Abstract
Background and objectives
Cardiovascular disease is the most important comorbidity affecting long-term survival in children with CKD.
Design, setting, participants, & measurements
The Cardiovascular Comorbidity in Children with CKD Study is a multicenter, prospective, observational study in children ages 6–17 years old with initial GFR of 10–60 ml/min per 1.73 m2. The cardiovascular status is monitored annually, and subclinical cardiovascular disease is assessed by noninvasive measurements of surrogate markers, including the left ventricular mass index, carotid intima-media thickness, and central pulse wave velocity. We here report baseline data at study entry and an explorative analysis of variables associated with surrogate markers.
Results
A total of 737 patients were screened from October of 2009 to August of 2011 in 55 centers in 12 European countries, and baseline data were analyzed in 688 patients. Sixty-four percent had congenital anomalies of the kidney and urinary tract; 26.1% of children had uncontrolled hypertension (24-hour ambulatory BP monitoring; n=545), and the prevalence increased from 24.4% in CKD stage 3 to 47.4% in CKD stage 5. The prevalence of left ventricular hypertrophy was higher with each CKD stage, from 10.6% in CKD stage 3a to 48% in CKD stage 5. Carotid intima-media thickness was elevated in 41.6%, with only 10.8% of patients displaying measurements below the 50th percentile. Pulse wave velocity was increased in 20.1%. The office systolic BP SD score was the single independent factor significantly associated with all surrogate markers of cardiovascular disease. The intermediate end point score (derived from the number of surrogate marker measurements >95th percentile) was independently associated with a diagnosis of congenital anomalies of the kidney and urinary tract, time since diagnosis of CKD, body mass index, office systolic BP, serum phosphorus, and the hemoglobin level.
Conclusions
The baseline data of this large pediatric cohort show that surrogate markers for cardiovascular disease are closely associated with systolic hypertension and stage of CKD.
Keywords: left ventricular hypertrophy; arteriosclerosis; pulse wave velocity; Biomarkers; blood pressure; Blood Pressure Monitoring, Ambulatory; Body Mass Index; Carotid Intima-Media Thickness; Child; Comorbidity; Europe; glomerular filtration rate; Hemoglobins; Humans; hypertension; Hypertrophy, Left Ventricular; Phenotype; Phosphorus; Phosphorus, Dietary; Prevalence; Prospective Studies; Pulse Wave Analysis; Renal Insufficiency, Chronic; vesico-ureteral reflux
Introduction
Previous studies in children, adolescents, and young adults with CKD have shown that cardiovascular disease (CVD) is the single most important comorbidity affecting long-term survival (1). CVD is usually subclinical in this age group, and the evolution of cardiovascular maladaptive changes with progression of CKD is unknown. Therefore, a consortium of pediatric nephrologists in Europe was formed to enroll a large cohort of children and adolescents with CKD in a long–term prospective observational study, the Cardiovascular Comorbidity in Children with CKD (4C) Study (2). Here, we present a descriptive analysis of the cardiovascular status at study entry of 688 children and adolescents with CKD and an explorative analysis of variables associated with surrogate markers for CVD: the left ventricular mass index (LVMI), the carotid intima-media thickness (cIMT), and the central pulse wave velocity (PWV).
Materials and Methods
Study Organization and Cardiovascular Monitoring
The study was approved by the institutional review boards at each participating institution (2). Written informed consent was obtained from all parents and patients as appropriate. Children ages 6–17 years old with GFR of 10–60 ml/min per 1.73 m2 not yet receiving RRT were eligible for the study. GFR was estimated using the 2009 cystatin C/creatinine-based formula (details are in Supplemental Appendix) (3). Exclusion criteria were the presence of active systemic vasculitis, renal vascular anomalies, coexisting primary cardiovascular anomalies, and anomalies of the limbs preventing diagnostic procedures.
In the ongoing 4C Study, all enrolled patients are followed by seven regional coordinators who visit the 55 participating centers in 12 European countries once per year. The coordinators were jointly trained in noninvasive imaging methodologies and are uniformly equipped with mobile cardiovascular assessment devices (Siemens p50 Vascular Ultrasound/Echocardiography; Siemens Medical Solutions USA, Inc. and Vicorder Oscillometric PWV devices). A full cardiovascular assessment comprising systolic and diastolic BP (median of three oscillometric measurements using locally available devices), carotid ultrasound, PWV measurement, echocardiography, and 24-hour ambulatory BP monitoring (ABPM) is carried out at baseline and each annual study visit. In addition, anthropometric data, the clinical status, a complete medication update, and any history of intercurrent medical events are recorded every 6 months, and blood and urine samples are collected. Physical activity is estimated by hours of physical activity (0/1–2/3–4/>4 h/wk) using a standardized questionnaire. Height, body mass index (BMI), office (4) and ambulatory BP (5), cIMT (6), and PWV values (7) are normalized for sex, age, and/or body size by calculation of SD scores (SDSs; normal range of −2–2; i.e., relating the measured values to the mean and variability of values in reference populations matched by sex, age, and/or body height). Biospecimens are collected according to a standardized protocol and stored in a central biobank. All laboratory measurements are performed centrally.
All ABPM measurements are performed using the same portable device type at all sites (Spacelabs 90207–2Q) as described previously (5). The time–averaged 24-hour mean arterial pressure (MAP) was used for the analyses presented in this study. The diagnosis of hypertension is made when 24-hour time–integrated MAP exceeds the 95th percentile (5). Patients on antihypertensive medication are referred to as having controlled or uncontrolled hypertension if their 24-hour MAP is below or above the 95th percentile, respectively. Masked hypertension is defined as normal office BP but elevated 24-hour MAP; white coat hypertension is defined as elevated office BP but normal 24-hour MAP.
Two–dimensional echocardiography images are obtained for the analysis of left ventricular (LV) volumes on three consecutive beats from apical four– and two–chamber views. Wall thickness and chamber dimensions are obtained from the two–dimensional parasternal long axis or M–mode short axis at the midventricular level. The LV mass is calculated according to the Devereux Equation (8) and indexed to height 2.7 (LVMI). The sex– and age–specific LVMI partition values of Khoury et al. (9) are applied to define left ventricular hypertrophy (LVH). For evaluation of LV geometry, relative wall thickness (RWT) is calculated as the ratio of myocardial thickness (posterior wall and septum) to the end diastolic LV diameter. RWT is normalized to age 10 years old (RWTa) using the formula RWTa = RWT−0.005×(age 10) as proposed by de Simone et al. (10), and a cutoff value of 0.38, the 95th percentile of the normalized RWT, is used to define concentric geometry (10).
