Abstract
Aging process is accompanied by hormonal changes characterized by an imbalance between catabolic hormones that remain stable and anabolic hormones (testosterone, insulin like growth factor-1 (IGF-1) and dehydroepiandrosterone sulphate (DHEAS), that decrease with age. Despite the multiple hormonal dysregulation occurring with age, the prevalent line of research in the last decades has tried to explain many age-related phenomena as consequence of one single hormonal derangement with disappointing results. In this review we will list the relationship between hormonal anabolic deficiency and frailty and mortality in older population, providing evidence to the notion that multiple hormonal dysregulation rather than change in single anabolic hormone is a powerful marker of poor health status and mortality. (www.actabiomedica.it)
Keywords: Anabolic hormones, frailty, mortality
Introduction
Aging has been conceptualized as the declining efficiency of the mechanisms that maintain the homeostatic equilibrium, which is continuously challenged by destabilizing events (1).
Among different pathways involved in the aging process of particular importance are the hormonal changes characterized by an imbalance between catabolic hormones that remain stable and anabolic hormones that decrease across age. All these changes contribute to the catabolic milieu typical of older and accelerated age (2).
Male aging is characterized by a progressive decline in anabolic hormones such as testosterone, insulin like growth factor-1 (IGF-1) and dehydroepiandrosterone sulphate (DHEAS).
The decline in one single anabolic hormone has been associated with specific symptoms and clinical signs. The term late onset hypogonadism (LOH) has been coined to indicate the symptoms possibly related to testosterone deficiency, somatopause to indicate the possible clinical implication of the decline in GH-IGF-1 activity and adrenopause to describe the clinical consequences of low DHEA biological activity (3).
Although there is a clear overlapping between these syndromes, the prevalent line of research in the past decades tried to explain many age-related phenomena as consequence of one single hormonal derangement.
For instance changes in body composition, higher risk of anaemia and metabolic syndrome in older men have been associated with low testosterone levels (4–5), IGF-1 signalling has been considered a determinant of longevity, possibly through beneficial effects on skeletal muscle, vasculature and metabolism (6) and finally DHEAS has been considered one of the mediators of the relationship between caloric restriction and longevity in both animals and humans (7).
However, the idea that a single change may explain the aging process strongly disagrees with the current concept of accelerated aging which implies a parallel dysregulation of multiple systems.
There has been recent interest on one specific model of accelerated aging, physical frailty, an highly prevalent condition in the geriatric population, and definable as a state of vulnerability to stressors and difficulty in maintaining homeostasis, due to decreased physiologic reserves. This condition correlates with many adverse health outcomes, including disability, dependency, falls, hospitalization, need for long-term care and mortality (8).
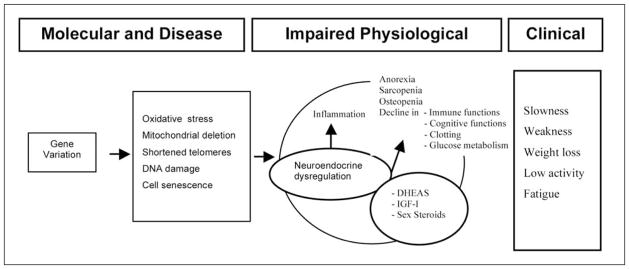
Multiple pathways are involved in the development of frailty syndrome including the hormonal dysregulation (figure 1) (9). According to this hypothesis one single hormonal derangement, namely single anabolic hormone deficiency, has been associated with accelerated aging (frailty) and mortality, with contrasting and often disappointing results.
Figure 1.
Overview of the hypothesized molecular, physiological, and clinical pathway to frailty focusing on the potential interaction between gene variation, oxidative stress and neuroendocrine dysregulation in the development of frailty (Adapted from Ref 9)
Single anabolic deficiency and frailty
DHEAS and frailty
In 494 women aged 70–79 years enrolled in the Women’s Health and Aging Studies I or II, DHEAS deficiency was not associated with frailty, defined according to Fried’s criteria (8, 10). By contrast two other studies found that frail (where frailty was defined according to different criteria) older men and women have lower DHEAS levels (11–12).
Testosterone and frailty
Recently low levels of bioavailable testosterone were independently associated with worse baseline frailty status (13). On the contrary, other studies found no association between total and free testosterone and frail phenotype in both men and women (14, 10).
IGF-1 and frailty
We have also similar and contradictory results for total IGF-1. Leng et al in 18 frail and 33 non frail community-dwelling older adults with complete data on serum levels of IGF-I, DHEA-S, and IL-6 found that age-adjusted serum levels of IGF-I (88±49 vs 122±47 [ng/mL], p<0.023) and DHEA-S (0.30±0.21 vs 0.53±0.25 [microg/mL], p=0.016) were significantly lower in frail vs non-frail individuals, respectively (11).
