Abstract
Efforts to improve care of patients with ESRD and the policies that guide those activities depend on evidence–based best practices derived from clinical trials and carefully conducted observational studies. Our review describes this process in the context of the translational research model (bench to bedside to populations), with a particular emphasis on bedside care. We illustrate some of its accomplishments and describe the limitations of the data and evidence supporting policy and practice.
Keywords: Quality improvement, Epidemiology and outcomes, surveillance, policy, guidelines, Translational epidemiology, Humans, Kidney Failure, Chronic, nephrology, Public Policy, Renal Insufficiency, Chronic, Translational, Medical Research
Introduction
The development and implementation of population–based health policy are complex and not a well understood process (1). We describe this complexity in the context of the development and implementation of evidence–based health policy in the Medicare ESRD Program. We focus on policy related to three dimensions of quality in ESRD care—adequacy of hemodialysis, vascular access, and transplant access—and situate this discussion in the broader context of translational medicine, which links research and population-based policy to ESRD practice. In particular, we focus on the translation of evidence–based practice guidelines into bedside care in ESRD treatment facilities. Throughout the article, we describe advantages and disadvantages of data available to inform this process.
Policy Development and Translation into Evidence-Based Practice
Figure 1 shows how population-based policy in ESRD relates to and guides this process of translation of evidence from the bench to bedside and populations. Experimental and clinical hypothesis testing studies generate new knowledge (discovery phase; T0/T1); these studies are aggregated, summarized (synthesis phase; T2), and translated into evidence-based guidelines with operationally defined measures (implementation phase; T3). Finally, these measures are used to assess the successful adoption of guidelines (surveillance phase; T4) (2). Each of these steps informs and is informed by policy and depends on high–quality, timely data.
Figure 1.
The public health–based translation medicine model as it applies to public policy–related research. Modified from reference 2, with permission.
Concerns about the translation of evidence-based care into bedside practice were first raised when multinational surveillance data for mortality in patients with ESRD were presented at the 1989 Dallas Conference on Morbidity and Mortality and Prescription of Dialysis. The data presented were novel and convincing evidence for unexplained excess mortality in United States patients on hemodialysis (T0 translation). The conclusion of the 3-day symposium included recommendations for both additional study and interventions to improve care (3).
The Medicare ESRD Program
The Medicare ESRD program, a comprehensive, population–based insurance program established in 1972, currently covers >90% of all United States citizens with ESRD; in 2013, this included 661,648 prevalent patients with ESRD (4). Although the annual age–adjusted incidence rates began to decline in 2010, the number of prevalent patients continues to increase because of aging of the United States population and improved survival. Direct program costs for the approximately 1% of Medicare beneficiaries with ESRD care now consume 7.1% of the Medicare budget (4).
From the program’s inception, policymakers were concerned about ensuring equitable access to high–quality ESRD care (5). These concerns were addressed in 1978 when the ESRD Networks were established (6). Each network contracts with the Centers for Medicaid and Medicare Services (CMS) to collect administrative data, oversee ESRD care quality, and provide technical assistance to ESRD providers and patients. The near-universal coverage afforded by the Medicare ESRD program makes it an important setting for examining the effectiveness and limitations of established processes for translating evidence-based care into effective practice.
ESRD Policymakers
The CMS, as the primary insurer for ESRD care, is the central driver of population–based ESRD policy. The CMS formed the Health Care Quality Improvement Program (HCQIP) to support quality improvement through policy driven by large data systems, evidence–based clinical practice guidelines, and statistical process control (continuous quality improvement) (7). The HCQIP policy initiative was extended to the ESRD Program in the 1994–1997 ESRD Network Scope of Work (8). Two of the quality targets identified for the initial ESRD HCQIP interventions were improving the adequacy of dialysis and appropriate vascular access use; these targets persist among the program’s quality issues today.
Clinical practice guideline developers also drive ESRD policy (Figure 1) by providing summaries of best care practices that policymakers can disseminate and promote. Initially, the ESRD HCQIP used reviews published by the Renal Physician’s Association (8,9); more recently, the program adopted the National Kidney Foundation Dialysis Outcome Quality Initiative’s clinical practice guidelines for many aspects of ESRD care (10).