The cIMT is measured according to the Mannheim cIMT consensus (11). The cIMT is obtained either by five averaged measurements on each side or semiautomatically using a portable ultrasound device (Acuson P50; Siemens Medical Solutions USA, Inc.) with integrated digital image evaluation software (Syngo US Workplace; Siemens Medical Solutions USA, Inc.). Interobserver variation studies showed an intraclass correlation coefficient of 0.42 and an interobserver coefficient of variation (CV) of 7.3% (6). Because cIMT in children changes with growth, reference values normalized for height and age were established in 1155 healthy children ages 6–18 years old (6).
The central PWV is measured with the Vicorder Oscillometric PWV device using the distance from the suprasternal notch to the femoral recording point via the umbilicus as path length. The method was validated against the gold standard of applanation tomonetry (12), and reference values normalized for height and age were established in a large European pediatric population (1003 healthy children ages 6–18 years old) (7). Intra- and interobserver variability studies showed CVs of 5.6% and 5.8%, respectively, and intraclass correlation coefficients of 0.8 and 1.0, respectively (7).
Statistical Analyses
Intermediate end points were defined as cardiovascular measurements exceeding the 95th percentile of healthy children.
Data are given as mean (SD), percentage, or median (interquartile range). Associations were assessed by Spearman correlation analysis. Analysis for linear trends of relative frequencies was conducted by the Cochran–Armitage trend test.
Linear regression modeling with stepwise variable selection was applied to identify factors associated with surrogate CV markers. The variable selection process was on the basis of a sequence of F tests, with variables added one by one to the model if the F statistic was significant at P≤0.20 (entry level) and kept in the model if still significant at P≤0.10 (stay level). Ordinal regression analysis with stepwise variable selection as described above was used to identify factors associated with the cumulative number of intermediate end points.
P values <0.05 were considered statistically significant. Data were analyzed using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Patient Characteristics
A total of 737 patients with CKD fulfilled the screening criteria; of these, 704 participated in the baseline examination. Nine patients were excluded because of eGFR>60 ml/min per 1.73 m2, and seven were excluded due to missing eGFR at the first study visit, leaving a final cohort of 688 patients with CKD stages 3–5. The key characteristics of the cohort are given in Table 1; a breakdown by country of residence is provided in Supplemental Table 1.
Table 1.
Patient characteristics of 688 children with CKD stages 3–5
| Patient Characteristics | Stage 3a | Stage 3b | Stage 4 | Stage 5 | All |
|---|---|---|---|---|---|
| N | 50 | 216 | 370 | 52 | 688 |
| Age, yr | 12.3 (2.9) | 12.2 (3.3) | 12.2 (3.4) | 12.1 (3.0) | 12.2 (3.3) |
| Time since CKD diagnosis, yr | 5.6 (4.5) | 6.3 (4.8) | 6.1 (4.5) | 4.2 (3.8) | 6.0 (4.6) |
| Men, % | 70.0 | 67.6 | 64.6 | 55.8 | 65.3 |
| CAKUT/glom.pathy/other, % | 70.0/10.0/20.0 | 72.2/4.6/23.1 | 68.1/9.5/22.4 | 57.7/13.5/28.8 | 68.8/8.3/23.0 |
| Birth history | |||||
| Gestational age, wk | 38.8 (2.0) | 38.5 (2.4) | 38.6 (2.5) | 38.7 (2.0) | 38.6 (2.4) |
| Birth weight, kg | 3.04 (0.64) | 3.15 (0.54) | 3.19 (0.65) | 3.11 (0.68) | 3.17 (0.61) |
| Birth length, cm | 48.4 (4.6) | 49.6 (3.6) | 50.0 (3.6) | 49.6 (3.1) | 49.8 (3.7) |
| Small for gestational age, % | 34.8 | 13.2 | 18.0 | 17.0 | 17.7 |
| eGFR, ml/min per 1.73 m2 | 51 (4) | 36 (4) | 22 (4) | 13 (1) | 28 (11) |
| Urine albumin-to-creatinine ratio, mg/g | 86 (309) | 212 (700) | 487 (1697) | 1034 (2819) | 359 (1170) |
| Height SDS | −0.81 (1.06) | −1.13 (1.34) | −1.54 (1.44) | −1.60 (1.43) | −1.36 (1.40) |
| Height less than third percentile, % | 16.0 | 25.5 | 35.9 | 36.5 | 31.3 |
| BMI SDS, % | 0.13 (1.02) | 0.04 (1.89) | 0.23 (1.41) | −0.01 (1.03) | 0.14 (1.53) |
| Malnourished | 4.0 | 10.2 | 7.6 | 5.8 | 8.0 |
| Overweight/obese | 20.0 | 24.1 | 23.8 | 17.3 | 23.1 |
| Physical activity, % | |||||
| 0 h/wk | 8.0 | 21.7 | 23.8 | 46.2 | 23.7 |
| 1–2 h/wk | 16.0 | 19.3 | 22.2 | 19.2 | 20.6 |
| 3–4 h/wk | 20.0 | 16.0 | 13.9 | 5.8 | 14.4 |
| >4 h/wk | 56.0 | 42.9 | 40.2 | 28.8 | 41.3 |
Numbers are presented as percentage, mean (SD), or for urine albumin-to-creatinine ratio, median (interquartile range). CAKUT, congenital anomalies of the kidney and urinary tract; glom.pathy, glomerulopathy; SDS, SD score; BMI, body mass index.
The distribution of underlying renal diseases is shown in Supplemental Figure 1; 10.4% of patients had a family history of renal disease. Parental consanguinity was reported in 19.4% of families. Defined syndromes were diagnosed in 40 patients (6.5%). Any comorbidities were present in 35.9% of patients; ocular abnormalities (n=57) and cognitive dysfunction (n=50) were the most frequent (Supplemental Figure 2). Reported cardiac abnormalities did not meet exclusion criteria and were without hemodynamic significance (anatomic variants) or secondary to hypertension.
A total of 105 patients (17.7%) were small for gestational age (SGA), 134 patients (21.3%) were born prematurely, and 13 (2.2%) were both born prematurely and SGA. Children with hypodysplastic kidneys had a significantly lower birth weight than children with other diagnoses (3.09±0.67 versus 3.20±0.58 kg; P=0.03).
