Abstract
AKI is an increasingly common disorder that is strongly linked to short- and long-term morbidity and mortality. Despite a growing heterogeneity in its causes, providing a timely and certain diagnosis of AKI remains challenging. In this review, we summarize the evolution of AKI biomarker studies over the past few years, focusing on two major areas of investigation: the early detection and prognosis of AKI. We highlight some of the lessons learned in conducting AKI biomarker studies, including ongoing attempts to address the limitations of creatinine as a reference standard and the recent shift toward evaluating the prognostic potential of these markers. Lastly, we suggest current gaps in knowledge and barriers that may be hindering their incorporation into care and a full ascertainment of their value.
Keywords: acute renal failure, biomarkers, acute kidney injury, creatinine, Early Diagnosis, Prognosis
Introduction
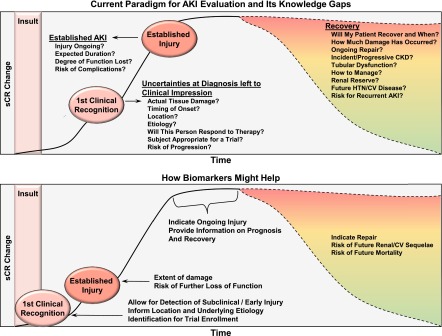
AKI is becoming increasingly common and is linked to progressive loss of kidney function, cardiovascular disease, and death (1–7). Despite often being reduced to its rudimentary manifestations (i.e., a change in serum creatinine), AKI is comprised of several diseases, highlighting a need to improve phenotyping beyond the circumstantial. Developing therapies will require a timely description of the onset, location, and severity of parenchymal injury, its underlying mechanisms, and prognosis (2,8). Next-generation biomarkers of AKI propose to fill these knowledge gaps (Figure 1). This review will discuss knowledge gained over the past decade and attempts to better integrate these markers into care.
Figure 1.
How biomarkers may improve the current approach to AKI. The top panel illustrates the current paradigm highlighting how serum creatinine does permit recognition of AKI, albeit delayed, and an estimate of severity based on loss of function. However, much is left to clinical impression, including its anatomic location, etiology, appropriateness for a trial, the presence and extent of tissue damage, and risk of progression. Similarly, tissue damage is presumed retrospectively only after loss of function becomes persistent and prospects for recovery often remain uncertain. The lower panel shows the complementary roles that AKI biomarkers may play, including aiding in detection of subclinical/early injury, providing information on its type and location with potential applications including as a tool for trial enrollment, detailing the extent of damage, as a beacon for ongoing injury during recovery or indicating repair/fibrosis, and risk stratifying for future complications. CV, cardiovascular, HTN, hypertension, sCR, serum creatinine.
The Growing Role of Biomarkers: From Detection to Prognosis
Appendix Table 1 lists the several putative biomarkers for AKI in humans, including enzymes (n-acetyl-β-d-glucosaminidase [NAG], α- and π-glutathione s-transferase [α-GST/π-GST], alkaline phosphates [AP], and alanine aminopeptidase [AAP]), proinflammatory mediators (IL-18; neutrophil gelatinase–associated lipocalin [NGAL]), and other structural upregulated proteins (kidney injury molecule-1 [KIM-1], liver-type fatty acid–binding protein [L-FABP], sodium–hydrogen (Na+/H+) exchanger isoform) that are released during tubular damage (9–20). Other markers of glomerular filtration reabsorbed by healthy tubular epithelium (cystatin-C [Cys-C], retinol binding protein [RBP], and α1/β2 microglobulin), and hormonal markers (angiotensinogen) have also been studied (9,15,21,22). Recently, markers thought to play a role in cell cycle regulation, including tissue inhibitor of metalloproteinases-2 (TIMP-2) and IGF-binding protein-7 (IGFBP7), have emerged and are available as a potential aid for AKI risk stratification (23).
Early Detection of AKI
Following early studies demonstrating the ability of several markers to identify established AKI (10,14,16,18,24–26), large prospective cohorts determined their capacity to provide an earlier diagnosis (Appendix Table 2). These investigations were inspired by the hypothesis that delays in intervention have hindered clinical trials and small studies demonstrating that markers such as NGAL could provide timelier identification of AKI (27,28). The landmark Translational Research Investigating Biomarkers and End Points for Acute Kidney Injury (TRIBE-AKI) consortium expanded upon this work by testing whether postoperative levels of urine NGAL (uNGAL), plasma NGAL (pNGAL), and urine IL-18 (uIL-18) could detect a doubling of baseline serum creatinine (sCR), acute dialysis, and other endpoints in 1219 adults and 311 children undergoing cardiac surgery in the United States and Canada (29,30). Postoperative biomarker levels were higher in patients who experienced AKI than those who did not, were associated with AKI after adjusting for clinical risk factors, and helped reclassify risk in some patients. However, discrimination was modest, yielding the following area under the receiver operating curves (AUCs): uNGAL, 0.67 and 0.71, pNGAL, 0.70 and 0.56, and uIL-18, 0.74 and 0.72 in adults and children, respectively (29,30). The observed incidence of AKI was 5% in adults and 17% in children, and respective cut-off values for uNGAL (102 ng/ml and 17 ng/ml), pNGAL (293 ng/ml and 154 ng/ml), and uIL-18 (60 pg/ml and 125 pg/ml) yielded negative predictive values (NPVs) of 86%–97% and positive predictive values (PPVs) of 11%–30%.
Among the critically ill, a study of 632 patients in Europe showed that pNGAL and uNGAL measured at intensive care unit admission discriminated between patients who developed AKI within 7 days and those who did not (pNGAL: AUCs of 0.77±0.05 and 0.80±0.06 and RIFLE risk [R] and injury [I]. uNGAL: AUCs of 0.80±0.04 and 0.85±0.04 for RIFLE Risk and Injury categories, respectively). Notably, 58% of patients fulfilled creatinine-based criteria for AKI on enrollment day (31). In another study of 451 critically ill adults in the US, uNGAL provided moderate discrimination for AKI developing within 24 and 48 hours of measurement (AUC, 0.71; 95% confidence interval [95% CI], 0.63 to 0.78; and AUC, 0.64; 95% CI, 0.57 to 0.71, respectively) (32). A subgroup analysis performed among patients with an eGFR≥75 ml/min per 1.73m2 to enrich for incident AKI improved discrimination to 0.77 (95% CI, 0.64 to 0.90) (32). However, a subsequent case-control study by the same authors did not confirm improvement in the performance of urine NGAL, Cys-C, and L-FABP for detecting AKI within 48 hours in patients with preserved kidney function (33). Several other studies evaluating NGAL, Cys-C, KIM-1, and IL-18 in the critically ill have also been performed, showing varying levels of performance (32–37).
Recently, a large multicenter study (SAPPHIRE) was conducted to identify novel biomarkers for AKI in critically ill adults (23). In a discovery cohort, the ability of 340 protein biomarker candidates to detect Kidney Disease Improving Global Outcomes (KDIGO) stage 2 or 3 injury within 12 hours was examined in 522 patients. Of the markers examined, the combined AUC of TIMP-2 and IGFBP7 was 0.77 (95% CI, 0.72 to 0.82), a finding subsequently replicated in a separate validation cohort of 728 patients (AUC, 0.80; 95% CI, 0.75 to 0.84). After adjusting for nine clinical covariates, TIMP-2*IGFBP7 was associated with a 4.1-fold increase in the risk of developing KDIGO stage 2 or 3 AKI. As one of the shared functions of these molecules is to induce G1 cell cycle arrest, the investigators hypothesized TIMP-2*IGFBP7 may serve a protective effect by minimizing the proliferation of damaged cells. In a separate study of 408 patients, a value of 0.3 ng/ml2/1000 for AKI demonstrated good discrimination (AUC, 0.82; 95% CI, 0.76 to 0.88) (38). The primary endpoint of stage 2 or 3 AKI occurred in 17% of patients yielding PPVs ranging from 27%–62% for cutoff values of 0.3 and 2.0 ng/ml2/1000. These observations recently led the Food and Drug Administration to approve the marketing of TIMP-2*IGFBP7 as a potential aid in the risk assessment for moderate or severe AKI in critically ill patients within 12 hours, when used in conjunction with clinical evaluation, including confirmation by alternative methods (38). Notably, NPVs for TIMP-2*IGFBP7 ranged from 88%–96%, suggesting that a strength might lie in the ability to identify patients at low risk for severe AKI. The 12-hour window in the SAPPHIRE study was also shorter than in other biomarker studies. Whether early knowledge of increased risk for severe AKI within this window can translate into improved outcomes remains to be determined (see Limitations and Future Directions, below).
Early detection has also been examined in other acute care settings (18,26,39–41). In one study of 121 hospitalized cirrhotic patients, uNGAL accurately discriminated patients who developed AKI from those who did not, with an AUC of 0.83 (95% CI, 0.76 to 0.91), a PPV of 54%, and an NPV of 89% (41). Similarly, in a single-center study of 91 patients with acute decompensated heart failure, elevated sNGAL levels (≥140 ng/ml) were associated with a 7.4-fold increase in the risk of developing AKI (39).
Challenges of Early Detection Studies
While showing some ability to provide a timelier diagnosis, interpretive and practical issues have emerged. One of these includes a need to expand the framework for assessing biomarker performance beyond sCR. This has been suggested by studies demonstrating that some injury markers increase in concert after percutaneous coronary intervention in patients with preserved kidney function without an increase in sCR (42), and in critically ill patients with prerenal injury (43). Whether this reflects nonspecific expression or unrecognized tubular damage due to renal functional reserve or other confounders is unknown. Conversely, low biomarker levels in the presence of elevated creatinine may not necessarily constitute a false-negative. These limitations were illustrated in a simulation study demonstrating that a biomarker with a 100% sensitivity in a 10% prevalent population may be seen as being only 47% sensitive when compared with sCR, assuming creatinine itself is 80% sensitive and 90% specific (44). Notably, the Predictive Safety Testing Consortium found that markers such as uKIM-1 and NAG outperformed sCR in preclinical studies when a tissue standard was used (45). However, tissue standards in human AKI have been limited to a few small studies (46–48). These results have suggested that AKI biomarkers might be more valuable when viewed as complementary rather than in competition with sCR, leading the Acute Dialysis Quality Initiative to propose a framework to characterize AKI using both functional and damage markers in 2014 (49). This delineation allows for subclinical AKI where damage markers are elevated without an accompanying change in function (biomarker+/sCR−), functional changes in the absence of damage (biomarker−/sCR+), and both functional and damage (biomarker+/sCR+). Subsequent proof of concept studies have illustrated that patients with elevated NGAL without elevated sCR were at higher risk for death, and that patients with elevations of both NGAL and sCR were at higher risk for death and dialysis than patients with elevated sCR alone (50,51).
The effect of differences in case mix on performance also remain poorly understood. Although the timing of tubular damage and their contributions to increases in sCR likely vary with the cause of AKI, clinical phenotyping remains limited in most studies. Some studies have shown improvement in biomarker discrimination when requiring more persistent increases in sCR (32,37), whereas others have shown some ability to distinguish prerenal versus intrinsic AKI (26,52–54). In one study of 1635 adults presenting to an emergency room, uNGAL, uL-FABP, and uKIM-1 showed moderate-to-good utility for discriminating between intrinsic and prerenal AKI, CKD, and normal kidney function (54). However, there were multiple contributors in the intrinsic AKI group, including, but not limited to, hypotension, obstruction, sepsis, nephrotoxicity, and GN/vasculitis. It remains unclear how biomarker expression patterns differ across these subtypes.
Clinical uncertainty as to when injury actually occurs and the kinetics of biomarker expression outside of cardiac surgery or planned nephrotoxin exposure may also affect performance. As AKI can occur at different times before creatinine rises, a biomarker measurement before injury or one that only transiently increases can reduce its observed sensitivity (55). The former would reflect more of a prediction rather than early detection scenario and not be expected to perform well. In 528 patients from the Early Intervention with Erythropoietin Does Not Affect the Outcome of Acute Kidney Injury (EARLY-ARF) trial, GGT, NGAL, Cys-C, KIM-1, and IL-18 were used to triage patients for enrollment but failed to adequately distinguish patients who developed AKI from those who did not (AUCs, 0.59–0.67) (56). However, performance of some biomarkers improved after stratifying by the timing of likely kidney insult determined by clinical adjudication (AUCs, 0.85–0.94) (56). Much also remains to be learned about the biologic variability of biomarker expression. For example, preclinical and clinical studies indicate that genetic background and comorbidities, such as CKD, can have a substantial effect on chronic and acute biomarker expression (57,58). An improved understanding and ability to quantify this variability is needed to better interpret values in the diagnosis and prognosis of AKI (59). Standardization of assays also remains an important goal. Although reported intra- and interassay coefficients of variation appear reasonable, studies comparing performance of commercially available platforms has been limited (60) and further collaboration is required to integrate and standardize different methodologies.
