Abstract
The objective of this study was to test the hypothesis that porcine circovirus type-2 (PCV2) vaccination is efficacious when administered in the first week of life. Three groups of pigs were vaccinated with Circumvent either early (at the end of week 1), late (at the end of week 4), or not at all. All 3 groups were later challenged intranasally with PCV2 (at the end of week 5). Two other groups were immunized with keyhole limpet hemocyanin (KLH) as a novel antigen at the end of either week 1 or week 4. Weight, PCV2 genome copy number in serum and saliva, anti-KLH antibody titer, and serum PCV2-neutralizing antibodies were measured weekly. Early PCV2 vaccination or KLH antigen exposure resulted in earlier humoral responses that were slower to develop than in older piglets, yet converged with the responses to later vaccination within 5 wk. Both groups of vaccinated piglets had periods of higher PCV2-neutralizing antibody titers and lower viral levels shortly after weaning and PCV2 challenge, thus supporting the recent labelling of 1 Canadian PCV2 vaccine for use in week 1 and suggesting that early PCV2 vaccination can reduce piglet handling without compromising vaccine efficacy.
Résumé
L’objectif de la présente étude était de vérifier l’hypothèse que la vaccination contre le circovirus porcin de type 2 (CVP2) est efficace lorsqu’administrée durant la première semaine de vie. Trois groupes de porcs ont été vaccinés avec Circumvent soit hâtivement (à la fin de la semaine 1), tardivement (à la fin de la semaine 4), ou pas du tout. Les trois groupes ont plus tard été inoculés par voie intranasale avec CVP2 (à la fin de la semaine 5). Deux autres groupes ont été immunisés avec de l’hémocyanine de patelle (KLH) à titre de nouvel antigène à la fin de soit la semaine 1 ou la semaine 4. Le poids, le nombre de copies du génome de CVP2 dans le sérum et la salive, le titre d’anticorps anti-KLH, et le titre d’anticorps sériques neutralisants CVP2 ont été mesurés à chaque semaine. La vaccination tôt contre CVP2 ou l’exposition à l’antigène KLH a donné des réponses humorales plus hâtives qui étaient plus lentes à se développer que chez les porcs plus vieux, mais qui convergeaient vers les réponses de la vaccination tardive à l’intérieur d’un délai de 5 sem. Les deux groupes de porcelets vaccinés avaient des périodes de titres d’anticorps neutralisants contre CVP2 plus élevés et des charges virales plus basses peu de temps après le sevrage et le challenge avec CVP2, soutenant ainsi l’étiquetage récent d’un vaccin canadien contre CVP2 pour utilisation dans la semaine 1 et suggérant qu’une vaccination tôt contre CVP2 peut réduire la manipulation des porcelets sans compromettre l’efficacité du vaccin.
(Traduit par Docteur Serge Messier)
Introduction
It is suspected that a high proportion of swine herds throughout Canada is seropositive for porcine circovirus type-2 (PCV2), a pathogenic variant of PCV first differentiated from non-pathogenic PCV type-1 (PCV1) in 1998 (1–4). Infection with PCV2 can result in a wide variety of diseases, known as porcine circovirus-associated diseases (PCVAD), including post-weaning multi-systemic wasting syndrome (PMWS), PCV2-systemic disease (PCV2-SD), PCV2-subclinical infection (PCV2-SI), PCV2-reproductive disease (PCV2-RD), and porcine dermatitis and nephropathy syndrome (PDNS) (3). The initiation of intensive vaccination by the Canadian swine industry in 2006 has greatly reduced the incidence of PCVAD and enhanced productivity for swine producers (5–7).
Four major PCV2 vaccines are licensed in Canada for pigs as young as 3 wk of age (3). This timing for vaccination can result in handling burdens for the producers and the piglets, particularly when the timing varies according to barn-specific protocols. Recent research showed that there was no significant difference in viral load or PCV2-associated lesions between PCV2-challenged piglets that had been vaccinated as newborns (3 to 5 d of age) or piglets vaccinated at weaning (21 d of age) (7,8). Based on the demand from producers and these promising findings, a commercial vaccine was recently re-released with an additional label claim for use in piglets at 3 d of age (9). The efficacy of early vaccination is influenced by the maturity of the immune system. In swine, there is evidence of in-utero immune system maturation and class-switching from immunoglobulin M (IgM) to immunoglobulin G (IgG) without the influence of environmental antigens (10). Specifically, early developing piglets in utero (< 70 d) die upon amniotic exposure to porcine parvovirus, whereas later developing piglets (> 70 d) are able to mount a protective immune response and survive the parvovirus challenge (11). This transition at around 70 d of gestation coincides with maturation of the immune system (10) and suggests that piglets might be developmentally able to respond effectively to vaccination soon after birth.
This concept is supported by a recent study on humoral responses to early PCV2 vaccination using sows naive to PCV2 and PCV2 vaccination, thereby eliminating the chance of interference from PCV2-specific maternal antibodies (7,12). Piglets vaccinated early responded equally well to PCV2 vaccination and challenge as older vaccinees (7,12). Given that most commercial barns are currently PCV2-seropositive and the majority of gilts will have received at least 1, if not 2, PCV2 vaccines in their lifetime; however, it is important to understand maternal antibody effects, which are transferred from the sow to piglets in colostrum (12–15), in order for early vaccination to be translated into practice.