Family history information was available from 82% of the families. Among these, 20% of fathers and 26% of mothers were obese, and 50% of fathers and 25% of mothers were smokers. Hypertension had been diagnosed in 16% of fathers and 12% of mothers, and coronary heart disease had been diagnosed in 3% of fathers and 1% of mothers. A history of cardiovascular events was present in 2.3% of fathers and 0.8% of mothers. Less than one half of the patients had self-reported physical activity for >4 h/wk, and almost one quarter of the study population (23.7%) reported no physical activity at all (Table 1).
Ten adolescent patients (1.4%) reported cigarette smoking. Thirteen patients (1.9%) received supplemental enteral feeding via a percutaneous endoscopic gastrostomy or nasogastric tube.
Cardiovascular Status
Hypertension.
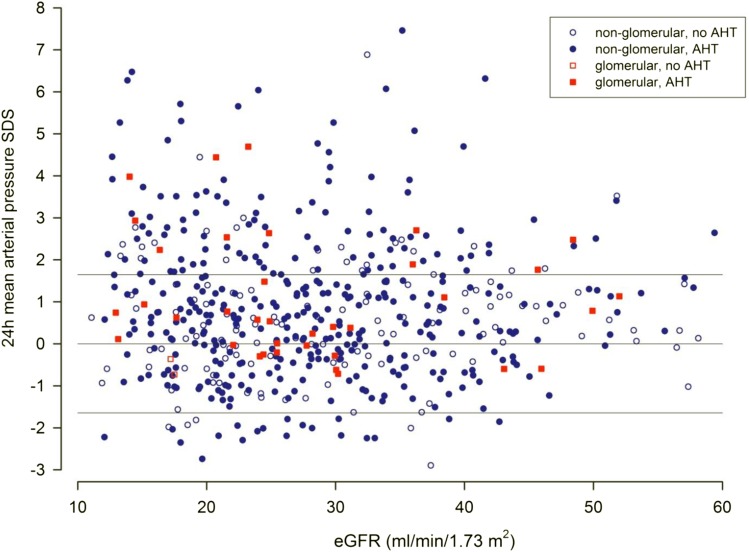
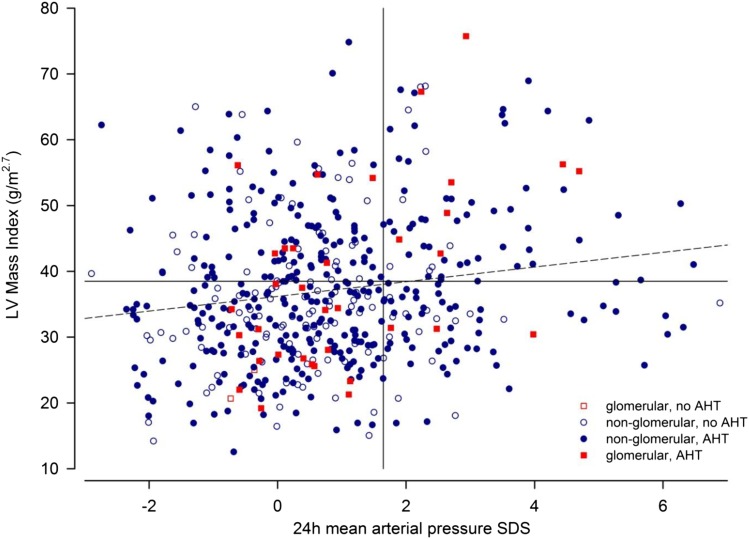
In addition to office BP measurements performed in all children at the time of cardiovascular assessment, ABPM profiles were obtained in 545 patients. Using the 95th percentile of 24-hour MAP as the diagnostic criterion (Table 2), a total of 142 patients (26.1%) were hypertensive (Figure 1). Although 24-hour MAP SDS was not significantly correlated with eGFR (Figure 1), it was significantly associated with LVMI (Figure 2), cIMT SDS, and PWV SDS (P<0.001). Seventy-nine patients (14.5%) had masked hypertension, and 61 patients (11.2%) had white coat hypertension. The prevalence was markedly different if the criteria proposed by the American Heart Association or the criteria applied by the Chronic Kidney Disease in Children (CKiD) Consortium were applied (Supplemental Figure 3, Supplemental Tables 2–4) (13–15).
Table 2.
Cardiovascular measures at study entry
| Measurements and Classifications | Stage 3a | Stage 3b | Stage 4 | Stage 5 | All |
|---|---|---|---|---|---|
| Systolic office BP | |||||
| Millimeters of mercury | 112 (15) | 114 (15) | 111 (15) | 117 (15) | 112 (15) |
| SDS | 0.60 (1.16) | 0.92 (1.39) | 0.67 (1.34) | 1.27 (1.39) | 0.79 (1.36) |
| Diastolic office BP | |||||
| Millimeters of mercury | 70 (11) | 70 (13) | 68 (12) | 74 (12) | 69 (12) |
| SDS | 0.67 (0.94) | 0.71 (1.12) | 0.59 (1.07) | 1.10 (1.08) | 0.67 (1.09) |
| 24-h Mean arterial pressure | |||||
| Millimeters of mercury | 88.2 (8.8) | 85.0 (9.5) | 85.0 (10.3) | 90.1 (12.0) | 85.6 (10.1) |
| SDS | 1.16 (1.61) | 0.71 (1.65) | 0.74 (1.73) | 1.59 (2.03) | 0.82 (1.73) |
| Confirmed normotension, N (%) | 25 (61.0) | 114 (63.3) | 189 (66.3) | 13 (34.2) | 341 (62.7) |
| White coat hypertension, N (%) | 6 (14.6) | 22 (12.2) | 26 (9.1) | 7 (18.4) | 61 (11.2) |
| Masked hypertension, N (%) | 7 (17.1) | 21 (11.7) | 43 (15.1) | 8 (21.1) | 79 (14.5) |
| Confirmed hypertension, N (%) | 3 (7.3) | 23 (12.8) | 27 (9.5) | 10 (26.3) | 63 (11.6) |
| No. of antihypertensive drugs | 1.2 (1.3) | 1.3 (1.1) | 1.6 (1.3) | 1.6 (1.5) | 1.4 (1.3) |
| Normotension without treatment, N (%) | 13 (31.7) | 35 (19.4) | 58 (20.4) | 6 (15.8) | 112 (20.6) |
| Controlled hypertension, N (%) | 18 (43.9) | 101 (56.1) | 157 (55.1) | 14 (36.8) | 290 (53.3) |
| Uncontrolled hypertension, N (%) | 10 (24.4) | 44 (24.4) | 70 (24.8) | 18 (47.4) | 142 (26.1) |
| LV mass index, g/m2.7 | 30.6 (8.1) | 35.3 (12.0) | 37.9 (11.5) | 40.8 (16.6) | 36.8 (12.1) |
| LV hypertrophy, N (%) | 5 (10.6) | 56 (28.1) | 126 (37.5) | 24 (48.0) | 211 (33.4) |
| Eccentric | 0 (0.0) | 19 (9.5) | 49 (14.6) | 7 (14.0) | 75 (11.9) |
| Concentric | 5 (10.6) | 37 (18.6) | 77 (22.9) | 17 (34.0) | 136 (21.5) |
| Concentric LV remodeling, N (%) | 18 (38.3) | 61 (30.7) | 74 (22.0) | 11 (22.0) | 164 (25.9) |
| Carotid intima-media thickness | |||||
| Millimeters | 0.46 (0.06) | 0.44 (0.06) | 0.45 (0.06) | 0.46 (0.07) | 0.45 (0.06) |
| SDS | 1.81 (1.36) | 1.39 (1.36) | 1.66 (1.46) | 1.87 (1.77) | 1.60 (1.45) |
| Elevated carotid intima-media thickness, N (%) | 20 (40.8) | 73 (34.0) | 162 (46.0) | 22 (44.0) | 277 (41.6%) |
| Pulse wave velocity | |||||
| Meters per second | 5.12 (1.01) | 4.91 (0.79) | 4.85 (0.82) | 5.07 (0.88) | 4.90 (0.83) |
| SDS | 0.37 (1.50) | 0.23 (1.51) | 0.31 (1.77) | 0.79 (1.67) | 0.33 (1.66) |
