulin-like dimer interface in the crystalline state. Based on existing structural data, we present a model of the TIGIT:nectin-2 complex and utilized complementary biochemical studies to map the nectin-binding interface on TIGIT. Our data provide important structural and biochemical determinants responsible for the recognition of nectin-2 by TIGIT. Defining the TIGIT:nectin-2 binding interface provides the basis for rational manipulation of this molecular interaction for the development of immunotherapeutic reagents in autoimmunity and cancer.
1. INTRODUCTION
T cell activation and differentiation requires two distinct synergistic signals. Antigen-specific interactions between peptide-MHC complexes on antigen presenting cells (APCs) and the cognate T cell receptors (TCR) provide the first signal for activation. A second antigen-independent signal results from the engagement of costimulatory receptors on T cells with their protein ligands on APCs. In absence of this second signal (costimulatory signal), T cells enter a state of non-responsiveness referred as anergy (1, 2). CD28 and CTLA-4 are the most extensively studied costimulatory molecules, which upon recognition of the B7-1/B7-2 ligands, deliver stimulatory and inhibitory signals, respectively (1, 2). However, there are numerous additional receptor-ligand interactions that regulate downstream signaling and contribute to the course, duration and strength of T cell responses (1-3). Defining the mechanisms responsible for these signaling events and the manner in which these processes are coordinated is critical for a complete understanding of T cell function and for the generation of new immune-based strategies to treat infectious diseases, autoimmunity and malignancies.
TIGIT is a recently identified immune receptor expressed on NK cells and specific T cell subsets, which interacts with the poliovirus receptor (PVR, also known as CD155 and nectin-like 5) and nectin-2 (also known as PVRL2 and CD112). Initial reports suggested that the interaction between TIGIT and PVR, expressed on dendritic cells (DCs), induces tolerogenic DCs that impair T cell proliferation and inhibit IFN-γ production from responding T cells (4). However, a recent study using antibody-mediated TIGIT blockade demonstrated direct inhibition of T cell responses, independent of APCs (5). The inhibitory function of TIGIT is attributed to the presence of an immune receptor tyrosine-based inhibitory motif (ITIM), 229LSYRSL234, in its cytoplasmic tail. Consistent with this notion, a point mutation in the putative ITIM (Y131A) abolishes its inhibitory function (6). It has also been demonstrated that upon engagement with either PVR or nectin-2, TIGIT can directly inhibit in vitro NK cell cytotoxicity through its ITIM (6).
In addition to TIGIT, another receptor CD226 expressed on T cells and NK cells also recognizes the same set of ligands, PVR and nectin-2, and delivers stimulatory signals to T and NK cells (7-9). These studies established TIGIT as a receptor of a newly emerging costimulatory network consisting of CD226/TIGIT:nectin-2/PVR, which exhibits significant parallels to the CD28/CTLA-4:B7-1/B7-2 pathways. Like the CD28/CTLA-4:B7 interactions, this set of molecules regulates the functional outcome of T cell activation and perturbation of the balance between activating and inhibitory signals results in increased susceptibility to infection or the induction of autoimmunity (5, 10). The genetic linkage of the CD226/TIGIT pathways to a number of human autoimmune diseases further supports an important role in controlling autoimmune responses (11). In addition, a recent study demonstrated that the homozygous deletion of TIGIT results in hyper-proliferative T cell responses and increased susceptibility to autoimmune diseases in mice (5).
Given the significance of CD226/TIGIT:nectin-2/PVR pathways in T cell and NK cell-mediated immunosurveillance, we examined the molecular basis for the TIGIT:nectin-2 interaction. In this study, we determined the high resolution crystal structure of TIGIT, and mapped its nectin-2 binding interface by structure-guided mutagenesis. Although, our analysis shows that TIGIT and nectin-2 interact through their IgV domain in the canonical front sheet-to-front sheet fashion observed in other complexes involving members of the immunoglobulin superfamily (IgSF), we also describe a distinctive lock-and-key mechanism that support this molecular interaction.
2. EXPERIMENTAL PROCEDURES
2.1. Cloning, expression and purification of TIGIT
The IgV domain of human TIGIT (residues 22 to 137) was cloned into pET3a (Novagen) and expressed in E. coli strain BL21 (DE3) pLysS as insoluble inclusion bodies. Protein expression was induced at OD600 of 0.5 with 1.0 mM IPTG, cells harvested by centrifugation and suspended in lysis buffer containing 50 mM Tris-HCl (pH 8.0), 100 mM NaCl, 20% (w/v) sucrose, 1 mM EDTA, and 10 mM dithiothreitol. DNase I (10 μg/ml) was added to the suspension, and then lysed by sonication, and insoluble protein pelleted by centrifugation. The inclusion bodies were washed three times with wash buffer (10 mM Tris-HCl (pH 8.0), 100 mM NaCl, 0.5% Triton X-100, 1 mM EDTA and 10 mM DTT). The detergent was removed by washing the inclusion bodies twice with wash buffer in which the Triton X-100 was omitted. Protein purity was confirmed by SDS-PAGE.
