Abstract
Objectives
Traditional evaluations of metabolic health may overlook underlying dysfunction in individuals who show no signs of insulin resistance or dyslipidemia. The purpose of this study was to characterize metabolic health in overweight and obese adults using traditional and non-traditional metabolic variables. A secondary purpose was to evaluate differences between overweight/obese and male/female cohorts, respectively.
Methods
Forty-nine overweight and obese adults (Mean ± SD; Age=35.0 ± 8.9 yrs; Body mass index=33.6 ± 5.2 kg·m−2; Percent body fat [%fat]=40.0 ± 7.3%) were characterized. Body composition (fat mass [FM], lean mass [LM], %fat) was calculated using a 4-compartment model; visceral adipose tissue (VAT) was quantified using B-mode ultrasound. Resting metabolic rate (RMR) and respiratory exchange ratio (RER) were evaluated using indirect calorimetry. Fasted blood and saliva samples were analyzed for total cholesterol (TC), high-density lipoproteins (HDL), low-density lipoproteins (LDL), triglycerides (TRG), glucose (GLUC), insulin, leptin, estradiol, and cortisol.
Results
The prevalence of individuals with two or more risk factors increased from 13% to 80% when non-traditional metabolic factors (list which factors) were considered in addition to traditional risk factors (GLUC, TRG, HDL). Between overweight and obese individuals, there were no significant differences in %fat (p=0.146), VAT (p=0.959), RER (p=0.493), blood lipids/GLUC (p>0.05), insulin (p=0.143), leptin (p=0.053), or cortisol (p=0.063); obese had higher FM, LM, RMR, and estradiol (p<0.001). Males had greater LM, RMR, and TRG (p<0.01); females had greater FM, %fat, HDL, and leptin (p<0.001). There were no significant sex differences in RER (p=0.638), estradiol (p=0.052), insulin (p=0.263), or cortisol (p=0.784).
Conclusions
Evaluating metabolic health beyond BMI and traditional cardio-metabolic risk factors can give significant insights into metabolic status. Due to high variability in metabolic health in overweight and obese adults and inherent sex differences, characterization based on body composition, metabolic factors, and hormonal profiles can improve early identification and approaches to disease prevention.
Keywords: Body composition, visceral fat, resting metabolic rate, fuel utilization, hormones, sex
Introduction
Metabolic syndrome is a chronic disease characterized by the presence of multiple risk factors that signify metabolic dysfunction [1]. Many of these risk factors, such as insulin resistance and dyslipidemia, are also associated with an increased risk for the development of cardiovascular disease and type 2 diabetes [1]. Body mass index (BMI) is the primary clinical method used in obesity classification [2]. When used in combination with blood lipid profiles and insulin resistance, BMI serves as a useful screening tool for health risk in clinical settings [2]. The diagnostic value of these measures, however, are limited by the inability to evaluate fat distribution or differentiate between fat mass (FM) and lean mass (LM), all of which have different physiological impacts on metabolic health [2]. In 2008, Wildman et al. [3] estimated that 51% of overweight and 32% of obese individuals had no more than one metabolic risk factor, classifying them as metabolically healthy. Despite their apparently normal metabolic status, subsequent studies suggest that these individuals are still at an increased risk of disease, indicating that other underlying signs of metabolic dysfunction are potentially being overlooked by standard clinical tools [4,5].
Regional distribution of body fat, or where an individual is predisposed to store body fat, may have stronger associations with risk of metabolic dysfunction than the overall presence of excess body fat [6,7]. Brochu et al. [8] previously demonstrated that obese women who were insulin resistant, had about 50% more visceral adipose tissue (VAT) than those who were not. Similarly, normal weight males and females with insulin resistance had 50% and 79% greater VAT than those who were not insulin resistant [6], suggesting more serious health implications associated with VAT as opposed to overall total body FM. Lean mass also has important associations with metabolic heath [9]. As a highly metabolically active tissue, LM has a significant influence on metabolic rate and fuel utilization [10,11]. Losses in LM are associated with insulin resistance, decreased metabolic rate, impaired fat oxidation, and poor functionality in activities of daily living, all of which are associated with weight gain and metabolic dysfunction [9,12]. Finally, hormonal imbalances are also associated with excess body fat [13,14]. Specifically related to insulin, leptin, estradiol, and cortisol, hormonal imbalances can lead to an unfavorable distribution of fat, as well as negative influences on energy expenditure [13]. Due to inherent hormonal differences between sexes, variations in hormone concentrations are likely to have different metabolic consequences for males and females, making sex-specific evaluations of metabolic health status an important consideration [14,15].
The associations between overweight and obesity and disease risk have resulted in decades of research with a primary focus on the reduction of body fat. While short-term success has been achieved mainly through caloric restriction and aerobic exercise interventions, success rates with long-term maintenance have been substantially lower [16]. Recent data suggest that a more comprehensive evaluation of metabolic health may be a more appropriate and sensitive approach to evaluating metabolic status, regardless of body weight [2]. Evaluation of non-traditional metabolic factors could provide valuable insight into aspects of metabolic health that may otherwise be overlooked, improving the detection, prevention, and treatment of metabolic health issues. Therefore, the purpose of this study was to characterize metabolic health in overweight and obese adults using traditional and non-traditional metabolic variables. A secondary purpose was to evaluate differences in metabolic characteristics between overweight and obese individuals as well as in men compared to women.
Materials and Methods
Subjects
Following CONSORT guidelines (Figure 1), approximately 114 individuals provided initial interest in study participation. Using electronic correspondence, 34 did not meet inclusion/exclusion criteria; an additional 16 did not respond after initial contact. Sixty-four individuals completed in-person inclusion/exclusion review at an initial enrollment visit. Eight individuals were excluded from participation based on violation of inclusion/exclusion criteria, resulting in a cohort of 56 individuals enrolled in the study, seven of whom withdrew from participation prior to testing. A total of 49 overweight and obese men (n = 23) and premenopausal women (n = 26), between the ages of 18 and 55 years (Mean ± standard deviation [SD]; Age=35.0 ± 8.9 years; BMI=33.6 ± 5.2 kg·m−2; %fat=40.0 ± 7.3%) were characterized for this study (Table 1). All subjects were healthy, having no history of medical or surgical events including cardiovascular disease, diabetes, renal, hepatic, or musculoskeletal disorders. Women were pre-menopausal, determined as reporting consistent menstruation for three months prior to enrollment. All subjects were considered weight-stable (± 4.5 kg) and had maintained dietary habits (no drastic changes in macronutrient or caloric intake) for three months prior to enrollment. Subjects were excluded from participation if they reported using a meal replacement or dietary supplement that may have influenced metabolism within eight weeks prior to the enrollment date, or had participated in another clinical trial within four weeks prior to enrollment. All subjects agreed to maintain their usual physical activity and dietary habits, and to abstain from smoking, caffeine, tobacco, and alcohol 24 hours before testing days.
Figure 1.
CONSORT flow diagram of subject recruitment and enrollment for study participation and analysis.
Table 1.