Currently, the CMS drives ESRD policy by requiring that dialysis units adhere to certain terms of participation in the Medicare ESRD program. For example, the CMS Conditions for Coverage for dialysis units mandate that patients must be informed and educated about kidney transplantation as a treatment option, be referred as appropriate, and have a documented plan for pursuing transplantation throughout the transplant process (11). Moreover, to address variation in access to kidney transplantation observed among dialysis facilities (12), in 2012, the CMS included the coordination of transplantation and transplant referral in the Statement of Work for ESRD Networks. The identification of adequate surveillance data for these dialysis facility transplant access measures is one challenge that illustrates how the quality and availability of ESRD data may influence ESRD policy.
Data Issues in the Translation of Evidence into ESRD Guidelines and Policy
A major limitation in the development of policy, programs, and guidelines for ESRD care (steps T1–T3 in Figure 1), which involves synthesis of evidence from clinical trials and observational studies, arises from a persistent paucity of clinical trials on which this process depends. Strippoli et al. (13) compared the publication of randomized clinical trials (RCTs) in nephrology with that in 12 other medical specialty areas between 1966 and 2002. Nephrology researchers had published the fewest RCTs among these clinical areas and had the third lowest frequency of cited RCTs, which were quite variable in quality. Among the three leading nephrology journals, the proportion of articles that were RCTs varied from 2.7% to 4.6%. The low frequency of RCTs in nephrology is a continuing problem (14) and may be inextricably linked to an overall decline in support for research in kidney disease (15) and nonpublication of relevant trials (16). Similar concerns were raised in the work by Deo et al. (17), which found that, among 196 RCTs concerning dialysis therapy or kidney transplantation published during 2007 and 2008, many had an inadequate description of primary outcomes and failed to adequately describe randomization and completeness of follow-up.
Beyond the relative lack of well designed and conducted clinical trials of care practices for patients with ESRD, other available evidence identifying best practices is often scarce or of low quality. Ross et al. (18) found that published observational studies comparing survival on hemodialysis versus peritoneal dialysis were highly variable with respect to the availability of information about numerous important patient and treatment characteristics. Furthermore, study characteristics were variably reported; as a consequence, Ross et al. (18) were unable to generate summary results.
Another vexing issue is that many guidelines and supporting policies, such as those for transplant access as described above, are on the basis of opinion rather than evidence. The recently published Kidney Disease Improving Global Outcomes (KDIGO) guidelines for CKD (19), which are currently used by the CMS to guide quality improvement policy, provide an example of this conundrum. The KDIGO guideline group assigned only 17% of the recommendations an A for strong evidence. They further underscored and emphasized the need for better rather than poor evidence or expert opinion alone to support nephrology guidelines when they stated that “…clinicians still need to make decisions in their daily practice, and they often ask, ‘what do the experts do in this setting?’ We opted to give guidance, rather than remain silent.”
Another relevant data issue is the often ad hoc nature of many ESRD guidelines. Guidelines should be transparent with respect to policy objectives, development process, and funding. The guideline writers should clearly state how data synthesis is conducted and how bias in data selection and interpretation is avoided. Principles governing participant conflict of interest, multidisciplinary involvement, and the inclusion of methodologic and clinical experts and public advocates should be stated. This process should be subject to external expert and public review and periodically updated. Some ESRD guidelines have been criticized for failure to meet these standards during their development (20).
Implementation of Evidence-Based Guidelines and Population Health: T4 Translation of Policy and Innovation
T4 translation is multidisciplinary and intended to ensure that the health care delivered to individuals and populations reflects the best evidence–based care available. Although the main focus of translational medicine is to expedite the bench to bedside delivery of innovative diagnostic tools and therapies, there is growing awareness that optimizing population health also entails T4 bedside to population translational strategies to promote the adoption and use of evidence-based medicine by community practitioners and health systems (21). The measurement of the success of T4 translation via surveillance is essential for identifying gaps in the quality of care of the ESRD population and ascertaining the success of guideline dissemination.
Measurement begins when contractors review and consolidate clinical practice guidelines into measurable quality indicators and field test these indicators using national samples of patients on hemodialysis stratified by ESRD Network (T2 and T3). Field test results are reported to the Technical Expert Panel (TEP) convened by the CMS and supported by the Arbor Research Collaborative for Health and the University of Michigan Kidney Epidemiology and Cost Center. The TEP prioritizes guidelines on the basis of the significance and strength of supporting evidence, the feasibility of developing performance measures, and the degree to which large gaps in care may be amenable to intervention. The TEPs, which include clinicians, statisticians, quality improvement methodologists, and consumers, advise measure development contractors on the selection and maintenance of measures.