The purified, detergent-free inclusion bodies were solubilized in buffer containing 6 M guanidine hydrochloride, 10 mM Na-acetate (pH 4.5), 5 mM EDTA, and 1 mM DTT to a final concentration of 1mg/ml. The solubilized material was refolded by rapidly diluting 15 ml into 1 liter of buffer composed of 400 mM arginine hydrochloride, 100 mM Tris-HCl (pH 8.0), 1 mM EDTA, 5 mM reduced glutathione and 0.5 mM oxidized glutathione (12, 13). This rapid dilution step was repeated at 4 hr intervals for a total of five additions, with the entire process being performed at 4°C with constant stirring. The refolded protein was purified by size-exclusion chromatography with a buffer composed of 20 mM HEPES (pH 7.0), 150 mM NaCl, and 1 mM EDTA. TIGIT mutants (T112A, Y113A and P114A) were generated by introducing point mutations using QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA), and purified by the same procedure described for wild type TIGIT. The IgV domain of human nectin-2 was refolded and purified as described previously (14).
2.2. Analytical ultracentrifugation sedimentation equilibrium analysis
Sedimentation equilibrium experiments were performed at 20 °C using a Beckman XL-I analytical ultracentrifuge, six-sector cells, and an AN-60Ti rotor. The 280 nm absorption scans (at 20,000 and 25,000 rpm) of TIGIT (concentrations of 9 μM, 21 μM and 47 μM) in 20 mM HEPES (pH 7.0), 150 mM NaCl, and 1 mM EDTA were globally analyzed using HeteroAnalysis ver 1.1.44 (15). Buffer density and the partial specific volume were calculated using SEDNTREP version 1.01 (16).
2.3. Crystallization and structure determination
TIGIT (13 mg/ml in 20 mM HEPES, pH 7.0, 150 mM NaCl, and 1 mM EDTA) was crystallized using the sitting drop vapor diffusion method at room temperature by mixing 0.5 ul of protein with 0.5 ul of precipitant. Distinct crystal forms were observed in (i) 0.1M MES pH 6.0, 1.3M (NH4)2SO4 (monoclinic form) and (ii) 0.1M Bis-Tris pH 5.5, 1.2M (NH4)2SO4, 1% PEG 3350 (hexagonal form). Crystals were cryoprotected in mother liquor supplemented with 15% glycerol prior to flash-cooling in liquid nitrogen. Diffraction was consistent with the space group P21 (a = 39.95, b = 74.67 Å, c =43.35 and β = 92°) for the monoclinic form and P6422 (a = b = 118.61 Å; c = 98.20 Å; and α = β = 90°; γ = 120°) for the hexagonal form, with two molecules per asymmetric unit in both cases. X-ray diffraction data were collected at the X29A beam line (National Synchrotron Light Source) and integrated and scaled with HKL2000 (17). The structure of the P21 crystal form was determined by molecular replacement with the program MOLREP (CCP4) using the CHAINSAW (CCP4) truncated model of 1IKF (25% identity) (18). Initial placement with MOLREP followed by rigid body refinement with REFMAC5 resulted in clear density for the additional residues of TIGIT that were missing in the truncated model (19). The resulting model was input to aRP/wARP with data extending up to 1.7Å resolution. The model was further improved by alternative cycles of manual revision with COOT and refinement using REFMAC5, yielding a final Rwork and Rfree of 20.9% and 23.7%, respectively. The structure of hexagonal crystal form was determined by a similar procedure to 2.7Å resolution using the monomer from the monoclinic crystal form as the search model, and refined to a final Rwork and Rfree of 20.8% and 25.3%, respectively. Atomic coordinates were deposited to the PDB with PDB ID: 3Q0H and 3RQ3, for monoclinic and hexagonal forms, respectively. The details of data and refinement statistics are provided in Table 1.
Table 1.
Crystallographic data, phasing, and refinement statistics
| PDB ID | 3RQ3 | 3Q0H |
|---|---|---|
| Source | NSLS X29A | NSLS X29A |
| Wavelength (Å) | 0.9790 | 0.9790 |
| Resolution limits (Å) | 37.82-2.7 | 39.9-1.7 |
| Space group | P 64 2 2 | P 21 |
| Unit cell a, b, c (Å) | 118.61, 118.61, 98.20 | 39.95, 74.67, 43.35 and β = 92°.4 |
| Number of observations | 159547 | 202847 |
| Number of unique reflections | 11655 | 27916 |
| Completeness (%) | 100(100) | 99.7 (100) |
| Mean I/σIc | 30.5(3.0) | 43.8/3.4 |
| Rmerge on Ia | 0.085(0.89) | 0.038(0.54) |
| Redundancy | 13.7(13.9) | 7.3(7.1) |
| Refinement | ||
| Resolution limits (Å) | 37.82-2.7 | 39.91-1.7 |
| Number of reflections (work/test) | 11255/536 | 27504/1376 |
| Rwork b | 0.208 | 0.207 |
| Rfree | 0.253 | 0.237 |
| Protein/H2O | 1636/38 | 1648/120 |
| Mean B values (Å2) | 40.8 | 18.7 |
| Bonds (Å) / angles (°) | 0.02/1.85 | 0.015/1.68 |
| Ramachandran plot (favored/allowed) | 98.1/1.9 | 98.8/1.2 |
Rmerge = ΣhklΣi|Ii(hkl) - <I(hkl)>|/ΣhklΣi Ii(hkl).