The current study was designed to determine the relationship between PCV2-antibody positive conditions, which are common in Canadian swine barns, and early vaccination with early PCV2 challenge. The hypotheses being tested are that piglets i) are equally able to produce antibodies to a novel antigen [keyhole limpet hemocyanin (KLH)] (16,17); and ii) respond equivalently to early and late PCV2 vaccination and challenge, resulting in similar serum viral load, salivary virus shedding, and PCV2-neutralizing antibody responses (nAb). If these hypotheses are supported by the results, the evidence base for translating early vaccination into industry practice will be strengthened.
Materials and methods
Animal source and housing
Five multiparous (3rd or 4th parity) sows (Genetiporc F25) and their 57 colostrum-fed piglets (aged 3 to 4 d; G-performer 4 boar as sire) were obtained from a western Canadian farm positive for PCV2. Sows were previously vaccinated against PCV2 with Ingelvac Circoflex at their weaning and again at their gilt selection. Throughout the study, animals were housed in a Biosafety Level-2 facility at the Veterinary Sciences Research Station of the University of Calgary, following modern pig production practices and biosafety and biosecurity protocols. Research was conducted with University of Calgary Animal Protocol Approval AC13-0150.
Five experimental groups
To ensure equal group size and to avoid bias due to different levels of maternal antibodies in the milk and genetic factors, groups were mixed from all 5 litters and each of the 5 groups was represented by at least 2 piglets from each litter. Two groups were immunized with a novel antigen, keyhole limpet hemocyanin (KLH) (16), that was mixed with the PCV2 vaccine, as detailed in this article. The Early-KLH group was immunized immediately after the first sampling in week 1 (6 to 7 d of age = days 0 to 2 of the study) and the Late-KLH group was immunized at the end of week 4 of sampling (27 to 28 d of age = days 21 to 23 of the study). Three additional groups were created to study the effects of early versus late PCV2 vaccination. The Early-VAC group was vaccinated against PCV2 after their first sampling in week 1 of the study, the Late-VAC group was vaccinated against PCV2 at the end of week 4 sampling, corresponding to industry-standard vaccination at > 3 wk of age, and the Never-VAC group received no treatment. At the end of week 5 (day 31), the Never-, Early-, and Late-VAC groups were exposed to PCV2 as described in the following sections and summarized in Table I.
Table I.
Experimental design
| Piglet age (wk) | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| 0 | 1 Early |
2 | 3 | 4 Late |
5 PCV2 challengeb |
|
| Treatment and groupa | KLHc (n = 12) | KLHc (n = 10) | KLH (n = 12) | |||
| VACd (n = 11) | VACd (n = 11) | VAC (n = 11) Never-VAC (n = 11) |
||||
All piglets were monitored and sampled twice weekly. Quantitative PCV2-PCR, ELISA for anti-KLH antibodies, and virus neutralization assay (VNA) for PCV2-neutralizing antibodies were carried out on most serum and saliva samples. All statistical analysis were conducted using JMP 12 software (SAS Institute), applying a critical alpha.
105 TCID50 PCV2 (intranasal).
~1.1 mg KLH in adjuvant per piglet (intramuscular).
A single dose of Circumvent PCV was used.
Biosafety/security procedures
All piglets were handled and sampled weekly, from week 0 through week 10 of the study. Foot traffic among rooms of different treatment groups was minimized. Footbaths with Virkon S (DuPont, Wilmington, Delaware, USA) were placed outside each room. If a room was re-entered, new boots, coveralls, and barrier gloves were donned. Further cleaning and traffic protocols were put into place including using separate or completely cleaned equipment for each treatment group that could not be replaced, disinfecting the hallway after every sampling event, completely disinfecting and cleaning the surgery room where sampling took place, and consistent sampling of each treatment group by the same students.
Sampling procedures
Each piglet was physically examined immediately before every sampling procedure. Weight (± 0.1 kg), heart rate, respiration rate, and capillary refill time were determined and recorded for each piglet. Before each sample collection, piglets up to approximately 8 kg were anesthetized with isoflurane gas administered using a Bain non-rebreathing anesthetic system. When piglets were too large for the mask induction procedure, they were sedated with 3.3 mg/kg body weight (BW) of azaperone (Stresnil; Vétoquinol, Lavaltrie, Quebec) injected into the neck muscle (3 mL-syringe with a 22-gauge × 1-in needle) during sample collection.
For the first serum collection, 1 to 2 mL of blood was collected from the tail stub when tails were docked (by #10 scalpel) or from the auricular vein if the stub sample was insufficient. Subsequent samples were collected from the cephalic, median cubital, lateral saphenous, or auricular veins using 21- or 23-gauge butterfly catheters and a 3-mL syringe. When pigs were large enough to be sedated with azaperone, blood was collected from dorsally recumbent pigs from the cranial vena cava using 18 to 20 gauge × 1.5-inch needles with a Vacutainer system (BD Diagnostics, Mississauga, Ontario). Collected blood was allowed to clot at room temperature for about 1 h and then refrigerated at 4°C. After clot removal, serum was separated and stored in 1-mL aliquots at −80°C for subsequent analyses.
In addition to the blood samples, pooled piglet saliva was collected from the pens for Early-VAC, Late-VAC, and Never-VAC groups, 3 times a week (Tuesday, Wednesday, and Thursday) from weeks 6 through 10. Saliva was collected by placing a 30 cm, 3-strand, twisted cotton rope in each group’s pen for 15 min (18). Piglets that explored the rope left saliva within the absorbent rope, which was extracted by wringing out (or twisting) the rope inside a clean ziplock bag, then decanting the liquid into a cryovial. Saliva samples were stored at −20°C for subsequent analyses.
