Abstract
Visceral adipose tissue (AT) obtained from surgical waste during routine ovariectomies was used as a source for isolating canine mesenchymal stem cells (MSCs). As determined by cytofluorimetry, passage 2 cells expressed MSC markers CD44 and CD90 and were negative for lineage-specific markers CD34 and CD45. The cells differentiated toward osteogenic, adipogenic, and chondrogenic directions. With therapeutic aims, 30 dogs (39 joints) suffering from elbow dysplasia (ED) and osteoarthritis (OA) were intra-articularly transplanted with allogeneic MSCs suspended in 0.5% hyaluronic acid (HA). A highly significant improvement was achieved without any medication as demonstrated by the degree of lameness during the follow-up period of 1 y. Control arthroscopy of 1 transplanted dog indicated that the cartilage had regenerated. Histological analysis of the cartilage biopsy confirmed that the regenerated cartilage was of hyaline type. These results demonstrate that transplantation of allogeneic adipose tissue-derived mesenchymal stem cells (AT-MSCs) is a novel, noninvasive, and highly effective therapeutic tool in treating canine elbow dysplasia.
Résumé
Du tissu adipeux viscéral (TA) obtenu de résidus chirurgicaux lors d’ovariectomies de routine a été utilisé comme source pour isoler des cellules souches mésenchymateuses canines (CSMs). Tel que déterminé par cytofluorométrie, les cellules du 2e passage exprimaient les marqueurs de CSM CD44 et CD90, et étaient négatives pour les marqueurs spécifiques de lignée CD34 et CD45. Les cellules se sont différenciées dans des directions ostéogéniques, adipogéniques, et chondrogéniques. À des fins thérapeutiques, 30 chiens (39 articulations) souffrant de dysplasie du coude (DC) et d’ostéoarthrite (OA) ont reçu une transplantation intra articulaire de CSMs allogéniques en suspension dans 0,5 % d’acide hyaluronique (AH). Une amélioration hautement significative a été obtenue sans aucune médication tel que démontré par le degré de boiterie durant la période de suivi de 1 an. Une arthroscopie de contrôle de un des chiens ayant reçu une transplantation montrait que le cartilage s’était régénéré. L’analyse histologique de la biopsie du cartilage a confirmé que le cartilage régénéré était de type hyalin. Ces résultats démontrent que la transplantation de cellules souches mésenchymateuses dérivées de tissu adipeux allogène est un outil thérapeutique novateur, non-invasif, et très efficace pour traiter la dysplasie du coude chez le chien.
(Traduit par Docteur Serge Messier)
Osteoarthritis (OA) is a progressive degenerative disease of the joint; its symptoms include synovial inflammation, cartilage loss, torn ligaments, osteophyte formation, and joint space narrowing (1). All of these lead to increased pain sensation, stiffness of the joint, lameness, and often a severely decreased quality of life. While the exact cause of OA is not known, it is associated with both environmental factors, such as obesity, age, and damage of the joint, as well as genetic factors, including dysplasia (2).
Regenerative medicine provides novel tools to repair damaged cartilage in joints as demonstrated by a plethora of research articles and clinical trials focusing on the role of chondrocytes and mesenchymal stem cells (MSCs) in treating OA (3). As MSCs are multipotent stromal cells that lack specific surface markers, the International Society for Cellular Therapy has declared that MSCs must meet 3 criteria, they should: i) grow as adherent cells; ii) express CD44, CD90, and CD105, while lacking endothelial and hematopoietic markers CD45, CD34, CD14 or CD11b, CD79α or CD19, as well as HLA-DR; and iii) be able to differentiate into chondrocytes, adipocytes, and osteocytes (4).
Most studies have analyzed the function of bone marrow MSCs (BM-MSCs) and adipose tissue-derived MSCs (AT-MSCs) (5). While AT-MSCs are similar to BM-MSCs in terms of their usefulness in regenerative medicine, they have an important advantage over BM-MSCs, namely, that they can be isolated in larger numbers using a minimally invasive procedure (5). As AT-MSCs are non-immunogenic and have a potent immunosuppressive activity, they can be used safely in allogeneic or even xenogeneic transplantation in OA and other models without the need for immunosuppressive medications (6,7). To enhance their effect, improve MSC survival, and ensure the prolonged presence of MSCs in a harsh microenvironment, MSCs can be combined with traditional treatments such as injecting hyaluronic acid (HA) (8,9).