Rwork = Σ|Fo - Fc|/ΣFo, where Fo and Fc are observed and calculated structure factors, respectively.
Values in parentheses correspond to the high resolution bin.
2.4. Surface plasmon resonance (SPR) for binding studies
Binding studies were performed with a BIAcore 3000 optical biosensor at 25°C. The IgV domain of human nectin-2 was immobilized on a research-grade CM5 sensor chip using amine-coupling chemistry to a level of ~1,000 RU (the concentration of protein used during immobilization was 10 nM to 20 nM consistent with monomer). During the course of the binding assay, different concentrations (1–100 μM) of human TIGIT were injected at a flow rate of 20 μl/min for 2 minutes in HBS-EP buffer (10 mM HEPES, pH 7.4, 150 mM NaCl, 3 mM EDTA, 0.005% Tween-20). Following injection, proteins were allowed to dissociate in protein-free HBS-EP buffer. The final response was obtained by subtracting the response of the blank channel (with no protein immobilized) from that of the experimental channel and plotting the difference against the corresponding concentration. The equilibrium dissociation constant (Kd) was obtained by fitting the data to the 1:1 Langmuir binding model using GraphPad Prism 5 (GraphPad). To assess the impact of point mutations on TIGIT activity, each mutant was injected at 10 μM and compared with the response of wild type TIGIT at the same concentration. Similarly, a series of concentrations of TIGIT (from 62.5 nM to 8 μM) was injected over the experimental flow cell containing immobilized human PVR (purchased from R&D systems) and the reference flow cell and the Kd determined as described above.
2.5. Thermal stability assay
Thermal denaturation of wild type and Y113A was performed using the iQ5 real-time PCR detection system (Biorad). Briefly, each protein (at 10 μM, which is consistent with monomer) was mixed with Sypro orange, loaded into the thermal cycler, and the temperature ramped from 25 °C to 99 °C in 1 °C increments with a dwell time of 6 seconds. The negative first derivative of the fluorescence change (−dRFU/dT) for each protein is plotted against temperature and the melting temperature is defined as the minimum in the −dRFU/dT curve.
2.6. Molecular modeling
A structural model of the TIGIT:nectin-2 complex was constructed with the MODELLER program (20), using TIGIT and nectin-2 (PDB accession codes: 3RQ3 and 3R0N, respectively) as structural templates.
3. RESULTS
3.1. TIGIT is predominantly a monomer in solution but forms a dimer at high concentrations
Bacterially expressed, refolded and purified human TIGIT IgV domain (calculated MW of 11.21 kDa (Fig 1A) eluted as monodisperse peak on a calibrated gel filtration column with apparent molecular mass < 17 kDa when loaded at a concentration of 10 μM (Fig 1B). Detailed analysis on the gel filtration profile revealed that TIGIT can form a dimer in solution when loaded at a concentration of 350 μM (Fig 1C). Analytical sedimentation equilibrium yields a Mw = 13563 Da, consistent with monomeric TIGIT in the 9 μM to 47 μM concentration range. Together, size exclusion chromatography and sedimentation equilibrium analysis show that the IgV domain of human TIGIT is predominantly a monomer at low concentrations in solution, with the propensity to form a dimer at higher concentration, consistent with the observation of dimers in the crystalline state (see below).
Figure 1.
Solution properties of the IgV domain of human TIGIT. (A) SDS-PAGE showing refolded and purified TIGIT (11.2 kDa, right lane; molecular weight standards are in the left lane). (B) TIGIT (blue) and molecular weight standards (dotted) were analyzed by size exclusion chromatography on a Superdex 200 column. The standard peaks are labeled with their molecular weights (in kDa). TIGIT elutes slightly after the 17 kDa peak which indicates a monomer in solution at the concentrations examined (10 uM). (C) Size exclusion chromatography showing the monomeric (blue, at low concentration), and dimeric (green, at high concentration) state of TIGIT. Dotted line represents the molecular weight standards, and all of them were analyzed on a preparative grade Superdex 200 column.
3.2. Overall structure of human TIGIT ectodomain
TIGIT is a single-pass type-1 membrane glycoprotein consisting of a single IgV domain, followed by a transmembrane region and a cytoplasmic tail (Fig 2A). The asymmetric unit of the hexagonal crystal form contains two copies of the molecule, each of which exhibits the classic two-layer β-sandwich topology present in other IgV structures (21) (Fig 2B). The structures of the two independent molecules are very similar, with an RMSD of 0.27 Å for 97 Cα atoms (Fig 2C). The front and back sheets of the domain are composed of the GFCC’C” and ABED strands, respectively (Fig 2B). Similar to other conventional Ig-super family (IgSF) members (21), the B and F strands of TIGIT are linked by a hallmark disulfide bond (Fig 2B), which is important for stabilizing the IgV domain (22). Molecules related by two-fold non-crystallographic symmetry generate a dimer, with the interface formed by the nearly orthogonal association of C, C’, C”, F, and G strands from the front sheets of the two engaging IgV domains (Fig. 2D). This organization results in a “kinked” dimer with an end-to-end distance of 62 Å, which is similar to the quaternary structure observed in numerous other dimers involving IgSF members. The overall fold of the two independent IgV domains in the asymmetric unit are also very similar in the monoclinic crystal form (PDB ID: 3Q0H), with an RMSD of 0.43 Å over 90 Cα atoms. Notably, the front sheet-to-front sheet dimer (as seen in the hexagonal form) is not observed in this form of crystal. The IgV domains present in hexagonal and monoclinic crystal forms are also similar with an RMSD of 0.72 Å for 92 Cα atoms when the respective A chains are compared. The conformation of front sheet β-strands and FG loop, which are known to be involved in canonical interactions between two engaging IgV domains, are nearly identical in these two crystal forms. The details of data and refinement statistics of these two structures are shown in Table 1.