Vaccination
Circumvent PCV vaccine (Intervet, Merck Animal Health, Summit, New Jersey, USA) was administered intramuscularly into the lateral aspect of the neck as a single 2-mL dose. The adjuvant of the product is Microsol Diluvac Forte (Merck Animal Health) in a base of mineral oil and dl-alpha-Tocopherol-acetate.
KLH antigen exposure
Keyhold limpet hemocyanin (KLH) was mixed with Circumvent PCV since the adjuvant of the vaccine could not be provided to us separately. Twenty milligrams of KLH (Sigma-Aldrich, St. Louis, Missouri, USA) were reconstituted in 5 mL of sterile water to a concentration of 4 mg/mL. Reconstituted KLH (3.75 mL, 15 mg) was added to 30 mL of Circumvent PCV vaccine in order to prepare 14 doses of 2.25 mL each. Treatments with KLH were administered intramuscularly into the left or right lateral aspect of the neck.
PCV2 challenge
A virus stock was derived from a molecular clone of PCV2 (Canadian strain 05-32650, GenBank accession no. EF394779), passaged several times through PK-15 cells, frozen and thawed 3 times, sonicated, and clarified at 400 × g for 5 min. The clarified stock was aliquoted and frozen at −80°C. Infectivity was 2.3 × 103 50% tissue culture infectious doses (TCID50) per milliliter as determined by endpoint titration on PK-15 cells. The PCV2 challenge consisted of a 2-mL inoculum of PCV2 administered intranasally to a sedated pig restrained with the head elevated. The upright position was maintained for 5 s to ensure contact with the nasal mucosa. Excess liquid was then wiped from the snout and disposed of as biohazardous material.
KLH antibody ELISA
The KLH antibody enzyme-linked immunosorbent assay (ELISA) was developed in-house using a peroxidase labelled rabbit anti-pig IgG (whole molecule) (Sigma Aldrich) as the secondary antibody. Keyhole limpet hemocyanin (KLH) (Sigma Aldrich) was first reconstituted in sterile double-distilled water to a concentration of 4 mg/mL and then diluted in carbonate-bicarbonate buffer (1.5 mM, pH 9.6) to a final concentration of 4 ng/μL. ELISA plates were coated with 400 ng per well KLH, incubated overnight at 4°C, and washed 3 times with phosphate-buffered saline (PBS) with 0.1% (v/v) Tween (PBST). Wells were blocked with 2% (w/v) skim milk (from powder) in PBST at 100 μL per well for 1.5 h at room temperature and washed again 3 times. The positive control consisted of pooled serum samples from the Early-KLH group, week 5, at a 1:10 dilution in PBST. The negative control consisted of pooled serum samples from the Early-VAC group, which did not get KLH antigen, week 5, at a 1:10 dilution. The conjugate control was 2% skim milk in PBST.
The serum samples from the Early- and Late-KLH piglets were diluted in 4 steps from 1:20 to 1:160 in 2% skim milk and all dilutions were tested in duplicates by ELISA. Samples that still tested positive at 1:160 were titrated out to a maximum dilution of 1:800. A volume of 100 μL of diluted sample per well was incubated overnight at 4°C. Wells were then washed 3 times with PBST. The secondary antibody, peroxidase-labelled rabbit anti-pig IgG (whole molecule) (Sigma Aldrich), was diluted 1:10 000 2% milk in PBST. One hundred microliters of the dilute antibody were used per well and incubated for 1.5 h at 37°C in a cell culture incubator. Wells were then washed 3 times with PBST and developed with 2,2-azino-bis (ABTS) (100 μL per well, room temperature, 20 min in the dark). Absorbance (optical density, OD) was read using a Microplate Absorbance Reader (“iMark,” Biorad, Mississauga, Ontario) with a 415 nm filter. Samples were run in duplicate, averaged, and reported as sample/positive ratios S/P = (sample OD — negative control OD)/(positive control OD — negative control OD). S/P values < 0.4 (mean + 2× SD of negative control samples) were scored as negative, while S/P values ≥ 0.4 were considered positive. The S/P values of positive samples were then translated to threshold titer values, i.e., the maximal dilution with a positive S/P value, and used in analyses.
Serum and saliva viral load
All serum and saliva samples were tested for the presence of PCV2 genomic deoxyribonucleic acid (DNA) by real-time quantitative polymerase reaction (qPCR) using the PerfeCTa SYBR Green SuperMix with Low ROX (Quanta BioSciences, Gaithersburg, Maryland, USA) on a CFX96 detection system (Bio-Rad, Hercules, California, USA). Nucleic acid was extracted from 100 μL of serum or saliva with the Mag-Bind Viral DNA/RNA Kit (Omega Bio-Tek, Norcross, Georgia, USA) on a MagMAX Express-96 magnetic particle processor (Life Technologies, Burlington, Ontario) (4). All tests were conducted in duplicate and only samples that were positive in both replicates were considered positive.