The Move toward More Meaningful Clinical Endpoints
Predicting AKI Severity
Given the debated significance and specificity of smaller or transient changes in sCR, studies have investigated whether biomarkers measured during early stages of AKI can predict progression to a higher stage (21,22,61,62). The TRIBE-AKI investigators tested if tubular injury markers could predict worsening of AKI stage in 380 cardiac surgery patients with stage 1 AKI. Although individual discrimination was moderate (AUCs, 0.63 for uIL-18 to 0.74 for pNGAL), the highest quintiles of uIL-18 and pNGAL associated with progression to a higher stage (adjusted odds ratios of 3.0 (95% CI, 1.3 to 7.3) and 7.7 (95% CI, 2.6 to 22.5), respectively) (61). All biomarkers improved risk classification, although uIL-18 was more effective in reclassifying nonevents to a lower level of risk whereas uNGAL and pNGAL appeared to help reclassify events to a higher level of risk (61).
Another study tested the ability of uL-FABP, uNGAL, uIL-18, and uKIM-1 to predict injury progression, dialysis, or death within 7 days in 152 critically ill patients with stage 1 AKI (62). Discrimination of uL-FABP was highest (AUC, 0.79; 95% CI, 0.70 to 0.86) with uNGAL, uIL-18, and uKIM-1 showing more modest performance (AUC range, 0.62–0.65). Adding uL-FABP raised the AUC of the clinical model from 0.74 (95% CI, 0.68 to 0.84) to 0.82 (95% CI, 0.75 to 0.90), an improvement not observed with the other biomarkers. uL-FABP correctly reclassified a higher proportion of nonevents and uNGAL reclassified a higher proportion of events than predicted by the clinical model. Comparable studies in patients with cirrhosis and hospitalized patients have shown similar results (24,63).
In children, the ability of biomarkers to enhance clinical risk prediction tools has been investigated. The renal angina index (RAI) is a clinical tool developed to identify critically ill children with evidence of early injury who are at risk for developing severe AKI (64). While effectively ruling out patients at low risk for severe AKI, the PPV of renal angina for predicting severe AKI was only 40% in the population studied, highlighting the need for additional tools beyond established clinical predictors. The authors hypothesized that RAI might also help guide biomarker measurement and improve performance by enriching the population for the outcome of interest and reduce false positives, a problem observed by less selective measurement of biomarkers, such as troponin (65–67). Subsequently, the investigators found that elevated matrix metalloproteinase-8 and neutrophil elastase increased the probability of a patient experiencing severe AKI compared with patients with RAI alone by 31% (68).
Informing Long-Term Prognosis of AKI.
Renal Recovery after AKI
The transition of care is an opportunity to improve longitudinal outcomes after AKI (69,70). As recovery is a key prognosticator (71), identifying poor recovery may help facilitate nephrology referral, inform clinical decision making, guide enrollment in trials, and, most importantly, provide key information to AKI survivors (72–75). AKI biomarkers may also be better suited to characterizing recovery compared with more peripherally associated outcomes, including mortality.
The Biologic Markers of Recovery for the Kidney study examined the ability of blood and urine biomarkers to predict recovery among patients with dialysis-requiring AKI (72–74). In 76 patients with urine available 1, 7, and 14 days after dialysis initiation, changes in urinary hepatocyte growth factor and uNGAL individually predicted dialysis-free survival by day 60 with AUCs of 0.74 (95% CI, 0.60 to 0.89) and 0.70 (95% CI, 0.55 to 0.85), respectively, and 0.84 (95% CI, 0.73 to 0.94) when combined (72). Similarly, the addition of biomarkers raised the c-statistic of the clinical model from 0.74 to 0.93. Among 817 patients with plasma available, each natural log increase in plasma IL-8 and TNF receptor-1 levels were associated with a lower likelihood of dialysis-free survival at 60 days (hazard ratios [HRs], 0.80 [95% CI, 0.70 to 0.91] and 0.63 [95% CI, 0.50 to 0.71], respectively) (73,74). Notably, these studies have been limited by use of highly select populations and variability in the timing of dialysis in relation to injury recognition. In addition, as these markers reflect mainly tubular injury, their ability to provide information on repair itself may be limited, highlighting the need to identify additional markers of recovery and prognosis (76,77).
Long-Term Outcomes
In cardiology, major adverse cardiac events (all-cause mortality, myocardial infarction or unplanned repeat revascularization, stroke, and heart failure) have been approved as a primary end point by the Food and Drug Administration for cardiovascular-outcomes studies (78,79). Similarly, others have advocated using long-term outcomes (e.g., major adverse kidney events—sustained loss of kidney function, dialysis, or death) for both intervention and biomarker studies in patients with established AKI (80,81).
The TRIBE-AKI study observed a graded risk for mortality between the highest and lowest tertiles of biomarker levels (uNGAL, uKIM-1, uIL-18, uL-FABP, albumin) and 3-year mortality in 1199 adults in patients with sCR+AKI (adjusted HR, 2.0 to 3.2) and in patients without sCR+AKI (adjusted HR, 0.9 to 1.8) (82). Notably, the risk of mortality in patients within the highest biomarker tertile in patients without sCR+AKI was similar to patients with sCR+AKI with the lowest tertiles of biomarker expression (except for uL-FABP). These results are consistent with the hypothesis that AKI biomarkers can provide additional prognostic information, although the extent to which elevated markers are specifically reflecting mortality risk attributable to kidney injury is uncertain.
Studies have also begun to examine the association between AKI biomarkers and long-term loss of kidney function. In a secondary analysis of 692 patients from the SAPPHIRE study, uTIMP-2*IGFBP7 levels above 2.0 (n=44) at intensive care unit admission were associated with death or dialysis at 9 months (HR, 2.16; 95% CI, 1.32 to 3.53) (83). Among patients receiving kidney transplant, median postoperative levels of uNGAL (>422 ng/ml) and uIL-18(>137 pg/ml) have been associated with a 6.0- and 5.5-fold adjusted risk for poor graft function (dialysis or eGFR<30) at 1 year (84).
Limitations and Future Directions
Despite progress, important challenges remain in determining whether biomarkers can improve outcomes in AKI. One key question is how to apply biomarkers in current practice. For example, while timelier evaluation may benefit some patients, recent studies of electronic alerting systems have failed to demonstrate that earlier knowledge of increased risk translates to better outcomes on a population level, possibly due to the lack of specific or effective interventions (85,86). A future trial that randomizes patients to biomarker measurement plus protocol-driven guidelines for AKI evaluation versus no biomarker measurement may be informative. Another potential application is their use in clinical trials. In the recent ELAIN study randomizing critically ill patients with stage II AKI to early versus delayed initiation of RRT, investigators excluded patients with plasma NGAL levels <150 ng/dl. Most patients had values of >400 ng/dl and >90% of patients in the delayed arm eventually received RRT, suggesting that high levels might help enrich populations for patients likely to progress or experience complications of severe AKI (87). In a similar study, the Standard versus Accelerated Initiation of RRT in Acute Kidney Injury STARRT-AKI trial researchers also measured NGAL in whole blood; however, they found that whole-blood NGAL was able to confirm the presence of severe AKI but had difficulty distinguishing between patients in the standard arm who did and did not go on to require RRT (88). The latter is consistent with observational studies, indicating that some of the stronger aspects of performance observed for several biomarkers may be in their NPV (29,33,68,89). Whether biomarkers have value as surrogate outcomes in interventional studies has not been studied.
To some extent, the use of markers such as TIMP2*IGFBP7 may be instructive. A strong association with more specific and persistent losses of kidney function (e.g., major adverse kidney events outcomes) may alone be sufficient to inform decision making; however, full realization of their potential will depend on broadening the scope of investigation. Preclinical and epidemiologic data now support the contention that the sequelae of AKI extend beyond eGFR and include potential mediators (e.g., hypertension) or alternative outcomes (e.g., cardiovascular disease). Yet studies that examine the association between injury biomarkers and related elements of tubular function, including sodium, water handling, solute transport, and novel imaging, remain lacking (90,91).
An important opportunity for biomarkers to help improve outcomes in AKI may lie in refining the current approach to clinical phenotyping. Determining whether biomarkers can help fill an unmet need to noninvasively align prospective therapies with target AKI subpopulations may benefit from tissue standards for comparison. In support of this idea, the National Institute of Diabetes and Digestive and Kidney Disease NIDDK recently highlighted in their Kidney Research National Dialogue the need for ‘more intensive efforts to procure human biologic samples’ (92). Specifically highlighted was the paucity of kidney biopsies for diseases unrelated to the glomerulus and a call for ‘reappraisal of the standard indications for biopsy.’ While the goals of such studies need to be delineated and the risk to patients carefully weighed, outcomes in this disease will depend on improving confidence in our ability to identify the types of underlying lesions seen in modern-day AKI. It is worth recalling that pilot biopsy studies in the African American Study of Kidney Disease and Hypertension (AASK) and Irbesartan Diabetic Nephropathy Trial (IDNT) helped to confirm that the clinical diagnoses of hypertensive nephrosclerosis and diabetic nephropathy were reliable enough to ensure enrollment of the target populations of interest (93,94). The extent to which heterogeneity in the diagnosis of AKI may be currently impacting the appropriate enrollment in clinical trials is unknown, although we know that considerable variability exists between clinicians in simply distinguishing between prerenal AKI versus acute tubular necrosis (95). The effect of even small degrees of clinical uncertainty can be illustrated by a hypothetical trial of an intervention with a 90% power (α of 0.05) to reduce dialysis dependence in sepsis-associated acute tubular necrosis by 10% (50%–40% reduction, n=518/arm). If the same study population contains even a 20% subpopulation of drug-induced AIN (assuming no response to intervention), the power of the study would be reduced to 73% (PS software, v3.0; Dupont and Plummer).
Similarly, developing future therapies will also depend on gaining improved mechanistic understanding of the pathogenesis of AKI. Whether current AKI markers can adequately capture these complexities remains to be examined. For example, the hypothesis that the expression of TIMP-2*IGFBP7 may be protective is provocative but has yet to be confirmed. Another recent study examining patients with septic shock who underwent rapid postmortem kidney biopsy found marked increases in KIM-1 immunohistochemical staining in corticomedullary junction and cortical labyrinth tubules that was more widespread than the amount of frank necrosis observed (48). Whether these results provide insight into a specific distribution of injury in sepsis or its ‘reversibility’ and the ability of urine KIM-1 to capture some of this information are worth exploring. Future use of rapidly evolving “omic” techniques and emerging systems biology approaches may facilitate the identification of novel markers that can provide greater biologic and mechanistic insights into the pathogenesis of clinical AKI (96–104) (Appendix Tables 2 and 3).
In conclusion, the investigation of next-generation AKI biomarkers over the past decade has prompted a needed reevaluation of long-standing knowledge gaps in the traditional approach to evaluating and treating AKI. Early experiences viewed within this traditional framework suggest potential value for prediction and prognosis that remains to be confirmed by ongoing use in practice and in clinical trials. However, advancing understanding of AKI and determining the full value of biomarkers will require creatively expanding the lens of investigation, including addressing fundamental questions of the etiology and underlying mechanisms that current methods of investigation alone may not elucidate. The latter may hold the key to eventually translating long-overdue care treatment strategies into routine practice.
Disclosures
None.
Acknowledgments
R.M. and E.D.S. are supported by the Vanderbilt Center for Kidney Disease. E.D.S. is also supported by grants from the National Institutes of Health NIDDK DK92192-07 and the Veterans Health Affairs HSRD & Merit Award IIR 13-073-3. At the time of preparation of this manuscript, R.M. was pursuing a nephrology fellowship at Vanderbilt University.
Appendix
Appendix Table 1.