| Elevated pulse wave velocity, N (%) | 9 (19.1) | 37 (17.3) | 76 (21.5) | 10 (20.4) | 133 (20.1) |
Values are presented as mean (SD) unless otherwise indicated. Ambulatory BP monitoring–derived data are on the basis of 545 patients. SDS, SD score; LV, left ventricular.
Figure 1.
24-hour blood pressure and eGFR in patients with or without antihypertensive treatment. The 24-hour mean arterial pressure SD scores (SDSs) in children with underlying glomerular (red squares) and nonglomerular nephropathies (blue circles) with (colored symbols) or without antihypertensive medication (outlined symbols) according to eGFR. Lines represent 5th, 50th, and 95th reference percentiles of 24-hour mean arterial pressure. AHT, Antihypertensive Treatment.
Figure 2.
24-hour blood pressure and left ventricular mass index in patients with or without antihypertensive treatment. The 24-hour mean arterial pressure SD scores (SDSs) and left ventricular (LV) mass index in children with underlying glomerular (red squares) and nonglomerular nephropathies (blue circles) with (colored symbols) or without antihypertensive medication (outlined symbols). Vertical and horizontal lines represent 95th reference percentiles of 24-hour mean arterial pressure and LV mass index, respectively. The dashed line represents the linear regression line. AHT, Antihypertensive Treatment.
Three quarters of the patients received antihypertensive therapy (33%, one drug; 24.2%, two drugs; and 17.2%, three or more drugs). Among the 406 patients receiving antihypertensive therapy, hypertension was uncontrolled in 116 (28.6%). However, there was no significant association of controlled versus uncontrolled hypertension and CKD stage (P=0.17 for trend) (Figure 1). The prevalence of hypertension was comparatively higher in CKD stage 5 than CKD stage 3, despite an increase in the mean number of antihypertensive drugs prescribed per patient (Table 2).
LVH.
LVH was observed in one third of the patients. The prevalence of LVH successively increased with CKD stage, from 14.9% in CKD stage 3a to 48% in CKD stage 5 (Table 2). Cardiac geometry was concentric in two thirds and eccentric in one third of the patients with LVH. There was no change in the amount of concentric versus eccentric hypertrophy with worsening levels of renal function (P=0.52 for trend). In addition, 26% of the patients showed cardiac remodeling with still normal LV mass. Variables showing significant univariate associations with LVMI, cIMT, and PWV are listed in Table 3.
Table 3.
Linear regression models of variables associated with surrogate markers at study entry after stepwise variable selection
| Variable | LVMI, n=493 | cIMT SDS, n=510 | PWV SDS, n=513 | |||
|---|---|---|---|---|---|---|
| β (95% CI) | P Value | β (95% CI) | P Value | β (95% CI) | P Value | |
| Intercept | 70.7 (61.3 to 80.1) | <0.001 | 0.58 (0.01 to 1.15) | 0.05 | 0.2 (−0.5 to 0.9) | 0.56 |
| Age, yr | −0.715 (−1.0 to −0.41) | 0.001 | — | −0.06 (−0.10 to −0.02) | 0.01 | |
| Girls | −2.54 (−4.58 to −0.49) | 0.02 | 0.31 (0.05 to 0.57) | 0.02 | — | |
| Systolic BP (SDS) | 1.15 (0.43 to 1.88) | <0.001 | 0.22 (0.13 to 0.31) | <0.001 | 0.37 (0.26 to 0.48) | <0.001 |
| Physical activity >2 h/wk | −3.94 (−5.95 to −1.93) | 0.001 | 0.25 (0.00 to 0.5) | 0.04 | ||
| BMI SDS | 1.43 (0.68 to 2.17) | 0.001 | 0.08 (−0.13 to 0.17) | 0.09 | — | |
| HDL cholesterol, mg/dl | −0.09 (−0.16 to −0.02) | 0.001 | — | — | ||
| LDL cholesterol, mg/dl | −0.03 (−0.06 to 0.00) | 0.02 | ||||
| Serum 25-OHD, ng/ml | — | −0.01 (−0.02 to 0.00) | 0.004 | −0.01 (−0.24 to 0.00) | 0.01 | |
| eGFR, ml/min per 1.73 m2 | −0.22 (−0.32 to −0.13) | <0.001 | — | — | ||
| Serum albumin, g/L | −0.20 (−0.37 to −0.04) | 0.07 | — | — | ||
| Log urine albumin-to-creatinine ratio | 0.10 (0.02 to 0.18) | 0.01 | ||||
| Serum phosphorus, mmol/L | — | 0.72 (0.4 to 1.0) | <0.001 | |||
| iPTH, ng/ml | — | — | 0.01 (0.00 to 0.01) | 0.002 | ||
Supplemental Table 5 shows full models. LVMI, left ventricular mass index; cIMT, carotid intima-media thickness; SDS, SD score; PWV, pulse wave velocity; 95% CI, 95% confidence interval; —, no significant correlation for this variable; BMI, body mass index; 25-OHD, 25-hydroxy vitamin D; iPTH, immunoreactive parathyroid hormone.
cIMT.