Serum PCV2-neutralizing antibodies (PCV2-nAb)
After 1 h of heat inactivation at 56°C, serum samples were serially diluted 3-fold in PBS, mixed with PCV2 (2.3 × 103 TCID50/well), incubated at 37°C for 1 h, and added to 96-well plates containing PK-15 cell monolayers seeded the previous day. After incubation on the cells for 90 min at 37°C, the supernatant was removed, cells were washed with sterile PBS, and 100 μL of fresh media were added. Plates were incubated at 37°C for 48 h. For indirect immunofluorescence staining, the supernatant was discarded, cells were washed with PBS and fixed with 4% (w/v) formaldehyde (from paraformaldehyde powder) in PBS at room temperature for 30 min and subsequently washed again with PBS. Fixed cells were treated with permeabilization buffer [PBS with 0.1% bovine serum albumin (BSA) and 0.1% (v/v) saponin] and then incubated with primary antibody (rabbit anti-PCV2-Cap) diluted in PBS followed by secondary antibody (goat anti-rabbit Alexa Fluor 568) and 4′6-diamidino-2-phenylindole (DAPI) for 30 min at room temperature. Plates were then analyzed using an automated fluorescence microscope (IN Cell Analyzer 2000; GE Healthcare, Mississauga, Ontario). Titers were defined as the highest serum dilution that reduced viral infectivity by 50% (4,19).
Statistical analyses
All statistical analyses were conducted using JMP 12 software (SAS Institute, Cary, North Carolina, USA), applying a critical alpha of 0.05. As detailed in the text for each test, non-parametric Wilcoxon and Kruskal-Wallis tests were applied to data sets with non-normal distributions, followed by post-hoc Chi-squared approximation or comparison against control (Dunn all pairs for joint ranks) as appropriate. Parameters with a normal distribution were analyzed using t-test or by analysis of variance (ANOVA) with post-hoc Tukey-Kramer as appropriate. Measures of titer were converted to log [titer + 1] so that titer = 0 was retained as data = 0, rather than being excluded from analyses as missing data.
Results
Piglet exclusion and inclusion
The 5 litters consisted of a total of 57 piglets (2 × 12, 3 × 11 piglets). One piglet in the Never-VAC group showed signs of PCVAD and was euthanized in week 9. Necropsy findings of this piglet included generalized lymphoid depletion and lesions consistent with acute streptococcal septicemia. All data for this piglet were retained in analyses. A second piglet assigned to the Early-VAC group was lost to sow rollover in week 1 and was excluded from all analyses. A third piglet in the Late-KLH group with acute lameness, fever, and neurological signs was euthanized in week 7, after failure to respond to treatment for Streptococcus suis. Postmortem diagnosis was polyserositis. This piglet was excluded from all analyses as the disease might have affected the immune response to KLH. In total, results for 55 piglets were included in the analyses.
Health status
There were no significant differences in initial piglet weight across groups in week 1 (F4, 55 = 1.86, P = 0.13). There were also no significant differences between control piglets (Never-VAC) and any other group in week 1 or week 9 (P = 0.23; P = 0.06). Weight gain from weeks 3 to 9 (0.43 ± 0.08 kg/d) was consistent with expectations for nursery pigs in the US from 2005 to 2010 (0.38 ± 0.05 kg/d from day 19 to day 65). With a mean piglet temperature of 38.9°C ± 0.1°C and a range from 37.4°C to 39.9°C, there was no evidence of hyperthermia (> 40°C) at the onset of the study and no significant difference among treatment groups (F4, 53 = 0.96, P = 0.50).
Antibody response to KLH
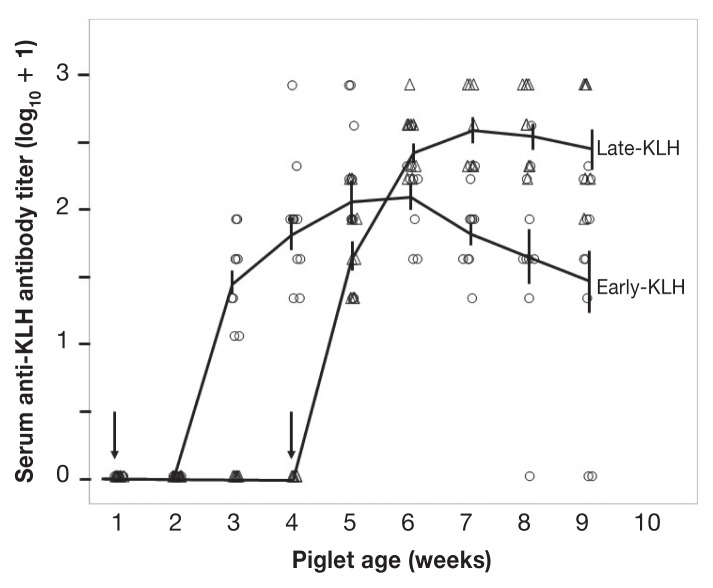
No antibodies to KLH were detected in animals in either the Early-KLH or the Late-KLH group before exposure to KLH antigen. All piglets immunized with KLH eventually developed specific antibodies (Figure 1). Specifically, at 1 wk after KLH antigen exposure, piglets in the Early-KLH group showed no KLH antibodies, whereas all piglets in the Late-KLH group had a positive KLH antibody titer, resulting in a significant difference between early and late exposure (Wilcoxon Chi-square approximation = 15.84, df = 1, P < 0.0001). By the second week post-immunization, all piglets were positive for KLH antibodies. In weeks 2 and 3 after exposure, the Late-KLH group (older at immunization) continued to demonstrate a stronger antibody response than the Early-KLH group (younger) (both P < 0.005). In week 4 after exposure, a significant but smaller difference remained (P < 0.025). However, the last comparable time-point (5 wk after the KLH exposure) did not show a significant difference between the 2 groups (P = 0.09). Thus, both groups achieved equivalent antibody titers against the KLH antigen within 5 wk.