Properties of Biomarkers of AKI
| Biomarker Types | Biomarkers | Sources Tested | Site of Expression | Known Functions | Biomarker Kinetics in Cardiac Surgery Setting | Factors Affecting Biomarker Levels | Assay Platform |
| Functional | Cys-C (9) | Urine, serum | Nucleated cell | 13-kDa cysteine protease inhibitor, freely filtered and further reabsorbed and metabolized in the PT | Detected 12–24 h postinjury, and peak levels at 24–48 h | Albuminuria increase urinary excretion of Cys-C (competitive reabsorption through megalin-facilitated endocytosis); blood Cys-C levels are affected by GFR | Nephelometry, turbidimetry, ELISA (assay analysis is affected by diabetes, large dose steroids, hyperthyroidism, inflammation, hyperbilirubinemia, rheumatoid factor, and hypertriglyceridemia) |
| Tubular injury | IL-18 (12) | Urine, serum | PT, mononuclear cells, macrophages, fibroblasts, dendritic cells, intestinal epithelia, adrenal cortex | 24-kDa molecule cleaved by caspase-1 to generate biologically active 18-kD molecule; upregulation in ischemic kidney injury and is proinflammatory | Elevation within first 6 h after injury, and peak levels at 12–18 h | IL-18 levels are also high in inflammation, sepsis, heart failure | ELISA |
| KIM-1 (10) | Urine | PT (KIM-1a: renal; KIM-1b: liver) | 38.7-kDa type-1 membrane glycoprotein; phosphatidylserine receptor; upregulated in tubular injury and activates immune cells TH-1, TH-2, and TH-17; promotes apoptotic and necrotic cell clearance and remodeling of injured epithelia | Detected 12–24 h postinjury and peak levels at 48–72 h | KIM-1 is also expressed at high levels in patients with clear cell–type renal cell carcinoma, chronic proteinuria, CKD, and sickle-cell nephropathy | ELISA; Luminex | |
| L-FABP (20) | Urine | PT, liver, small intestine, pancreas, lung, stomach | 15-kDa cytoplasmic protein which transports free fatty acids to mitochondria and peroxisomes for metabolism; protects against damage caused by reactive oxygen species; upregulated during ischemia–reperfusion injury | Detected within 1 h after injury, and peak levels within 6 h | L-FABP levels are also elevated in liver disease, sepsis, polycystic kidney disease, CKD | ELISA | |
| NGAL (13) | Urine, serum | Kidney (PT>DT), neutrophils, liver, spleen | 25-kDa lipocalin protein associated with gelatinase from neutrophils; NGAL chelates labile Fe released from damaged tubules and prevents formation of hydroxy radicals; also upregulates the reno-protective enzyme heme-oxygenase-1 | Detected as early as 3 h after injury, peak at 6 h, and can have sustained elevation as long as 5 d | Plasma NGAL influenced by CKD (really?), chronic hypertension (really?), systemic infections, inflammatory conditions, anemia, hypoxia, and malignancies | ELISA | |
| NAG (19) | Urine | PT | 140-kDa proximal tubular lysosomal enzyme, not filtered | Detected 12 h after renal injury | Urinary NAG is inhibited by urea, heavy metals, industrial solvents | Colorimetric (false positive in rheumatoid arthritis, hyperthyroidism, and impaired glucose tolerance; degraded over time even when stored at -80°C) | |
| α-/π-GST (11,17) | Urine | α-GST: PT; π-GST: DT | 47–51 kDa cytoplasmic enzymes; increased after tubular injury | Detected 12 h after renal injury | — | ELISA | |
| Cell cycle arrest | TIMP-2 (23) | Urine | All epithelial cells; kidney tubular epithelial cells | TIMP-2 stimulates p27 expression and IGFBP7 increases the expression of p53 and p21, which block cyclin-dependent protein kinase complexes on cell cycle promotion, thereby resulting in transient cell cycle arrest | Detected within 12 h after renal injury | — | ELISA; Luminex |
| IGFBP7 (23) |
PT, proximal tubule; KIM-1, kidney injury molecule-1; TH, T-helper cells; L-FABP, liver-type fatty acid–binding protein; NGAL, neutrophil gelatinase–associated lipocalin; DT, distal tubule; Fe, iron; NAG, n-acetyl-β-d-glucosaminidase; α-GST, α-glutathione s-transferase; π-GST, π-glutathione s-transferase; TIMP-2, tissue inhibitor of metalloproteinases-2; IGFBP7, IGF-binding protein 7.
Appendix Table 2.
Biomarkers Studies for Early Diagnosis of AKI
| Clinical Settings | Authors and Year of Publications | Biomarkers | Sample Size | Study Design | Serial Biomarkers | End Point (Urine/Serum) | Summary of Findings |
| ICU | Bell et al. (105), 2015 | uTIMP-2*IGFBP7, uNGAL, uCys-C | 94 adults | Prospective, single-center | No | KDIGO stage 1, 2, and 3 within 48 h | AUCs for prediction of AKI by uTIMP-2*IGFBP7, uNGAL, and uCys-C were 0.40, 0.51, and 0.43, respectively. |
| Zwiers et al. (106), 2015 | uNGAL, uKIM-1 | 100 children (age 1 d to 1 yr) | Prospective, single-center | Yes (0–6 h, 6–12 h, 12–24 h, 24–36 h, 36–48 h, and 48–72 h) | RIFLE criteria within 48 h after ICU admission | AUCs for predicting AKI: uNGAL: 0–6 h, 0.81; 6–12 h, 0.78; 12–24 h, 0.71; 24 h peak level, 0.81; uKIM-1: at 12–24 h, 0.74. | |
| Wong et al. (107), 2015 | ELA2, FGF13, MMP-8, OLFM4, PRTN3 | 241 children (derivation); 200 children (validation) | Prospective, multicenter | No | Modified KDIGO stage 2 classification (at least 2-fold increase of sCR from baseline serum creatinine), mortality | Multibiomarker-based model to predict SAKI at day 3 had an AUC of 0.95 (95% CI, 0.91 to 0.99) in derivation and 0.83 (95% CI, 0.72 to 0.95) in test cohort. AUC of SAKI risk model to predict mortality was 0.90 (95% CI, 0.85 to 0.96). | |
| Bihorac et al. (38), 2014 | uTIMP-2*IGFBP7 | 408 adults | Prospective, multicenter | No | KDIGO Stage 2 and 3 criteria within 12 h after the enrollment | AUCs for predicting AKI for uTIMP-2*IGFBP7, 0.82; sCR, 0.63. Clinical model had an AUC of 0.70; clinical model + uTIMP-2*IGFBP7 improved AUC to 0.86. Patients with uTIMP-2*IGFBP7 values between 0.3 and 2.0 had 5-fold risk of AKI whereas it was 17-fold for value above 2.0. | |
| Frank AJ et al. (100), 2012 | GWAS | 1264 adults (887 with genotype data) | Retrospective, single-center | No | 0.3 mg/dl or ≥50% increase in sCR from baseline in the first 72 h after ICU admission | 2 SNPs in the B-cell CLL/Lymphoma 2 (BCL2): rs8094315 (OR 0.68 per additional copy of minor G allele; P<0.01) and rs12457893 gene (OR 0.68 per additional copy of minor C allele; P<0.01) and a SNP in the serpin peptidase inhibitor, clade A (α-1 antiproteinase, antitrypsin), member 4 (SERPINA4) gene: rs2093266 (OR 0.53 per additional copy of minor A allele; P<0.01) were associated with a decreased risk of developing AKI. | |
| Kashani et al. (23), 2013 | uTIMP-2*IGFBP7, uKIM-1, IL-18, uL-FABP, uNGAL, pNGAL, pCys-C, α-/π-GST | 522 adults (discovery) | Prospective, multicenter | No | Three KDIGO stage 2 and 3 criteria within 12 h of sample collection | AUCs for predicting AKI: uTIMP-2*IGFBP7, 0.80; IGFBP7, 0.76; uTIMP-2, 0.79; sCR, 0.75. Clinical-model AUC for AKI stage 2/3 was 0.81. Clinical model + uTIMP-2*IGFBP7 improved AUC to 0.87, IDI to 0.08, and NRI to 68%. | |
| 728 adults (validation) | |||||||
| Nejat et al. (35), 2010 | pCys-C, sCR | 444 adults | Prospective, multicenter | Yes (0 h, 12 h, 24 h, and daily for the next 6 d) | AKIN criteria (50% or 0.3 mg/dl increase in sCR), sustained AKI, dialysis, and mortality within 30 d | AUCs for predicting AKI: pCys-C, 0.78; sCR ,0.87. pCys-C predicted sustained AKI (AUC, 0.80) and need of dialysis or death (AUC, 0.68). | |
| Siew et al. (37), 2010 | uIL-18 | 451 adults | Prospective, single-center | No | AKIN criteria (50% or 0.3 mg/dl increase in sCR), AKI severity, dialysis within 28 d, mortality | AUCs for predicting AKI (uIL-18: at 24 h 0.62, 48 h 0.60, AKIN stage 1 0.59, AKIN stage 2/3 0.62). uIL-18 independently predicts need of dialysis or death (OR 1.86). Addition of uIL-18 did not improve ability of uNGAL to predict AKI as assessed by change in AUC curve. | |
| Cruz et al. (34), 2010 | pNGAL | 301 adults | Prospective, single-center | Yes (daily for 4 d) | RIFLE criteria, need of dialysis during ICU stay | AUCs for predicting AKI within 48 h (pNGAL 0.78, sensitivity 73%, specificity 81%, cut-off 150 ng/ml). pNGAL was good predictor for RRT (AUC 0.82); whereas moderate predictor for ICU mortality (AUC 0.67). | |
| Siew et al. (32), 2009 | uNGAL | 451 adults | Prospective, single-center | No | AKIN criteria (50% or 0.3 mg/dl increase in sCR), dialysis within 28 d, mortality | AUCs for predicting AKI: clinical model, 0.81; uNGAL at 24 h, 0.71; 48 h, 0.64; clinical model + uNGAL, 0.82. uNGAL independently predicts severe AKI during hospitalization (HR, 2.6). | |
| Cardiac surgery | Elmariah S et al. (99), 2016 | Mass spectrometry (metabolite profiling) | 44 adults | Prospective, single-center | No | Increase in plasma creatinine to 150%–199% of baseline or increase of ≥0.3 mg/dl within 7 d of TAVR | Baseline levels of 5‐adenosylhomocysteine predicted AKI after adjustment for eGFR (OR per 1 SD increase, 5.97; 95% CI, 1.62 to 22.0; P<0.001). |
| Zacharias et al. (104), 2015 | NMR spectra | 85 adults | Prospective single-center | No | AKIN staging 1,2, and 3 | A random forests classifier prognosticated AKI across all stages with an average accuracy of 80±0.9% and an AUC of 0.87. | |