Subject characteristics (Mean ± standard deviation [SD])
| Total | Male | Female | Overweight | Obese | |
|---|---|---|---|---|---|
| N | 49 | 23 | 26 | 13 | 36 |
| Age (yrs) | 35.0 ± 8.9 | 37.4 ± 10.3 | 32.8 ± 6.9 | 37 ± 8.8 | 34.2 ± 8.9 |
| Height (cm) | 170.7 ± 9.7 | 178.1 ± 6.4* | 164.1 ± 7.1* | 169.7 ± 9.6 | 171.0 ± 9.9 |
| Weight (kg) | 97.9 ± 17.7 | 102.2 ± 15.1 | 94.2 ± 19.3 | 81.6 ± 9.9# | 103.8 ± 16.2# |
| BMI (kg·m-2) | 33.6 ± 5.2 | 32.1 ± 3.3 | 34.9 ± 6.2 | 28.2 ± 1.3# | 35.5 ± 4.7# |
| FM (kg) | 39.2 ±10.8 | 34.8 ± 8.8* | 43.1 ± 11.0* | 30.1 ± 4.0# | 42.5 ± 10.6# |
| %fat | 40.0 ± 7.3 | 33.8 ± 4.8* | 45.5 ± 4.1* | 37.4 ± 6.3 | 40.9 ± 7.5 |
| LM (kg) | 58.7 ± 12.1 | 67.4 ± 8.6* | 51.0 ± 9.4* | 51.4 ± 10.7# | 61.3 ± 11.6# |
| VAT (cm) | 6.0 ± 1.8 | 6.5 ± 1.9 | 5.5 ± 1.6 | 6.0 ± 1.7 | 6.0 ± 1.9 |
| RMR (kcal·d1) | 1813.2 ± 311.2 | 2011.1 ± 259.1* | 1638.2 ± 242.8* | 1587.5 ± 216.5# | 1894.8 ± 301.6# |
| RER (a.u.) | 0.9 ± 0.1 | 0.9 ± 0.0 | 0.8 ± 0.1 | 0.8 ± 0.0 | 0.9 ± 0.0 |
| TC (mg/dL) | 185.5 ± 39.5 | 191.4 ± 35.0 | 179.4 ± 44.5 | 187.9 ± 36.8 | 184.6 ± 41.5 |
| LDL (mg/dL) | 118.8 ± 34.3 | 124.3 ± 29.6 | 113.0 ± 38.4 | 120.0 ± 33.7 | 118.3 ± 35.0 |
| HDL (mg/dL) | 44.6 ± 12.9 | 39.0 ± 7.9* | 50.5 ± 14.5* | 45.8 ± 13.2 | 44.2 ± 12.9 |
| TRG (mg/dL) | 110.7 ± 73.5 | 140.7 ± 87.7* | 79.3 ± 35.2* | 110.6 ± 56.6 | 110.7 ± 79.5 |
| GLUC (mg/dL) | 87.2 ± 22.2 | 92.8 ± 29.6 | 81.4 ± 7.0 | 81.5 ± 6.2 | 89.3 ± 25.5 |
| Insulin (uIU/mL) | 9.7 ± 7.4 | 8.5 ± 4.9 | 10.9 ± 9.2 | 7.0 ± 3.8 | 10.6 ± 8.1 |
| Cortisol (μg/dL) | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.2 ± 0.1 | 0.3 ± 0.2 |
| Estradiol (pg/mL) | 2.0 ± 0.8 | 1.7 ± 0.8 | 2.2 ± 0.8 | 1.6 ± 0.6# | 2.1 ± 0.9# |
| Leptin (ng/mL) | 36.1 ± 28.9 | 15.0 ± 10.9* | 58.1 ± 25.2* | 22.3 ± 16.4 | 41.1 ± 31.0 |
significant difference between male/female (p<0.05).
significant difference between overweight/obese (p<0.05).
Experimental Design
In a cross-sectional design, subjects completed measures of total and regional body composition, resting metabolic rate (RMR), respiratory exchange ratio (RER), a blood draw, and a saliva sample. Prior to testing, all subjects provided written informed consent approved by the University’s Institutional Review Board, a medical history questionnaire, and received standardized dietary recommendations based on a 4-day dietary log (two weekdays, two weekend days). A two-week run-in period, followed by a 24-hour dietary log, proceeded testing to allow for normalization of potential dietary changes that may occur at the beginning of a trial [17]. All resting measures were performed in the morning following an eight-hour fast.
Body Composition
Fat mass, %fat, and LM were calculated using a four compartment (4C) gold standard model previously described by Wang et al. [18] (Equation 1), where BV is total body volume, TBW is total body water, Mo is total body bone mineral density, and BM is body mass measured in kilograms.
| Equation 1 |
Test-retest reliability for the 4C model from our lab in a similar population are as follows: FM intraclass correlation coefficient (ICC)=0.994, standard error of measure (SEM)=0.830 kg, and minimum difference (MD)=2.30 kg; %fat ICC=0.988, SEM=0.868%, MD=2.40%, and LM ICC=0.996, SEM=0.842 kg, MD=2.33 kg. Multi-compartment body composition evaluation techniques are considered the current gold standard for composition estimations (REFS).
Body volume was estimated using air-displacement plethysmography (BodPod®, COSMED USA, Inc., Concord, CA, USA). While wearing tight-fitting clothing, such as a bathing suit or spandex and swimming cap, subjects were weighed using the device scale (Tanita Corp, Tokyo Japan) to determine BM (kg). Subjects were asked to enter the BodPod, breathe normally, and remain still for the duration of the test. Body volume was determined from the average of two measurements; lung volume was predicted from the default software (Software Version 5.4.1, COSMED USA, Inc.).
Total body water was estimated using bioelectrical impedance spectroscopy (BIS; SFB7, ImpediMed, Queensland, Australia) immediately following RMR testing. Following manufacturer recommendations, leads were connected to four electrodes placed on 1) the right wrist (bisecting the ulnar head), 2) five centimeters distally on the hand, 3) the right ankle (bisecting the malleoli), and 4) five centimeters distally on the foot while lying supine with separation between limbs. The average of two measurements was recorded as TBW.
Dual-energy X-ray absorptiometry (DEXA; GE Lunar iDXA, GE Medical Systems Ultrasound & Primary Care Diagnostics, Madison, WI, USA) was used to estimate total body bone mineral content (BMC) to calculate total body bone mineral density (Mo = BMC (kg) × 1.0436). Total body scans were performed by trained DEXA technicians and analyzed using default software (enCORE Software Version 16). Following manufacturer recommendations, subjects were positioned in the center of the scanning table with the head at the top of the scanning parameter. Hands were placed at the sides with palms facing the body. Limbs were separated as much as possible, while staying within the scanning parameters. For participants wider than the scanning parameter (n= 6), the subject was shifted left-of-center to ensure a full scan of the right side of the body, and left limbs were estimated from the right limbs according to manufacturer guidelines. A strap was secured around the ankles for comfort and subjects were instructed to relax and breathe normally for the duration of the scan (7-13 min). For all body composition tests, subjects wore lightweight athletic clothing and removed all metal items that may disrupt the measures.
Visceral Adipose Tissue
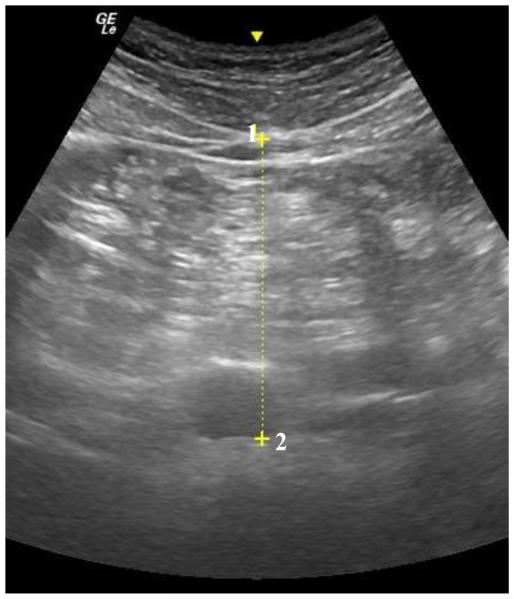
Brightness-mode (B-mode) ultrasound (US) (GE LOGIQ-e, Software version R8.0.7, GE Healthcare, Wisconsin, USA) with standardized settings (Frequency: 4.0 MHz, Gain: 45) was used to quantify visceral adipose tissue thickness (VAT). While lying supine, a wide-band convex array ultrasound transducer (GE: C1-5 RS) with transducer gel was applied to the abdomen at the site of the linea alba, approximately 5 cm proximal to the umbilicus. The depth of the scan was adjusted as necessary to ensure full image capture of visceral adiposity. A still image of the abdomen was captured at the moment of complete exhale (Figure 2). Visceral adipose tissue thickness was defined and quantified as the perpendicular distance between the interior border of the rectus abdominis and the posterior wall of the aorta [19,20]. The average of two measurements were recorded as VAT. Test-retest reliability for VAT produced an ICC=0.99, SEM=0.35 cm, and MD=0.69 cm.