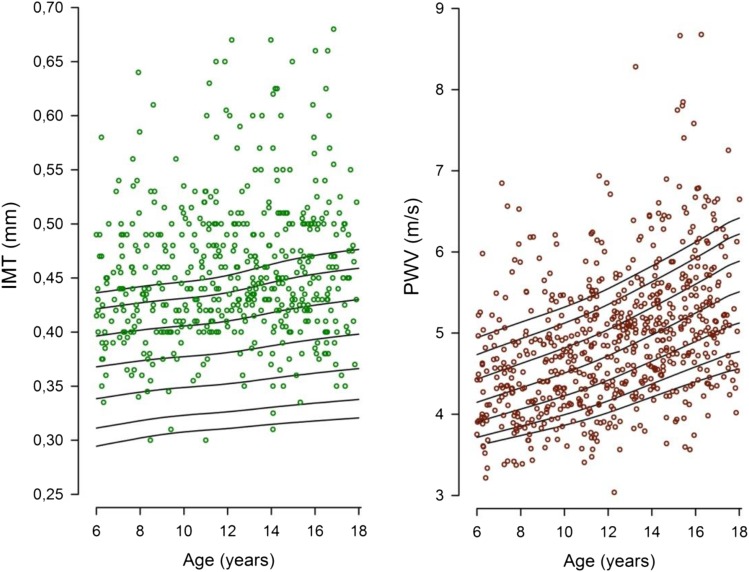
cIMT was elevated (in 41.6% of patients) or clustered in the upper normal range, with only 10.8% of patients displaying measurements below the 50th percentile (Figure 3, Table 2). No clear correlation with eGFR was evident (r=−0.06; P=0.15).
Figure 3.
Surrogate marker measurements. Distribution of carotid intima-media thickness (IMT; left panel) and pulse wave velocity (PWV; right panel). Curves represent 5th, 10th, 25th, 50th, 75th, 90th, and 95th reference percentiles.
PWV.
PWV was increased in 20.1% of patients (Figure 3), independent of eGFR (r=−0.03; P=0.44) (Table 2). PWV was moderately correlated with cIMT (r=0.23; P<0.001). Supplemental Tables 5 shows the full statistical models for surrogate markers and associated variables.
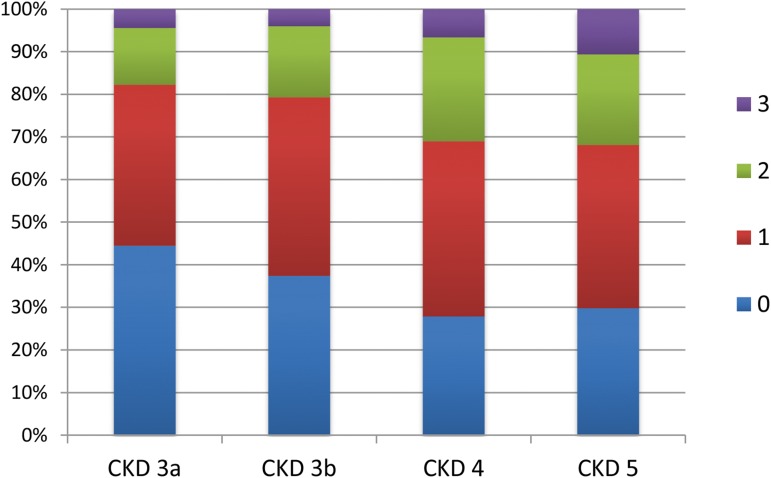
The number of intermediate end points present per patient increased significantly (r=−0.15; P=0.001) with declining eGFR. Between CKD stages 3a and 5, the fraction of patients without intermediate end points successively decreased from 44% to 30%, whereas the fraction with two or more intermediate end points increased from 18% to 32% (Figure 4). The number of intermediate end points per patient was independently associated with congenital anomalies of the kidney and urinary tract (CAKUT) diagnosis, time since diagnosis of CKD, BMI SDS, office systolic BP SDS, serum phosphorus, and hemoglobin (Table 4).
Figure 4.
Intermediate end points by CKD stage. Percentages of patients presenting with zero, one, two, or three intermediate cardiovascular end points by CKD stage. Intermediate end points are left ventricular mass index, carotid intima-media thickness, or central pulse wave velocity exceeding the 95th percentile for height.
Table 4.
Multiple ordinal regression of cumulative intermediate cardiovascular end point score at study entry
| Variable | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| CAKUT diagnosis | 1.59 | 1.09 to 2.33 | 0.02 |
| Time since CKD diagnosis, yr | 0.94 | 0.90 to 0.98 | 0.002 |
| BMI SDS | 1.32 | 1.15 to 1.51 | <0.001 |
| Systolic BP SDS | 1.37 | 1.20 to 1.56 | <0.001 |
| Serum phosphorus, mmol/L | 1.83 | 1.16 to 2.89 | <0.01 |
| Hemoglobin, g/dl | 0.83 | 0.74 to 0.92 | 0.001 |
The score comprised left ventricular mass index, carotid intima-media thickness, and central pulse wave velocity exceeding the 95th percentile. The variables included in the model after stepwise selection are presented. Supplemental Table 6 shows the full model. CAKUT, congenital anomalies of the kidney and urinary tract; BMI, body mass index; SDS, SD score.
Discussion
This cross-sectional analysis measured the extent of subclinical CVD in a large pediatric CKD cohort. LVMI was the surrogate marker with greatest plasticity, with an incremental increase at each stage of CKD; the distribution of cIMT measurements was markedly shifted to higher percentiles and above normal in almost one half of the patients, whereas PWV was elevated in only 20% of patients. With each CKD stage, we observed a stepwise increase in the fraction of patients with measurements of LVMI, cIMT, or PWV exceeding the 95th percentile (intermediate end points) and a steady decrease of the fraction of patients with measurements in the normal range. Therefore, these cross-sectional data suggest progressive development of subclinical CVD with loss of GFR. BP, measured as office systolic BP SDS or 24-hour MAP SDS, was the single independent factor significantly associated with all measured surrogate markers.