| Torregrosa et al. (108), 2015 | uNGAL, uKIM-1, uL-FABP | 193 adults | Prospective, single-center | No | RIFLE criteria | In coronary angiography group, AUCs for predicting AKI: uNGAL, 0.96; uKIM-1, 0.71, whereas L-FABP had poor prediction. In CABG group, AUCs for predicting AKI: uNGAL, 0.92; uKIM-1, 0.72; and uL-FABP, 0.74. | |
| Zhang et al. (109), 2015 | IL-6, IL-10 | 960 adults | Prospective, multicenter | Yes (preoperative and daily up to day 5) | AKIN criteria (50% or 0.3 mg/dl increase in sCR from baseline), severe AKI, all-cause mortality | Elevated first postoperative IL-16 and IL-10 were significantly associated with AKI (OR, 2.13 and 1.57, respectively; 3rd tertile versus 1st tertile). Elevated IL-10 was associated with lower risk of mortality (adjusted HR, 0.72). | |
| Meersch et al. (110), 2014 | uTIMP-2*IGFBP7, uNGAL, uKIM-1 | 51 children | Prospective, single-center | Yes (preoperative, 4 h, and 24 h after initiation of CPB) | RIFLE criteria within 72 h after surgery | AUCs for predicting AKI: uTIMP-2*IGFBP7, 0.85; uNGAL, 0.87; and uKIM-1, 0.64. Adding uTIMP-2*IGFBP7 to clinical model improved AKI risk prediction significantly (P<0.001). | |
| Pilarczyk et al. (111), 2015 | uTIMP-2*IGFBP7 | 60 adults | Prospective, single-center | Yes (4 h after surgery and then every 12 h up to 4 d) | KDIGO stage 2 or 3 within 48 h after surgery | AUC for predicting AKI stage 2/3: uTIMP-2*IGFBP7, 0.86, whereas sCR had poor prediction (AUC, 0.36). | |
| Ho et al. (112), 2015 | NGAL, KIM-1, NAG, Cys-C, IL-18, L-FABP, Hepcidin-25, uTIMP-2, uIGFBP7, u-α-GST, u-π-GST | 28 studies | Meta-analysis | — | RIFLE (eight studies), AKIN (16 studies), or KDIGO criteria three studies); 1 study used combined AKIN/RIFLE | Composite AUCs for AKI: NGAL, KIM-1, and L-FABP, 0.72; and Cys-C, IL-18, u-α-GST, u-π-GST, <0.70). | |
| Sabbisetti et al. (113), 2014 | Blood KIM-1, uKIM-1 | 92 adults | Prospective, single-center | No | KDIGO criteria within 48 h | AUCs for predicting AKI: blood KIM-1, 0.96; normalized uKIM-1, 0.98; uKIM-1, 0.91. | |
| Koyner et al. (114), 2013 | uCys-C | 1203 adults, 299 children | Prospective, multicenter | Yes (preoperative, 0–6 h, and 6–12 h postoperative) | AKIN criteria (50% or 0.3 mg/dl increase in sCR), severe AKI (doubling of sCR or need of dialysis) | uCys-C was not associated with the development of AKI after cardiac surgery in both adults and children. Addition of uCys-C to clinical model did not significantly increase AUC (adults: mild AKI: clinical model, 0.67; uCys-C, 0.68; clinical model + uCys-C, 0.68; severe AKI clinical model, 0.72; uCys-C, 0.71; clinical model + uCys-C, 0.72). | |
| Parikh et al. (115), 2013 | uKIM-1, uL-FABP, uNGAL, uIL-18 | 1219 adults, 311 children | Prospective, multicenter | Yes (preoperative and every 6 h for the first 24 h after surgery) | AKIN stage 2/RIFLE-I or dialysis, progression to higher stage, mortality | Clinical model for AKI had an AUC of 0.69 in adults and 0.78 in children. Addition of uKIM-1 to clinical model marginally improved discrimination and classification in adults (AUC, 0.73; NRI, 19%). uKIM-1 and uL-FABP were not significantly associated with AKI in adults or children after adjusting for other kidney injury biomarkers (uNGAL and uIL-18). | |
| Parikh et al. (29), 2011 | uNGAL, pNGAL, uIL-18 | 1219 adults | Prospective, multicenter | Yes (preoperative and every 6 h for the first 24 h after surgery) | AKIN stage 2/RIFLE-I or dialysis | AUCs for predicting AKI: clinical model, 0.69; uNGAL, 0.67; pNGAL, 0.70; and uIL-18, 0.74. Adding uIL-18 and pNGAL separately to clinical model improved AUCs (0.76 and 0.75, respectively), NRI, and IDI. | |
| Devarajan P et al. (98), 2010 | SELDI-TOF MS (proteomics) | 30 children (discovery), 365 children (validation) | Prospective, single-center | ≥50% increase in sCR from baseline within 72 h of CPB | AUCs for urine α1-microglobulin, α1-acid glycoprotein, and albumin measured 6 h post-CPB were 0.84 (95% CI, 0.79 to 0.89), 0.87 (95% CI, 0.83 to 0.91), and 0.76 (95% CI, 0.71 to 0.81), respectively. | ||
| Liangos et al. (116), 2009 | uKIM-1, uNAG, uNGAL, uCys-C, uIL-18, urinary α-1 microglobulin | 103 adults | Prospective, multicenter | Yes (preoperative, 2 h, 24 h, and 48 h after discontinuation of CPB) | ≥50% increase in sCR in the first 72 h after termination of CPB | AUCs for predicting AKI: clinical model, 0.83; uKIM-1, 0.78; uNAG, 0.62; uIL-18, 0.66. Adding uKIM-1 to clinical model improved AUC to 0.88. | |
| Bennett et al. (27), 2008 | uNGAL | 196 children | Prospective, single-center | Yes (preoperative, 2 h, 4 h, 6 h, 8 h, 12 h, up to 72 h) | 50% increase in sCR, dialysis, mortality | AUCs for predicting AKI: uNGAL, 0.93 sensitivity, 82%, specificity, 90%, cut-off 100 ng/ml. Need of dialysis: AUC, 0.86; and mortality: AUC, 0.91. | |
| Stafford-Smith et al. (103), 2005 | GWAS | 1671 adults | Prospective, single-center | No | Delta sCR | Two alleles (IL-6 −572C and angiotensinogen 842C) showed a strong association with renal injury in white patients (P<0.001). Adding genetic to clinical factors provided 2–4-fold improvement in prediction of renal injury post–cardiac surgery. | |
| Cardiac catheterization | Hirsch et al. (117), 2007 | uNGAL, pNGAL | 91 children | Prospective, single-center | Yes (precontrast, 2 h, and 6 h) | 50% increase in sCR above baseline | AUCs for predicting CIN at 2 h: uNGAL, 0.92; and pNGAL, 0.91; PPV 100% and 80%; at 6 h: uNGAL, 0.97; and pNGAL, 0.95; PPV 90%; and 100%; for cut-off 100 ng/ml. |
| Hospitalized | Vaidya et al. (18), 2008 | uKIM-1, uIL-18, uNGAL, uNAG, uVEGF, uHGF, uCys-C, uCXCL10 | 204 adults | Cross-sectional | No | a ≥50% increase in sCR from admission value or known baseline, dialysis, mortality | AUCs for predicting AKI: uKIM-1, 0.93; uNAG, 0.83; uNGAL, 0.89; uIL-18, 0.83; uCys-C, 0.85). |
| Ferguson et al. (25), 2010 | uL-FABP | 154 adults | Cross-sectional | No | AKI was defined as a ≥50% increase in sCR from admission value or known baseline, dialysis, mortality | AUC for predicting AKI: uL-FABP, 0.93. uL-FABP was also significant predictor of RRT (P=0.02) and composite end point of death/RRT (P=0.03). Higher levels of uL-FABP were seen in ATN and sepsis AKI, whereas lower levels in contrast nephropathy. | |
| Cirrhosis | Treeprasertsuk et al. (41), 2015 | uNGAL | 121 adults | Prospective, single-center | No | AKIN criteria (50% or 0.3 mg/dl increase in sCR from baseline), 30-d mortality | AUCs for predicting AKI: uNGAL, 0.83; sensitivity, 77.1%; specificity 73.3%; cut-off, 56 ng/ml; baseline sCR, 0.58. In multiadjusted model, uNGAL could not predict 30-d mortality. |
| ICU, NICU, cardiac surgery, cardiac catheterization, hospitalized,ER | Shao et al. (118), 2014 | uKIM-1 | 2979 patients | Meta-analysis (five prospective, two cross-sectional, and four case-control studies) | —- | AKIN/RIFLE/KDIGO | Pooled analysis of studies showed an AUC of 0.86 for prediction of AKI; sensitivity, 74%; and specificity, 86%. |
uTIMP-2, urinary tissue inhibitor of metalloproteinases-2; IGFBP7, IGF-binding protein 7; uNGAL, urinary neutrophil gelatinase-associated lipocalin; uCys-C, urinary cystatin-C; KDIGO, Kidney Disease Improving Global Outcomes; uKIM-1, urinary kidney injury molecule-1; RIFLE, risk, injury, failure, loss of kidney function, and ESRD; ICU, intensive care unit; AUC, area under curve; ELA2,elastase 2; FGF13, fibroblast growth factor 13; MMP-8, matrix metalloproteinases 8; OLFM4, olfactomedin 4; PRTN3, proteinase; sCR, serum creatinine; 95% CI, 95% confidence interval; SAKI, septic acute kidney injury; GWAS, genome-wide association studies; SNP, single-nucleotide polymorphism; OR, odds ratio; uL-FABP, urinary liver-type fatty acid–binding protein; pNGAL, plasma neutrophil gelatinase-associated lipocalin; pCys-C, plasma cystatin-C; α-GST, α-glutathione s-transferase; π-GST, π-glutathione s-transferase; IDI, integrated discrimination improvement; NRI, net-reclassification index; uIL-18, urinary IL-18; AKIN, Acute Kidney Injury Network; HR, hazard ratio; TAVR, transcatheter aortic valve replacement; NMR, nuclear magnetic resonance spectroscopy; CABG, coronary artery bypass graft; NGAL, neutrophil gelatinase-associated lipocalin; NAG, n-acetyl-β-d-glucosaminidase; L-FABP, liver-type fatty acid–binding protein; uIGFBP7, urinary IGF-binding protein 7; u-α-GST, urinary α-glutathione s-transferase; u-π-GST, urinary π-glutathione s-transferase; KIM-1, kidney injury molecule-1; SELDI-TOF MS. surface-enhanced laser desorption/ionization time-of-flight mass spectrometry; CPB, cardio-pulmonary bypass; CIN, contrast nephropathy; PPV, positive predictive value; uVEGF-A, urinary vascular endothelial growth factor-A; uHGF, urinary hepatocyte growth factor; uCXCL10, urinary chemokine (c-x-c motif) ligand 10; ATN, acute tubular necrosis; NICU, neonatal intensive care unit; ER, emergency room.
Appendix Table 3.