Figure 2.
Abdominal ultrasound scan. Visceral adipose tissue thickness was measured between the interior border of the rectus abdominis (1) and the posterior wall of the aorta (2).
Resting Metabolic Rate
Resting metabolic rate and RER were evaluated using a ventilated canopy with indirect calorimetry (TrueOne 2400 Canopy System, ParvoMedics, Inc., Sandy, UT). While lying supine, the canopy was placed over the head and secured comfortably around the head and arms to prevent air penetration. For the duration of the test (30 minutes) subjects were asked to relax and breathe normally, without falling asleep. Respiratory gases, oxygen uptake, and carbon dioxide production were analyzed breath-by-breath with a metabolic cart (TrueOne 2400, ParvoMedics, Inc., Sandy, UT). During the first five minutes of the test, the dilution rate was adjusted until the fraction of expired carbon dioxide was 1.0-1.2% in accordance with manufacturer’s guidelines. This portion of the test was excluded and the mean RMR and RER were recorded from the remaining 25 minutes. Test-retest reliability from our lab produced an RMR ICC=0.94, SEM=125.6 kcal·day−2 and MD=244.3 kcal·day−2, and RER ICC=0.83, SEM=0.03 arbitrary units (a.u.) and MD=0.05 a.u.
Fasted Blood Draw and Saliva Sample
A 4 ml blood sample from the region of the antecubital vein was collected and analyzed for fasting blood glucose (GLUC), total cholesterol (TC), triglycerides (TG), high-density lipoproteins (HDL), low-density lipoproteins (LDL), insulin, and leptin. Potential insulin resistance was assessed by calculating HOMA-IR, using the HOMA2 model described by Levy et al. [21] (insulin [pmol/L] and glucose [mmol/L]) [22]. A 1.0-2.0 ml saliva sample was collected via passive drool and analyzed for estradiol and cortisol concentrations. To avoid blood contamination, subjects were asked to avoid brushing their teeth for 45 minutes prior and undergoing dental work for 48 hours prior to giving saliva samples. Immediately upon arrival and prior to sample collection, subjects were asked to rinse their mouth with water to remove any residues. All samples were analyzed by the Biobehavioral Lab (Chapel Hill, NC) using commercially available enzymatic assays. Blood samples were analyzed for lipid profiles within 15 minutes of the draw. Saliva samples were kept frozen at −20°C until analysis. For all assays, the coefficient of variation was below 15%.
Dietary Intake Assessment
To account for dietary influences on RER, average calorie (CAL), carbohydrate (CHO), fat (FAT), and protein (PRO) intake were evaluated from 4-day dietary food logs using nutrition analysis software (The Food Processor, version 10.12.0, Esha Research, Salem, OR, USA). For the entire group, average CAL=2235.1 ± 651.1 kcal, CHO=257.7 ± 82.3 g (47% of caloric intake), FAT=88.1 ± 31.6 g (36%), and PRO=87.6 ± 27.7 g (17%).
Statistical Analysis
To characterize metabolic health, individuals were stratified into risk categories (i.e. low, moderate, high) for each metabolic variable based on accepted reference ranges; the rate of occurrence for each stratification was then calculated. Individuals were then evaluated for potential metabolic risk using a hierarchal order of: 1) BMI, 2) common clinical markers of metabolic syndrome criteria (TRG, HDL, GLUC), 3) metabolic syndrome criteria with the addition of VAT, and 4) all potential metabolic variables (VAT, LMI, RMR, RER, TRG, TC, LDL, HDL, GLUC, HOMA-IR), respectively [1]. Individuals were categorized as overweight (25-29.9 kg·m−2), obese (30-34.9 kg·m−2), obese II (35-39.9 kg·m−2), or obese III (≥ 40.0 kg·m−2) to assess metabolic disease risk based on BMI [23]. Based on %fat, individuals were categorized as moderate (M<25%; F<38%) or high risk (M≥25%; F≥38%) [24]. Visceral adipose thickness was substituted for the traditional waist circumference risk factor and was stratified as low (<7cm), moderate (M=7-9cm; W=7-8 cm), or high (M>9cm; W>8cm) [19]; moderate and high VAT were considered at risk. Lean mass index (LMI; kg·m−2) was used to stratify LM measured by DEXA based on normative values for sex, race, and age [25]; LMI<10th percentile was considered at risk. Resting metabolic rate was classified as low (>125.6 kcal·d−1 below predicted), normal (±125.6 kcal·d−1 of predicted), or high (>125.6 kcal·d−1 above predicted) based on the difference between predicted and measured metabolic rate (predicted – measured) using the Harris-Benedict equation [26]. High and low cutoff values were set at the SEM for RMR, with an RMR below the SEM considered low and at-risk. Resting RER was stratified as low (<0.82 a.u.), normal (0.85 ± 0.03 a.u.) or high (>0.88 a.u.). High and low cutoff values were set at the SEM for RER, with an RER above the SEM considered at-risk. Glucose, lipids, and hormones were stratified according to standard clinical metabolic diagnoses and reference data (Table 3) [1,21,22,27-31]. For insulin resistance, individuals were categorized as normal (≤2.5) or high (>2.5) according to their calculated HOMA-IR [32]; high HOMA-IR was considered at risk. Four individuals were excluded from metabolic risk prevalence calculations due to the inability to obtain a blood sample.
Table 3.
Stratification and frequencies based on metabolic and hormonal factors.