Figure 2.
Structural organization of TIGIT. (A) TIGIT consists of a single extracellular IgV domain followed by a transmembrane region and a cytoplasmic tail that contains the inhibitory ITIM motif. (B) Structure of the IgV domain of TIGIT showing a classical two-layer β-sandwich topology. The front and back sheets of the domain are composed of the GFCC’C” and AA’BED strands, respectively. The conserved disulfide bond between the B and F strands is marked by an arrow. (C) Superimposition of the two protomers present in the asymmetric unit of the hexagonal crystal form reflects their similar structural organization (RMSD of 0.27 Å for 97 Cα atoms). (D) Two monomers (gray and cyan) of the hexagonal crystal form interact in a nearly orthogonal fashion to form the dimer, resulting in an end-to-end distance of ~ 62 Å. The dimer interface is formed by the orthogonal association of the front β sheets with predominant contribution from the residues of the FG loop, and C, C’, F, and G strands of each monomer.
3.3. The homodimer interface of TIGIT
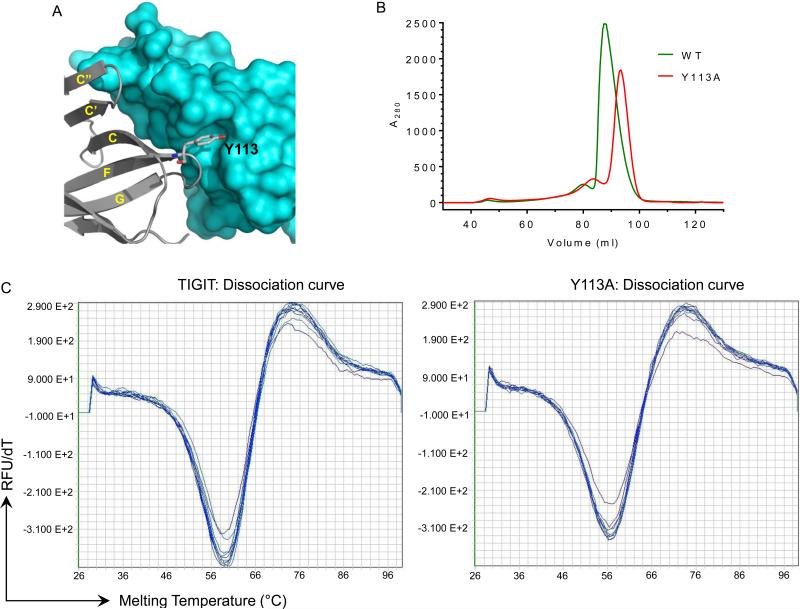
In agreement with our solution studies demonstrating that TIGIT forms a dimer at high concentration and a previous crystallographic report that suggests the dimeric association of this molecules (23, PDB: 3UCR), here we also observed a canonical dimeric association of TIGIT in the crystalline state. In the hexagonal crystal form (PDB ID: 3RQ3), molecules related by two-fold non-crystallographic symmetry generate a dimer typical of the IgSF, which buries a total of 1330 A2 of surface area at the dimer interface. Fourteen residues (Thr-55, Gln-56, Asp-72, Trp-75, His-76, Thr-112, Tyr-113 and the symmetry-related residues from the engaging partner) are involved in sixteen potential hydrogen bonds. Several residues contribute van der Waals contacts to this homophilic interface, including Asn-58, Leu-65, Ile-68, Leu-73, His-76, His-111, Tyr-113, Pro-114 and Gly-116, which are partially or completely buried at the dimer interface. Most of the residues contributing to the dimer interface are polar, except for Ile-68 and Leu-73, which form a small hydrophobic core at the interface together with Tyr-113 from the interacting partner. Leu-73, being situated on the C” strand (edge of the front sheet), is only partially buried in the interface. The side chain of Tyr-113 is buried in a largely hydrophobic pocket formed by the Leu-73 and His-76 on the C” strand, and Ile-68 and Asn-70 on the C’ strand of the engaging molecule (Fig 3A).
Figure 3.
Tyr-113 is an important residue at the TIGIT homodimer interface. (A) Tyr-113 (shown by ball-and-stick model) is buried in a hydrophobic pocket of the engaging molecule (represented by surface). (B) The contribution of Tyr-113 is confirmed by size-exclusion chromatography. The elution profile of wild type TIGIT is shown by green and Y113A by red lines. The elution volume of Y113A increases significantly. (C) Fluorescence-monitored thermal denaturation assay shows the dissociation curve of wild type TIGIT and Y113A mutant.