On the contrary, IGF-1 was not associated and was not predictive of frailty syndrome in older men and women (15), and consistently women with IGF-1 deficiency were not more likely to be frail (10).
Multiple hormonal dysregulation and frailty
Although the relationship between a single hormonal derangement and frailty or accelerated aging (LOH, somatopause, adrenopause) is uncertain, the connection between parallel changes in multiple hormonal axes (multiple hormonal derangement) and the accelerating aging are more interesting. There is strong evidence that neuroendocrine dysregulation has an important role in the clinical development of frailty.
Therefore to weigh the impact of hormonal pathway on aging process and age-related syndromes is probably to consider the multiple and parallel hormonal dysregulation (Figure 1) (9).
By providing notion to this hypothesis, plurihormonal dysregulation rather than single hormonal derangement has been associated with frailty syndrome in both men and women (2, 10–11).
Maggio et al selected from old male population of the InCHIANTI Study 403 non frail subjects mean age ±SD of 73.2±6.2 and 34 frail men with 79.7±7.5, and with complete data on testosterone (total, free, bioavailable), cortisol, DHEAS IGF-1 (total and free), insulin (fasting) and leptin. In this population criteria for diagnosis of frailty syndrome according to Fried et al were applied. They investigated the prevalence of the frailty syndrome according to age-adjusted quintiles of hormonal levels with no evidence of significant trend for any of these hormones (2).
However, the prevalence of frailty increased with the number of hormones dysregulated.
Participants with 2–3 hormones dysregulated were 2.2 times more likely to be frail, while those with more than 4 hormones dysregulated were more than 6 times to be frail.
Accordingly, Cappola et al investigated the relationship between anabolic deficiency (total IGF-1, DHEAS, and free testosterone) and frailty in 494 women aged 70–79 years enrolled in the Women’s Health and Aging Studies I or II. As expected, the prevalence of hormonal dysregulation according to frail status and the percentage of hormonal deficit was higher in the frail group.
In adjusted analyses examining the ability of the hormonal deficiency count to predict frailty, women with one hormonal deficiency were not more likely to be frail than women with no hormonal deficiency. However, women with two or more deficiencies were significantly more likely to be frail than their counterparts with no hormonal deficiency, suggesting a nonlinear relationship between the burden of hormonal deficiencies and frailty (10).
Similarly Leng et al in 18 frail and 33 non-frail community-dwelling older adults with complete data on serum levels of IGF-I, DHEA-S, and IL-6 found that age-adjusted serum levels of IGF-I (88±49 vs 122±47 [ng/mL], p<0.023) and DHEA-S (0.30±0.21 vs 0.53±0.25 [microg/mL], p=0.016) were significantly lower in frail vs non-frail individuals, respectively (11).
The concept that a single hormonal deficiency is not a powerful marker of poor heath status is enforced by studies testing the link between anabolic deficiency (DHEAS, testosterone, IGF-1) and mortality.
Relationship between DHEA e DHEAS and mortality
Table 1 shows the studies testing the relationship between DHEAS and mortality. The first study is the 12 year longitudinal population study of Barrett-Connor et al performed in 242 men (age range 50–79 years) of Rancho Bernardo Study. In this study DHEA levels were related to cardiovascular and all cause mortality. Low DHEAS levels were predictors of cardiovascular but not of all cause mortality. A multivariate analysis adjusted for several confounders (age, BMI, smoking, fasting glucose, cholesterol, systolic blood pressure, history of coronary artery disease) showed that an increase in DHEA concentration is associated with 36% reduction in all cause mortality and 48% decrease in cardiovascular mortality (16). Accordingly, Mazat et al in 290 older men and women of Pasquid study underlined the role of low DHEA level as predictor of all cause mortality, but only in male subjects aged between 65 and 69 years (17). By contrast Cappola, et al, in 5-year follow up period analysis performed in 539 women (mean age 77.6 years) of Women’s Health and Aging Study I (WHAS I), showed a U shape relationship between DHEAS and mortality. DHEAS in the lowest quartile was predictive of cardiovascular mortality and DHEAS levels in the highest quartile were predictive of cancer mortality (18). In a more recent perspective study, Cappola et al in 952 subjects of both sexes followed during 8 –year follow-up period, showed that steep decline and extreme variability in DHEAS levels had a significantly higher death rate than those with neither pattern (141 vs 48 deaths per 1,000 person-years, p < .001) (19). In contrast with these studies, Tilvis et al in 5 year follow-up period longitudinal study conducted in 571 subjects of both sexes did not show any significant relationship between DHEAS and all cause mortality (20).
Table 1.