| Stratification | Total (%) | Males | Females | |
|---|---|---|---|---|
| VAT | Low (< 7cm) | 36 (73.5%) | 15 | 21 |
|
| ||||
| Moderate (M 7-9cm; W 7-8cm) | 10 (20.4%) | 6 | 4 | |
| High (M>9 cm; W >8cm) | 3 (6.1%) | 2 | 1 | |
|
| ||||
| LM | Low (<10%) | 0 (0%) | 0 | 0 |
| Normal (10th - 90th %) | 33 (67.3%) | 18 | 15 | |
| High >90% | 11 (22.4%) | 5 | 6 | |
| >97% | 5 (10.2%) | 0 | 5 | |
|
| ||||
| RMR | Low (<125.6 kcal/d) | 22 (44.9%) | 14 | 8 |
| Normal (±125.6 kcal/d) | 24 (49.0%) | 6 | 18 | |
| High (>125.6 kcal/d) | 3 (6.1%) | 3 | 0 | |
|
| ||||
| RER | Low (<0.82) | 13 (26.5%) | 4 | 9 |
| Normal (0.85±0.03) | 28 (57.1%) | 16 | 12 | |
| High (>0.88) | 8 (16.3%) | 3 | 5 | |
|
| ||||
| TRG | Normal (<150 mg/dL) | 38 (84.4%) | 17 | 21 |
| High (≥ 150 mg/dL) | 7 (15.6%) | 6 | 1 | |
|
| ||||
| TC | Low (<100 mg/dL) | 4 (8.2%) | 0 | 0 |
| Normal (100-199 mg/dL) | 30 (61.2%) | 13 | 17 | |
| High (>200 mg/dL) | 15 (30.6%) | 10 | 5 | |
|
| ||||
| LDL | Normal (<100 mg/dL) | 13 (28.9%) | 4 | 9 |
| Above normal (100-160 mg/dL) | 26 (57.8%) | 15 | 11 | |
| High (>160 mg/dL) | 6 (13.3%) | 4 | 2 | |
|
| ||||
| HDL | Low (M< 40 mg/dL; F< 50 mg/dL) | 25 (55.6%) | 12 | 13 |
| Normal (M≥40 mg/dL; F≥50 mg/dL) | 20 (44.4%) | 11 | 9 | |
|
| ||||
| Glucose | Low (<70 mg/dL) | 1 (2.2%) | 0 | 1 |
| Normal (70-110 mg/dL) | 42 (93.3%) | 21 | 21 | |
| High (≥110 mg/dL) | 2 (4.4%) | 2 | 0 | |
|
| ||||
| HOMA-IR | Normal (≤2.5) | 44 (97.8%) | 23 | 21 |
| High (>2.5) | 1 (2.2%) | 0 | 1 | |
|
| ||||
| Leptin | Low (<10th percentile) | 0 (0.0%) | 0 | 0 |
| Normal (10th - 90th percentile) | 14 (31.1%) | 12 | 2 | |
| High (>90th percentile) | 31 (68.9%) | 11 | 22 | |
|
| ||||
| Estradiol | Low (M<0.10 pg/mL; F<1.35 pg/mL) | 3 (6.1%) | 0 | 3 |
| Normal (M=0.10-1.94 pg/mL; F=1.35-2.97 pg/mL) | 37 (75.5%) | 15 | 22 | |
| High (M>1.94 pg/mL; F>2.97 | 9 (18.4%) | 8 | 1 | |
|
| ||||
| Cortisol | Low | 7 (14.3%) | 1 | 6 |
| Normal | 42 (85.7%) | 22 | 20 | |
| High | 0 (0%) | 0 | 0 | |
Note: VAT, glucose, triglycerides, and HDL are considered risk factors for metabolic syndrome.
One-way analyses of variance (ANOVAs) were used to evaluate differences in all variables between overweight/obese and male/female cohorts, respectively. Separate analyses of covariance (ANCOVAs) were used to evaluate differences between overweight/obese and male/female when covarying for LM. Separate ANCOVAs were used to evaluate differences in RER between overweight/obese and male/female cohorts when covarying separately for CAL, CHO, FAT, and PRO. All statistical analyses were completed using SPSS (Version 21, IBM, Armonk, NY, USA), using an α level of 0.05 to determine statistical significance.
Results
Individual variable risk stratifications for the whole group and for men/women are presented in Table 2. Based on BMI and %fat, all individuals were considered to be at an increased risk of metabolic dysfunction. Twenty-two individuals (45.3%) were considered to be at a very high risk, with a BMI ≥ 35 kg·m−2. When evaluating traditional metabolic syndrome risk factors (GLUC, TRG, HDL), 39 individuals (86.7%) had no more than one risk factor, while six individuals (13.3%) had two risk factors. With the addition of VAT, the number of individuals with two or more risk factors increased to 13 (28.9%). The most common metabolic syndrome risk factor for both men and women was low HDL (55.6%). When considering all metabolic factors (VAT, LMI, RMR, RER, TRG, TC, LDL, HDL, GLUC, HOMA-IR), individuals with no more than one risk factor decreased to 20%, while individuals with at least two risk factors increased to 80%. For men, the most common risk factors were high LDL (82.6%), low RMR (60.9%), low HDL (47.8%), and high TC (43.5%) (Table 3). For women, low HDL (50.0%) and high LDL (50.0%) were the most common risk factors, followed by RMR (30.8%), VAT (19.2%), and RER (19.2%) (Table 3).
Table 2.
Risk stratification and frequencies based on BMI, clinical metabolic syndrome risk factors, and metabolic syndrome risk factors.
| Stratification | N (%) | Males | Females | |
|---|---|---|---|---|
| BMI | Overweight (25.0-29.9) | 13 (26.5%) | 6 (26.1%) | 7 (26.9%) |
| Obese (30-34.9) | 14 (28.6%) | 11 (47.8%) | 3 (11.5%) | |
| Obese II (35-39.9) | 18 (36.73%) | 6 (26.1%) | 12 (46.2%) | |
| Obese III (≥40) | 4 (8.6%) | 0 (0.0%) | 4 (15.4%) | |
|
| ||||
|
Metabolic Syndrome
(GLUC+TRG+HDL) |
0 Risk Factors | 17 (37.8%) | 8 (34.8%) | 9 (40.9%) |
| 1 Risk Factor | 22 (48.9%) | 10 (43.5%) | 12 (54.5%) | |
| 2 Risk Factors | 6 (13.3%) | 5 (21.7%) | 1 (4.5%) | |
| 3 Risk Factors | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
|
| ||||
| Metabolic Syndrome + VAT | 0 Risk Factors | 14 (31.1%) | 6 (26.1%) | 8 (36.4%) |
| 1 Risk Factor | 18 (40.0%) | 7 (30.4%) | 11 (50.0%) | |
| 2 Risk Factors | 12 (26.7%) | 9 (39.1%) | 3 (37.5%) | |
| 3 Risk Factors | 1 (2.2%) | 1 (4.3%) | 0 (0.0%) | |
| 4 Risk Factors | 0 (0%) | 0 (0.0%) | 0 (0.0%) | |
|
| ||||
|
All Metabolic Risk Factors
(VAT, LMI, RMR, RER, TRG, TC, LDL, HDL, GLUC, HOMA-IR) |
0 Risk Factors | 2 (4.4%) | 1 (4.3%) | 1 (4.5%) |
| 1 Risk Factor | 7 (15.6%) | 0 (0.0%) | 7 (31.8%) | |
| 2 Risk Factors | 14 (31.1%) | 7 (30.4%) | 7 (31.8%) | |
| 3 Risk Factors | 11 (24.4%) | 6 (26.1%) | 5 (22.7%) | |
| 4 Risk Factors | 4 (8.9%) | 4 (17.4%) | 0 (0.0%) | |
| 5 Risk Factors | 6 (13.3%) | 4 (17.4%) | 2 (9.1%) | |
| 6 Risk Factors | 1 (2.2%) | 1 (4.3%) | 0 (0.0%) | |
Note: Metabolic syndrome defined as the presence of 2+ risk factors including: elevated TRG (≥150 mg/dl), low HDL (men<40 mg/dl, women<50 mg/dl), and elevated GLUC (≥110 mg/dl) and VAT (>7cm). Additional metabolic risk factors included LMI (<10th percentile), RMR (<125.6 kcal from predicted), RER (>0.88 a.u.), TC (>200 mg/dL), LDL (>100 mg/dL), and HOMA-IR (>2.5).
Body Composition
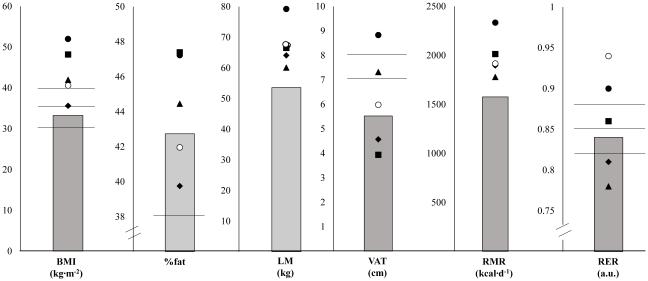
For the entire group, FM ranged from 24.54 - 75.05 kg, LM ranged from 39.10 - 85.48 kg, %fat ranged from 25.42 - 52.78%, and VAT ranged from 2.21 - 10.55 cm. Five individuals had a LMI greater than two standard deviations from the population mean (>97th percentile; LM: 56.05 – 75.15 kg), suggesting higher than average LM for their height, sex, age, and race. All five individuals were females, with a %fat >40%, and normal metabolic rate, TC, TRG, and GLUC; four of the five had normal LDL and low cortisol levels (Figure 3). No individuals were categorized with low LM.