Figure 1.
Antibody response to immunization with keyhole limpet hemocyanin (KLH). Piglets were immunized with KLH at 6 to 7 (○) and 27 to 28 (△) d of age, as indicated by vertical arrows. KLH-specific IgG titers are shown as the base-2 logarithm after adding 1 to the original values to demonstrate a base line at 0. Vertical bars represent the standard error of the mean (SEM) of a given group on a specific sampling day. Means of each group are connected by a line.
PCV2 genome load in serum
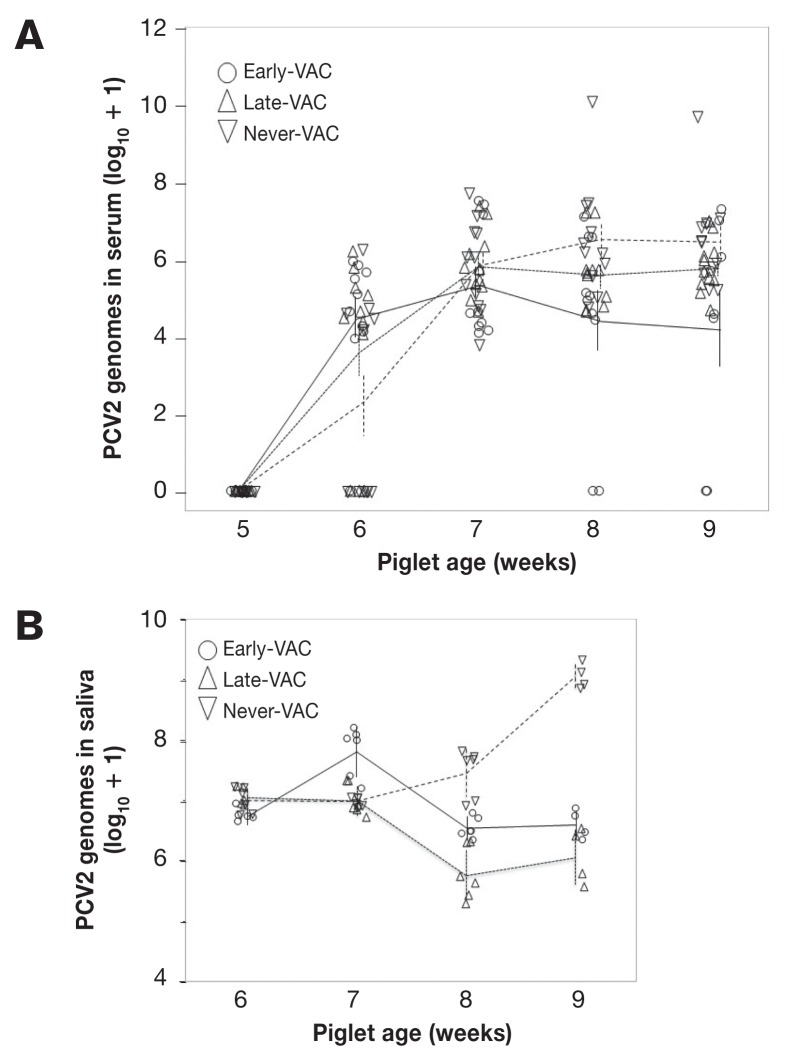
Sera from both the Early- and Late-KLH groups were negative in the PCV2 qPCR over the entire course of the study. All 3 PCV2 groups remained negative up to and including the day before they were exposed to PCV2 (after the week 5 sample). This confirmed the effectiveness of our biosafety procedures. One week (5 or 6 d) after PCV2 challenge, 24/34 piglets were positive for PCV2 in the serum by qPCR (Figure 2A), with 1 Early-VAC (sampled 6 d post-challenge), 3 Late-VAC (5 d post-challenge), and 6 Never-VAC (6 d post-challenge) piglets negative. By week 2 after challenge (age 6 wk), sera from all (34/34) piglets were positive in the PCV2 qPCR. There was no difference between the PCV2 genome copy numbers in sera from Never-VAC, Early-VAC, and Late-VAC piglets (Kruskal-Wallis, Chi-square approximation, df = 2, P > 0.4) in weeks 2 to 4 post challenge. Over weeks 8 and 9, 3 piglets (5 samples) had no detectable virus genome in serum, although the same piglets had been positive in week 7. All samples with no detectable virus genome came from the Early-VAC group. The 1 piglet with the highest viral load in weeks 8 and 9 of the Never-VAC group was later euthanized, but retained in analyses. Thus, all challenged piglets became positive for PCV2 genome in serum, 1 progressed to clinical disease and death, and 3 reverted to qPCR-negative status before the end of the study.
Figure 2.
PCV2 genome load in serum (A) and saliva (B). Viral load is shown for piglets from Early-VAC (○), Late-VAC (△), and Never-VAC (▽) groups. PCV2 genome copy numbers from qPCR are shown as the base-10 logarithm after adding 1 to the original values to demonstrate a base line at 0. Pooled saliva was collected from each group 3 times a week and quantified in duplicate, with the mean qPCR copy number presented for each sample. Vertical bars represent the standard error of the mean (SEM) on a given group at a specific sampling day. Means of each group are connected by a line.