After measures are updated with the TEP input, they are submitted to the National Quality Forum (NQF) for review and endorsement (22). The NQF applies a consensus–based, rigorous, transparent process for endorsing evidence–based quality of care measures. The role of the NQF in this process is critical, because United States law requires that endorsed measures be used instead of alternative measures when appropriate.
Data Issues in T4 Translation
During 2015, the NQF assessed 14 previously endorsed and 11 new quality measures for kidney disease care proposed by the CMS and other organizations (22). Only 13 measures were endorsed. Three additional measures, determined to be important quality indicators for which high levels of performance had already been attained and that should remain in the renal quality portfolio, were included as reserve measures. The CMS proposed eight of these 16 endorsed and retained measures.
After reviewing these measures, the NQF committee “identified numerous areas where additional measure development is needed” (22). These included transitions in care, palliative therapy/comfort therapy, patient experience of care, consumer assessment of health care providers and systems, quality of life as measured using the Kidney Disease Quality of Life instrument, depression screening, informed decision making for pregnant patients with ESRD about birth control and family planning, medicine reconciliation, appropriateness of medications, patient follow-up duration after transplant, care of patients with incident ESRD (i.e., early outcomes), patient engagement and participation in care planning, infection associated with peritoneal dialysis, comorbid anxiety, dialysis center staffing ratios, and patient malnutrition (22). Although data to support these patient–centered, goal–oriented concepts and associated guidelines and indicators remain sparse (23), the ESRD community believes that they are high-priority areas for which the CMS and the National Institute of Diabetes and Digestive and Kidney Diseases should support future research and development of relevant evidence–based guidelines.
Among the endorsed quality indicators included ESRD treatment center–level proportion of patients on prevalent hemodialysis using an arteriovenous fistula (AVF). The proportion of adult patients whose average single–pool Kt/V–delivered dose of hemodialysis was ≥1.2 was considered a reserve measure, an indicator that no longer varies substantially but is judged to warrant continued surveillance (22). Both measures are currently reported in the Dialysis Facility Compare (DFC) public program. Measures related to access to transplant are currently under development and have not yet been approved by the NQF. Of note, no transplant measures are currently reported in the publicly available DFC program, because facility–level standardized transplant ratios were abandoned in 2014.
Surveillance and Assessment of T4 Translation
Surveillance systems support systematic and ongoing collection, aggregation, analysis, and interpretation of data in a defined population and disseminate the results (24). There are multiple surveillance systems relevant to kidney disease. These include the US Renal Data System (USRDS); the Centers for Disease Control and Prevention’s National Healthcare Safety Network, which tracks infection control and water safety in dialysis facilities; and the Organ Procurement and Transplantation Network–based registries the Scientific Registry of Transplant Recipients and the United Network for Organ Sharing.
The CMS quality of care surveillance activities have evolved from manual data collection to electronic data transfer from dialysis facilities (25). The current system, CROWNWeb, is a secure, Health Insurance Portability and Accountability Act of 1996–compliant, web–based data collection system implemented in 2012 (26). The surveillance information generated by CROWNWeb has multiple uses, including populating the DFC website (27). The DFC provides a public reporting portal for dialysis facility–specific summary information relevant to guideline–based quality metrics, such as hemodialysis adequacy, AVF use rates, and standardized rate ratios for mortality and other outcomes. The accessibility and intelligibility of the DFC site have been assessed and found to be satisfactory (28,29).
Data Issues with Kidney Disease Surveillance Systems
First, one major issue that is evident in all surveillance systems is that of missing data. For example, Figure 2 shows the distribution of facility-specific adequacy of dialysis in 2014 but excludes the 12% or more of United States facilities that provided no data. Second, another issue is that data reported to the CMS may be inaccurate. Furthermore, the USRDS data derived from the Medical Evidence report submitted to the CMS are often inaccurate (30–34). The effects of increased use of financial incentives to improve the accuracy of dialysis facilities’ self-reported data are also unclear. Missing and inaccurate data, which are the joint responsibilities of the clinicians and staff who enter the data and the surveillance systems, represent a higher–level quality improvement issue that needs both policy consideration and programmatic remediation. Third, although CROWNWeb surveillance is in real time and the DFC data are relatively recent, most surveillance data, especially patient-level data, have time lags of 18–24 months or more, precluding the ideal of continuous quality improvement. Fourth, although many large– and medium–sized dialysis organizations collect quality improvement data that may be useful to assess best practices, these data are proprietary and rarely published. These data limitations inhibit the discovery of suboptimal care processes and outcomes as well as any unintended consequences of established policy that should be explored through new research (T0 and T1). Because most of the information about processes and outcomes of care are derived from these data, it is reasonable to suggest that the USRDS and ESRD leadership organizations, like the American Society of Nephrology, the National Kidney Foundation, the American Nephrology Nurses Association, the American Association of Kidney Patients, and others, might raise this quality issue with the CMS policymakers.