Studies testing the association between DHEA(S) and mortality
| Author, Year, Ref | Time of follow up (years) | Number and age of Subjects | Results |
|---|---|---|---|
| PROS* | |||
| Barrett-Connor (1986) ref 16 |
12 years | 242 men 50–79 years |
Low DHEAS is a predictor of death It is inversely related with all-cause mortality (36%) and with cardiovascular mortality (48%) |
| Cappola (2006) ref 19 |
8 years | 952 subjects of both sexes (Cardiovascular Health Study) age >65 yr (median ♂ 72.5 yr, median ♀ 73.2 yr) |
DHEA is predictor of mortality in women with significant decline in DHEA levels DHEA is predictor of mortality in men with significant decline in DHEA levels |
| CONS** | |||
| Tilvis (1999) ref 20 |
5 years | 571 subjects 75 years and older |
DHEAS is not predictor of 5 yr mortality, but subjects who died had lower DHEA levels |
| Mazat (2001) ref 17 |
8 years | 290 subjects (119 ♂, 171 ♀) 65 years older |
DHEAS is not predictor of 8 –yr mortality in women. DHEA is predictor of mortality only in smokers subjects and 65–69 yr old men. |
| Cappola (2009) ref 18 |
5 years | 539 women WHAS I Study age 65–101 yr (median 77.6 yr) |
DHEAS is predictor of cardiovascular mortality in women with lower DHEAS levels. DHEAS is predictor of cancer mortality in women with higher DHEAS levels |
| Maggio (2007) ref 28 |
6 years | 410 men (InCHIANTI Study) age > 65 yr |
DHEAS, Testosterone, IGF-I (in the lowest quartiles of the population), were an independent predictor of all cause mortality. On the contrary, serum levels of each of these hormones considered separately were not associated with mortality |
PROS: supporting the hypothesis that single hormonal derangement is predictor of mortality
CONS: against this hypothesis
Testosterone and mortality
The relationship between testosterone levels and risk of death (Table 2) was first analyzed in a small study conducted in a geriatric rehabilitation unit. Shores et al. found that men with low testosterone levels had an increased 6-month mortality compared with men with normal testosterone levels (21).
Table 2.
Studies testing the association between testosterone and mortality
| Author Year Ref | Time of follow up (years) | Number and age of Subjects | Results |
|---|---|---|---|
| PROS | |||
| Shores MM (2006) ref 22 |
4.3 | 858 aged 3 40 yr |
Low testosterone levels are associated with an increased mortality risk either in an unadjusted model or in partially and fully adjusted model |
| Knaw KT (2007) ref 23 |
7 | 11606 40–79 yr |
Lowest quartile of TT levels are inversely related to mortality due to all-causes, cardiovascular and cancer causes, but not in chronic heart disease mortality |
| Laughlin GA (2008) ref 24 |
11.8 | 794 50–91 yr (median 73,6) |
Lowest quartile of TT and BioT levels are inversely related to mortality due to all-causes, cardiovascular and respiratory causes, but are not significantly related with cancer cause |
| Menke A (2010) ref 25 |
9 yr | 1114 men age 3 20 yr |
Men with low free and bioavailable testosterone levels may have a higher risk of mortality within 9 years of hormone measurement |
| CONS | |||
| Smith GD (2005) ref 26 |
16.5 | 2512 45–59 yr |
Positive Association between Cortisol/Testosterone Ratio and ischemic heart disease mortality, but no association between testosterone and CV death, incident ischemic heart disease |
| Araujo AB 2007) ref 27 |
15.3 | 1686 40–70 yr |
In multivariate-adjusted models higher FT and lower DHT levels are associated with ischemic heart disease. Moreover higher FT levels are associated with respiratory mortality, but TT e SHBG level not associated with all-cause mortality |
| Maggio M (2007) ref 28 |
6 | 410 aged 3 65 yr |
Low circulating levels of multiple anabolic hormones, including BioT, IGF-I and DHEAS (in the lowest quartiles), are independent predictor of mortality |
After these preliminary findings, Shores et al in a retrospective cohort study conducted in 858 middle-age and elderly male veterans, showed an inverse association between testosterone levels and mortality (22).
Khaw et al also examined the prospective relationship between endogenous testosterone concentrations and all cause, cardiovascular and cancer mortality in a case-control study including 11606 men aged 40 to 79, during 7 year follow up period. Endogenous testosterone levels at baseline resulted inversely related to all cause, cancer, and cardiovascular death, but not with chronic heart failure mortality (23).
Additionally, Laughlin et al reported the association of serum testosterone with all cause and cause-specific mortality among 794 community-dwelling older men from the Rancho Bernardo Study, who were followed for 11,8 years. In this multi-adjusted-analysis, low total and bioavailable testosterone were each significantly associated with elevated cardiovascular mortality and death due to respiratory disease but not with cancer or all cause death (24).
Recently Menke et al analyzed the relationship between testosterone levels and all-causes mortality in 1114 US men during 9 years follow up period, showing that men with low free and bioavailable testosterone have higher mortality risk (25). Contrasting data come from 3 other prospective studies (26–28).