Figure 3.
Five females with LMI>97th percentile based on age, gender, and race, (individual points) compared with the female group average (grey bars) and risk stratification cut-off values (black lines). Of the five women considered to have above average LM, all were considered to be in the obese II or obese III categories and had a %fat>40%; two were considered to have moderate (7-8cm) and high VAT (>8cm). All five women had normal metabolic rates (±125.6 kcal/d from predicted); two had an RER>0.88, potentially indicating increased reliance on carbohydrate at rest; two had an RER<0.82, potentially indicating greater reliance on fat at rest; one had an RER=0.86, potentially indicating equal contribution of carbohydrate and fat at rest. All women had normal TC, TRG, and GLUC while four of the five had normal LDL.
Obese individuals had greater FM (p<0.001) and LM (p=0.010) than overweight individuals. There were no significant differences in %fat (p=0.146) or VAT (p=0.959) between overweight and obese cohorts. Females had greater FM (p=0.006) and %fat (p<0.001) compared to males, while males had greater LM than females (p<0.0001). Although not statistically significant (p=0.053), males had more VAT than females.
Resting Metabolic Rate and Respiratory Exchange Ratio
For the entire group, RMR ranged from 1292 – 2466 kcal·d−1 and RER ranged from 0.77 - 0.98 a.u. Twenty-two individuals (44.9%) had lower than predicted RMR, while three individuals had higher than predicted RMR (6.1%). Eight individuals (16.3%) had a RER greater than 0.88 a.u. indicating higher carbohydrate oxidation at rest, compared to 13 individuals (26.5%) who had a RER less than 0.82 a.u., indicating greater fat oxidation at rest.
Individuals categorized as obese had a higher RMR than overweight individuals (p=0.002). Males had significantly higher RMR than females (p<0.001). There was no significant difference in RER between overweight and obese (p=0.493), or male and female (p=0.638) cohorts, respectively. When accounting for quantity of LM, obese individuals still had a higher RMR than overweight individuals, though the difference was not statistically significant (p=0.055). When accounting for LM, there was no significant difference in RMR between males and females (p=0.873). There were no significant differences in RER between overweight and obese (p=0.949) or males and females (p=0.328) when accounting for LM. There were no significant differences in dietary intake between overweight and obese (p>0.05), while males consumed more calories (p=0.010), FAT (p=0.050), and PRO (p=0.015), than females. When accounting separately for differences in CAL, CHO, FAT, and PRO, there were no significant differences in RER between overweight/obese and male/female cohorts (p>0.05), respectively.
Glucose, Lipids, and Hormones
Average GLUC, lipid, and hormonal values are presented in Table 1. There was no significant difference in GLUC or blood lipids between overweight and obese individuals (p>0.05). Males had significantly higher TRG (p=0.004), while females had significantly higher HDL (p=0.002). There was no significant difference in TC (p=0.320), LDL (p=0.274), or GLUC (p=0.086) between males and females.
For the entire group, insulin ranged from 1.20 – 46.50 uIU/mL, leptin ranged from 3.90 – 103.17 ng/mL, estradiol ranged from 0.63 – 5.46 pg/mL, and cortisol ranged from 0.08 – 0.71 μg/dL. Obese individuals had higher estradiol levels than overweight individuals (p=0.035) and differences in leptin approached significance (p=0.053; OB=41.09 ± 31.03 ng/mL; OW=22.33 ± 16.41 ng/mL). There were no significant differences in insulin (p=0.143) or cortisol (p=0.063) between overweight and obese. Females had significantly higher leptin than males (p<0.0001; F=58.11 ± 25.23 ng/mL; M=15.02 ± 10.93 ng/mL). Though not statistically significant (p=0.052), females had higher estradiol levels than males. There was no significant difference in insulin (p=0.263) or cortisol (p=0.784) between males and females.
Discussion
Clinical identification of disease risk has been heavily reliant on the use of BMI, which is still recommended as a primary indicator of health risk [2]. Although higher BMI classifications (≥30 kg·m−2) have been associated with markers of metabolic dysfunction, such as dyslipidemia, high fasting glucose, and insulin resistance [33], an estimated 51% of overweight and 32% of obese adults have no more than one metabolic risk factor [3]. Further, the use of BMI as a primary risk classification may overlook an estimated 23.5% of normal-weight adults who have metabolic dysfunction [3]. Previous research has supported the use of insulin resistance or other variations of the metabolic syndrome criteria, as a means of stratifying individual health risk to ultimately improve treatment intervention recommendations [3,7,8]. However, it has been proposed that metabolic health in obesity is a transient state, and even these methods may significantly overlook underlying metabolic dysfunction [4,5]. As previously suggested by Müller et al. [2], evaluating metabolic health using components of body composition and metabolic function, such as VAT, LM, RMR, and RER, in addition to hormonal and metabolic profiles, can give significant insights into metabolic status regardless of body weight. In support, the present study demonstrates that when evaluating traditional metabolic syndrome risk factors alone (GLUC, TRG, HDL), 38% of individuals had no metabolic risk factors and 87% had no more than one, despite a high average BMI (33.6 kg·m−2). Yet when accounting for additional risk factors, the proportion of individuals who could be considered to be considered at-risk for metabolic dysfunction increased from about 13% to 80%, indicating that more extensive assessment tools may be necessary for appropriate evaluation of metabolic health in overweight and obese individuals.
Previous literature has sought to identify characteristics of obese individuals that are associated with healthy or abnormal metabolic markers [7,8,34,35]. Despite associations between excess body fat and compromised metabolic health, recent studies have identified individuals with up to 50% body fat who have normal blood lipid profiles and are insulin sensitive, indicating that other factors beyond excess FM have significant influences on metabolic health [8]. In contrast, VAT has shown stronger associations with cardiometabolic risk than BMI, total body fat, or weight [36], and is consistently associated with signs of metabolic dysfunction in both obese and normal weight individuals [6-8,36]. Despite its significance, evaluation of VAT at the clinical level is non-existent in practice, and instead is reduced to a global measure of waist circumference, which does not differentiate between subcutaneous fat and VAT [37]. Due to the lack of precision, waist circumference is often omitted in the metabolic evaluation of obese individuals who have a large waist regardless of health status [7,37]. Although the present study did not incorporate waist circumference, which is a limitation, B-mode ultrasound was used as a potentially feasible clinical method to quantify VAT [19,38]. Using previously established risk cut-offs for VAT assessed by ultrasound [19], values from the present study classified 74% of the present cohort at a low metabolic risk, reflective of their relatively healthy status. Further, there was no significant difference in VAT between overweight and obese individuals, reiterating the limitations of solely relying on BMI and/or waist circumference to evaluate metabolic health in overweight and obese individuals.