The objective of this study was to evaluate the long-term effects of administering MSCs dispersed in an HA solution on regeneration of osteoarthritic elbow joints in dogs. Allogeneic AT-MSCs were isolated from surgical waste obtained from the spaying of female mixed-breed dogs and were characterized by marker analysis and differentiation. The cells were suspended in HA and injected into joints of dogs suffering from elbow dysplasia with OA. The treatment efficacy was assessed using questionnaires completed by the dog owners based on changes in the dogs’ lameness. Transplantation of MSCs resulted in highly significant long-term improvement (over a 1-year period) in the condition of the involved animals.
This study was approved by the National Scientific Ethical Committee of the National Food Chain Safety Office. All the dog owners signed an informed consent authorizing treatment and were informed of the possible risks of joint injections and potential complications of the procedure.
Visceral adipose tissue (AT) was collected by a veterinarian from 13 healthy, small, mixed-breed dogs that underwent all routine vaccinations and were regularly surveyed by veterinarians. Adipose tissue was digested with 0.1% collagenase (Sigma Aldrich, St. Louis, Missouri, USA) in Hank’s buffer for 30 min at 37°C. The resulting stromal vascular fraction (SVF) was plated at 104/cm2 in DMEM/F12 containing 10% fetal calf serum (FCS), 2 mM glutamine, and 50 U/mL penicillin and streptomycin (complete medium) (all from Thermo Fisher Scientific, Waltham, Massachusetts, USA). Cultures were grown up to 80% confluency, diluted twice to reach stage passage 2 (P2), and stored in liquid nitrogen until further usage.
Passage 2 (P2) cells from 8 donors were analyzed for surface markers by cytofluorimetry (FACSCalibur; BD Biosciences, San José, California, USA) using the following antibodies: fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse/human CD44 (BioLegend, San Diego, California, USA); Phycoerythrin (PE)-conjugated rat anti-canine CD90 (eBioscience, San Diego, California, USA); FITC-conjugated rat anti-canine CD45 (eBioscience); and FITC-conjugated mouse anti-canine CD34 (R&D Systems, Minneapolis, Minnesota, USA). The data were evaluated using CellQuest software (BD Biosciences).
The differentiation capacity of P2 cells was tested as follows. The cells were fixed in 8% formaldehyde in phosphate-buffered saline (PBS) for 15 min after culturing cells in osteogenic differentiation medium (10 mM β-glycerophosphate, 50 μg/mL L-ascorbic acid 2-phosphate, and 10 nM dexamethasone, all from Sigma Aldrich) for 21 d (10) or adipogenic differentiation medium (15% rabbit serum, 2 mM glutamine, and 50 U/mL penicillin and streptomycin containing DMEM/F12) for 4 d (11). The cultures were then stained with 1% Alizarin Red S for 45 min or AdipoRed (Lonza, Basel, Switzerland) for 15 min and analyzed with an Olympus CKX41 microscope at 200× magnification or a fluorescent Olympus microscope (Olympus IX81) at 100× magnification, respectively. Photos were taken with an Olympus C-5060 digital camera from 5 non-overlapping regions for osteogenic and adipogenic differentiation.
Chondrogenic differentiation was induced according to Bosnakovski et al (12) in 3-dimensional pellet culture without any growth factor for 21 d. Fifteen chondrogenic pellets were collected and digested with 0.3% collagenase (Sigma Aldrich) in Hank’s buffer containing 2 U/μL RNase inhibitor (RNasin Promega, Madison, Wisconsin, USA) for 30 min at 37°C in a water bath. Total ribonucleic acid (RNA) was isolated from the pellet cultures and from non-induced cells as control using a Perfect Pure RNA Kit (5Prime, Gaithersburg, Maryland, USA) according to the manufacturer’s instructions. A RevertAid H Minus First-Strand cDNA Synthesis Kit (Thermo Fisher Scientific) was used to obtain complementary deoxyribonucleic acid (cDNA) from 94 ng of total RNA/reaction. Quantitative real-time polymerase chain reaction (qPCR) was carried out using an AccuPower 2X Greenstar qPCR Master Mix (Bioneer, Daejeon, Republic of Korea) in a RotoGene3000 instrument (Qiagen, Hilden, Germany).