Figure 2.
Cumulative count of the number of centers with facility-level percentage of adult (≥18 years old) patients on in-center hemodialysis (HD) with Kt/V≥1.2 in 2015 from Dialysis Facility Compare (DFC; 5727 of 6376 [89.8%] facilities reporting). STD, SD.
Finally, data relevant to policy may not be collected. One illustrative example is the limited data available on dialysis facility transplant referral. The CMS convened a TEP in 2004 and 2005 to develop a transplant referral quality measure for dialysis facilities (35). However, inadequate surveillance data prevented the national measurement of referral data. A second TEP established in 2015 proposed that dialysis facilities be held accountable for the percentage of patients who are waitlisted rather than the percentage referred for transplant evaluation. However, because national data on waitlisting but not referral are currently collected, this recommendation will not take into account the subjective factors outside of dialysis facilities’ control that lead to successful referral (36). This lack of surveillance data has significant policy implications; ESRD policymakers typically rely on the best available data, even if it may not lead to the development of an optimal quality measure.
Guideline Implementation and Outcomes of the Policy Development Translational Process: Examples
This complex and loosely connected system of translational medicine and supporting population–based health policy lends itself to a simple question: does it work to improve processes and outcomes of ESRD care? We will use two examples to address this question: adequacy of the delivered hemodialysis dose and use of an AVF for vascular access during dialysis.
Adequacy of Dialysis
The ESRD HCQIP quality targets for the 1994–1997 Scope of Work for the ESRD Networks were on the basis of a broader CMS quality improvement strategy and explicitly included adequacy of hemodialysis dose (8). The translational infrastructure necessary to implement the adequacy quality improvement goal included clinical practice guidelines that established evidence-based recommendations for best practice with respect to dialysis dose. These guideline-based recommendations were then systematically reviewed, translated into quality metrics, and field tested (37).
Surveillance data for assessing these recommendations revealed substantial variation among treatment centers in these quality metrics. This information was used to implement targeted, multifaceted interventions to assist poorly performing treatment centers to conduct quality of care improvement (24). The subsequent change in adequacy of hemodialysis dose uniformly observed after implementation of the ESRD HCQIP has been described and cited as evidence of success of the CMS policy (38).
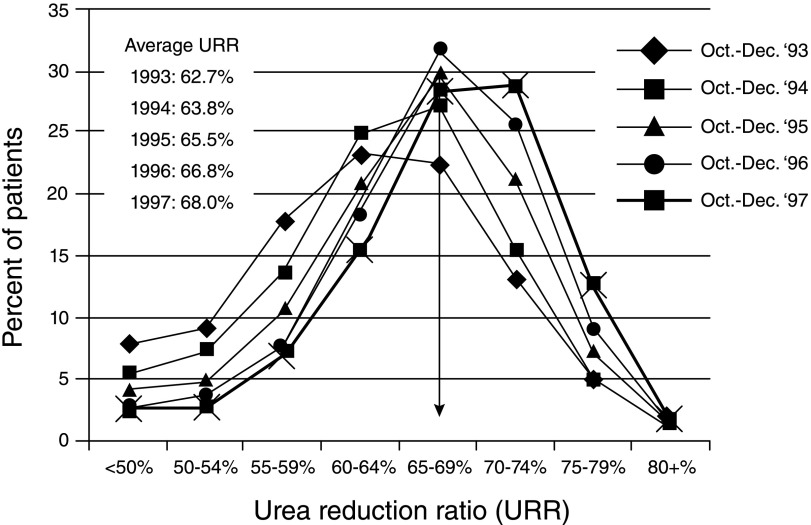
During the initial iteration of the policy, the prevalence of adequately treated patients increased substantially. Figure 3 shows the distribution of hemodialysis adequacy over the first 5 years of the ESRD HCQIP. The population mean for dialysis adequacy, as measured by the urea reduction ratio, increased from 63% to 68% among all patients on hemodialysis in the program. This improvement has continued as have the CMS programs to improve hemodialysis adequacy. Moreover, as shown in recent facility reports on the DFC website, adequate hemodialysis dose is essentially uniform throughout reporting facilities, with a mean percentage of patients with adequate dialysis among reporting centers of 89.4% (Figure 2). Additional support for the success of the ESRD HCQIP is provided by small, group–randomized evaluations, which were consistent with an intervention effect leading to improvement in adequacy over that observed in comparison groups (39,40).