Lean mass is another characteristic that is rarely evaluated clinically [9]. As a major site for glucose uptake and substrate oxidation within the body, LM may improve insulin sensitivity, lower blood lipids, and increase metabolic rate [9,10]. Abnormally low LM, as well as poor quality LM, similar to that observed in sarcopenic obesity, have been previously associated with metabolic dysfunction and insulin resistance, even in normal weight individuals [39,40]. However, as supported by results of the present study, obese individuals often have more LM associated with supporting a greater body mass [41]. All individuals in the present study had normal to above-average LM with five individuals above the 97th percentile for LM relative to their height, age, gender, and race. The subjects within the top 97th percentile were all females and all had normal TC, TRG, and GLUC, while four of the five had normal LDL and insulin sensitivity despite being amongst those with the highest BMIs and %fat (Figure 3). A previous study by Camhi et al. [42] found no significant differences in LM between males with and without metabolic dysfunction, but in contrast to the current study, found that females with metabolic dysfunction had greater LM than those considered healthy. Other studies in postmenopausal women have reported similar findings of greater LM in women with metabolic dysfunction, despite no significant differences in FM [8,43]. Further, evidence suggests that despite greater LM, the quality of the tissue, characterized by the presence of intramuscular fat, may be a more important characteristic to evaluate in relation to metabolic syndrome [9,44]. Although the current study did not measure muscle quality, and observations from the present study cannot be extrapolated to the larger population, the potential positive benefit of LM to metabolic health in women should be further investigated. Overall, the present results in combination with previous results, support the importance of evaluating LM when characterizing metabolic health in overweight and obese adults.
Lean mass is also more metabolically active than FM, accounting for 20-30% of an individual’s RMR and 63% of inter-individual variability in RMR [9,10,45]. Low relative metabolic rate is associated with unfavorable weight gain making it an important factor in the early identification of metabolic dysfunction [12,46]. As supported by results of the present study, obese individuals often have a higher absolute metabolic rate than overweight individuals, by association of greater body mass [47]. However, in the context of obesity, absolute metabolic rate may be deceptive. Metabolic rate is highly variable in obese individuals and as recent literature suggests, metabolic rate may decrease significantly in response to chronic caloric restriction or previous weight loss attempts, resulting in a metabolic rate that is lower than expected [48,49]. Despite obese individuals having a higher metabolic rate in the current study, individual analysis revealed that 45% of the entire group (14 males and 8 females) had lower-than-predicted RMR. Additionally, the five females with above-average LM were all considered to have normal metabolic rates. Therefore, overall, a majority of the sample (94%) had low-to-normal metabolic rates. Evaluating metabolic rate on an individual basis is critical when characterizing metabolic status, especially in the early identification and prevention of metabolic dysfunction.
In addition to low metabolic rate, higher reliance on carbohydrate oxidation at rest is associated with weight gain and insulin resistance [12,46,50,51]. Previous studies have suggested that obese individuals may have impaired fat metabolism, which could place them at a greater risk for metabolic disease [44,52,53]. Rosenkilde et al. [52] found that moderately overweight males with a low RER (greater fat oxidation at rest) had fewer metabolic syndrome risk factors compared to those with a high RER (greater carbohydrate oxidation at rest), despite no differences in age, body composition, fitness, energy expenditure, or dietary intake. In the current study, there was no significant difference in RER between overweight and obese adults, even when controlling for LM and dietary intake, but the inter-individual variability was high (range = 0.77 - 0.98 a.u.). Other studies have found RER in obese and normal weight individuals to be highly variable [50,54]. Zurlo et al. [50] observed respiratory quotients in obese individuals ranging from 0.799 to 0.903 a.u., similar to the range in the current study (0.77 – 0.98 a.u.). In the same study, there were also no significant differences in fuel utilization between men and women [50]. Though women are typically more efficient at fat oxidation than men [55,56], results of the current study found no significant differences in RER between men and women. Chronic dietary intake and exercise are known to have significant impacts on RER [57]. Specifically, carbohydrate reliance has been shown to increase with chronic overfeeding, especially with increased carbohydrate intake [58,59]. In contrast, chronic consumption of both high fat/low carbohydrate diets, as well as regular physical activity, are associated with a decrease in RER [51,54]. Due to high individual variability in dietary and exercise practices, RER may be best evaluated on an individual basis when evaluating metabolic status.
Blood lipid profiles are routinely assessed in clinical settings and commonly used as primary markers of metabolic health. Hormonal imbalances can have profound effects on fat distribution, metabolic regulation, hunger, and satiety [13,14,60,61], but are less often evaluated in standard clinical evaluations of metabolic health. In the current study, there were no significant differences in blood lipids or glucose between overweight and obese individuals. In contrast, when evaluating hormonal profiles, obese individuals had significantly higher estradiol levels irrespective of sex. The obese cohort also had higher insulin and leptin levels; though due to high subject variability, there was not a significant difference from overweight individuals. High insulin, leptin, estradiol, and cortisol have all been previously associated with obesity, but may have stronger associations with fat distribution than total body fat [14,15,62]. Specifically, insulin and cortisol may have stronger associations with VAT compared to leptin and estradiol, which seem to have stronger associations with subcutaneous fat [14,15,62]. Women typically have greater subcutaneous fat distribution, in addition to greater leptin and estradiol levels compared to men [13,63]. In the current study, women had significantly higher leptin levels, with no significant differences in estradiol between men and women. Based on individual evaluation, eight men potentially had high estradiol levels (Table 3). High estradiol levels have previously been observed in obese men and may be an indication of excess fat accumulation, as well as an early sign of metabolic dysregulation [64-66]. When examining individual leptin levels, half of the men and almost all of the women had high leptin levels, with no subject exhibiting low levels, potentially indicating leptin resistance in a proportion of the sample.
Overall, the purpose of this study was to characterize a relatively healthy overweight and obese population using additional physiological variables that could potentially be addressed with lifestyle interventions. Overweight and obese individuals are known to be at an increased risk for developing metabolic disease, such as type 2 diabetes and cardiovascular disease. However, as results of the current study support, evaluation of health risk based on BMI and blood lipid profiles alone do not give a complete picture of metabolic status. Characterization solely on these traditional criteria may not identify early warning signs of metabolic dysfunction, whereas specific measurements of body composition and metabolic variables provide information that can be used to individualize interventions for disease prevention. Specifically, measurements of body composition and VAT with portable equipment may improve the accuracy of evaluating abdominal obesity-related risk, compared to waist circumference [19,67]. Further, quantification of metabolic rate, substrate oxidation, and hormonal profiles may also be beneficial as an initial risk matrix.
Results of the current study are not meant to replace current diagnostic criteria for metabolic disease, but rather highlight the importance of evaluating otherwise healthy overweight and obese individuals beyond traditional diagnostic criteria. It is recognized that, due to time constraints and training requirements, not all of these variables can realistically be implemented in a standard clinical setting. However, with over 60% of the US population considered to be overweight or obese, and high rates of cardiovascular disease and type 2 diabetes, early identification of those who are at risk can help connect them to specialists to further support disease prevention. Traditional metabolic syndrome risk factors also commonly include elevated waist circumference and high blood pressure, in addition to the factors used in the current study (TRG, HDL, GLUC). The inability to make a direct comparison to these risk factors is a limitation of the current study, but as previously mentioned, waist circumference is often excluded in the evaluation of overweight and obese individuals due to the commonality of large waist circumferences in this population. Similarly, while it cannot be assumed that all individuals had normal blood pressure, none reported being on blood pressure-lowering medications, suggesting that evaluation of blood pressure would result in similar outcomes. Finally, the lack of comparison to a normal weight population and cross sectional design of the study limits the ability to evaluate direct associations with health risk. However, future evaluation of these factors in normal-weight adults without surface-level risk may be useful in identifying individuals who have underlying metabolic concerns. Additionally, assessing the feasibility of integrating some of these measures into a patient centered medical home would be of value. 2
Conclusion
In conclusion, characterization of metabolic health using traditional and non-traditional variables may be necessary for appropriate evaluation of metabolic health in overweight and obese individuals. Especially in the absence of traditional risk factors, characterization based on total and regional body composition, metabolic rate, fuel utilization, and hormonal profiles can improve early identification and approaches to disease prevention. These factors may also prove valuable for identifying at-risk normal weight individuals who are not identified using traditional disease criteria.