Biomarker AKI Prognostic Studies
| Clinical Settings | Authors and Year of Publication | Biomarkers | Sample Size | Study Design | Patient Type at Time of Biomarker Measurement | Serial Biomarkers | End Point | Summary of Findings |
| ICU | Parr et al. (62), 2015 | uL-FABP, uIL-18, uKIM-1, uNGAL | 152 adults | Prospective, single-center | Stage 1 AKI | No | Composite outcome was comprised of persistent doubling of sCR (≥2 d), dialysis, and mortality | AUCs for predicting composite outcome (uL-FABP 0.79, uIL-18 0.64, uKIM-1 0.62, uNGAL 0.65, and combination of biomarkers 0. 81). Clinical-model AUCs for composite outcome was 0.74; adding uL-FABP to clinical model improved AUC (0.82), NRI (31%) and IDI (0.09). |
| Pike et al. (73), 2015 | IL-6, IL-8, IL-10, IL-18, MMIF, TNFR-I, TNFR-II, DR-5 | 817 adults | Prospective, nested observational cohort, multicenter | AKI with RRT | No | Renal recovery was defined as being alive and independent from RRT by day 60 after hospital discharge; 60-d mortality | AUCs for renal recovery (IL-6 0.61, IL-8 0.63, IL-10 0.57, IL-18 0.58, MIF 0.57). Clinical-model AUCs for renal recovery (0.73) and mortality (0.74); Adding IL-8 to clinical model improved the prediction of renal recovery and mortality (AUCs 0.76 and 0.78, respectively). IL-8 improved IDI and NRI for renal recovery and mortality. | |
| Koyner et al. (83), 2015 | uTIMP-2*IGFBP7 | 692 adults | Prospective, multicenter | No AKI/stage 1 AKI | No | 9-mo composite endpoint of all-cause mortality and/or RRT | UTIMP-2*IGFBP7>2.0 and sCR showed (adjusted HRs of 2.16 and 1.40, respectively) for death or RRT within 9 mo; Clinical model + uTIMP-2*IGFBP7 did not improved AUC (0.70), but did improve NRI by 23% and IDI (0.01). | |
| Koyner et al. (119), 2015 | uNGAL, pNGAL, uKIM-1, uIL-18, uTIMP-2*IGFBP7, FST | 77 adults | Prospective, multicenter | Stage 1/stage 2 AKI | No | Progression to AKIN stage 3, need of RRT, mortality | AUCs for prediction of progression to AKI stage 3 (uNGAL 0.65, pNGAL 0.75, uKIM-1 0.63, uIL-18 0.65, uTIMP-2*IGFBP7 0.69, and FST 0.87), Combining FST with uTIMP-2*IGFBP7 resulted in nonsignificant improvement in AUC 0.90. FST (2 h urine output) had AUCs of 0.86 and 0.70 for RRT and mortality. | |
| Dewitte et al. (75), 2015 | uTIMP-2*IGFBP7, pNGAL | 57 adults | Prospective, single-center | Stage 1/stage 2/stage 3 | Yes (at inclusion and 24 h) | Recovery defined as return to sCR<1.5×baseline or 0.35 mg/dl above the baseline with reversal of oliguria within 48 h; major adverse kidney events was defined as death, RRT, or persistence of renal dysfunction (sCR≥200% above baseline) at hospital discharge | uTIMP-2*IGFBP7 and pNGAL AUCs for early renal recovery (0.70 and 0.78, respectively); uTIMP-2*IGFBP7 showed good ability to predict major adverse kidney events, with AUC-ROC values close to 0.8; where as pNGAL had AUCs 0.68–0.76. Clinical Model AUC for renal recovery was 0.87; adding uTIMP-2*IGFBP7+pNGAL to clinical model improved AUC (0.89), NRI, and IDI. | |
| Murugan et al. (74), 2014 | IL-1β, IL-6, IL-8, IL-10, IL-18, MIF, TNF, TNFR-I, TNFR-II, DR-5, GM-CSF | 817 adults | Prospective, nested observational cohort, multicenter | AKI with RRT | No | Renal recovery was defined as being alive and independent from RRT by day 60 after hospital discharge; 60-d mortality | Increased concentrations of plasma IL-8, IL-18, MIF, and TNFR-I were associated with slower renal recovery and increased mortality. | |
| Aregger et al. (120), 2014 | uIGFBP7, pNGAL | 64 adults (includes 12 adults as control) | Prospective, multicenter | Stage 1/stage 2/stage 3 | No | Predicting early recovery (defined as not classifying for any RIFLE class during 7-d follow-up), RRT, mortality | AUCs of uIFGBP-7 for early recovery 0.74, need of RRT 0.65, 30-d mortality 0.65 and in-hospital mortality 0.68. Addition of uIGFBP-7 to clinical model improved prediction of renal recovery from 76.6%–82.1% concordant values (IDI: 0.081±0.04). Clinical Model + uIGFBP-7+pNGAL did not improved AUCs. | |
| Yamashita et al. (121), 2014 | uTIMP-2, uNAG, pNGAL, pIL-6 | 98 adults | Prospective, single-center | No AKI/stage 1/stage 2 | No | AKI was defined on the basis of KDIGO criteria; progression of AKI stage (non-AKI to AKI any stage, stage 1–2, stage 2–3) | AUCs for prediction of severe AKI: uTIMP-2, 0.80; pNGAL, 0.87; pIL-6, 0.70; uNAG, 0.83; and progression of AKI: uTIMP-2, 0.73; pNGAL, 0.76; pIL-6, 0.74; uNAG, 0.77. uTIMP-2 showed the highest AUCs for 7-d (0.83) and in-hospital mortality (0.74), whereas sCR had AUCs of 0.67 and 0.61, respectively. AUC of the clinical model for severe AKI was 0.87; adding uTIMP-2 to the clinical model improved AUC (0.89), NRI (41%), and IDI (0.04). | |
| Kashani et al. (23), 2013 | uTIMP-2*IGFBP7, uKIM-1, IL-18, uL-FABP, uNGAL, pNGAL, pCys-C, π-GST | 522 adults (discovery) | Prospective, multicenter | No AKI/stage 1 AKI | No | MAKE30 includes death, dialysis and persistent renal dysfunction (sCR ≥ 200% above baseline at hospital discharge) | MAKE30 elevated sharply for uTIMP-2*IGFBP7 above 0.30 and doubled for values above 2.0. | |
| 728 adults (validation) | ||||||||
| Zhang et al. (122), 2012 | sCys-C | 232 adults | Retrospective, single-center | AKI with RRT | No | Renal recovery was defined as dialysis independence and final sCR<50% above baseline value | sCys-C showed better performance in predicting renal function recovery than sCR (AUC, 0.87 versus 0.63; P<0.01). | |
| Srisawat et al. (72), 2011 | uNGAL, uCys-C, uIL-18, uHGF, uNGAL/MMP-9, and urinary creatinine | 76 adults | Prospective, multicenter | AKI with RRT | Yes (days 1, 7, and 14) | Renal recovery was defined as alive and free of dialysis at 60 d from the start of RRT | AUC for renal recovery: uNGAL, 0.70; uCys-C, 0.61; uIL-18, 0.42; uHGF, 0.74; uNGAL/MMP-9, 0.53; urinary creatinine, 0.66. Clinical model had an AUC of 0.74; clinical model + relative change of uHGF + uNGAL + uCys-C + uNGAL/MMP-9+uIL-18 improved AUC (0.94) and NRI (63.3%). | |
| Lorenzen JM et al. (102), 2011 | mRNAs (transcriptome) | 77 adults, 30 age-matched controls | Prospective, single-center | AKI with RRT | No | Survival 4 wk after initiation of AKI | MIR-210 was identified as an independent prognostic factor for 28-d survival with AUC of 0.7 (95% CI, 0.53 to 0.78; P=0.03), PPV 0.41, and NPV 1.0. | |
| Kumpers et al. (123), 2010 | pNGAL | 109 adults | Prospective, single-center | AKI with RRT | Yes | Renal recovery was defined as no need for RRT at day 28 after the study enrollment, mortality | AUCs for predicting 14-d mortality: pNGAL, 0.74; cut-off, 360 ng/ml); no association was found between renal recovery and pNGAL. | |
| Cardiac surgery | Moledina et al. (124), 2015 | pNGAL | 1191 adults | Prospective, multicenter | No AKI | Yes (preoperative, 0–6 h, peak 1–3 d postsurgery) | All-cause mortality (3-y follow-up) | Elevated first preoperative and postoperative pNGAL levels were significantly associated with mortality (adjusted HR, 1.48 and 1.31, respectively; 3rd tertile versus first tertile). No association persists between postoperative NGAL and mortality after adjusting for perioperative sCR changes. |
| Arthur et al. (22), 2014 | uL-FABP, uIL-18, uKIM-1, uNGAL, 32 biomarkers | 95 adults | Prospective, multicenter | Stage 1 AKI | No | AKI progression was defined as worsening of renal dysfunction (AKIN stage 1 to a higher AKIN stage 2/3) within 10 d or mortality within 30 d; secondary outcome was AKIN stage 3 or death | AUCs for primary and secondary outcomes: clinical model, 0.63 and 0.68; uIL-18, 0.74 and 0.89; uL-FABP, 0.67 and 0.85; uKIM-1, 0.73 and 0.81; uNGAL, 0.72 and 0.83. Combination of uIL-18 and uKIM-1 improves prediction of AKIN-3 or death with an AUC of 0.93. | |
| Coca et al. (82), 2014 | uL-FABP, uIL-18, uKIM-1, uNGAL, albumin | 1199 adults | Prospective, multicenter | No AKI | Yes (preoperative, 0–6 h postoperative, daily for up to 5 d) | 3-y mortality | Among patients with AKI, adjusted HRs for 3-year mortality for individual urinary biomarkers were: uNGAL, 2.52; uIL-18, 3.16; uKIM-1, 2.01; uL-FABP, 2.35 and urine albumin 2.85 (highest tertile versus lowest tertile). Addition of uIL-18, uL-FABP, and uKIM-1 to clinical model did not change AUC (0.69–0.71), but improved NRI (44%, 44%, and 18%, respectively). | |
| Meersch et al. (125), 2014 | uTIMP-2*IGFBP7, pNGAL | 50 adults | Prospective, single-center | No AKI | Yes (preoperative, 4 h, 12 h, and 24 h after CPB) | Renal recovery was defined as sCR value at hospital discharge equal to or lower than the preoperative creatinine value | AUCs for prediction of renal recovery for uTIMP-2*IGFBP7 was 0.79. Combination of NGAL and uTIMP-2*IGFBP7 did not improve AUC. | |
| Alge et al. (21), 2013 | Urinary angiotensinogen | 97 adults | Prospective, single-center | Stage 1 AKI | No | Progression to a higher AKIN stage, AKIN stage 3, RRT, mortality | uAnCR AUCs for worsening of AKI (0.70), AKIN stage 3 (0.71), RRT (0.71), AKIN 3 or mortality (0.75) and RRT or mortality (0.71). Prognostic predictive power of uAnCR was improved when only patients with AKIN stage 1 were analyzed. Adding uAnCR to clinical model improved prediction of worsening of AKI (NRI, 45.7%). | |
| Kidney transplant | Hall et al. (126), 2010 | uNGAL, IL-18, KIM-1 | 91 adults | Prospective, single-center | Transplant | Yes (0 h, 6 h, 12 h, 18 h, 1st, and 2nd postoperative day) | Graft recovery was defined in three categories: DGF, SGF, and IGF; RRT within 1 wk of kidney transplant | AUCs for predicting dialysis: uNGAL 0.82, IL-18 0.82; whereas KIM-1 had poor prediction (AUC 0.50); Clinical model + uNGAL+ IL-18 did improve reclassification by 110% (P<0.001). |
| Reese et al. (127), 2015 | uNGAL, uKIM-1, uIL-18, uL-FABP, urinary microalbumin | 2441 adults | Prospective, multicenter | Transplant | No | DGF was defined as a requirement for RRT within 7 d after transplant, 6-mo eGFR | High donor uNGAL levels were significantly associated with recipient DGF with RR 1.21 (highest versus lowest tertiles). Addition of urinary biomarkers does not improve AUC, IDI, and NRI. At 6-mo, donor urinary biomarkers added minimal value in predicting recipient allograft function. | |
| Pianta et al. (128), 2015 | uTIMP-2*IGFBP7, VEGF-A, MMIF, MCP-1, TFF3, CXCL16, sCR | 56 adults | Prospective, single-center | Transplant | Yes (4 h, 8 h, and 12 h) | DGF was defined as requirement for RRT within 7 d | AUCs for predicting DGF: uTIMP-2*IGFBP7, 0.76; VEGF-A, 0.81; uTIMP-2, 0.73; and uIGFBP7, 0.71; whereas sCR showed poor prediction for DGF (AUC 0.56). Clinical model AUC for DGF was 0.70; adding TIMP-2 (0.81 and 0.11) and VEGF-A (0.85 and 0.19) separately to clinical model improved AUC and IDI. | |
| Hospitalized | Wang et al. (129), 2015 | uL-FABP | 114 adults | Prospective, single-center | Stage 3 AKI | No | Recovery was defined as alive and neither requiring RRT nor having persistent AKIN 3 with a minimum crcl of 20 mL/min at hospital discharge | AUCs for renal recovery: uL-FABP, 0.91; sensitivity, 85.5; and specificity, 86.4% for cut-off 102.1 ng/mg). uL-FABP modestly predicted hospital death (AUC, 0.83) and RRT (AUC, 0.71). u-FABP improved renal recovery classification compared with clinical model (NRI, 35%; P=0.02) |
| Westhoff et al. (130), 2015 | uTIMP-2*IGFBP7 | 46 children | Prospective, single-center | Stage 1/stage 2/stage 3 | No | Predicting mortality and need of RRT | AUCs for 30-d and 90-d mortality: uTIMP-2*IGFBP7, 0.79 and 0.84, respectively). AUC for RRT was 0.67. | |
| Singer et al. (63), 2011 | uNGAL | 145 adults | Prospective, single-center | Stage 1/stage 2/stage 3 | No | Progression to a higher AKIN stage, dialysis, mortality within 7 d of ICU admission; type of AKI | uNGAL had an AUC of 0.71 (95% CI, 0.62 to 0.8), as compared with sCR level, which had an AUC of 0.61 (95% CI, 0.51 to 0.71). | |
| Srisawat et al. (89), 2011 | pNGAL | 189 adults | Prospective, multicenter | Stage 3 | No | Recovery was defined as alive and not requiring RRT during hospitalization and nor having a persistent RIFLE-F classification at hospital discharge | pNGAL AUCs for renal recovery was 0.74. Clinical model + pNGAL did not improve AUC, but did improve NRI by 17%. | |
| Cirrhosis | Belcher et al. (24), 2014 | uL-FABP, uIL-18, uKIM-1, uNGAL | 188 adults | Prospective, multicenter | Stage 1/stage 2/stage 3 | Yes (daily for 3 d) | Progression to a higher AKIN stage, dialysis, mortality | AUCs for AKI progression and death: uL-FABP, 0.76; uIL-18, 0.71; uKIM-1, 0.66; uNGAL, 0.77. IL-18 independently improved NRI (51%). |
| Heart Failure | Verbrugge et al. (131), 2013 | uIL-18, uKIM-1, uNGAL | 83 adults | Prospective, single-center | No AKI | No | Persistent renal impairment was defined as persistently elevated sCR levels (≥0.3 mg/dl) after 6 mo compared with baseline; all-cause mortality | uIL-18 was a predictor of persistent renal impairment with an AUC of 0.67. uIL-18 was also associated with all-cause mortality (HR 1.48). |
uL-FABP, urinary liver-type fatty acid–binding protein; uIL-18, urinary IL-8; uKIM-1, urinary kidney injury molecule-1; uNGAL, urinary neutrophil gelatinase–associated lipocalin; sCR, serum creatinine; AUC, area under curve; NRI, net-reclassification index; IDI, integrated discrimination improvement; MMIF, macrophage migration inhibitory factor; TNFR-I, TNF receptor 1; TNFR-II, TNF receptor II; DR-5, death receptor-5; MIF, migration inhibitory factor; uTIMP-2, urinary tissue inhibitor of metalloproteinases-2; IGFBP7, IGF-binding protein 7; pNGAL, plasma neutrophil gelatinase–associated lipocalin; uIL-18, urinary IL-18; FST, furosemide stress test; AKIN, Acute Kidney Injury Network; AUC-ROC, area under the receiver operating characteristic curve; GM-CSF, granulocyte macrophage stimulating factor; RIFLE, risk, injury, failure, loss of kidney function, and ESRD; uIGFBP7, urinary IGF-binding protein 7; uNAG, urinary n-acetyl-β-d-glucosaminidase; pIL-6, plasma IL-6; KDIGO, Kidney Disease Improving Global Outcomes; pCys-C, plasma cystatin-C; π-GST, π-glutathione s-transferase; MAKE, major adverse kidney events; sCys-C, serum cystatin-C; uHGF, urinary hepatocyte growth factor; MMP-9, matrix metalloproteinase-9; NPV, negative predictive value; CPB, cardio-pulmonary bypass; HR, hazard ratio; NGAL, neutrophil gelatinase–associated lipocalin; uAnCR, urinary-angiotensinogen-creatinine ratio; DGF, delayed graft function; SGF, slow graft function; IGF, immediate graft function; KIM-1, kidney injury molecule-1; RR, relative risk; VEGF-A, vascular endothelial growth factor-A; MCP-1, monocyte chemotactic protein 1; TFF3, trefoil factor 3; CXCL16, chemokine (c-x-c motif) ligand 16; TIMP-2, tissue inhibitor of metalloproteinases-2; crcl, creatinine clearance; ICU, intensive care unit.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related commentary, “Commentary on Biomarkers for Early Detection and Prognosis of AKI” on pages 174–175.