Relative quantification of gene expression was done by comparing threshold values. All results were normalized to Gapdh (Gapdh: forward: TGGCAAAGTGGATATTGTCG reverse: AGATGGACTTCCCGTTGATG) and qPCR primers (Sox9: forward: ACGACTACACTGACCACCAGAAC reverse: GTAGGTGAAGGTGGAGTAGAGGC, Aggrecan: forward: TTGCACTCAGGAGAGGAGAC reverse: CCACGCAGGTGGCTCCATTC) were used as in Lee et al (13) and Zang et al (14), with slight modifications.
Osteogenic, adipogenic, and chondrogenic differentiation assays were carried out on MSC cultures derived from 3 donors for osteogenic, 1 donor for adipogenic and 1 donor for chondogenic differentiation.
Preparation of AT-MSCs for therapy
Passage 2 AT-MSCs from 2 different adipose tissue donors were thawed, cultured for 3 d, mixed, and suspended in 0.5% sodium hyaluronate (TRB Chemedica International S.A. Geneva, Switzerland). Adipose tissue-derived MSCs (12 × 106 ± 3.2 × 106 cells/injection) were transported to the veterinarian clinics in syringes. In total, 6 different MSC cultures derived from 6 donors were used for transplantation.
Patient selection
Thirty-nine elbow joints from 30 dogs were included in the study. The inclusion criteria were recurrent lameness and pain attributed to OA after conventional treatment of dogs suffering from elbow dysplasia and cartilage degeneration (nonsteroidal anti-inflammatory drugs, intra-articular injection of hyaluronic acid, arthroscopy, or traditional surgery). Five dogs with osteoarthritic elbows were excluded as they were receiving pain relief medication for additional hip dysplasia or were undergoing analgesic treatment for spinal disc herniation. During this study, 6 dogs with elbow dysplasia died due to diseases unrelated to transplantation. Anti-inflammatory drugs (carprofen or meloxicam) were administered 10 d before transplantation.
The health status of the treated dogs was evaluated by the owners using a questionnaire modified from Black et al (15), as well as a survey by the veterinarians. The veterinarians taking part in the survey also collected data regularly from the owners and the results at 6, 9, and 12 mo were used to assess the effectiveness of stem cell treatment.
Arthroscopic record samples were taken from 1 dog before cell injection and 12 mo after transplantation when the biopsy sample was also taken. As the dog’s diagnosis was fissured medial coronoid process, a subtotal coronoidectomy was carried out and the torn part was removed. Images were copied from the video, which referred to the same site of the ulna. As the original video was taken at low resolution, we were not able to present better quality images. The biopsy specimen was taken from the regenerated area of the cartilage of 1 dog during arthroscopic survey. After fixation in 10% neutral buffered formalin for 24 h at room temperature, tissue samples were dehydrated in a series of ethanol and xylene baths and embedded in paraffin wax. Sections (3 to 4 μm) were stained with hematoxylin and eosin or Safranin O. The slides were analyzed using an Olympus BX53 light microscope and images were taken with an Olympus SC100 digital camera using the Olympus cellSense software. Data were calculated as mean ± standard error of mean (SEM) and groups were compared using the nonparametric Wilcoxon test.