The dimer interface observed in the hexagonal crystal form (PDB ID: 3RQ3) is consistent with the results of mutagenesis experiments. For example, the Y113A mutant exhibited a significantly decreased molecular weight as judged by size exclusion chromatography (Fig 3B), even though the stability of this mutant protein is comparable to the wild type protein as assessed by fluorescence monitored thermal denaturation (Fig 3C). These experiments were performed with protein concentrations of 10 μM, where both should be monomeric.
3.4. TIGIT:nectin-2 interaction is weaker than the TIGIT:PVR interaction
In addition to the homophilic interaction, nectin-2 also participates in heterophilic interactions with nectin-3 to mediate cell-cell adhesion, as well as with the TIGIT and CD226 immune receptors. On the basis of cell-based experiments, it has been reported that TIGIT exhibits a much higher affinity for PVR than nectin-2 (4, 6). Using SPR methods, we determined the Kds for the TIGIT:nectin-2 and TIGIT:PVR interactions to be 11.5 (±1.1) μM and .36 μM (±.025) μM, respectively (Figs 4A and 4B). The difference in binding affinity between these two interactions is consistent with the previous report that nectin-2 is a low affinity ligand for TIGIT compared to PVR (4).
Figure 4.
Equilibrium dissociation constants of TIGIT:nectin-2 and TIGIT:PVR interactions as assessed by surface plasmon resonance (A) Steady-state analysis reveals that the IgV domain of TIGIT binds nectin-2 with a Kd value of about 11.5 μM. (B) Steady-state binding data shows that TIGIT binds PVR with a Kd value of about 0.36 μM.
3.5. Mapping the nectin-2 binding sites on TIGIT reveals a “lock-and-key” mechanism
The homodimeric structures of the IgV domains of nectin-2 (14) and TIGIT observed in the hexagonal form share a considerable similarity with an RMSD of 2.07 Å for 166 Cα atoms (Fig 5A). On this basis, we hypothesized that the TIGIT:nectin-2 heterophilic interface is similar to the homophilic interfaces utilized by these molecules and generated a molecular model of the TIGIT:nectin-2 interaction, in which the heterodimer interface is formed by interactions between the front β-sheets of each molecule (Fig 5B). The conserved motif 112TYP114, located in the FG loop region of TIGIT, is predicted to play a major role in this interaction, as all of these residues are significantly buried at the proposed interface (Fig 5C). Of particular importance, Tyr-113 acts as the “key” for this molecular recognition, fitting nicely into a hydrophobic pocket formed by His-86, Met-73, Ala-84 and Leu-67 from the engaging nectin-2 molecule (Fig 5C). A similar “lock-and-key” mechanism was also described in the structure of the TIGIT:PVR interaction (23). To assess the contribution of these FG loop residues, we evaluated the binding of TIGIT mutants (T112A, Y113A, and P114A) with nectin-2 by SPR. All of these three mutants exhibited reduced binding to nectin-2 when compared with the same concentration of wild type TIGIT, supporting an important role for the 112TYP114 motif in the recognition of nectin-2 (Fig 5D).
Figure 5.
Mapping of nectin-2 binding interface on TIGIT. (A) Structure alignment study shows the overall structural similarity between the TIGIT (gray) and nectin-2 (violet) homodimers with an RMSD of 2.07 Å for 166 Cα atoms (B) Molecular model of the heterophilic interaction between TIGIT and nectin-2 based on a canonical front sheet-to-front sheet interaction. (C) The modeling also suggests that the FG loop residues are crucial for this interaction. TIGIT FG loop residues 112TYP114 are represented by ball-and-stick model, while nectin-2 is represented by a surface representation. Tyr-113 is buried in a hydrophobic pocket formed by the engaging nectin-2 molecule. (D) Wild type TIGIT and three mutants (T112A, Y113A, and P114A) were injected over experimental (containing immobilized human nectin-2) and reference flow cells at 10 μM concentration. The final response of each protein was obtained by subtraction of the response of the reference cell from the experimental cell.
4. DISCUSSION
The nectins are a group of type-I membrane glycoproteins involved in cell adhesion processes, which act independently or in concert with other cell adhesion molecules such as cadherins (24). Recent structural reports have proposed mechanisms for the homophilic, as well as selective heterophilic interactions among nectins that contribute cell-cell adhesion. (14, 25, 26). Several studies also demonstrated that nectins are capable of a range of heterophilic interactions involving proteins outside the nectin family (4, 6, 27). In particular, nectin-2 plays an important role in immune modulation due to its ability to interact with TIGIT and CD226 expressed on T cells and NK cells. The homophilic interaction of nectin-2 and its heterophilic interaction with CD226 have been previously characterized (14, 28). However, in the absence of either the apo structure of CD226 or its complex together with Nectin-2 or PVR and in the absence of mutational/biophysical studies to define the CD226 interaction with its cognate partners Nectin-2 and PVR, it is difficult to propose the exact organization of these complexes. Present study defines the molecular basis of TIGIT:nectin-2 recognition. In addition to reporting high resolution crystal structures and the solution properties of the human TIGIT ectodomain, we biochemically characterized the heterophilic interaction between TIGIT and nectin-2, and mapped the nectin-2 binding surface on TIGIT based on structure-guided molecular modeling and complementary mutagenesis studies. A recent study involving structural, biochemical and cell-based analyses of the TIGIT:PVR interaction suggests that TIGIT can self-associate through the back β-sheet (i.e., A, B, E, and D strands) of its ectodomain and assemble as cis-homodimers on the cell surface (23). This laterally associated TIGIT cis-homodimer forms a canonical trans interaction with PVR via their front β-sheets (23).