In the Caerphilly Study, Smith et al, demonstrated a positive association between Cortisol:Testosterone ratio and ischemic death and incidence of ischemic heart disease, but not with other causes of death. In the same study no association was found between testosterone levels and mortality (26).
Araujo et al, examined the relationship between serum testosterone, dihydrotestosterone and sex hormone binding globulin (SHBG) and all cause mortality in 1709 older adult men of Massachusetts Male Aging Study. During 15 year follow up period free testosterone level was positively associated with ischemic heart disease (IHD) mortality, but negatively and strongly associated with respiratory disease death. Moreover, dihydrotestosterone levels were inversely related with IHD mortality (27).
Finally, Maggio et al. in 410 old male subjects from InCHIANTI study, followed during 6 year follow up, found that low circulating levels of multiple anabolic hormones were independent predictors of all cause mortality. On the contrary, low bioavailable testosterone level alone was not significant predictor of mortality (28).
IGF-I and mortality
The age-related decline in IGF-I levels is associated with changes in body composition, altered cognitive and immune function, worse cardiovascular profile and cancer progression (6).
The IGF-I plays an important role in the regulation of structure and function of the cardiovascular system. IGF-I can directly oppose endothelial dysfunction in a number of ways: by interacting with high-affinity endothelial binding sites that lead to nitric oxide (NO) production, by promoting insulin sensitivity and potassium-channel opening. IGF-I also modulates macrophage activation, chemo-taxis and cytokine release, enhances cellular LDL uptake and degradation, and promoting endothelial cell migration and regulating angiogenesis. Therefore IGF-I induces vasodilatation and other beneficial effects at the vessel wall, like enhance glucose uptake, anti-platelet action, free oxygen radical scavenging (29–30).
A negative role for IGF-I has been demonstrated in carcinogenesis. High serum concentration of IGF-I is associated with an increased risk of breast, prostate, colorectal and lung cancer. Thus IGF-I has a strong influence on cell proliferation and differentiation and is a potent inhibitor of apoptosis. The action of IGF-I is predominantly mediated through the IGF-I receptor (IGF1R) which is overexpressed by many tumor cell lines (31–33).
All these data suggest a potential association between IGF-I bioactivity and mortality from cardiovascular or cancer disease (Table 3).
Table 3.
Studies testing the relationship between IGF-1 and mortality
| Author Year Ref | Time of follow up (years) | Number and age of Subjects | Results |
|---|---|---|---|
| Pros | |||
| Brugts MP (2008) ref 40 |
8,6 yr | 376 men age: 73–94 yr |
There is positive relationship between IGF-I bioactivity and survival in older male subjects. Negative relationship between IGF-I bioactivity and cardiovascular risk. |
| Cappola AR (2003) ref 42 |
5 yr | 718 women age >65 yr (77,6 yr) |
The combination of low IGF-I and high IL-6 levels confers a high risk for progressive disability and death in women. |
| Friedrich N (2009) ref 38 |
8,5 yr | 1988 men 2069 women age 20–79 yr |
No association between IGF-I levels and mortality in women. In men an inverse associations between IGF-I levels and all cause mortality, cardiovascular mortality or cancer death. |
| Roubenoff R (2003) ref 35 |
4 yr | 525 subjects 202 men 323 women age 72–92 |
Low IGF-I levels is associated with increased mortality in community-dwelling elderly adults. The relationship remains significant after adjustment for multiple confounders. |
| Arai Y (2008) ref 36 |
6,2 yr | 252 subjects 197 women 55 men age > 100 yr |
The IGF-I axis may be potentially important for maintaining health and function and promoting survival at an extremely old age. The relationship between low IGF-I levels and mortality remains significant after adjusting for multiple confounders. When adjusted for covariates and for conventional risk factors, like serum levels of albumin, HDL-C, and IL-6 the relationship loses significance. |
| Laughlin GA (2004) ref 39 |
13 yr | 633 men 552 women 51–98 yr |
Low IGF-I levels are predictors of mortality for all causes and for non-ischemic heart disease (IHD) cardiovascular mortality. Negative correlation between IGF-I levels and mortality for ischemic heart disease in men and women, independent of cardiovascular risk factors. |
| Saydah S (2007) ref 37 |
12 yr | 6056 subjects 2741 men 3315 women age 43,9 yr |
Mortality decreased with increasing IGF-I quartiles for deaths from all causes, heart disease and cancer but the trend was not statistically significant for adjusted models. |
| Yamaguchi H (2008) ref 41 |
90 days | 54 patients with acute myocardial infarction | Low concentration of serum IGF-I on admission was associated with a poor early prognosis of acute myocardial infarction. |
| Cons | |||
| Andreassen M (2009) ref 43 |
30 months | 363 subjects: 194 cases 169 controls (105 women, 258 men) age 68 ±10 |
IGF-I levels were not reduced in patients with CHF and did not influence cardiac status at baseline or the prognosis |
| Hu D (2009) ref 44 |
6,2 yr | 625 subjects: age > 70 yr |
No association between IGF-I levels and all cause mortality |
| Kaplan R (2008) ref 45 |
8 yr | 1122 subjects: 725 women 397 men age >65 yr |
Low total IGF-I had a marginal association with weaker hand-grip strength, but total IGF-I levels did not predict walking speed, incident decline in functional status or mortality. |
| Raynaud Simon (2001) ref 46 |
6 yr | 256 subjects age 65–101 yr |
Highest IGF-I levels were associated with higher risk of short term mortality |
| Andreassen (2009) ref 47 |
5 yr (median) | 642 men and women 50–89 68.3±10.8 years |
High IGF-I levels were independently associated with increased all cause mortality and risk of development of CHF |
| Major J (2010) ref 48 |
18 yr | 633 men age>50 yr (mean 73) |
Higher serum IGF-I in older men is associated with increased risk cancer of death |
Although studies have analyzed the relationship between IGF-I levels and mortality in older subject, the role of IGF-I as a single determinant of longevity is still debated (34).