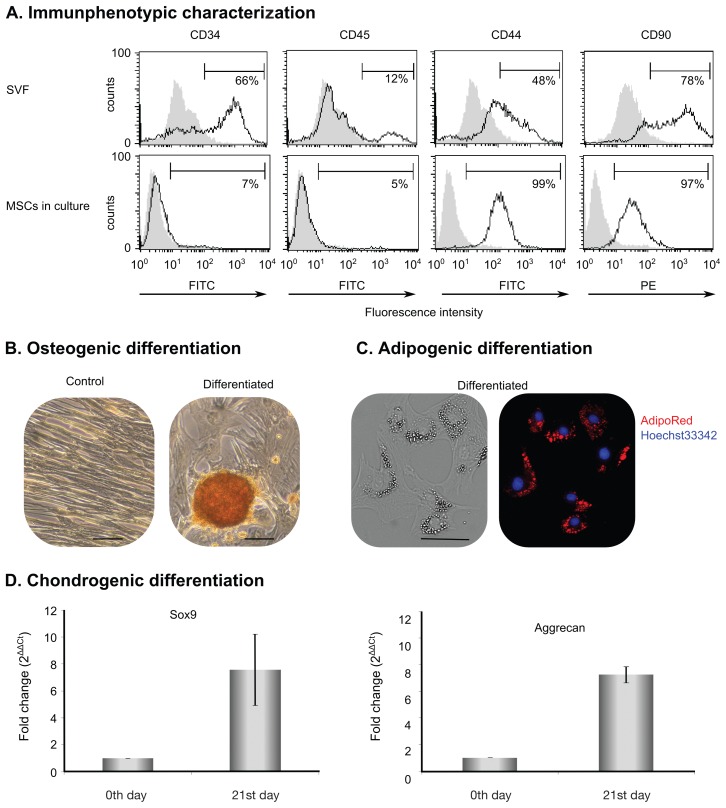
Cell surface markers of the initial SVF and cultured MSCs were analyzed. The stromal vascular fraction (SVF) contained cells with lineage-specific markers (66% CD34+ hematopoietic progenitor and endothelial cells and 12% CD45+ leucocytes) and cells with MSC markers (48% CD44 and 78% CD90). After culturing, the contaminating cell lineages disappeared and the resulting homogenous CD44+ and CD90+ cell population was referred to as AT-MSCs (Figure 1A). Passage 2 AT-MSCs were analyzed for differentiation capacity. The results showed that AT-MSCs differentiated into osteoblasts (Figure 1B) and adipocytes (Figure 1C), as verified by Alizarin Red for extracellular calcium and AdipoRed for lipid droplet staining. Elevation of aggrecan and Sox9 messenger RNA (mRNA) expression was detected when chondrocyte differentiation was induced (Figure 1D).
Figure 1.
Characterization of adipose tissue-derived mesenchymal stem cells (AT-MSCs). A — The presence of CD34+, CD45+, CD44+, and CD90+ cells was analyzed in isolated stromal vascular fraction (SVF) (top row) and cultured AT-MSC (bottom row) with flow cytometer. Grey histograms represent control samples without any antibody and black-lined histograms show fluorochrome-conjugated, antibody-stained samples. Percentages indicate the proportion of positive cells to the analyzed surface marker in the cell population. B — Osteogenic differentiation. Extracellular calcium was stained with Alizarin Red S solution. Representative figure shows matrix mineralization in an induced passage 2 cell culture. Original magnification: 200×, scale bar = 500 μm. C — Adipogenic differentiation. Accumulated lipid droplets were stained with AdipoRed (red) and nuclei were dyed with Hoechst 33342 (blue). Original magnification: 100×, scale bar 100 μm. D — Chondrogenic differentiation. Chondrogenic induction was carried out in micromass culture and quantitative polymerase chain reaction (qPCR) was conducted for mRNA expression of Sox9 and aggrecan. Cells on day 0 of micromass induction served as control. Results are expressed as mean ± standard deviation.
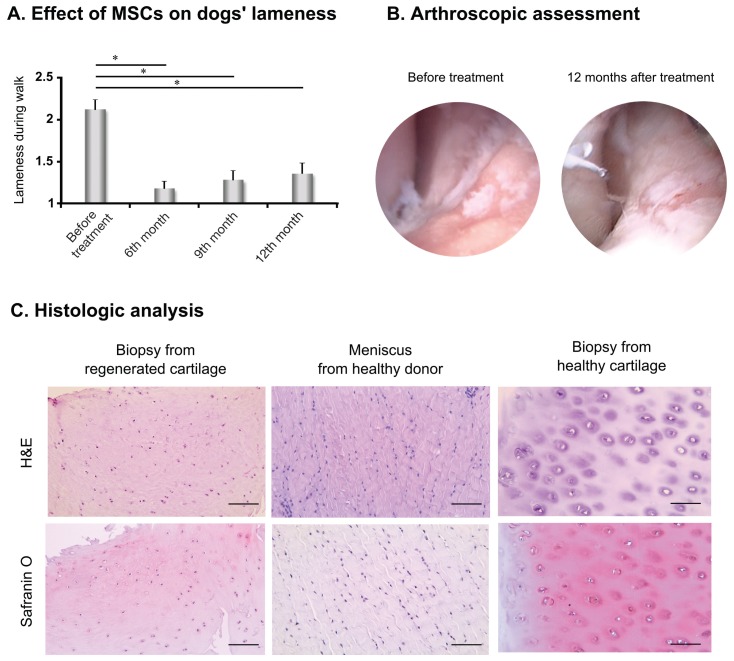
Thirty dogs (39 joints) suffering from elbow dysplasia resulting in OA and cartilage degeneration were treated with AT-MSCs and followed for 1 y by veterinarians and the owners. Based on the owners’ evaluations, AT-MSCs had significant effect (31 cases out of 39) on the dogs’ lameness during walking (Figure 2A) without any further medication for at least a 1-year period. Three further joints from the 39 showed stable improvement in lameness, with only occasional application of painkillers required.