About two thirds of the 4C Study participants are boys (65%), and three quarters were diagnosed with defined congenital anomalies (i.e., CAKUT and cystic kidney diseases). These findings are quite similar to the epidemiologic data of the CKiD Study in the United States in 586 children with CKD stages 1–4, where a preponderance of boys (62%) and congenital anomalies (78% nonglomerular diseases) was found (16). Both studies show further similarities in the prevalence of growth failure (16% each) and obesity (20% versus 24%, respectively). The high concordance is remarkable considering the different ethnic composition of the two cohorts.
In the general population, physical activity is favorably associated with vascular health in children (17) and lower cIMT in adolescents (18). Young patients with CKD are inactive compared with their peers (19), and the significance of this risk factor seems to be confirmed by our study showing that increased physical activity was strongly associated with a lower LVMI.
In previous studies (20,21), we identified a high prevalence of SGA patients with CKD (17.7%), whereas the average gestational age was normal. Low birth weight has been identified as a risk factor for CKD (22) and CVD. It is, therefore, of interest that all birth parameters, including SGA status, were not associated with any surrogate markers measured in our study. Similarly, birth parameters had no significant association with ABPM results or eGFR in the CKiD Study (23). It is likely that postnatal factors (e.g., CKD symptoms and their treatment) supersede putative effects of birth history in these populations.
ABPM is the reference standard for classification of hypertension and accurate assessment of cardiovascular risk (14). In our study, about 25% of patients with CKD stages 3 and 4 and 47% with CKD stage 5 had uncontrolled hypertension. Our findings illustrate the major discrepancies between office and ambulatory BP: <50% of the patients with ambulatory hypertension were identified by office BP, and almost 50% of the patients diagnosed as hypertensive in office were found normotensive by ABPM. Unfortunately, a uniform definition of ambulatory hypertension has not been agreed on to date. We found prevalence figures to be influenced to a major degree by the consideration of elevated BP load. The prevalence of masked and confirmed hypertension more than doubled by defining patients with isolated high BP load as hypertensive (Supplemental Appendix). The inclusion of elevated BP load as a diagnostic criterion entirely explained the higher prevalence of ambulatory hypertension observed in the CKiD Study (58% versus 26%) (15). In a recent meta-analysis of ten long–term outcome studies performed in >8000 adults, BP load did not add significantly to the cardiovascular risk associated the mean 24-hour BP level (24). Also, the usefulness of 24-hour MAP, irrespective of BP load, as a target of therapeutic intervention was impressively shown for a renal survival end point in the Effect of Strict Blood Pressure Control and ACE-Inhibition on Progression of Chronic Renal Failure in Pediatric Patients (ESCAPE) Trial (25). There is an obvious need to harmonize the diagnostic criteria of ambulatory hypertension in adults and children with CKD.
Notwithstanding the difficulties of categorizing BP measurements as normo- or hypertensive, our multivariate regression analyses showed strong linear relationships of BP—measured by either office or ambulatory readings—with major intermediate cardiovascular end points. Hypertension is considered a modifiable risk factor as shown by slowing of CKD progression and regression of LVH with improved BP control (25–27). Our findings support the notion that such benefits will depend primarily on absolute BP lowering rather than achieving an arbitrary hypertension cutoff level.
The presence of LVH indicates substantial cardiac remodeling and is associated with high CVD morbidity and mortality in adults (28). Our study shows a high prevalence of LVH of both the concentric and eccentric types in children, with an incremental increase of the LVMI in each stage of CKD. The LVMI was positively associated with systolic BP and BMI and negatively associated with patient age, girls, physical activity, eGFR, HDL and LDL cholesterol levels, and serum albumin. Previous smaller studies have found variable prevalence of LVH, a similar association with women (29,30), or different associations with other patient–related variables, such as 25-hydroxy-vitamin D (25-OHD) levels (31) or anemia, a significant risk factor for LVH in most studies of adults (32). However, a correlation with systolic BP has been observed in almost all of these studies.
Subclinical cardiac disease in children with CKD is not confined to ventricular remodeling and LVH but may result in ventricular dysfunction (33). In a recent analysis of cardiac mechanics by strain analysis of echocardiography images obtained in a subgroup of the children participating in this study, we showed a high prevalence of cardiac dysfunction (34).
In >40% of the children in this study, cIMT exceeded the 95th percentile without a significant difference between CKD stages 3 and 5. cIMT was independently associated with girls, physical activity, BMI, systolic BP SDS, and serum phosphorus. The observed negative correlation with 25-OHD levels is consistent with the putative protective cardiovascular effects of nutritional vitamin D in the general population (35). There was no significant association of cIMT with LDL cholesterol, in contrast to other studies (36,37).
Aortic stiffness has independent predictive values for all-cause and cardiovascular mortality in adults with ESRD (38). Our study shows a normal PWV in 80% of patients and a similar PWV in CKD stages 3a, 3b, and 4 but an increase of the mean PWV SDS in patients with CKD stage 5. PWV was positively associated with BP SDS, proteinuria, and parathyroid hormone, whereas age and 25-OHD levels were negative predictors. Interestingly, cystatin C levels, which have been found to be associated with CVD outcomes independent of GFR (39), were not significantly associated with surrogate markers in our study.
Defining the number of intermediate end points as a putative indicator of the extent of cardiovascular comorbidity permitted further exploration of factors with predictive value for the cumulative presence of intermediate end points. Interestingly, the intermediate end point score was associated with a somewhat different set of variables then found in the single–surrogate end point models. Thus, the serum phosphorus level, the presence of CAKUT, systolic BP SDS, and BMI SDS were positive predictors, and time since diagnosis of CKD and the hemoglobin level were negative predictors. The favorable association with time since diagnosis of CKD is probably not due to (long) duration of CKD but might reflect the influence of late diagnosis/referral (i.e., undertreatment of CKD symptoms) or the presence of a subpopulation of patients with faster progression; this needs further clarification by longitudinal analysis. Higher serum phosphorus, higher systolic BP, higher BMI SDS, and lower hemoglobin are well described risk factors for CVD in adult and pediatric patients with CKD, and this study confirms their importance in a representative population. It is likely that the variables associated with the cumulative presence of intermediate end points are determinants of progressive cardiovascular morbidity, but this notion awaits confirmation by prospective follow-up.