References
- 1.Singbartl K, Joannidis M: Short-term Effects of Acute Kidney Injury. Crit Care Clin 31: 751–762, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Hsu RK, McCulloch CE, Heung M, Saran R, Shahinian VB, Pavkov ME, Burrows NR, Powe NR, Hsu CY; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team: Exploring Potential Reasons for the Temporal Trend in Dialysis-Requiring AKI in the United States. Clin J Am Soc Nephrol 11: 14–20, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu CY, Liu KD: Cardiovascular events after AKI: a new dimension. J Am Soc Nephrol 25: 425–427, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu CY: Where is the epidemic in kidney disease? J Am Soc Nephrol 21: 1607–1611, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honoré PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA: Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 41: 1411–1423, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 81: 442–448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chawla LS, Kimmel PL: Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int 82: 516–524, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Nadkarni GN, Coca SG: Temporal Trends in AKI: Insights from Big Data. Clin J Am Soc Nephrol 11: 1–3, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahlström A, Tallgren M, Peltonen S, Pettilä V: Evolution and predictive power of serum cystatin C in acute renal failure. Clin Nephrol 62: 344–350, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV: Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 62: 237–244, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Harrison DJ, Kharbanda R, Cunningham DS, McLellan LI, Hayes JD: Distribution of glutathione S-transferase isoenzymes in human kidney: basis for possible markers of renal injury. J Clin Pathol 42: 624–628, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melnikov VY, Ecder T, Fantuzzi G, Siegmund B, Lucia MS, Dinarello CA, Schrier RW, Edelstein CL: Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest 107: 1145–1152, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P: Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D’Agati V, Devarajan P, Barasch J: Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest 115: 610–621, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostermann M, Philips BJ, Forni LG: Clinical review: Biomarkers of acute kidney injury: where are we now? Crit Care 2012Available at http://ccforum.biomedcentral.com/articles/10.1186/cc11380. Accessed December 4, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL: Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis 43: 405–414, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Sundberg AG, Appelkvist EL, Bäckman L, Dallner G: Urinary pi-class glutathione transferase as an indicator of tubular damage in the human kidney. Nephron 67: 308–316, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Vaidya VS, Waikar SS, Ferguson MA, Collings FB, Sunderland K, Gioules C, Bradwin G, Matsouaka R, Betensky RA, Curhan GC, Bonventre JV: Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci 1: 200–208, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wellwood JM, Ellis BG, Price RG, Hammond K, Thompson AE, Jones NF: Urinary N-acetyl- beta-D-glucosaminidase activities in patients with renal disease. BMJ 3: 408–411, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto T, Noiri E, Ono Y, Doi K, Negishi K, Kamijo A, Kimura K, Fujita T, Kinukawa T, Taniguchi H, Nakamura K, Goto M, Shinozaki N, Ohshima S, Sugaya T: Renal L-type fatty acid--binding protein in acute ischemic injury. J Am Soc Nephrol 18: 2894–2902, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Alge JL, Karakala N, Neely BA, Janech MG, Tumlin JA, Chawla LS, Shaw AD, Arthur JM; SAKInet Investigators: Urinary angiotensinogen and risk of severe AKI. Clin J Am Soc Nephrol 8: 184–193, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arthur JM, Hill EG, Alge JL, Lewis EC, Neely BA, Janech MG, Tumlin JA, Chawla LS, Shaw AD; SAKInet Investigators: Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney Int 85: 431–438, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM, Chawla LS, Davison DL, Feldkamp T, Forni LG, Gong MN, Gunnerson KJ, Haase M, Hackett J, Honore PM, Hoste EA, Joannes-Boyau O, Joannidis M, Kim P, Koyner JL, Laskowitz DT, Lissauer ME, Marx G, McCullough PA, Mullaney S, Ostermann M, Rimmelé T, Shapiro NI, Shaw AD, Shi J, Sprague AM, Vincent JL, Vinsonneau C, Wagner L, Walker MG, Wilkerson RG, Zacharowski K, Kellum JA: Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013. Available at http://ccforum.biomedcentral.com/articles/10.1186/cc12503. Accessed December 6, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belcher JM, Garcia-Tsao G, Sanyal AJ, Thiessen-Philbrook H, Peixoto AJ, Perazella MA, Ansari N, Lim J, Coca SG, Parikh CR; TRIBE-AKI Consortium: Urinary biomarkers and progression of AKI in patients with cirrhosis. Clin J Am Soc Nephrol 9: 1857–1867, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson MA, Vaidya VS, Waikar SS, Collings FB, Sunderland KE, Gioules CJ, Bonventre JV: Urinary liver-type fatty acid-binding protein predicts adverse outcomes in acute kidney injury. Kidney Int 77: 708–714, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nickolas TL, O’Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, Buchen C, Khan F, Mori K, Giglio J, Devarajan P, Barasch J: Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med 148: 810–819, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Syed H, Ali S, Barasch J, Devarajan P: Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol 3: 665–673, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P: Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Swaminathan M, Garg AX; TRIBE-AKI Consortium: Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 22: 1748–1757, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parikh CR, Devarajan P, Zappitelli M, Sint K, Thiessen-Philbrook H, Li S, Kim RW, Koyner JL, Coca SG, Edelstein CL, Shlipak MG, Garg AX, Krawczeski CD; TRIBE-AKI Consortium: Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol 22: 1737–1747, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Geus HR, Bakker J, Lesaffre EM, le Noble JL: Neutrophil gelatinase-associated lipocalin at ICU admission predicts for acute kidney injury in adult patients. Am J Respir Crit Care Med 183: 907–914, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Siew ED, Ware LB, Gebretsadik T, Shintani A, Moons KG, Wickersham N, Bossert F, Ikizler TA: Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol 20: 1823–1832, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siew ED, Ware LB, Bian A, Shintani A, Eden SK, Wickersham N, Cripps B, Ikizler TA: Distinct injury markers for the early detection and prognosis of incident acute kidney injury in critically ill adults with preserved kidney function. Kidney Int 84: 786–794, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cruz DN, de Cal M, Garzotto F, Perazella MA, Lentini P, Corradi V, Piccinni P, Ronco C: Plasma neutrophil gelatinase-associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med 36: 444–451, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nejat M, Pickering JW, Walker RJ, Westhuyzen J, Shaw GM, Frampton CM, Endre ZH: Urinary cystatin C is diagnostic of acute kidney injury and sepsis, and predicts mortality in the intensive care unit. Crit Care 2010. Available at http://ccforum.biomedcentral.com/articles/10.1186/cc9014. Accessed December 8, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL: Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol 16: 3046–3052, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Siew ED, Ikizler TA, Gebretsadik T, Shintani A, Wickersham N, Bossert F, Peterson JF, Parikh CR, May AK, Ware LB: Elevated urinary IL-18 levels at the time of ICU admission predict adverse clinical outcomes. Clin J Am Soc Nephrol 5: 1497–1505, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bihorac A, Chawla LS, Shaw AD, Al-Khafaji A, Davison DL, Demuth GE, Fitzgerald R, Gong MN, Graham DD, Gunnerson K, Heung M, Jortani S, Kleerup E, Koyner JL, Krell K, Letourneau J, Lissauer M, Miner J, Nguyen HB, Ortega LM, Self WH, Sellman R, Shi J, Straseski J, Szalados JE, Wilber ST, Walker MG, Wilson J, Wunderink R, Zimmerman J, Kellum JA: Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med 189: 932–939, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Aghel A, Shrestha K, Mullens W, Borowski A, Tang WH: Serum neutrophil gelatinase-associated lipocalin (NGAL) in predicting worsening renal function in acute decompensated heart failure. J Card Fail 16: 49–54, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirsch R, Dent C, Pfriem H, Allen J, Beekman RH 3rd, Ma Q, Dastrala S, Bennett M, Mitsnefes M, Devarajan P: NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol 22: 2089–2095, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Treeprasertsuk S, Wongkarnjana A, Jaruvongvanich V, Sallapant S, Tiranathanagul K, Komolmit P, Tangkijvanich P: Urine neutrophil gelatinase-associated lipocalin: a diagnostic and prognostic marker for acute kidney injury (AKI) in hospitalized cirrhotic patients with AKI-prone conditions. BMC Gastroenterol 2015. Available at http://bmcgastroenterol.biomedcentral.com/articles/10.1186/s12876-015-0372-5. Accessed December 8, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Dobrzycki S: Neutrophil-gelatinase-associated lipocalin and renal function after percutaneous coronary interventions. Am J Nephrol 26: 287–292, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Nejat M, Pickering JW, Devarajan P, Bonventre JV, Edelstein CL, Walker RJ, Endre ZH: Some biomarkers of acute kidney injury are increased in pre-renal acute injury. Kidney Int 81: 1254–1262, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waikar SS, Betensky RA, Emerson SC, Bonventre JV: Imperfect gold standards for kidney injury biomarker evaluation. J Am Soc Nephrol 23: 13–21, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, Perentes E, Cordier A, Vonderscher J, Maurer G, Goering PL, Sistare FD, Bonventre JV: Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol 28: 478–485, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mishra J, Ma Q, Kelly C, Mitsnefes M, Mori K, Barasch J, Devarajan P: Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol 21: 856–863, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Zhang PL, Rothblum LI, Han WK, Blasick TM, Potdar S, Bonventre JV: Kidney injury molecule-1 expression in transplant biopsies is a sensitive measure of cell injury. Kidney Int 73: 608–614, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, Jarman S, Efimov IR, Janks DL, Srivastava A, Bhayani SB, Drewry A, Swanson PE, Hotchkiss RS: Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med 187: 509–517, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murray PT, Mehta RL, Shaw A, Ronco C, Endre Z, Kellum JA, Chawla LS, Cruz D, Ince C, Okusa MD; ADQI 10 workgroup: Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int 85: 513–521, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, Krawczeski CD, Koyner JL, Murray P, Zappitelli M, Goldstein SL, Makris K, Ronco C, Martensson J, Martling CR, Venge P, Siew E, Ware LB, Ikizler TA, Mertens PR: The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol 57: 1752–1761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moledina DG, Parikh CR, Garg AX, Thiessen-Philbrook H, Koyner JL, Patel UD, Devarajan P, Shlipak MG, Coca SG; TRIBE-AKI Consortium: Association of Perioperative Plasma Neutrophil Gelatinase-Associated Lipocalin Levels with 3-Year Mortality