Figure 2.
Therapeutic effect of adipose tissue-derived mesenchymal stem cells (AT-MSCs). A — Lameness was rated by the owners using a questionnaire modified from Black et al (15). Results were substantiated by the nonparametric Wilcoxon test. *P < 0.001, N = 39. B — Images were excised from videos taken during arthroscopic surgery 3 wk before MSC injection (left image) and control arthroscopy 1 y after transplantation (right image). Images represent the same area of the joint. C — Images of tissue samples at 200× magnification, stained with hematoxylin and eosin (H&E, upper row) and Safranin O (lower row), obtained from the regenerated cartilage (left column), a healthy meniscus cartilage (middle), and healthy cartilage (right column). Scale bar = 50 μm.
Comparison of arthroscopic images taken before and after transplantation showed regenerated cartilage islets in the MSC-treated joint (Figure 2B). Analysis of hematoxylin-eosin stained slides revealed that the repaired tissue was hyaline-like cartilage with fibrous elements (upper left, Figure 2C) compared to hyaline cartilage from a healthy joint (upper right, Figure 2C) and it was morphologically very different than fibrocartilage obtained from a healthy dog, which is typically found in the meniscus (upper middle, Figure 2C). The regenerated cartilage and healthy hyaline cartilage had lower cell density and less fibrous elements than fibrocartilage (Figure 2C). Moreover, Safranin O staining indicated higher glycosaminoglycan (GAG) content in the regenerated cartilage (lower left, Figure 2C) and healthy hyaline cartilage (lower right, Figure 2C) compared to fibrocartilage (lower middle, Figure 2C).
Damage from osteoarthritis and cartilage does not heal spontaneously due to poor vascularization and high density synovial fluid. There is therefore a great need for novel therapies to enhance formation of proper cartilage as well as to inhibit and cure OA. This is especially the case for elbow dysplasia, for which there are far fewer surgical solutions than for hip dysplasia. Recent developments include the local transplantation of MSCs into the articular cavity. This study shows that visceral adipose tissue is an excellent source of canine MSCs. After isolation, MSCs showed the expected characteristics, including the expression of cell surface markers, CD90 and CD44. Moreover, they were free of the lineage-specific markers CD45 and CD34 and retained their differentiation capability, since they differentiated toward osteogenic, adipogenic, and chondrogenic directions.
Intra-articular injection of AT-MSCs resulted in highly significant and sustained improvement in the condition of the patients. Lameness was ameliorated after 3 mo of treatment (data not shown) without any further medication and this improvement persisted at least during the 1-year survey. To our knowledge, this is the longest survey carried out with the largest patient population in the literature. It is worth mentioning that several additional dogs suffering from osteoarthritis in joints other than the elbow have also been treated with MSCs with encouraging results. The dogs’ conditions improved after MSC transplantation in 3 out of 4 hip cases, 2 out of 2 shoulder cases, 3 out of 3 knee cases, and 3 out of 3 hock joints (data not shown). Side effects were rare, with swelling for several days in 2 of 39 treated joints. Comparison of surgical and control arthroscopy confirmed newly regenerated cartilage after treatment. Although fibrocartilage tissue is formed during spontaneous repair of damaged hyaline cartilage, our results clearly indicate that MSC transplantation induces hyaline-like cartilage formation, which is more adequate for proper joint function. Our results strongly indicate that the significant improvement in lameness is attributable to the formation of long-lasting (sustained), hyaline-like cartilage repair tissue due to AT-MSC transplantation.