Several data revealed an association between low IGF-I levels and increased mortality.
For instance, Roubenoff et al. have demonstrated an association between low IGF-I levels and an increased all-cause mortality in 525 community-dwelling older adults (35).
Analysing 252 centenarians during 6,2 year follow up period, Arai et al observed that low IGF-I levels were associated with increased mortality, suggesting that IGF-I axis may be potentially important for maintaining health and promoting survival at an extremely old age (36).
Consistently, Saydah et al. (37) and Friedrich et al. (38) showed that the decline in IGF-I levels is associated with all-causes of death, and with cardiovascular and cancer mortality.
The association with cardiovascular mortality was also analyzed by Laughlin et al (39). In this study, the association of serum IGF-I and all cause, ischemic heart disease (IHD) and non-IHD cardiovascular mortality was examined in 633 men and 552 post-menopausal women, aged 51–98 yr, during 13 year follow up period. The authors showed that IGF-I levels are not related with all cause or non-IHD mortality, but low IGF-I levels increased the risk of fatal IHD among elderly subjects, independent of cardiovascular risk factors.
Similarly Brugts et al suggested that high IGF-I bioactivity in elderly men is associated with extended survival and with reduced cardiovascular risk (40).
Hypothesizing an association between serum IGF-I levels and poor clinical outcomes in patients affected by acute myocardial infarction (AMI), Yamaguchi et al. examined the impact of serum IGF-I in acute phase of AMI on 90-day mortality. Patients with low IGF-I levels at hospital admission had significantly lower survival rate (41).
Moreover, Cappola et al. (42) demonstrated, in a cohort of 718 women aged 65 or older enrolled in the Women’s Health and Aging Study I, that the combination of low IGF-I and high IL-6 levels confers a high risk for death, suggesting an aggregate effect of dysregulation in endocrine and immune systems on mortality.
On the contrary, three different studies found no association between IGF-I levels and mortality (43–45).
Recently, Andreassen et al (43) examining 363 subjects aged 68 ± 10 yr affected by chronic heart failure (CHF) found no evidence of association between IGF-I levels and adverse outcomes.
Hu et al. evaluated the relationship between IGF-I levels and mortality in 725 healthy men and women aged 70 and older. In this study, IGF-I levels were not predictive of mortality (44).
Similarly, Kaplan et al, showed no association between IGF-I and all cause mortality in 1122 subjects aged >65 yr, evaluated during 8-yr follow up period (45).
Moreover Raynaud Simon et al in 256 community-dwelling subjects aged 65–101 years, enrolled in the Paquid study observed a positive association between high IGF-I levels and short term mortality (46). Consistently, in a population based study of 642 individuals, aged 50–89 years and with a median of 5 year follow-up period, Andreassen et al found that high IGF1 levels were independently associated with increased all cause mortality and risk of development of CHF (47).
And recently Major et al examining 633 older community-dwelling men aged 50 yr and older (mean age=73) showed a significant positive quadratic association between IGF-I and all-cancer mortality (P=0.039) after adjusting for age, IGF-binding protein-1, adiposity, exercise, current smoking, and previous cancer (48).
In conclusion the role of single hormonal derangement as risk factor for death is matter of debate and more attention should be devoted to multiple hormonal dysregulation.
Multiple hormonal dysregulation as predictor of mortality
Maggio et al in a large study of older men, the Aging in the CHIANTI Area (InCHIANTI) study showed the important role of multiple hormonal dysregulation and particularly anabolic deficiency as important predictor of mortality in 6 year longitudinal study. In this study testosterone, IGF-1, DHEAS, were evaluated in a representative sample of 410 men 65 years and older. Men were divided into four groups: no hormone in the lowest quartile (reference) and one, two and three hormones in the lowest quartiles. Thresholds for lowest quartile definitions were identified as 70 ng/dl for bioavailable testosterone, 63.9 ng/ml for total IGF-1 and 50 ug/dl for DHEAS.