after Cardiac Surgery: A Prospective Observational Cohort Study. PLoS One 10: e0129619, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Belcher JM, Sanyal AJ, Peixoto AJ, Perazella MA, Lim J, Thiessen-Philbrook H, Ansari N, Coca SG, Garcia-Tsao G, Parikh CR; TRIBE-AKI Consortium: Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology 60: 622–632, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang CH, Yang CH, Yang HY, Chen TH, Lin CY, Chang SW, Chen YT, Hung CC, Fang JT, Yang CW, Chen YC: Urinary Biomarkers Improve the Diagnosis of Intrinsic Acute Kidney Injury in Coronary Care Units. Medicine (Baltimore) 94: e1703, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nickolas TL, Schmidt-Ott KM, Canetta P, Forster C, Singer E, Sise M, Elger A, Maarouf O, Sola-Del Valle DA, O’Rourke M, Sherman E, Lee P, Geara A, Imus P, Guddati A, Polland A, Rahman W, Elitok S, Malik N, Giglio J, El-Sayegh S, Devarajan P, Hebbar S, Saggi SJ, Hahn B, Kettritz R, Luft FC, Barasch J: Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol 59: 246–255, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krawczeski CD, Goldstein SL, Woo JG, Wang Y, Piyaphanee N, Ma Q, Bennett M, Devarajan P: Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J Am Coll Cardiol 58: 2301–2309, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Endre ZH, Pickering JW, Walker RJ, Devarajan P, Edelstein CL, Bonventre JV, Frampton CM, Bennett MR, Ma Q, Sabbisetti VS, Vaidya VS, Walcher AM, Shaw GM, Henderson SJ, Nejat M, Schollum JB, George PM: Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int 79: 1119–1130, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carter JL, Parker CT, Stevens PE, Eaglestone G, Knight S, Farmer CK, Lamb EJ: Biological Variation of Plasma and Urinary Markers of Acute Kidney Injury in Patients with Chronic Kidney Disease. Clin Chem 62: 876–883, 2016 [DOI] [PubMed] [Google Scholar]
- 58.Harrill AH, Desmet KD, Wolf KK, Bridges AS, Eaddy JS, Kurtz CL, Hall JE, Paine MF, Tidwell RR, Watkins PB: A mouse diversity panel approach reveals the potential for clinical kidney injury due to DB289 not predicted by classical rodent models. Toxicol Sci 130: 416–426, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vasile VC, Saenger AK, Kroning JM, Jaffe AS: Biological and analytical variability of a novel high-sensitivity cardiac troponin T assay. Clin Chem 56: 1086–1090, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Cruz DN, Virzì GM, Brocca A, Ronco C, Giavarina D: A comparison of three commercial platforms for urinary NGAL in critically ill adults. Clin Chem Lab Med 54: 353–362, 2016 [DOI] [PubMed] [Google Scholar]
- 61.Koyner JL, Garg AX, Coca SG, Sint K, Thiessen-Philbrook H, Patel UD, Shlipak MG, Parikh CR; TRIBE-AKI Consortium: Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol 23: 905–914, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parr SK, Clark AJ, Bian A, Shintani AK, Wickersham NE, Ware LB, Ikizler TA, Siew ED: Urinary L-FABP predicts poor outcomes in critically ill patients with early acute kidney injury. Kidney Int 87: 640–648, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singer E, Elger A, Elitok S, Kettritz R, Nickolas TL, Barasch J, Luft FC, Schmidt-Ott KM: Urinary neutrophil gelatinase-associated lipocalin distinguishes pre-renal from intrinsic renal failure and predicts outcomes. Kidney Int 80: 405–414, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Basu RK, Zappitelli M, Brunner L, Wang Y, Wong HR, Chawla LS, Wheeler DS, Goldstein SL: Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int 85: 659–667, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siew ED, Furth SL: Acute kidney injury: a not-so-silent disease. Kidney Int 85: 494–495, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jaffe AS: Elevations in cardiac troponin measurements: false false-positives: the real truth. Cardiovasc Toxicol 1: 87–92, 2001 [DOI] [PubMed] [Google Scholar]
- 67.Dupont J, Fromonot J, Franceschi F, Deharo JC, Boucraut J, Quilici J, Bonnet JL, Monserrat C, Guieu R: A case of false positive troponin elevation: role of the biological laboratory. Int J Cardiol 162: e66–e67, 2013 [DOI] [PubMed] [Google Scholar]
- 68.Basu RK, Wang Y, Wong HR, Chawla LS, Wheeler DS, Goldstein SL: Incorporation of biomarkers with the renal angina index for prediction of severe AKI in critically ill children. Clin J Am Soc Nephrol 9: 654–662, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sawhney S, Mitchell M, Marks A, Fluck N, Black C: Long-term prognosis after acute kidney injury (AKI): what is the role of baseline kidney function and recovery? A systematic review. BMJ Open 5: e006497, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goldstein SL, Jaber BL, Faubel S, Chawla LS; Acute Kidney Injury Advisory Group of American Society of Nephrology: AKI transition of care: a potential opportunity to detect and prevent CKD. Clin J Am Soc Nephrol 8: 476–483, 2013 [DOI] [PubMed] [Google Scholar]
- 71.Pannu N, James M, Hemmelgarn B, Klarenbach S; Alberta Kidney Disease Network: Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol 8: 194–202, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Srisawat N, Wen X, Lee M, Kong L, Elder M, Carter M, Unruh M, Finkel K, Vijayan A, Ramkumar M, Paganini E, Singbartl K, Palevsky PM, Kellum JA: Urinary biomarkers and renal recovery in critically ill patients with renal support. Clin J Am Soc Nephrol 6: 1815–1823, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pike F, Murugan R, Keener C, Palevsky PM, Vijayan A, Unruh M, Finkel K, Wen X, Kellum JA; Biological Markers for Recovery of Kidney (BioMaRK) Study Investigators: Biomarker Enhanced Risk Prediction for Adverse Outcomes in Critically Ill Patients Receiving RRT. Clin J Am Soc Nephrol 10: 1332–1339, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murugan R, Wen X, Shah N, Lee M, Kong L, Pike F, Keener C, Unruh M, Finkel K, Vijayan A, Palevsky PM, Paganini E, Carter M, Elder M, Kellum JA; Biological Markers for Recovery of Kidney (BioMaRK) Study Investigators: Plasma inflammatory and apoptosis markers are associated with dialysis dependence and death among critically ill patients receiving renal replacement therapy. Nephrol Dial Transplant 29: 1854–1864, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dewitte A, Joannès-Boyau O, Sidobre C, Fleureau C, Bats ML, Derache P, Leuillet S, Ripoche J, Combe C, Ouattara A: Kinetic eGFR and Novel AKI Biomarkers to Predict Renal Recovery. Clin J Am Soc Nephrol 10: 1900–1910, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Basile DP, Bonventre JV, Mehta R, Nangaku M, Unwin R, Rosner MH, Kellum JA, Ronco C; ADQI XIII Work Group: Progression after AKI: Understanding Maladaptive Repair Processes to Predict and Identify Therapeutic Treatments. J Am Soc Nephrol 27: 687–697, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV: Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16: 535–543, 2010 [DOI] [PMC free article] [PubMed]
- 78.Fleming TR, Powers JH: Biomarkers and surrogate endpoints in clinical trials. Stat Med 31: 2973–2984, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hirshberg B, Katz A: Cardiovascular outcome studies with novel antidiabetes agents: scientific and operational considerations. Diabetes Care 36[Suppl 2]: S253–S258, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Billings FT 4th, Shaw AD: Clinical trial endpoints in acute kidney injury. Nephron Clin Pract 127: 89–93, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Palevsky PM, Molitoris BA, Okusa MD, Levin A, Waikar SS, Wald R, Chertow GM, Murray PT, Parikh CR, Shaw AD, Go AS, Faubel SG, Kellum JA, Chinchilli VM, Liu KD, Cheung AK, Weisbord SD, Chawla LS, Kaufman JS, Devarajan P, Toto RM, Hsu CY, Greene T, Mehta RL, Stokes JB, Thompson AM, Thompson BT, Westenfelder CS, Tumlin JA, Warnock DG, Shah SV, Xie Y, Duggan EG, Kimmel PL, Star RA: Design of clinical trials in acute kidney injury: report from an NIDDK workshop on trial methodology. Clin J Am Soc Nephrol 7: 844–850, 2012 [DOI] [PubMed] [Google Scholar]
- 82.Coca SG, Garg AX, Thiessen-Philbrook H, Koyner JL, Patel UD, Krumholz HM, Shlipak MG, Parikh CR; TRIBE-AKI Consortium: Urinary biomarkers of AKI and mortality 3 years after cardiac surgery. J Am Soc Nephrol 25: 1063–1071, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koyner JL, Shaw AD, Chawla LS, Hoste EA, Bihorac A, Kashani K, Haase M, Shi J, Kellum JA; Sapphire Investigators: Tissue Inhibitor Metalloproteinase-2 (TIMP-2)⋅IGF-Binding Protein-7 (IGFBP7) Levels Are Associated with Adverse Long-Term Outcomes in Patients with AKI. J Am Soc Nephrol 26: 1747–1754, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hall IE, Doshi MD, Reese PP, Marcus RJ, Thiessen-Philbrook H, Parikh CR: Association between peritransplant kidney injury biomarkers and 1-year allograft outcomes. Clin J Am Soc Nephrol 7: 1224–1233, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.James MT, Hobson CE, Darmon M, Mohan S, Hudson D, Goldstein SL, Ronco C, Kellum JA, Bagshaw SM; Acute Dialysis Quality Initiative (ADQI) Consensus Group: Applications for detection of acute kidney injury using electronic medical records and clinical information systems: workgroup statements from the 15(th) ADQI Consensus Conference. Can J Kidney Health Dis 2016Available at http://cjkhd.biomedcentral.com/articles/10.1186/s40697-016-0100-2. Accessed January 28, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilson FP, Shashaty M, Testani J, Aqeel I, Borovskiy Y, Ellenberg SS, Feldman HI, Fernandez H, Gitelman Y, Lin J, Negoianu D, Parikh CR, Reese PP, Urbani R, Fuchs B: Automated, electronic alerts for acute kidney injury: a single-blind, parallel-group, randomised controlled trial. Lancet 385: 1966–1974, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zarbock A, Kellum JA, Schmidt C, Van Aken H, Wempe C, Pavenstädt H, Boanta A, Gerß J, Meersch M: Effect of Early vs Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients With Acute Kidney Injury: The ELAIN Randomized Clinical Trial. JAMA 315: 2190–2199, 2016 [DOI] [PubMed] [Google Scholar]
- 88.Wald R, Adhikari NK, Smith OM, Weir MA, Pope K, Cohen A, Thorpe K, McIntyre L, Lamontagne F, Soth M, Herridge M, Lapinsky S, Clark E, Garg AX, Hiremath S, Klein D, Mazer CD, Richardson RM, Wilcox ME, Friedrich JO, Burns KE, Bagshaw SM; Canadian Critical Care Trials Group: Comparison of standard and accelerated initiation of renal replacement therapy in acute kidney injury. Kidney Int 88: 897–904, 2015 [DOI] [PubMed] [Google Scholar]
- 89.Srisawat N, Murugan R, Lee M, Kong L, Carter M, Angus DC, Kellum JA; Genetic and Inflammatory Markers of Sepsis (GenIMS) Study Investigators: Plasma neutrophil gelatinase-associated lipocalin predicts recovery from acute kidney injury following community-acquired pneumonia. Kidney Int 80: 545–552, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Herrera J, Rodríguez-Iturbe B: Stimulation of tubular secretion of creatinine in health and in conditions associated with reduced nephron mass. Evidence for a tubular functional reserve. Nephrol Dial Transplant 13: 623–629, 1998 [DOI] [PubMed] [Google Scholar]
- 91.Rodríguez-Iturbe B, Herrera J, Marín C, Mañalich R: Tubular stress test detects subclinical reduction in renal functioning mass. Kidney Int 59: 1094–1102, 2001 [DOI] [PubMed] [Google Scholar]