Although the TIGIT:PVR interaction (Kd ~ 350 nM) is much stronger than the TIGIT/nectin-2 interaction (Kd ~ 11 μM), we propose that the overall recognition mode is similar in both cases. The 112TYP114 motif of TIGIT plays a significant role in these interactions, where Tyr-113 is buried in a hydrophobic pocket formed by residues of the engaging molecule. Point mutations of this motif in TIGIT reduce the affinity for both nectin-2 and PVR (Fig 5D and (23)). The structure of the TIGIT:PVR complex also revealed that the 127TFP129 motif present in the FG loop of PVR (the equivalent of TIGIT 112TYP114) is important for this molecular recognition, where Phe-128 is similarly buried in a hydrophobic pocket formed by the engaging TIGIT (23). Moreover, the observed conformations of the T-(Y/F)-P loops are highly similar, including the side chain conformations, suggesting that this loop makes analogous contacts in both the TIGIT:PVR and TIGIT:Nectin-2 heterodimers. In addition to the homophilic interface of TIGIT involving canonical heterophilic front sheet-to-front-sheet contacts, we also observed back sheet-to-back sheet contacts of TIGIT molecules related by 2-fold crystallographic symmetry in both the monoclinic and hexagonal forms; these back sheet-to-back sheet interactions are nearly identical to those described in the TIGIT:PVR complex (3UDW) (Fig 6A). The observation of this back sheet-to-back sheet association in both TIGIT and the TIGIT:PVR complex (3UDW) is consistent with the previously proposed biological significance of this non-canonical interaction (23). Moreover, based on our structural observations, we constructed a model in which two TIGIT molecules interact via their back β-sheets at the center, with each of them additionally interacting with nectin-2 through canonical front sheet-to-front sheet contacts (Fig 6B), similar to the TIGIT:PVR interaction.
Figure 6.
Structural interpretation of TIGIT:TIGIT and TIGIT:nectin-2 recognition. (A) Two TIGIT molecules located at the center interact through their canonical front sheet-to front sheet contacts, and each of these two molecules also interacts with another TIGIT through their back sheet-to-back sheet contact. This assembly causes the formation of a homotetramer in the crystal. (B) Based on the tetrameric assembly of TIGIT (3RQ3) and the TIGIT:PVR complex (3UDW), the TIGIT:nectin-2 interaction was modeled, in which two TIGIT molecules located at the center interact through back sheet-to-back sheet contacts, while the front sheet of these two molecules recognizes nectin-2 at the periphery via canonical front sheet interactions.
Most canonical interactions between the IgV domains possess certain key features, including orthogonal packing of the front sheets and in particular the packing of the FG strands and the FG loop of one protomer against the front face of other (predominantly involving C, C’, C”, F and G strands). This mode of recognition relies on complementary interactions between the two engaging front faces and results in substantial burial of elements of these surfaces. Although the conformation and residue composition (similarity) of the front-face is conserved among TIGIT, Nectin-2 and PVR, the CC’ loops are slightly longer in both PVR and Nectin-2. PVR and Nectin-2 have two and five residue insertions, respectively, in the CC’ loop compared to TIGIT (Fig 7A). These insertions and the residue composition in the PVR CC’ loop appears to support efficient engagement, positioning the CC’ loop (residues 72SGS74) ~4.5 Å from the G-strand (residues 115DGT117) of the interacting TIGIT molecule, resulting in three backbone-backbone and backbone-side chain interactions (Fig 7B). In the case of TIGIT:nectin-2 (modeled) and TIGIT:TIGIT dimers, the closest approaches of the CC’ loop to the G-strand of the interacting partner (TIGIT residues 115-117) is ~ 8.7 Å and 11.8 Å, respectively, which preclude the additional interactions involving CC’ loop observed in TIGIT:PVR complex.
Figure 7.
Contribution of CC’ loop in the recognition of TIGIT:TIGIT/nectin-2/PVR. (A) Structure-based sequence alignment study shows the insertion of two and five residues at the CC’ loop of PVR and nectin-2 respectively, in comparison to the CC’ loop of TIGIT. (B) The CC’ loop of PVR contributes to the heterophilic interaction. In particular, 72SGS74 from the CC’ loop of PVR makes several contacts with G strand residues (115DGT117) of the engaging TIGIT.
Moreover, the homodimer interface of TIGIT buries about 1330 A2, while both TIGIT:nectin-2 and TIGIT:PVR interactions result in the burial of approximately 1600 A2 at their heterophilic binding interface. Furthermore, the homodimeric interface of TIGIT is mostly composed of polar residues, in contrast to the TIGIT:PVR and TIGIT:nectin-2 heterodimeric interfaces where hydrophobic interactions are prevalent, which is consistent with the relatively weak homodimerization of TIGIT. As mentioned earlier, the closer association of the CC’ loop of PVR with the G-strand of the TIGIT also appear to contribute to the greater affinity observed in the TIGIT:PVR complex compared to TIGIT:Nectin-2 complex. The chemical and physical determinants underlying the homophilic and heterophilic interactions of TIGIT appear to explain the spectrum of affinities among these interacting molecules, which is required for achieving optimal biological functions.