Several limitations of this study should be noted. Comparison with epidemiologic data shows that patients with CAKUT were overrepresented; thus, conclusions of the study may not apply to patients with other diagnoses. Comparability with other studies on hypertension may be limited, because local oscillometric devices were used for office BP measurements in participating centers. Moreover, the observed prevalence of ambulatory hypertension strongly depends on the diagnostic criteria applied. Because the absence of true CVD end points in the pediatric age group necessitates studies of surrogate markers and in the case of cIMT, pediatric percentiles of normal data merge with adult percentiles (6), the predictive value of pediatric data for CKD–related CVD events in adulthood is unknown. Moreover, the value of intermediate end points in clinical trials (40) and cardiovascular risk prediction by current models on the basis of atherosclerosis research (41) has recently been questioned, and prediction may be especially difficult for patients with CKD, in whom sudden cardiac death is the leading cause of CVD-related mortality (42).
In conclusion, baseline data of the 4C Study show the presence of subclinical CVD in an increasing portion of patients with each CKD stage and significant associations with modifiable risk factors, such as hypertension, physical activity, BMI, and serum phosphorus levels, delineating therapeutic targets for the prevention of cardiovascular comorbidity in children with CKD.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was made possible by grants from the European Renal Association–European Dialysis and Transplant Association (www.era-edta.org), the Kuratorium für Dialyse und Nierentransplantation (KfH) Foundation for Preventive Medicine, the German Federal Ministry of Education and Research (reference no. 01EO0802), and Pfizer Deutschland GmbH.
Supplemental Appendix has information on the Cardiovascular Comorbidity in Children with CKD Study Collaborators.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01090216/-/DCSupplemental.
References
- 1.Mitsnefes MM: Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol 23: 578–585, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Querfeld U, Anarat A, Bayazit AK, Bakkaloglu AS, Bilginer Y, Caliskan S, Civilibal M, Doyon A, Duzova A, Kracht D, Litwin M, Melk A, Mir S, Sözeri B, Shroff R, Zeller R, Wühl E, Schaefer F; 4C Study Group : The cardiovascular comorbidity in children with chronic kidney disease (4C) study: Objectives, design, and methodology. Clin J Am Soc Nephrol 5: 1642–1648, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents : The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114[Suppl 4th Report]: 555–576, 2004 [PubMed] [Google Scholar]
- 5.Wühl E, Witte K, Soergel M, Mehls O, Schaefer F; German Working Group on Pediatric Hypertension : Distribution of 24-h ambulatory blood pressure in children: Normalized reference values and role of body dimensions. J Hypertens 20: 1995–2007, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Doyon A, Kracht D, Bayazit AK, Deveci M, Duzova A, Krmar RT, Litwin M, Niemirska A, Oguz B, Schmidt BM, Sözeri B, Querfeld U, Melk A, Schaefer F, Wühl E; 4C Study Consortium : Carotid artery intima-media thickness and distensibility in children and adolescents: Reference values and role of body dimensions. Hypertension 62: 550–556, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Thurn D, Doyon A, Sözeri B, Bayazit AK, Canpolat N, Duzova A, Querfeld U, Schmidt BM, Schaefer F, Wühl E, Melk A, Consortium CS; 4C Study Consortium : Aortic pulse wave velocity in healthy children and adolescents: Reference values for the vicorder device and modifying factors. Am J Hypertens 28: 1480–1488, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N: Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol 57: 450–458, 1986 [DOI] [PubMed] [Google Scholar]
- 9.Khoury PR, Mitsnefes M, Daniels SR, Kimball TR: Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr 22: 709–714, 2009 [DOI] [PubMed] [Google Scholar]
- 10.de Simone G, Daniels SR, Kimball TR, Roman MJ, Romano C, Chinali M, Galderisi M, Devereux RB: Evaluation of concentric left ventricular geometry in humans: Evidence for age-related systematic underestimation. Hypertension 45: 64–68, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Desvarieux M, Ebrahim S, Fatar M, Hernandez R, Kownator S, Prati P, Rundek T, Taylor A, Bornstein N, Csiba L, Vicaut E, Woo KS, Zannad F; Advisory Board of the 3rd Watching the Risk Symposium 2004, 13th European Stroke Conference : Mannheim intima-media thickness consensus. Cerebrovasc Dis 18: 346–349, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Kracht D, Shroff R, Baig S, Doyon A, Jacobi C, Zeller R, Querfeld U, Schaefer F, Wühl E, Schmidt BM, Melk A; 4C Study Consortium : Validating a new oscillometric device for aortic pulse wave velocity measurements in children and adolescents. Am J Hypertens 24: 1294–1299, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Urbina E, Alpert B, Flynn J, Hayman L, Harshfield GA, Jacobson M, Mahoney L, McCrindle B, Mietus-Snyder M, Steinberger J, Daniels S; American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee : Ambulatory blood pressure monitoring in children and adolescents: Recommendations for standard assessment: A scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the council on cardiovascular disease in the young and the council for high blood pressure research. Hypertension 52: 433–451, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Flynn JT, Daniels SR, Hayman LL, Maahs DM, McCrindle BW, Mitsnefes M, Zachariah JP, Urbina EM; American Heart Association Atherosclerosis, Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young : Update: Ambulatory blood pressure monitoring in children and adolescents: A scientific statement from the American Heart Association. Hypertension 63: 1116–1135, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samuels J, Ng D, Flynn JT, Mitsnefes M, Poffenbarger T, Warady BA, Furth S; Chronic Kidney Disease in Children Study Group : Ambulatory blood pressure patterns in children with chronic kidney disease. Hypertension 60: 43–50, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furth SL, Abraham AG, Jerry-Fluker J, Schwartz GJ, Benfield M, Kaskel F, Wong C, Mak RH, Moxey-Mims M, Warady BA: Metabolic abnormalities, cardiovascular disease risk factors, and GFR decline in children with chronic kidney disease. Clin J Am Soc Nephrol 6: 2132–2140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Idris NS, Evelein AM, Geerts CC, Sastroasmoro S, Grobbee DE, Uiterwaal CS: Effect of physical activity on vascular characteristics in young children. Eur J Prev Cardiol 22: 