After adjusting for confounders including age, body mass index, cognitive and mood function, physical activity, hypertension, coronary heart disease and congestive heart failure, stroke, diabetes, parkinson disease, peripheral artery disease, pulmonary disease, and cancer no association was found between single hormone and mortality. However, the HR for mortality increased significantly with the number of hormones dysregulated but it was significant only for the group having all the hormones in the lowest quartile (28).
Similar results were found by Jankowska et al. in men affected by chronic heart failure. In this manuscript, participants with normal levels of all anabolic hormones had the best 3-year survival rate (83%, 95% CI 67% to 98%) compared with those with deficiencies in 1 (74% survival rate, 95% CI 65% to 84%), 2 (55% survival rate, 95% CI 45% to 66%), or all 3 (27% survival rate, 95% CI 5% to 49%) anabolic endocrine axes (P<0.0001) (49).
Conclusions
All these studies suggest that multiple hormonal dysregulation is a more important predictor of frailty and mortality than a single anabolic hormonal derangement in the elderly. This paradigm underlines the importance to examine multiple hormonal axes simultaneously in this population (50–51). A key feature of homeostasis is the interrelatedness of systems, which is a departure from the one disease/one organ system approach. We believe that this is particularly applicable to the hormonal paradigm, since hormones circulate in the bloodstream to target receptors throughout the body, resulting in multisystem effects. The endocrine system and its network of hormonal pathways is one network that itself operates as part of a much broader network. This paradigm could also be further expanded to include additional networks outside the endocrine system, most notably inflammation, which can affect and be affected by multiple hormones. Despite the unclear therapeutic implications of the paradigm of multiple hormonal dysregulation, these findings suggest to move beyond the “one deficiency, one replacement” model into an integrated approach to multiple hormonal dysregulation during accelerated aging.
References
- 1.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 59(3):255–63. doi: 10.1093/gerona/59.3.m255. 200. [DOI] [PubMed] [Google Scholar]
- 2.Maggio M, Cappola AR, Ceda GP, et al. The hormonal pathway to frailty in older men. J Endocrinol Invest. 2005;28(11 Suppl Proceedings):15–9. Review. [PubMed] [Google Scholar]
- 3.Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science. 1997;278(5337):419–24. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- 4.Ferrucci L, Maggio M, Bandinelli S, et al. Low testosterone levels and the risk of anemia in older men and women. Arch Intern Med. 2006;166(13):1380–8. doi: 10.1001/archinte.166.13.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez A, Muller DC, Metter EJ, et al. Aging, androgens, and metabolic syndrome in a longitudinal study of aging. J Clin Endocrinol Metab. 2007;92(9):3568–72. doi: 10.1210/jc.2006-2764. [DOI] [PubMed] [Google Scholar]
- 6.Ceda GP, Dall’Aglio E, Maggio M, et al. Clinical implications of the reduced activity of the GH-IGF-I axis in older men. J Endocrinol Invest. 2005;28(11 suppl Proceedings):96–100. Review. [PubMed] [Google Scholar]
- 7.Roth GS, Lane MA, Ingram DK, et al. Biomarkers of caloric restriction may protect longevity in humans. Science. 2002;297(5582):811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 9.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on aging research conference on frailty in older adults. J Am Geriatr Soc. 2006;54(6):991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 10.Cappola AR, Xue QL, Fried LP. Multiple hormonal deficiencies in anabolic hormones are found in frail older women: the Women’s Health and Aging studies. J Gerontol A Biol Sci Med Sci. 2009;64(2):243–8. doi: 10.1093/gerona/gln026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leng SX, Cappola AR, Andersen RE, et al. Serum levels of insulin like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S) and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16(2):153–7. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- 12.Voznesensky M, Walsh S, Dauser D, Brindisi J, Kenny AM. The association between dehydroepiandrosterone and frailty in older men and women. Age Ageing. 2009;38(4):401–6. doi: 10.1093/ageing/afp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cawthon PM, Ensrud KE, Laughlin GA, et al. Sex hormones and frailty in older men: the osteoporotic fractures in men (MrOS) study. J Clin Endocrinol Metab. 2009;94(10):3806–15. doi: 10.1210/jc.2009-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohr BA, Bhasin S, Kupelian V, Araujo AB, O’Donnell AB, McKinlay JB. Testosterone, sex hormone-binding globulin and frailty in older men. J Am Geriatr Soc. 2007;55(4):548–55. doi: 10.1111/j.1532-5415.2007.01121.x. [DOI] [PubMed] [Google Scholar]