The recognition of nectin-2 and PVR by two receptors TIGIT and CD226 with opposing functions is reminiscent of CTLA-4/CD28:B7-1/B7-2 network where the CTLA-4 inhibitory receptor binds the same ligands (B7-1 and B7-2) as the CD28 activating receptor (Fig 8). Enhanced expression of nectin-2 is observed in various tumors (8, 29, 30) and several reports suggest that nectin-2 expression on tumors might enhance NK cell cytotoxicity (8, 31, 32). Furthermore, it was recently demonstrated that CD226 knockout mice exhibit enhanced tumorigenicity (29). In contrast, TIGIT knockout mice showed uncontrolled T cell proliferation and susceptibility to autoimmune diseases (5). Therefore, manipulation of CD226/TIGIT:nectin-2/PVR pathways might provide important therapeutic strategies against malignancies and autoimmune diseases (33-36). The structural and biochemical features of the receptor-ligand interactions discussed here further define the molecular basis of these interactions and offer insights for the manipulation of these emerging pathways for therapeutic purposes. For example, the structure-guided engineering of ligands (either nectin-2 or PVR) that preferentially interact with CD226 or TIGIT to provide stimulatory or inhibitory signals in the tumor microenvironment or in the context autoimmunity will be of great interest for mechanistic analysis and may possibly represent new therapeutic strategies.
Figure 8.
Schematic comparison of TIGIT/CD226:nectin-2/PVR pathways with the CTLA-4/CD28:B7-1/B7-2 pathways. TIGIT is an inhibitory receptor like CTLA-4, whereas CD226 is an activating receptor like CD28. Both sets of molecules are engaged by two sets of counter-ligands, TIGIT and CD226 by nectin-2 and PVR, and CTLA-4 and CD28 by B7-1 and B7-2. Activation is indicated by ‘+’ and inhibition is indicated by ‘–’ symbol.
Highlights.
T-cell stimulatory and inhibitory signals control mammalian immune system
TIGIT:nectin-2 interaction delivers inhibitory signals to T cell.
This study reveals structural and biochemical basis of TIGIT:nectin-2 recognition
A distinctive “lock-and-key” mechanism supports this molecular interaction
This study provides basis for rational manipulation of TIGIT:nectin2 interaction.
ACKNOWLEDGMENT
Authors gratefully acknowledge the contribution of the late Prof. Stanley G. Nathenson to the early aspects of this work. We thank the staff of the X29A beam line at the National Synchrotron Light Source; Michael Brenowitz for help with analytical ultracentrifugation.
Funding Information:
This work was supported by National Institutes of Health Grants AI007289 (to Prof. Nathenson and SCA), GM094662 (SCA) and GM094665 (SCA). The Albert Einstein Cancer Center is supported by NIH P30CA013330.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:2233–2258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 2.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 3.Chattopadhyay K, Lazar-Molnar E, Yan Q, Rubinstein R, Zhan C, et al. Sequence, structure, function, immunity: structural genomics of costimulation. Immunol Rev. 2009;229:356–386. doi: 10.1111/j.1600-065X.2009.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 5.Joller N, Hafler JP, Brynedal B, Kassam N, Spoerl S, et al. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol. 2011;186:1338–1342. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci USA. 2009;106:17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shibuya A, Campbell D, Hannum C, Yssel H, Franz-Bacon K, et al. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity. 1996;4:573–581. doi: 10.1016/s1074-7613(00)70060-4. [DOI] [PubMed] [Google Scholar]
- 8.Lakshmikanth T, Burke S, Ali TH, Kimpfler S, Ursini F, et al. NCRs and DNAM-1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J Clin Invest. 2009;119:1251–1263. doi: 10.1172/JCI36022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlsten M, Björkström NK, Norell H, Bryceson Y, van Hall T, et al. DNAX accessory molecule-1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells. Cancer Res. 2007. 2007;67:1317–1325. doi: 10.1158/0008-5472.CAN-06-2264. [DOI] [PubMed] [Google Scholar]
- 10.Levin SD, Taft DW, Brandt CS, Bucher C, Howard ED, et al. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur J Immunol. 2011;41:902–915. doi: 10.1002/eji.201041136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hafler JP, Maier LM, Cooper JD, Plagnol V, Hinks A, et al. CD226 Gly307Ser association with multiple autoimmune diseases. Genes Immun. 2009;10:5–10. doi: 10.1038/gene.2008.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garboczi DN, Ghosh P, Utz U, Fan QR, Biddison WE, et al. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996;384:134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Schwartz JC, Almo SC, Nathenson SG. Expression, refolding, purification, molecular characterization, crystallization, and preliminary X-ray analysis of the receptor binding domain of human B7-2. Protein Expr Purif. 2002;25:105–113. doi: 10.1006/prep.2002.1616. [DOI] [PubMed] [Google Scholar]