Figure 3.
Distribution of urea reduction ratio (URR) values for adult (≥18 years old) patients on in-center hemodialysis from October to December of 1997 compared with from October to December 1993–1996. Reprinted from reference 51, with permission.
Vascular Access
Another focus on encouraging AVF as the primary vascular access used by patients on hemodialysis has been termed the Fistula First Initiative (41). The Fistula First Initiative was on the basis of available clinical practice guidelines, used the translational infrastructure and techniques used in the HCQIP, and was evaluated using ESRD surveillance data. After the inception of the project, there was an increase in the prevalence of AVF use among hemodialysis centers (Figure 4), and regional variation in use diminished (42).
Figure 4.
Trends in vascular access type among United States patients on prevalent dialysis from 1995 to 2000 (42). AV, arteriovenous; AVF, arteriovenous fistula.
Population Health Outcomes Associated with ESRD Policy
Subsequent analyses of mortality trends in the ESRD program suggested that the improved quality of care after the inception of the program was associated with a reduction in mortality in the ESRD hemodialysis population (43). The declines in mortality rates noted after the inception of the ESRD HCQIP have continued during subsequent ESRD quality initiatives in the program (4). Furthermore, regional ESRD HCQIP interventions were associated with reduced racial and sex disparities in care (44). Finally, meta-analyses, which included reports of the ESRD HCQIP interventions conducted by the ESRD Networks, have found that the quality improvement approaches adopted in the program are consistently associated with improvements of care over comparison populations (45,46).
It is important to note that the success of T4 translational activities depends on support and leadership of the dialysis community (47). In particular, it is emphasized that physician leadership must master quality improvement concepts that include evidence-based guidelines and statistical quality control techniques to improve and maintain high levels of patient care (48).
Finally, it is recognized that the translation process is iterative; some quality indicators must be revised or abandoned as new evidence emerges from additional clinical trials, observational research, or new surveillance data. This can be illustrated best by the evolution of efforts to develop and implement guideline-based care for anemia in ESRD, which has been extensively described in the 10th edition of Brenner and Rector’s The Kidney and illustrates that additional investigation of the basic mechanisms of erythropoietin-stimulating agents in the correction of anemia is warranted (49).
Evolving CMS Quality Policy
The CMS continues to use the T4 translational apparatus to promote quality improvement in the ESRD program. The CMS quality policy has broadened to incorporate a first of its kind value–based purchasing initiative for bundled dialysis services in a program termed the ESRD Quality Incentive Program (ESRD-QIP). The ESRD-QIP links a portion of facility reimbursement to performance on quality measures that currently include eight clinical measures (concerning adequacy of dialysis, vascular access use, level of hemoglobin, bloodstream infections, and hypercalcemia) and three reporting measures (patient quality of life, management of bone and mineral metabolism, and anemia management). The ESRD-QIP measures are scored for each facility, and those facilities failing to meet quality of care goals are subjected to reductions in reimbursement up to 2%. The CMS publicly reports the facility data driving the ESRD-QIP on the DFC website. Additionally, facilities will have to display a DFC Performance Score Certificate listing both its overall performance and its component quality scores, similar to public health inspection reports that the public routinely encounters. The newly awarded Scope of Work for the ESRD Networks includes reduction in permanent catheter use for dialysis, reduction in bloodstream infection rates, and improvement in patient quality of life as evaluation measures. By extension, these measures will be the translational goals for the 5-year contract. This expanding program and changes in incentives underscore the urgent need to rethink how and what data are collected.
Conclusion
We have described a system of ESRD policy and related translation that has evolved since the inception of the Medicare ESRD program. Furthermore, to focus on the quality of care, we have ignored major areas of policy that are integral to understanding the ESRD program, including significant economic and political factors. However, we contend that the established policy/translational approach has successfully served the ESRD program over several decades, and we feel that this experience should be considered by policymakers seeking to understand and reduce variation in patient care.