- 15.Puts MT, Visser M, Twisk JW, Deeg DJ, Lips P. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol (Oxf) 2005;63(4):403–11. doi: 10.1111/j.1365-2265.2005.02355.x. [DOI] [PubMed] [Google Scholar]
DHEAS and mortality
- 16.Barrett-Connor E, Khaw KT, Yen SS. A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. N Engl J Med. 1986;315(24):1519–24. doi: 10.1056/NEJM198612113152405. [DOI] [PubMed] [Google Scholar]
- 17.Mazat L, Lafont S, Berr C, et al. Prospective measurements of dehydroepiandrosterone sulfate in a cohort of elderly subjects: relationship to gender, subjective health, smoking habits, and 10-year mortality. Proc Natl Acad Sci. 2001;98(14):8145–50. doi: 10.1073/pnas.121177998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cappola AR, Xue QL, Walston JD, et al. DHEAS levels and mortality in disabled older women: the Women’s Health and Aging Study I. J Gerontol A Biol Sci Med Sci. 2006;61(9):957–62. doi: 10.1093/gerona/61.9.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cappola AR, O’Meara ES, Guo W, Bartz TM, Fried LP, Newmann AB. Trajectories of dehydroepiandrosterone sulfate predict mortality in older adults: the cardiovascular health study. J Gerontol A Biol Sci Med Sci. 2009;64(12):1268–74. doi: 10.1093/gerona/glp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tilvis RS, Kähönen M, Härkönen M. Dehydroepiandrosterone sulfate, diseases and mortality in a general aged population. Aging. 1999;11(1):30–4. [PubMed] [Google Scholar]
Testosterone and mortality
- 21.Shores MM, Moceri VM, Gruenewald DA, Brodkin KI, Matsumoto AM, Kivlahan DR. Low testosterone is associated with decreased function and increased mortality risk: a preliminary study of men in geriatric rehabilitation unit. J Am Geriatr Soc. 2004;52:2077–81. doi: 10.1111/j.1532-5415.2004.52562.x. [DOI] [PubMed] [Google Scholar]
- 22.Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med. 2006;166:1660–65. doi: 10.1001/archinte.166.15.1660. [DOI] [PubMed] [Google Scholar]
- 23.Khaw KT, Dowsett M, Folkerd E, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: european prospective investigation into cancer Norfolk (EPIC-Norfolk) prospective population study. Circulation. 2007;116:2694–701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- 24.Laughlin GA, Barrett-Connors E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menke A, Guallar E, Rohrmann S, et al. Sex Steroid hormone concentrations and risk of death in US men. Am J Epidemiol. 2010 Jan 18; doi: 10.1093/aje/kwp415. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith GD, Ben-Shlomo Y, Beswick A, Yarnell J, Lightman S, Elwood P. Cortisol, testosterone, and coronary heart disease: prospective evidence from the Caerphilly Study. Circulation. 2005;112:332–40. doi: 10.1161/CIRCULATIONAHA.104.489088. [DOI] [PubMed] [Google Scholar]
- 27.Araujo A, Kupelian V, Page ST, Handelsman DJ, Bremner J, McKinlay JB. Sex steroids and all-cause and cause-specific mortality in men. Arch Intern Med. 2007;167:1252–60. doi: 10.1001/archinte.167.12.1252. [DOI] [PubMed] [Google Scholar]
- 28.Maggio M, Lauretani F, Ceda GP, et al. Relationship between low levels of anabolic hormones and 6-year mortality in older men: the aging in the Chianti Area (In-CHIANTI) study. Arch Intern Med. 2007;167(20):2249–54. doi: 10.1001/archinte.167.20.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
IGF-1 and mortality
- 29.Bayes-Genis A, Conover CA, Schwartz RS. The insulin like growth factor axis: a review of atherosclerosis and restenosis. Circ Res. 2000;86:25–130. doi: 10.1161/01.res.86.2.125. [DOI] [PubMed] [Google Scholar]
- 30.Conti E. Insulin like growth factor 1 as a vascular protective factor. Circulation. 2004 doi: 10.1161/01.CIR.0000144309.87183.FB. [DOI] [PubMed] [Google Scholar]
- 31.Baserga R. The insulin like growth factor 1 receptor: a key to tumor growth? Cancer Res. 1995;55(2):249–52. [PubMed] [Google Scholar]
- 32.Fürstenberger G. Insulin like growth factors and cancer. Lancet Oncol. 2002;3(5):298–302. doi: 10.1016/s1470-2045(02)00731-3. Review. [DOI] [PubMed] [Google Scholar]
- 33.Samani A. The role of IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28(1):20–47. doi: 10.1210/er.2006-0001. Review. [DOI] [PubMed] [Google Scholar]