- 92.Bonventre JV, Boulware LE, Dember LM, Freedman BI, Furth SL, Holzman LB, Ketchum CJ, Little MH, Mehrotra R, Moe SM, Sands JM, Sedor JR, Somlo S, Star RA, Rys-Sikora KE; Kidney Research National Dialogue (KRND): The kidney research national dialogue: gearing up to move forward. Clin J Am Soc Nephrol 9: 1806–1811, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fogo A, Breyer JA, Smith MC, Cleveland WH, Agodoa L, Kirk KA, Glassock R; AASK Pilot Study Investigators: Accuracy of the diagnosis of hypertensive nephrosclerosis in African Americans: a report from the African American Study of Kidney Disease (AASK) Trial. Kidney Int 51: 244–252, 1997 [DOI] [PubMed] [Google Scholar]
- 94.Schwartz MM, Lewis EJ, Leonard-Martin T, Lewis JB, Batlle D; The Collaborative Study Group: Renal pathology patterns in type II diabetes mellitus: relationship with retinopathy. Nephrol Dial Transplant 13: 2547–2552, 1998 [DOI] [PubMed] [Google Scholar]
- 95.Koyner JL, Garg AX, Thiessen-Philbrook H, Coca SG, Cantley LG, Peixoto A, Passik CS, Hong K, Parikh CR; TRIBE-AKI Consortium: Adjudication of etiology of acute kidney injury: experience from the TRIBE-AKI multi-center study. BMC Nephrol 2014. Available at http://bmcnephrol.biomedcentral.com/articles/10.1186/1471-2369-15-105. Accessed December 14, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Berthier CC, Zhang H, Schin M, Henger A, Nelson RG, Yee B, Boucherot A, Neusser MA, Cohen CD, Carter-Su C, Argetsinger LS, Rastaldi MP, Brosius FC, Kretzler M: Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes 58: 469–477, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Devarajan P: Genomic and Proteomic Characterization of Acute Kidney Injury. Nephron 131: 85–91, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Devarajan P, Krawczeski CD, Nguyen MT, Kathman T, Wang Z, Parikh CR: Proteomic identification of early biomarkers of acute kidney injury after cardiac surgery in children. Am J Kidney Dis 56: 632–642, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Elmariah S, Farrell LA, Daher M, Shi X, Keyes MJ, Cain CH, Pomerantsev E, Vlahakes GJ, Inglessis I, Passeri JJ, Palacios IF, Fox CS, Rhee EP, Gerszten RE: Metabolite Profiles Predict Acute Kidney Injury and Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement. J Am Heart Assoc 5: e002712, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frank AJ, Sheu CC, Zhao Y, Chen F, Su L, Gong MN, Bajwa E, Thompson BT, Christiani DC: BCL2 genetic variants are associated with acute kidney injury in septic shock*. Crit Care Med 40: 2116–2123, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ju W, Nair V, Smith S, Zhu L, Shedden K, Song PX, Mariani LH, Eichinger FH, Berthier CC, Randolph A, Lai JY, Zhou Y, Hawkins JJ, Bitzer M, Sampson MG, Thier M, Solier C, Duran-Pacheco GC, Duchateau-Nguyen G, Essioux L, Schott B, Formentini I, Magnone MC, Bobadilla M, Cohen CD, Bagnasco SM, Barisoni L, Lv J, Zhang H, Wang HY, Brosius FC, Gadegbeku CA, Kretzler M; ERCB, C-PROBE, NEPTUNE, and PKU-IgAN Consortium: Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med 7: 316ra193, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lorenzen JM, Kielstein JT, Hafer C, Gupta SK, Kümpers P, Faulhaber-Walter R, Haller H, Fliser D, Thum T: Circulating miR-210 predicts survival in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol 6: 1540–1546, 2011 [DOI] [PubMed] [Google Scholar]
- 103.Stafford-Smith M, Podgoreanu M, Swaminathan M, Phillips-Bute B, Mathew JP, Hauser EH, Winn MP, Milano C, Nielsen DM, Smith M, Morris R, Newman MF, Schwinn DA; Perioperative Genetics and Safety Outcomes Study (PEGASUS) Investigative Team: Association of genetic polymorphisms with risk of renal injury after coronary bypass graft surgery. Am J Kidney Dis 45: 519–530, 2005 [DOI] [PubMed] [Google Scholar]
- 104.Zacharias HU, Hochrein J, Vogl FC, Schley G, Mayer F, Jeleazcov C, Eckardt KU, Willam C, Oefner PJ, Gronwald W: Identification of Plasma Metabolites Prognostic of Acute Kidney Injury after Cardiac Surgery with Cardiopulmonary Bypass. J Proteome Res 14: 2897–2905, 2015 [DOI] [PubMed] [Google Scholar]
- 105.Bell M, Larsson A, Venge P, Bellomo R, Martensson J: Assessment of cell-cycle arrest biomarkers to predict early and delayed acute kidney injury. Dis Markers, 2015: 158658, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zwiers AJ, de Wildt SN, van Rosmalen J, de Rijke YB, Buijs EA, Tibboel D, Cransberg K: Urinary neutrophil gelatinase-associated lipocalin identifies critically ill young children with acute kidney injury following intensive care admission: a prospective cohort study. Crit Care, 19: 181, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wong HR, Cvijanovich NZ, Anas N, Allen GL, Thomas NJ, Bigham MT, Weiss SL, Fitzgerald J, Checchia PA, Meyer K, Shanley TP, Quasney M, Hall M, Gedeit R, Freishtat RJ, Nowak J, Raj SS, Gertz S, Dawson E, Howard K, Harmon K, Lahni P, Frank E, Hart KW, Lindsell CJ: A Multibiomarker-Based Model for Estimating the Risk of Septic Acute Kidney Injury. Crit Care Med, 43: 1646–1653, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Torregrosa I, Montoliu C, Urios A, Andres-Costa MJ, Gimenez-Garzo C, Juan I, Puchades MJ, Blasco ML, Carratala A, Sanjuan R, Miguel A: Urinary KIM-1, NGAL and L-FABP for the diagnosis of AKI in patients with acute coronary syndrome or heart failure undergoing coronary angiography. Heart Vessels, 30: 703–711, 2015 [DOI] [PubMed] [Google Scholar]
- 109.Zhang WR, Garg AX, Coca SG, Devereaux PJ, Eikelboom J, Kavsak P, McArthur E, Thiessen-Philbrook H, Shortt C, Shlipak M, Whitlock R, Parikh CR, Consortium T-A: Plasma IL-6 and IL-10 Concentrations Predict AKI and Long-Term Mortality in Adults after Cardiac Surgery. J Am Soc Nephrol, 26: 3123–3132, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Meersch M, Schmidt C, Van Aken H, Rossaint J, Gorlich D, Stege D, Malec E, Januszewska K, Zarbock A: Validation of Cell-Cycle Arrest Biomarkers for Acute Kidney Injury after Pediatric Cardiac Surgery. Plos One, 9, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pilarczyk K, Edayadiyil-Dudasova M, Wendt D, Demircioglu E, Benedik J, Dohle DS, Jakob H, Dusse F: Urinary [TIMP-2]*[IGFBP7] for early prediction of acute kidney injury after coronary artery bypass surgery. Ann Intensive Care, 5: 50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ho J, Tangri N, Komenda P, Kaushal A, Sood M, Brar R, Gill K, Walker S, MacDonald K, Hiebert BM, Arora RC, Rigatto C: Urinary, Plasma, and Serum Biomarkers' Utility for Predicting Acute Kidney Injury Associated With Cardiac Surgery in Adults: A Meta-analysis. Am J Kidney Dis, 66: 993–1005, 2015 [DOI] [PubMed] [Google Scholar]
- 113.Sabbisetti VS, Waikar SS, Antoine DJ, Smiles A, Wang C, Ravisankar A, Ito K, Sharma S, Ramadesikan S, Lee M, Briskin R, De Jager PL, Ngo TT, Radlinski M, Dear JW, Park KB, Betensky R, Krolewski AS, Bonventre JV: Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol, 25: 2177–2186, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Koyner JL, Garg AX, Shlipak MG, Patel UD, Sint K, Hong K, Devarajan P, Edelstein CL, Zappitelli M, Thiessen-Philbrook H, Parikh CR, Translational Research Investigating Biomarker Endpoints in AKIC: Urinary cystatin C and acute kidney injury after cardiac surgery. Am J Kidney Dis, 61: 730–738, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Parikh CR, Thiessen-Philbrook H, Garg AX, Kadiyala D, Shlipak MG, Koyner JL, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Coca SG, Consortium T-A: Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol, 8: 1079–1088, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liangos O, Tighiouart H, Perianayagam MC, Kolyada A, Han WK, Wald R, Bonventre JV, Jaber BL: Comparative analysis of urinary biomarkers for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers, 14: 423–431, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hirsch R, Dent C, Pfriem H, Allen J, Beekman RH 3rd, Ma Q, Dastrala S, Bennett M, Mitsnefes M, Devarajan P: NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol, 22: 2089–2095, 2007 [DOI] [PubMed] [Google Scholar]
- 118.Shao X, Tian L, Xu W, Zhang Z, Wang C, Qi C, Ni Z, Mou S: Diagnostic value of urinary kidney injury molecule 1 for acute kidney injury: a meta-analysis. PLoS One, 9: e84131, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Koyner JL, Davison DL, Brasha-Mitchell E, Chalikonda DM, Arthur JM, Shaw AD, Tumlin JA, Trevino SA, Bennett MR, Kimmel PL, Seneff MG, Chawla LS: Furosemide Stress Test and Biomarkers for the Prediction of AKI Severity. J Am Soc Nephrol, 26: 2023–2031, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aregger F, Uehlinger DE, Witowski J, Brunisholz RA, Hunziker P, Frey FJ, Jorres A: Identification of IGFBP-7 by urinary proteomics as a novel prognostic marker in early acute kidney injury. Kidney International, 85: 909–919, 2014 [DOI] [PubMed] [Google Scholar]
- 121.Yamashita T, Doi K, Hamasaki Y, Matsubara T, Ishii T, Yahagi N, Nangaku M, Noiri E: Evaluation of urinary tissue inhibitor of metalloproteinase-2 in acute kidney injury: a prospective observational study. Crit Care, 18: 716, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Z, Xu X, Ni H, Jin N: Serum cystatin C is associated with renal function recovery in critically ill patients undergoing continuous renal replacement therapy. Nephron Clin Pract, 122: 86–92, 2012 [DOI] [PubMed] [Google Scholar]
- 123.Kumpers P, Hafer C, Lukasz A, Lichtinghagen R, Brand K, Fliser D, Faulhaber-Walter R, Kielstein JT: Serum neutrophil gelatinase-associated lipocalin at inception of renal replacement therapy predicts survival in critically ill patients with acute kidney injury. Crit Care, 14: R9, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Moledina DG, Parikh CR, Garg AX, Thiessen-Philbrook H, Koyner JL, Patel UD, Devarajan P, Shlipak MG, Coca SG, Consortium T-A: Association of Perioperative Plasma Neutrophil Gelatinase-Associated Lipocalin Levels with 3-Year Mortality after Cardiac Surgery: A Prospective Observational Cohort Study. PLoS One, 10: e0129619, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Meersch M, Schmidt C, Van Aken H, Martens S, Rossaint J, Singbartl K, Görlich D, Kellum JA, Zarbock A. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One, 9(3):e93460, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hall IE, Yarlagadda SG, Coca SG, Wang Z, Doshi M, Devarajan P, Han WK, Marcus RJ, Parikh CR: IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol, 21: 189–197, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reese PP, Hall IE, Weng FL, Schroppel B, Doshi MD, Hasz RD, Thiessen-Philbrook H, Ficek J, Rao V, Murray P, Lin H, Parikh CR: Associations between Deceased-Donor Urine Injury Biomarkers and Kidney Transplant Outcomes. J Am Soc Nephrol, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pianta TJ, Peake PW, Pickering JW, Kelleher M, Buckley NA, Endre ZH: Evaluation of biomarkers of cell cycle arrest and inflammation in prediction of dialysis or recovery after kidney transplantation. Transpl Int, 28: 1392–1404, 2015 [DOI] [PubMed] [Google Scholar]
- 129.Wang L, Xue J, Chen C, Zhang Z, Deng Z, Sun Z, Xing C: Urinary liver-type fatty acid-binding protein predicts recovery from acute kidney injury. Clin Nephrol, 84: 255–261, 2015 [DOI] [PubMed] [Google Scholar]
- 130.Westhoff JH, Tonshoff B, Waldherr S, Poschl J, Teufel U, Westhoff TH, Fichtner A: Urinary Tissue Inhibitor of Metalloproteinase-2 (TIMP-2) * Insulin-Like Growth Factor-Binding Protein 7 (IGFBP7) Predicts Adverse Outcome in Pediatric Acute Kidney Injury. PLoS One, 10: e0143628, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Verbrugge FH, Dupont M, Shao Z, Shrestha K, Singh D, Finucan M, Mullens W, Tang WH: Novel urinary biomarkers in detecting acute kidney injury, persistent renal impairment, and all-cause mortality following decongestive therapy in acute decompensated heart failure. J Card Fail, 19: 621–628, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]