Virus shedding in saliva
The PCV2 genome load in pooled saliva was determined weekly, from weeks 6 through 9, based on 3 samples per week from the Early-, Late-, and Never-VAC pens. Across treatment groups, piglets from the Never-VAC group shed significantly more PCV2 than those from the Late-VAC group (ANOVA with post-hoc Tukey-Kramer testing P < 0.005). No overall effect of week was seen (P = 0.39). Within week 7, shedding of PCV2 by Early-VAC piglets exceeded that of Late-VAC and Never-VAC piglets (P < 0.05). Within weeks 8 and 9, Never-VAC piglets had higher PCV2 genome loads in saliva than those from both Early- and Late-VAC groups (both P < 0.005) (Figure 2B). Thus, vaccination reduced virus shedding in saliva compared to the Never-VAC group, although the Early-VAC group did have a phase of higher shedding.
PCV2-neutralizing antibodies in serum
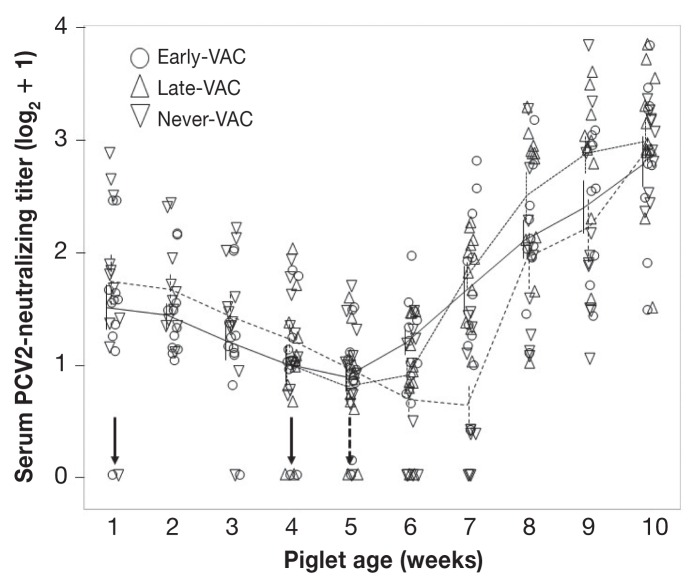
All piglets had detectable levels of PCV2-neutralizing antibodies in the first week of sampling and before PCV2 vaccination. These antibodies were regarded as transferred from mothers through colostrum and their titers declined until exposure to PCV2 at day 32 of the study (Figure 3). Vaccination, whether at an early or older age, did not induce substantial amounts of PCV2-neutralizing antibodies before PCV2 challenge.
Figure 3.
Development of serum PCV2-neutralizing antibodies. Titers of PCV2-neutralizing IgG (nAb) are shown for piglets from Early-VAC (○), Late-VAC (△), and Never-VAC (▽) groups. Vertical arrows indicate day of immunization (solid) and of PCV2 challenge (dashed). Titers of nAb are shown as the base-2 logarithmic after adding 1 to the original values to demonstrate a base line at 0. Vertical bars represent the standard error of the mean (SEM) on a given group at a specific sampling day. Means of each group are connected by a line (Early-VAC — solid line; Late-VAC — narrow dashes; Never-VAC — wider dashes).
All animals challenged with PCV2 showed increasing neutralizing antibody levels across kinetics of PCV2-neutralizing antibodies after PCV2 challenge (P < 0.0001), indicating that neither age nor presence of maternal antibodies interfered with development of neutralizing antibody in vaccinated piglets. In week 7, piglets in the Never-VAC group had a significantly lower titer than both the Early-and Late-VAC piglets (both P < 0.001). Thus, the Never-VAC group was delayed by at least 1 wk in mounting a neutralizing antibody response compared to both groups that were vaccinated. Once the Never-VAC piglets began to respond with the production of PCV2-nAB, they rapidly caught up to the Early-VAC piglets.
Discussion
This experimental study was conducted to determine whether piglets could be successfully vaccinated against PCV2 at a very young age and whether maternal antibodies would interfere with vaccination efficacy. Our results indicate that PCV2 vaccination of piglets less than 6 days old produced results similar to those obtained from older vaccinees. As reported in a previous study, however, PCV2-neutralizing antibodies were not induced by vaccination per se but only upon infection with PCV2 (4). Further study is required to determine whether this lack of induction of PCV2-neutralizing antibody resulted from a weakness of the PCV2 vaccine formulation and/or from circulating maternal antibodies against PCV2 that inhibited the vaccination. Certainly, the lack of suitable levels of neutralizing antibodies before PCV2 exposure is a major reason for the high prevalence of PCV2 on many pig farms today (4).
Maturity of immune system in newborn piglets
It has been demonstrated in a previous study that PCV2 vaccination of less than 6-day-old piglets is as efficacious as vaccination of older pigs (7). It was thus not surprising to find antibody responses of KLH vaccinees immunized early in life to be very similar to those of older vaccinees. Since our enzyme-linked immunosorbent assay (ELISA) detected exclusively IgG, we can only assume that a class switch from IgM to IgG occurred very soon after KLH immunization. The kinetics of the humoral immune response and the inferred immunoglobulin class switch indicate that pigs already possess a mature immune system at birth. Our data extend previous findings (7), but further studies are needed to determine whether the immune system of younger pigs requires similar or different amounts of immunogen and/or adjuvant than older pigs to be equally efficacious.