Acknowledgments
The authors thank Karolina Korom and Marietta Görög for collecting information from dogs’ owners and liaising with the veterinarians involved in the program. We are grateful to the veterinarians, Drs. Ottó Sebő (Small Animal Clinic, Makó), Péter Pál Muka (Profivet Veterinary Surgery Center and Animal Hospital, Göd), Péter Makai (MiniVet Small Animal Clinic, Budapest), György Pelle (Teaching Animal Hospital, Nyíregyháza), and András Bánfi (PrimaVet Small Animal Clinic, Budapest), for surgery, transplantation, and supervision of the dogs. The authors also thank Ákos Hornung for helpful advice about statistical evaluation and Mrs. Andrea Gercsó and Katalin Kovács for their excellent assistance. This work was supported by grants, GINOP-2.3.2-15-2016-00020 and GINOP-221-5-2016-00007.
References
- 1.Man GS, Mologhianu G. Osteoarthritis pathogenesis — A complex process that involves the entire joint. J Med Life. 2014;7:37–41. [PMC free article] [PubMed] [Google Scholar]
- 2.Poulet B, Staines KA. New developments in osteoarthritis and cartilage biology. Curr Opin Pharmacol. 2016;28:8–13. doi: 10.1016/j.coph.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W, Ouyang H, Dass CR, Xu J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016;4:15040. doi: 10.1038/boneres.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 5.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 6.Gutiérrez-Fernández M, Rodríguez-Frutos B, Ramos-Cejudo J, et al. Comparison between xenogeneic and allogeneic adipose mesenchymal stem cells in the treatment of acute cerebral infarct: Proof of concept in rats. J Transl Med. 2015;13:4–13. doi: 10.1186/s12967-015-0406-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai S-Y, Huang Y-C, Chueh L-L, Yeh L-S, Lin C-S. Intra-articular transplantation of porcine adipose-derived stem cells for the treatment of canine osteoarthritis: A pilot study. World J Transplant. 2014;4:196–205. doi: 10.5500/wjt.v4.i3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen PY, Huang LLH, Hsieh HJ. Hyaluronan preserves the proliferation and differentiation potentials of long-term cultured murine adipose-derived stromal cells. Biochem Biophys Res Commun. 2007;360:1–6. doi: 10.1016/j.bbrc.2007.04.211. [DOI] [PubMed] [Google Scholar]
- 9.Altman AM, Abdul Khalek FJ, Seidensticker M, et al. Human tissue-resident stem cells combined with hyaluronic acid gel provide fibrovascular-integrated soft-tissue augmentation in a murine photoaged skin model. Plast Reconstr Surg. 2010;125:63–73. doi: 10.1097/PRS.0b013e3181c2a59a. [DOI] [PubMed] [Google Scholar]
- 10.Vieira NM, Brandalise V, Zucconi E, Secco M, Strauss BE, Zatz M. Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transplant. 2010;19:279–289. doi: 10.3727/096368909X481764. [DOI] [PubMed] [Google Scholar]
- 11.Diascro DD, Vogel RL, Johnson TE, et al. High fatty acid content in rabbit serum is responsible for the differentiation of osteoblasts into adipocyte-like cells. J Bone Miner Res. 1998;13:96–106. doi: 10.1359/jbmr.1998.13.1.96. [DOI] [PubMed] [Google Scholar]
- 12.Bosnakovski D, Mizuno M, Kim G, et al. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells in pellet cultural system. Exp Hematol. 2004;32:502–509. doi: 10.1016/j.exphem.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Lee KS, Cha SH, Kang HW, et al. Effects of serial passage on the characteristics and chondrogenic differentiation of canine umbilical cord matrix derived mesenchymal stem cells. Asian-Australas J Anim Sci. 2013;26:588–595. doi: 10.5713/ajas.2012.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang N, Dietrich MA, Lopez MJ. Canine intra-articular multipotent stromal cells (MSC) from adipose tissue have the highest in vitro expansion rates, multipotentiality, and MSC immunophenotypes. Vet Surg. 2013;42:137–146. doi: 10.1111/j.1532-950X.2013.01091.x. [DOI] [PubMed] [Google Scholar]
- 15.Black LL, Gaynor J, Gahring D, et al. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: A randomized, double-blinded, multicenter, controlled trial. Vet Ther. 2007;8:272–284. [PubMed] [Google Scholar]