Acknowledgements
This study was funded by Scivation, Inc., Burlington, NC. The project described was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR001109. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest Statement
The authors report no conflicts of interest.
References
- 1.Grundy SM, Brewer HB, Jr., Cleeman JI, et al. Definition of metabolic syndrome: Report of the national heart, lung, and blood institute/american heart association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 2.Müller MJ, Lagerpusch M, Enderle J, et al. Beyond the body mass index: Tracking body composition in the pathogenesis of obesity and the metabolic syndrome. Obesity Reviews. 2012;13:6–13. doi: 10.1111/j.1467-789X.2012.01033.x. [DOI] [PubMed] [Google Scholar]
- 3.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering. Archives of internal medicine. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 4.Kuk JL, Ardern CI. Are metabolically normal but obese individuals at lower risk for all-cause mortality? Diabetes Care. 2009;32:2297–2299. doi: 10.2337/dc09-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Annals of internal medicine. 2013;159:758–769. doi: 10.7326/0003-4819-159-11-201312030-00008. [DOI] [PubMed] [Google Scholar]
- 6.Cnop M, Landchild MJ, Vidal J, Havel PJ, Knowles NG, Carr DR, Kahn SE. The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations. Diabetes. 2002;51:1005–1015. doi: 10.2337/diabetes.51.4.1005. [DOI] [PubMed] [Google Scholar]
- 7.Karelis AD, Brochu M, Rabasa-Lhoret R. Can we identify metabolically healthy but obese individuals (mho)? Diabetes & metabolism. 2004;30:569–572. doi: 10.1016/s1262-3636(07)70156-8. [DOI] [PubMed] [Google Scholar]
- 8.Brochu M, Tchernof A, Dionne IJ, et al. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab. 2001;86:1020–1025. doi: 10.1210/jcem.86.3.7365. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe RR. The underappreciated role of muscle in health and disease. The American journal of clinical nutrition. 2006;84:475–482. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 10.Johnstone AM, Murison SD, Duncan JS, et al. Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am J Clin Nutr. 2005;82:941–948. doi: 10.1093/ajcn/82.5.941. [DOI] [PubMed] [Google Scholar]
- 11.Goodpaster BH, Wolf D. Skeletal muscle lipid accumulation in obesity, insulin resistance, and type 2 diabetes. Pediatric diabetes. 2004;5:219–226. doi: 10.1111/j.1399-543X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 12.Ravussin E, Gautier J. Metabolic predictors of weight gain. International Journal of Obesity & Related Metabolic Disorders. 1999:23. doi: 10.1038/sj.ijo.0800793. [DOI] [PubMed] [Google Scholar]
- 13.Shi H, Seeley RJ, Clegg DJ. Sexual differences in the control of energy homeostasis. Front Neuroendocrinol. 2009;30:396–404. doi: 10.1016/j.yfrne.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi H, Clegg D. Sex differences in the regulation of body weight. Physiology & behavior. 2009;97:199–204. doi: 10.1016/j.physbeh.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pradhan AD. Sex differences in the metabolic syndrome: Implications for cardiovascular health in women. Clinical Chemistry. 2014;60:44–52. doi: 10.1373/clinchem.2013.202549. [DOI] [PubMed] [Google Scholar]
- 16.Wu T, Gao X, Chen M, et al. Long-term effectiveness of diet-plus-exercise interventions vs. Diet-only interventions for weight loss: A meta-analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2009;10:313–323. doi: 10.1111/j.1467-789X.2008.00547.x. [DOI] [PubMed] [Google Scholar]
- 17.Dennis BH. Well-controlled diet studies in humans : A practical guide to design and management. American Dietetic Association; Chicago: 1999. [Google Scholar]
- 18.Wang Z, Pi-Sunyer FX, Kotler DP, et al. Multicomponent methods: Evaluation of new and traditional soft tissue mineral models by in vivo neutron activation analysis. Am J Clin Nutr. 2002;76:968–974. doi: 10.1093/ajcn/76.5.968. [DOI] [PubMed] [Google Scholar]
- 19.Leite CC, Wajchenberg BL, Radominski R, et al. Intra-abdominal thickness by ultrasonography to predict risk factors for cardiovascular disease and its correlation with anthropometric measurements. Metabolism. 2002;51:1034–1040. doi: 10.1053/meta.2002.34035. [DOI] [PubMed] [Google Scholar]
- 20.Stoner L, Chinn V, Cornwall J, et al. Reliability tests and guidelines for b-mode ultrasound assessment of central adiposity. European Journal of Clinical Investigation. 2015;45:1200–1208. doi: 10.1111/eci.12540. [DOI] [PubMed] [Google Scholar]
- 21.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (homa) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 22.Wallace TM, Levy JC, Matthews DR. Use and abuse of homa modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 23.American College of Sports Medicine. Thompson WR, Gordon NF, et al. Acsm's guidelines for exercise testing and prescription. Lippincott Williams & Wilkins; Philadelphia: 2010. [Google Scholar]
- 24.Antonio J, Kalman D, Stout JR, et al. Essentials of sports nutrition and supplements. Springer Science & Business Media; 2009. [Google Scholar]
- 25.Fan B, Shepherd JA, Levine MA, et al. National health and nutrition examination survey whole-body dual-energy x-ray absorptiometry reference data for ge lunar systems. Journal of Clinical Densitometry. 2014;17:344–377. doi: 10.1016/j.jocd.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 26.Jésus P, Achamrah N, Grigioni S, et al. Validity of predictive equations for resting energy expenditure according to the body mass index in a population of 1726 patients followed in a nutrition unit. Clinical Nutrition. 2015;34:529–535. doi: 10.1016/j.clnu.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Panel NCEPNE Third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) final report. Circulation. 2002;106:3143. [PubMed] [Google Scholar]
- 28.Aardal E, Holm AC. Cortisol in saliva--reference ranges and relation to cortisol in serum. Eur J Clin Chem Clin Biochem. 1995;33:927–932. doi: 10.1515/cclm.1995.33.12.927. [DOI] [PubMed] [Google Scholar]
- 29.Ruhl CE, Everhart JE. Leptin concentrations in the united states: Relations with demographic and anthropometric measures. Am J Clin Nutr. 2001;74:295–301. doi: 10.1093/ajcn/74.3.295. [DOI] [PubMed] [Google Scholar]
- 30.High sensitivity salivary 17β-estradiol enzyme immunoassay kit. Press Release. 2015 [Google Scholar]
- 31.Shirtcliff EA, Granger DA, Schwartz EB, et al. Assessing estradiol in biobehavioral studies using saliva and blood spots: Simple radioimmunoassay protocols, reliability, and comparative validity. Hormones and behavior. 2000;38:137–147. doi: 10.1006/hbeh.2000.1614. [DOI] [PubMed] [Google Scholar]
- 32.Matthews D, Hosker J, Rudenski A, et al. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 33.Han TS, Lean ME. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc Dis. 2016;5 doi: 10.1177/2048004016633371. 2048004016633371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dvorak RV, DeNino WF, Ades PA, Poehlman ET. Phenotypic characteristics associated with insulin resistance in metabolically obese but normal weight young women. Diabetes. 1999;48:2210–2214. doi: 10.2337/diabetes.48.11.2210. [DOI] [PubMed] [Google Scholar]