656–664, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Pahkala K, Heinonen OJ, Simell O, Viikari JS, Rönnemaa T, Niinikoski H, Raitakari OT: Association of physical activity with vascular endothelial function and intima-media thickness. Circulation 124: 1956–1963, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Akber A, Portale AA, Johansen KL: Pedometer-assessed physical activity in children and young adults with CKD. Clin J Am Soc Nephrol 7: 720–726, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenbaum LA, Muñoz A, Schneider MF, Kaskel FJ, Askenazi DJ, Jenkins R, Hotchkiss H, Moxey-Mims M, Furth SL, Warady BA: The association between abnormal birth history and growth in children with CKD. Clin J Am Soc Nephrol 6: 14–21, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franke D, Völker S, Haase S, Pavicic L, Querfeld U, Ehrich JH, Zivicnjak M: Prematurity, small for gestational age and perinatal parameters in children with congenital, hereditary and acquired chronic kidney disease. Nephrol Dial Transplant 25: 3918–3924, 2010 [DOI] [PubMed] [Google Scholar]
- 22.White SL, Perkovic V, Cass A, Chang CL, Poulter NR, Spector T, Haysom L, Craig JC, Salmi IA, Chadban SJ, Huxley RR: Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis 54: 248–261, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Flynn JT, Ng DK, Chan GJ, Samuels J, Furth S, Warady B, Greenbaum LA, Chronic Kidney Disease in Children S : The effect of abnormal birth history on ambulatory blood pressure and disease progression in children with chronic kidney disease. J Pediatr 165: 154–162.e1, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Thijs L, Boggia J, Asayama K, Hansen TW, Kikuya M, Björklund-Bodegård K, Ohkubo T, Jeppesen J, Torp-Pedersen C, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Filipovsky J, Imai Y, Ibsen H, O’Brien E, Wang J, Staessen JA; International Database on Ambulatory blood pressure in relation to Cardiovascular Outcomes (IDACO) Investigators* : Blood pressure load does not add to ambulatory blood pressure level for cardiovascular risk stratification. Hypertension 63: 925–933, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen AM, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Möller K, Wigger M, Peruzzi L, Mehls O, Schaefer F, Schaefer F; ESCAPE Trial Group : Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361: 1639–1650, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Matteucci MC, Chinali M, Rinelli G, Wühl E, Zurowska A, Charbit M, Pongiglione G, Schaefer F, Group ET; ESCAPE Trial Group : Change in cardiac geometry and function in CKD children during strict BP control: A randomized study. Clin J Am Soc Nephrol 8: 203–210, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kupferman JC, Aronson Friedman L, Cox C, Flynn J, Furth S, Warady B, Mitsnefes M, Group CKS; CKiD Study Group : BP control and left ventricular hypertrophy regression in children with CKD. J Am Soc Nephrol 25: 167–174, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, Barre PE: Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int 47: 186–192, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Matteucci MC, Wühl E, Picca S, Mastrostefano A, Rinelli G, Romano C, Rizzoni G, Mehls O, de Simone G, Schaefer F, Group ET; ESCAPE Trial Group : Left ventricular geometry in children with mild to moderate chronic renal insufficiency. J Am Soc Nephrol 17: 218–226, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Gruppen MP, Groothoff JW, Prins M, van der Wouw P, Offringa M, Bos WJ, Davin JC, Heymans HS: Cardiac disease in young adult patients with end-stage renal disease since childhood: A Dutch cohort study. Kidney Int 63: 1058–1065, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Patange AR, Valentini RP, Gothe MP, Du W, Pettersen MD: Vitamin D deficiency is associated with increased left ventricular mass and diastolic dysfunction in children with chronic kidney disease. Pediatr Cardiol 34: 536–542, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Parfrey PS, Lauve M, Latremouille-Viau D, Lefebvre P: Erythropoietin therapy and left ventricular mass index in CKD and ESRD patients: A meta-analysis. Clin J Am Soc Nephrol 4: 755–762, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mencarelli F, Fabi M, Corazzi V, Doyon A, Masetti R, Bonetti S, Castiglioni L, Pession A, Montini G: Left ventricular mass and cardiac function in a population of children with chronic kidney disease. Pediatr Nephrol 29: 893–900, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Chinali M, Matteucci MC, Franceschini A, Doyon A, Pongiglione G, Rinelli G, Schaefer F: Advanced parameters of cardiac mechanics in children with CKD: The 4C Study. Clin J Am Soc Nephrol 10: 1357–1363, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juonala M, Voipio A, Pahkala K, Viikari JS, Mikkilä V, Kähönen M, Hutri-Kähönen N, Jula A, Burgner D, Sabin MA, Marniemi J, Loo BM, Laitinen T, Jokinen E, Taittonen L, Magnussen CG, Raitakari OT: Childhood 25-OH vitamin D levels and carotid intima-media thickness in adulthood: The cardiovascular risk in young Finns study. J Clin Endocrinol Metab 100: 1469–1476, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Brady TM, Schneider MF, Flynn JT, Cox C, Samuels J, Saland J, White CT, Furth S, Warady BA, Mitsnefes M: Carotid intima-media thickness in children with CKD: Results from the CKiD study. Clin J Am Soc Nephrol 7: 1930–1937, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khandelwal P, Murugan V, Hari S, Lakshmy R, Sinha A, Hari P, Bagga A: Dyslipidemia, carotid intima-media thickness and endothelial dysfunction in children with chronic kidney disease. Pediatr Nephrol 31: 1313–1320, 2016 [DOI] [PubMed] [Google Scholar]
- 38.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB: Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 63: 636–646, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tangri N, Inker LA, Tighiouart H, Sorensen E, Menon V, Beck G, Shlipak M, Coresh J, Levey AS, Sarnak MJ: Filtration markers may have prognostic value independent of glomerular filtration rate. J Am Soc Nephrol 23: 351–359, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeboah J, Herrington DM: Intermediate end point cardiovascular clinical trials: An excellent idea based on an obsolete paradigm. Expert Rev Cardiovasc Ther 12: 161–165, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Ruwanpathirana T, Owen A, Reid CM: Review on cardiovascular risk prediction. Cardiovasc Ther 33: 62–70, 2015 [DOI] [PubMed] [Google Scholar]
- 42.Kanbay M, Solak Y, Covic A, Goldsmith D: Sudden cardiac death in patients with chronic kidney disease: Prevention is the sine qua non. Kidney Blood Press Res 34: 269–276, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.