Effects of maternal antibodies
We found substantial levels of PCV2-neutralizing antibody in almost all newborn piglets after ingestion of colostrum. These antibodies waned over a period of approximately 5 wk (Figure 3). As all sows of our experimental piglets had been vaccinated against PCV2 at least twice, these perinatal antibodies most likely represent maternal antibodies. It has been suggested that maternal antibodies protect piglets from PCVAD but not entirely from PCV2 infection (20,21). On the other hand, it is not clear whether maternal anti-PCV2 antibodies interfere with PCV2 vaccination as occurs with various other vaccinations, both in humans and animals [for review, see (22)]. Opriessnig et al (8) found no evidence that maternal antibodies inhibited PCV2 vaccination. Only 1 commercial vaccine was tested in that study, however, and outcomes could vary depending on the type of vaccine used.
Our results indicate that generation of PCV2-neutralizing antibodies was very similar in young and old vaccinees. Notably, most of the young vaccinees had maternal PCV2-specific antibodies in their sera at the time of vaccination and were thus immunized in the presence of anti-PCV2 antibodies. Our data indicate that maternal antibodies did not interfere with PCV2 vaccination under the given circumstances. While these results are concordant with published data (8), they would require higher statistical power for a conclusive statement about whether maternal antibodies do or do not inhibit PCV2 vaccination.
Efficacy of vaccination
The ultimate goal of prophylactic vaccination is prevention of infection. Since all pigs became infected after exposure to PCV2, this goal was not met. Our data do not differ from those of numerous other experimental and clinical studies and highlight a profound weakness of current PCV2 vaccines. However, all currently commercially available PCV2 vaccines do reliably prevent PCVAD (23). We assessed the efficacy of PCV2 vaccination by determining the levels of PCV2 genomes in sera and saliva and by the de novo generation of PCV2-neutralizing antibody. We observed no difference between the PCV2 genome copy numbers in sera from Never-VAC, Early-VAC, and Late-VAC piglets in weeks 2 to 4 post challenge. Only piglets in the Early-VAC treatment group had undetectable PCV2 viremia by the end of the study and those piglets also fell at the low end of copy numbers relative to other piglets in the previous week. It is therefore possible that the longer interval between vaccination and challenge (4 wk versus 1 wk) enhanced the booster effect of the PCV2 challenge. Our study allowed only 1 wk for the Late-VAC piglets to respond to vaccination before challenge. As a result, our study simulated the risk of PCV2 exposure in early life and was able to identify benefits of early vaccination.
Porcine circovirus type-2 (PCV2) is shed and transmitted via oronasal secretions. During the final 2 wk of our experiment, virus shedding in saliva was significantly lower in the 2 vaccinated groups than in the Never-VAC group, in which shedding continued to increase over time. Again, this result demonstrated that vaccination of both young and older piglets was equally efficacious. This is further supported by the kinetics of PCV2-neutralizing antibody production. After viral challenge, all groups and piglets developed higher PCV2-neutralizing titers. The groups were similar in week 6, 1 wk after challenge, and were also similar in weeks 8 through 10, which indicates that the cumulative immune response to PCV2 challenge was the same in the presence or absence of vaccination and confirms the findings of O’Neill et al (7) that early vaccination does not alter the neutralizing antibody response relative to later vaccination. The Never-VAC piglets did not increase their neutralizing capacity in serum between weeks 6 and 7, however, which resulted in a 1-week delay in the response of the Never-VAC piglets. Thus, vaccination did accelerate the development of the neutralizing antibody response (24), but was not necessary for a strong response over time.
In conclusion, the findings of this study were complementary in many ways to those of O’Neil et al (7). Both studies found strong evidence of a humoral response to PCV2 vaccination in the first week of life and confirmed that the responses of early- and late-vaccinated piglets tend to converge over time. The current study adds evidence that exposure to KLH, a novel antigen, follows a similar time course. With earlier experimental infection in the current study, these data therefore suggest that early vaccination reduces virus shedding in saliva and is preferable to traditional vaccination if the infectious challenge is likely to be experienced quite early in life.
Acknowledgments
The UCVM Class of 2015 acknowledges the exceptional efforts of Dr. Jessica Law in publishing these findings and the generosity of Prairie Swine Health Service (Red Deer, Alberta) in granting her time to do so. This research project was integral to the undergraduate DVM curriculum at the University of Calgary, Faculty of Veterinary Medicine (UCVM), with components in the Investigative Medicine Area of Emphasis and the Professional Skills curriculum (http://vet.ucalgary.ca). The authors are grateful for the skilled technical contributions of the staff at the Veterinary Sciences Research Station of the University of Calgary. Preliminary results from this research were presented at the International Pig Veterinary Society Congress in Cancun, Mexico in 2014 and Dublin, Ireland in 2016.