- 14.Samanta D, Ramagopal UA, Rubinstein R, Vigdorovich V, Nathenson SG, Almo SC. Structure of Nectin-2 reveals determinants of homophilic and heterophilic interactions that control cell-cell adhesion. Proc Natl Acad. Sci USA. 2012;109:14836–14840. doi: 10.1073/pnas.1212912109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole JL. Analysis of heterogeneous interactions. Methods Enzymol. 2004;384:212–232. doi: 10.1016/S0076-6879(04)84013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glasel JA. Introduction to Biophysical Methods for Protein and Nucleic Acid Research. Academic Press; San Diego: 1995. [Google Scholar]
- 17.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 18.Altschuh D, Vix O, Rees B, Thierry JC. A conformation of cyclosporin A in aqueous environment revealed by the X-ray structure of a cyclosporin-Fab complex. Science. 1992;256:92–94. doi: 10.1126/science.1566062. [DOI] [PubMed] [Google Scholar]
- 19.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 20.Eswar N, Webb B, Marti-Renom MA, Madhusudhan MS, Eramian D, et al. Comparative protein structure modeling with MODELLER. Current Protocols in Bioinformatics. 2006 doi: 10.1002/0471250953.bi0506s15. Chapter 5: Unit 5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bork P, Holm L, Sander C. The immunoglobulin fold. Structural classification, sequence patterns and common core. J Mol Biol. 1994;242:309–320. doi: 10.1006/jmbi.1994.1582. [DOI] [PubMed] [Google Scholar]
- 22.Proba K, Honegger A, Plückthun A. A natural antibody missing a cysteine in VH: consequences for thermodynamic stability and folding. J Mol Biol. 1997;265:161–172. doi: 10.1006/jmbi.1996.0726. [DOI] [PubMed] [Google Scholar]
- 23.Stengel KF, Harden-Bowles K, Yu X, Rouge L, Yin J, et al. Structure of TIGIT immunoreceptor bound to poliovirus receptor reveals a cell-cell adhesion and signaling mechanism that requires cis-trans receptor clustering. Proc Natl Acad Sci USA. 2012;109:5399–5404. doi: 10.1073/pnas.1120606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takai Y, Ikeda W, Ogita H, Rikitake Y. The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu Rev Cell Dev Biol. 2008;24:309–342. doi: 10.1146/annurev.cellbio.24.110707.175339. [DOI] [PubMed] [Google Scholar]
- 25.Narita H, Yamamoto Y, Suzuki M, Miyazaki N, Yoshida A, et al. Crystal Structure of the cis-Dimer of Nectin-1: implications for the architecture of cell-cell junctions. J Biol Chem. 2011;286:12659–12669. doi: 10.1074/jbc.M110.197368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrison OJ, Vendome J, Brasch J, Jin X, Hong S, et al. Nectin ectodomain structures reveal a canonical adhesive interface. Nat Struct Mol Biol. 2012;19:906–915. doi: 10.1038/nsmb.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan CJ, Andrews DM, Smyth MJ. Receptors that interact with nectin and nectin-like proteins in the immunosurveillance and immunotherapy of cancer. Curr Opin Immunol. 2012;24:246–251. doi: 10.1016/j.coi.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Qian X, Chen Z, Xu X, Gao F, et al. Crystal structure of cell adhesion molecule nectin-2/CD112 and its binding to immune receptor DNAM-1/CD226. J Immunol. 2012;188:5511–5520. doi: 10.4049/jimmunol.1200324. [DOI] [PubMed] [Google Scholar]
- 29.Iguchi-Manaka A, Kai H, Yamashita Y, Shibata K, Tahara-Hanaoka S, et al. Accelerated tumor growth in mice deficient in DNAM-1 receptor. J Exp Med. 2008;205:2959–2964. doi: 10.1084/jem.20081611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oshima T, Sato S, Kato J, Ito Y, Watanabe T, et al. Nectin-2 is a potential target for antibody therapy of breast and ovarian cancers. Mol Cancer. 2013;12:60. doi: 10.1186/1476-4598-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pende D, Bottino C, Castriconi R, Cantoni C, Marcenaro S, et al. PVR (CD155) and Nectin-2 (CD112) as ligands of the human DNAM-1 (CD226) activating receptor: involvement in tumor cell lysis. Mol Immunol. 2005;42:463–469. doi: 10.1016/j.molimm.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 32.Pende D, Spaggiari GM, Marcenaro S, Martini S, Rivera P, et al. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112). Blood. 2005;105:2066–2073. doi: 10.1182/blood-2004-09-3548. [DOI] [PubMed] [Google Scholar]
- 33.Samanta D, Almo SC. Nectin family of cell-adhesion molecules: structural and molecular aspects of function and specificity. Cell Mol Life Sci. 2015;72:645–658. doi: 10.1007/s00018-014-1763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pauken KE, Wherry EJ. TIGIT and CD226: tipping the balance between costimulatory and coinhibitory molecules to augment the cancer immunotherapy toolkit. Cancer Cell. 2014;26:785–787. doi: 10.1016/j.ccell.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26:923–937. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H, et al. TIGIT and PD-1 impair tumor antigen-specific CD8+ T cells in melanoma patients. J Clin Invest. 2015 doi: 10.1172/JCI80445. doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]