- 34.Anversa P. Aging and longevity: the Igf-1 enigma. Circ Res. 2005;97(5):411–4. doi: 10.1161/01.RES.0000182212.09147.56. [DOI] [PubMed] [Google Scholar]
- 35.Roubenoff R, Parise H, Payette HA, et al. Cytokines, insulin like growth factor 1, sarcopenia and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med. 2003;115:429–35. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Arai Y, Takayama M, Gondo Y, et al. Adipose endocrine function, insulin like growth factor-1 axis and exceptional survival beyond 100 years of age. J Gerontol A Biol Sci Med Sci. 2008;63(11):1209–18. doi: 10.1093/gerona/63.11.1209. [DOI] [PubMed] [Google Scholar]
- 37.Saydah S, Graubard B, Ballard-Barbash R, Berrigan D. Insulin like growth factors and subsequent risk of mortality in the United States. Am J Epidemiol. 2007;166(5):518–26. doi: 10.1093/aje/kwm124. [DOI] [PubMed] [Google Scholar]
- 38.Friedrich N, Haring R, Nauck M, et al. Mortality and serum insulin like growth factor-I and IGF binding protein 3 concentration. J Clin Endocrinol Metab. 2009;94(5):1732–9. doi: 10.1210/jc.2008-2138. [DOI] [PubMed] [Google Scholar]
- 39.Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D. The prospective association of serum insulin lik growth factor-I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: The Rancho Bernardo Study. J Clin Endocrinol Metab. 2004;89(1):114–20. doi: 10.1210/jc.2003-030967. [DOI] [PubMed] [Google Scholar]
- 40.Brugts MP, van den Beld AW, Hofland LJ, et al. Low circulating insulin like growth factor-I bioactivity in elderly men is associated with increased mortality. J Clin Endocrinol Metab. 2008;93(7):2515–22. doi: 10.1210/jc.2007-1633. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi H, Komamura K, Choraku M, et al. Impact of serum insulin like growth factor-1 on early prognosis in acute myocardial infarction. Intern Med. 2008;47(9):819–25. doi: 10.2169/internalmedicine.47.0736. [DOI] [PubMed] [Google Scholar]
- 42.Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried L. Insulin like growth factor I and Interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab. 2003;88(5):2019–25. doi: 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]
- 43.Andreassen M, Kistorp C, Raymond I, et al. Plasma insulin like growth factor I as predictor of progression and all cause mortality in chronic heart failure. Growth Horm IGF Res. 2009;19(6):486–90. doi: 10.1016/j.ghir.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Hu D, Pawlikowska L, Kanaya A, et al. Serum insulin like growth factor-1, binding protein 1 and 2 and mortality in older adults: the health, aging, and body composition study. J Am Geriatr Soc. 2009;57:1213–8. doi: 10.1111/j.1532-5415.2009.02318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaplan R, McGinn AP, Pollak MN, et al. Total insulin like growth factor-I and insulin like growth factor binding protein levels, functional status, and mortality in older adults. J Am Geriat Soc. 2008;56:652–60. doi: 10.1111/j.1532-5415.2007.01637.x. [DOI] [PubMed] [Google Scholar]
- 46.Raynaud Simon A, Lafort S, Berr C, Dartigues JF, Baulieu EE, Le Bouc Y. Plasma insulin like growth factor I levels in the elderly: relation to plasma dehydroepiandrosterone sulfate levels, nutritional status, health and mortality. Gerontology. 2001;47(4):198–206. doi: 10.1159/000052799. [DOI] [PubMed] [Google Scholar]
- 47.Andreassen M, Raymond I, Kistorp C, Hildebrandt P, Faber J, Kristensen LØ. IGF1 as predictor of all cause mortality and cardiovascular disease in an elderly population. Eur J Endocrinol. 2009;160(1):25–31. doi: 10.1530/EJE-08-0452. [DOI] [PubMed] [Google Scholar]
- 48.Major JM, Laughlin GA, Kritz-Silvestein D, Wingard DL, Barrett-Connor E. Insulin like growth factor I and cancer mortality in older men. J Clin Endocrinol Metab. 2010 doi: 10.1210/jc.2009-1378. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Multiple Hormonal Dysregulation and accelerated aging
- 49.Jankowska EA, Biel B, Majda J, et al. Anabolic deficiency in men with chronic heart failure: prevalence and detrimental impact on survival. Circulation. 2006;114(17):1829–37. doi: 10.1161/CIRCULATIONAHA.106.649426. [DOI] [PubMed] [Google Scholar]
- 50.Cappola AR, Maggio M, Ferrucci L. Is research on hormones and aging finished? No! Just started! J Gerontol A Biol Sci Med Sci. 2008;63(7):696–7. doi: 10.1093/gerona/63.7.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valenti G, Schwartz RS. Anabolic decline in the aging male: a situation of unbalanced syncrinology. Aging Male. 2008;11(4):153–6. doi: 10.1080/13685530802571100. [DOI] [PubMed] [Google Scholar]