References
- 1.Allan G, Krakowka S, Ellis J, Charreyre C. Discovery and evolving history of two genetically related but phenotypically different viruses, porcine circoviruses 1 and 2. Virus Res. 2012;164:4–9. doi: 10.1016/j.virusres.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Dulac GC, Afshar A. Porcine circovirus antigens in PK-15 cell line (ATCC CCL-33) and evidence of antibodies to circovirus in Canadian pigs. Can J Vet Res. 1989;53:431–433. [PMC free article] [PubMed] [Google Scholar]
- 3.Segales J, Kekarainen T, Cortey M. The natural history of porcine circovirus type 2: From an inoffensive virus to a devastating swine disease? Vet Microbiol. 2013;165:13–20. doi: 10.1016/j.vetmic.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 4.Worsfold CS, Dardari R, Law S, et al. Assessment of neutralizing and non-neutralizing antibody responses against Porcine circovirus 2 in vaccinated and non-vaccinated farmed pigs. J Gen Virol. 2015;96:2743–2748. doi: 10.1099/vir.0.000206. [DOI] [PubMed] [Google Scholar]
- 5.Gillespie J, Opriessnig T, Meng XJ, Pelzer K, Buechner-Maxwell V. Porcine circovirus type 2 and porcine circovirus-associated disease. J Vet Intern Med. 2009;23:1151–1163. doi: 10.1111/j.1939-1676.2009.0389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kixmöller M, Ritzmann M, Eddicks M, Saalmüller A, Elbers K, Fachinger G. Reduction of PMWS-associated clinical signs and co-infections by vaccination against PCV2. Vaccine. 2008;26:3443–3451. doi: 10.1016/j.vaccine.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 7.O’Neill KC, Shen HG, Lin K, et al. Studies on porcine circovirus type 2 vaccination of 5-day-old piglets. Clin Vaccine Immunol. 2011;18:1865–1871. doi: 10.1128/CVI.05318-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Opriessnig T, Patterson AR, Elsener J, Meng XJ, Halbur PG. Influence of maternal antibodies on efficacy of porcine circovirus type 2 (PCV2) vaccination to protect pigs from experimental infection with PCV2. Clin Vaccine Immunol. 2008;15:397–401. doi: 10.1128/CVI.00416-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Intervet Inc., Merck Animal Health. Porcine circovirus vaccine, type 2, killed baculovirus vector. 2013. [Last accessed October 15, 2016]. Available from: http://www.circumvent-g2.ca/pdfs/Circumvent-G2-Product-Info-Full-Labels-PCV-M-G2.pdf.
- 10.Sinkora M, Butler JE. The ontogeny of the porcine immune system. Dev Comp Immunol. 2009;33:273–283. doi: 10.1016/j.dci.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachmann PA, Sheffy BE, Vauhan JT. Experimental in utero infection of fetal pigs with a porcine parvovirus. Infect Immun. 1975;12:455–460. doi: 10.1128/iai.12.3.455-460.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraile L, Grau-Roma L, Sarasola P, et al. Inactivated PCV2 one shot vaccine applied in 3-week-old piglets: Improvement of production parameters and interaction with maternally derived immunity. Vaccine. 2012;30:1986–1992. doi: 10.1016/j.vaccine.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Butler JE, Zhao Y, Sinkora M, Wertz N, Kacskovics I. Immunoglobulins, antibody repertoire and B cell development. Dev Comp Immunol. 2009;33:321–333. doi: 10.1016/j.dci.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Opriessnig T, Patterson AR, Madson DM, et al. Comparison of the effectiveness of passive (dam) versus active (piglet) immunization against porcine circovirus type 2 (PCV2) and impact of passively derived PCV2 vaccine-induced immunity on vaccination. Vet Microbiol. 2010;142:177–183. doi: 10.1016/j.vetmic.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 15.Pejsak Z, Podgórska K, Truszczyński M, Karbowiak P, Stadejek T. Efficacy of different protocols of vaccination against porcine circovirus type 2 (PCV2) in a farm affected by postweaning multisystemic wasting syndrome (PMWS) Comp Immunol Microbiol Infect Dis. 2010;33:e1–5. doi: 10.1016/j.cimid.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Harris JR, Markl J. Keyhole limpet hemocyanin (KLH): A biomedical review. Micron. 1999;30:597–623. doi: 10.1016/s0968-4328(99)00036-0. [DOI] [PubMed] [Google Scholar]
- 17.UVVM Class of 2014. McCorkell R, Horsman SR, et al. Acute BVDV-2 infection in beef calves delays humoral responses to a non-infectious antigen challenge. Can Vet J. 2015;56:1075–1083. [PMC free article] [PubMed] [Google Scholar]
- 18.Ramirez A, Wang C, Prickett JR, et al. Efficient surveillance of pig populations using oral fluids. Prev Vet Med. 2012;104:292–300. doi: 10.1016/j.prevetmed.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Eschbaumer M, Law S, Solis C, Chernick A, van der Meer F, Czub M. Rapid detection of neutralizing antibodies against bovine viral diarrhoea virus using quantitative high-content screening. J Virol Methods. 2014;198:56–63. doi: 10.1016/j.jviromet.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 20.McKeown NE, Opriessnig T, Thomas P, et al. Effects of porcine circovirus type 2 (PCV2) maternal antibodies on experimental infection of piglets with PCV2. Clin Diagn Lab Immunol. 2005;12:1347–1351. doi: 10.1128/CDLI.12.11.1347-1351.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostanello F, Caprioli A, Di Francesco A, et al. Experimental infection of 3-week-old conventional colostrum-fed pigs with porcine circovirus type 2 and porcine parvovirus. Vet Microbiol. 2005;108:179–186. doi: 10.1016/j.vetmic.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Niewiesk S. Maternal antibodies: Clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front Immunol. 2014;5:446. doi: 10.3389/fimmu.2014.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chae C. Commercial porcine circovirus type 2 vaccines: Efficacy and clinical application. Vet J. 2012;194:151–157. doi: 10.1016/j.tvjl.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 24.Seo HW, Han K, Park C, Chae C. Clinical, virological, immunological and pathological evaluation of four porcine circovirus type 2 vaccines. Vet J. 2014;200:65–70. doi: 10.1016/j.tvjl.2014.02.002. [DOI] [PubMed] [Google Scholar]