- 35.Goncalves CG, Glade MJ, Meguid MM. Metabolically healthy obese individuals: Key protective factors. Nutrition. 2016;32:14–20. doi: 10.1016/j.nut.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Shah RV, Murthy VL, Abbasi SA, et al. Visceral adiposity and the risk of metabolic syndrome across body mass index. JACC: Cardiovascular Imaging. 2014;7:1221–1235. doi: 10.1016/j.jcmg.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shuster A, Patlas M, Pinthus JH, et al. The clinical importance of visceral adiposity: A critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85:1–10. doi: 10.1259/bjr/38447238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stolk R, Wink O, Zelissen P, et al. Validity and reproducibility of ultrasonography for the measurement of intra-abdominal adipose tissue. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2001;25:1346–1351. doi: 10.1038/sj.ijo.0801734. [DOI] [PubMed] [Google Scholar]
- 39.Kohara K, Ochi M, Tabara Y, et al. Leptin in sarcopenic visceral obesity: Possible link between adipocytes and myocytes. PLoS One. 2011;6:e24633. doi: 10.1371/journal.pone.0024633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conus F, Allison DB, Rabasa-Lhoret R, et al. Metabolic and behavioral characteristics of metabolically obese but normal-weight women. The Journal of Clinical Endocrinology & Metabolism. 2004;89:5013–5020. doi: 10.1210/jc.2004-0265. [DOI] [PubMed] [Google Scholar]
- 41.Lang PO, Trivalle C, Vogel T, et al. Markers of metabolic and cardiovascular health in adults: Comparative analysis of dexa-based body composition components and bmi categories. Journal of cardiology. 2015;65:42–49. doi: 10.1016/j.jjcc.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Camhi SM, Katzmarzyk PT. Differences in body composition between metabolically healthy obese and metabolically abnormal obese adults. International Journal of Obesity. 2014;38:1142–1145. doi: 10.1038/ijo.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brochu M, Mathieu ME, Karelis AD, et al. Contribution of the lean body mass to insulin resistance in postmenopausal women with visceral obesity: A monet study. Obesity. 2008;16:1085–1093. doi: 10.1038/oby.2008.23. [DOI] [PubMed] [Google Scholar]
- 44.Kelley DE, Goodpaster B, Wing RR, et al. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. American Journal of Physiology-Endocrinology And Metabolism. 1999;277:E1130–E1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 45.Dulloo AG, Jacquet J, Solinas G, et al. Body composition phenotypes in pathways to obesity and the metabolic syndrome. International Journal of Obesity. 2010;34(Suppl 2):S4–17. doi: 10.1038/ijo.2010.234. [DOI] [PubMed] [Google Scholar]
- 46.Ravussin E, Lillioja S, Knowler WC, et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. New England Journal of Medicine. 1988;318:467–472. doi: 10.1056/NEJM198802253180802. [DOI] [PubMed] [Google Scholar]
- 47.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. New England Journal of Medicine. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 48.Weyer C, Snitker S, Rising R, et al. Determinants of energy expenditure and fuel utilization in man: Effects of body composition, age, sex, ethnicity and glucose tolerance in 916 subjects. International Journal of Obesity & Related Metabolic Disorders. 1999:23. doi: 10.1038/sj.ijo.0800910. [DOI] [PubMed] [Google Scholar]
- 49.Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after "the biggest loser" competition. Obesity. 2016 doi: 10.1002/oby.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zurlo F, Lillioja S, Esposito-Del Puente A, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: Study of 24-h rq. American Journal of Physiology-Endocrinology And Metabolism. 1990;259:E650–E657. doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]
- 51.Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes. 2003;52:2191–2197. doi: 10.2337/diabetes.52.9.2191. [DOI] [PubMed] [Google Scholar]
- 52.Rosenkilde M, Nordby P, Nielsen LB, et al. Fat oxidation at rest predicts peak fat oxidation during exercise and metabolic phenotype in overweight men. International Journal of Obesity. 2010;34:871–877. doi: 10.1038/ijo.2010.11. [DOI] [PubMed] [Google Scholar]
- 53.Ellis AC, Hyatt TC, Hunter GR, et al. Respiratory quotient predicts fat mass gain in premenopausal women. Obesity. 2010;18:2255–2259. doi: 10.1038/oby.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goedecke JH, Gibson ASC, Grobler L, et al. Determinants of the variability in respiratory exchange ratio at rest and during exercise in trained athletes. American Journal of Physiology-Endocrinology And Metabolism. 2000;279:E1325–E1334. doi: 10.1152/ajpendo.2000.279.6.E1325. [DOI] [PubMed] [Google Scholar]
- 55.Horton TJ, Pagliassotti MJ, Hobbs K, et al. Fuel metabolism in men and women during and after long-duration exercise. Journal of Applied Physiology. 1998;85:1823–1832. doi: 10.1152/jappl.1998.85.5.1823. [DOI] [PubMed] [Google Scholar]
- 56.Tarnopolsky L, MacDougall J, Atkinson S, et al. Gender differences in substrate for endurance exercise. Journal of Applied Physiology. 1990;68:302–308. doi: 10.1152/jappl.1990.68.1.302. [DOI] [PubMed] [Google Scholar]
- 57.Achten J, Jeukendrup AE. Optimizing fat oxidation through exercise and diet. Nutrition. 2004;20:716–727. doi: 10.1016/j.nut.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt SL, Kealey EH, Horton TJ, et al. The effects of short-term overfeeding on energy expenditure and nutrient oxidation in obesity-prone and obesity-resistant individuals. International Journal of Obesity. 2013;37:1192–1197. doi: 10.1038/ijo.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kahlhofer J, Lagerpusch M, Enderle J, et al. Carbohydrate intake and glycemic index affect substrate oxidation during a controlled weight cycle in healthy men. European Journal of Clinical Nutrition. 2014;68:1060–1066. doi: 10.1038/ejcn.2014.132. [DOI] [PubMed] [Google Scholar]
- 60.Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obesity Reviews. 2007;8:21–34. doi: 10.1111/j.1467-789X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 61.Niswender KD, Baskin DG, Schwartz MW. Insulin and its evolving partnership with leptin in the hypothalamic control of energy homeostasis. Trends in Endocrinology & Metabolism. 2004;15:362–369. doi: 10.1016/j.tem.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 62.Björntorp P. Endocrine abnormalities of obesity. Metabolism. 1995;44:21–23. doi: 10.1016/0026-0495(95)90315-1. [DOI] [PubMed] [Google Scholar]
- 63.Minocci A, Savia G, Lucantoni R, et al. Leptin plasma concentrations are dependent on body fat distribution in obese patients. Int J Obes Relat Metab Disord. 2000;24:1139–1144. doi: 10.1038/sj.ijo.0801385. [DOI] [PubMed] [Google Scholar]
- 64.Zumoff B. Hormonal abnormalities in obesity. Acta Medica Scandinavica. 1987;222:153–160. doi: 10.1111/j.0954-6820.1987.tb05939.x. [DOI] [PubMed] [Google Scholar]
- 65.Schneider G, Kirschner MA, Berkowitz R, et al. Increased estrogen production in obese men. J Clin Endocrinol Metab. 1979;48:633–638. doi: 10.1210/jcem-48-4-633. [DOI] [PubMed] [Google Scholar]
- 66.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. The Journal of Clinical Endocrinology & Metabolism. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 67.Smith-Ryan AE, Fultz SN, Melvin MN, et al. Reproducibility and validity of a-mode ultrasound for body composition measurement and classification in overweight and obese men and women. PLoS One. 2014;9:e91750. doi: 10.1371/journal.pone.0091750. [DOI] [PMC free article] [PubMed] [Google Scholar]