Perhaps the most significant threats to the continued success of the policy translational process in ESRD policy are presented by limitations in evidence (e.g., paucity of RCTs) and data. Another significant barrier to understanding the value and limitations of this process is its foundation in population health sciences rather than clinical medicine. However, as clinicians and their educators become more conversant and proficient with the core competencies relating to population-based health, practice-based improvement, and systems-based practice (50), we expect this barrier to break down (50).
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Woolf SH, Purnell JQ, Simon SM, Zimmerman EB, Camberos GJ, Hayley A, Fields RP: Translating evidence into population health improvement: Strategies and barriers. In: Annual Review of Public Health, Vol. 36, edited byFielding JE, Palo Alto, CA, Annual Reviews, 2015, pp 463–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khoury MJ, Gwinn M, Ioannidis JP: The emergence of translational epidemiology: From scientific discovery to population health impact. Am J Epidemiol 172: 517–524, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anonymous: Introduction and summary. Proceedings from the Morbidity, Mortality and Prescription of Dialysis Symposium, Dallas, TX, September 15 to 17, 1989. Am J Kidney Dis 15: 375–383, 1990 [PubMed] [Google Scholar]
- 4.USRDS: USRDS 2015 Annual Data Report, Bethesda, MD, USRDS, 2015
- 5.Rettig RA, Sadler JH: Measuring and improving the health status of end stage renal disease patients. Health Care Financ Rev 18: 77–82, 1997 [PMC free article] [PubMed] [Google Scholar]
- 6.Krisher J, Pastan S: Promoting quality of care for ESRD patients: The role of the ESRD Networks. Adv Ren Replace Ther 8: 138–143, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Jencks SF, Wilensky GR: The health care quality improvement initiative. A new approach to quality assurance in Medicare. JAMA 268: 900–903, 1992 [PubMed] [Google Scholar]
- 8.McClellan WM, Helgerson SD, Frederick PR, Wish JB, McMullan M: Implementing the Health Care Quality Improvement Program in the Medicare ESRD Program: A new era of quality improvement in ESRD. Adv Ren Replace Ther 2: 89–94, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Hornberger JC; Renal Physicians Association Working Committee on Clinical Guidelines: The hemodialysis prescription and quality-adjusted life expectancy. J Am Soc Nephrol 4: 1004–1020, 1993 [DOI] [PubMed] [Google Scholar]
- 10.National Kidney Foundation: KDOQI clinical practice guidelines and clinical practice recommendations for 2006 updates: Hemodialysis adequacy, peritoneal dialysis adequacy and vascular access. Am J Kidney Dis 48[Suppl 1]: S1–S322, 2006. 17045862 [Google Scholar]
- 11.Register F: Conditions for Coverage for ESRD Facilities, Washington, DC, US Government Printing Office, 2008 [Google Scholar]
- 12.Patzer RE, Plantinga L, Krisher J, Pastan S: Dialysis facility and network factors associated with low kidney transplantation rates among United States dialysis facilities. Am J Transplant 14: 1562–1572, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strippoli GFM, Craig JC, Schena FP: The number, quality, and coverage of randomized controlled trials in nephrology. J Am Soc Nephrol 15: 411–419, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Palmer SC, Sciancalepore M, Strippoli GFM: Trial quality in nephrology: How are we measuring up? Am J Kidney Dis 58: 335–337, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Bryan L, Ibrahim T, Zent R, Fischer MJ: The kidney research predicament. J Am Soc Nephrol 25: 898–903, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moorthi RN, Lam A, Tangri N, Sood MM, Wagner M, Alam A: Predictors of publication of randomized controlled trials in nephrology. Clin Nephrol 80: 280–285, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Deo A, Schmid CH, Earley A, Lau J, Uhlig K: Loss to analysis in randomized controlled trials in CKD. Am J Kidney Dis 58: 349–355, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross S, Dong E, Gordon M et al. : Meta-analysis of outcome studies in end-stage renal disease. Kidney Int 57: S28–S38, 2000 [Google Scholar]
- 19.KDIGO: KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Coyne DW: Influence of industry on renal guideline development. Clin J Am Soc Nephrol 2: 3–7, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Sugarman JR, Frederick PR, Frankenfield DL, Owen WF Jr., McClellan WM; Dialysis Outcomes Quality Initiative Clinical Practice Guidelines: Developing clinical performance measures based on the Dialysis Outcomes Quality Initiative Clinical Practice Guidelines: Process, outcomes, and implications. Am J Kidney Dis 42: 806–812, 2003 [DOI] [PubMed] [Google Scholar]
- 22.NQF: NQF-Endorsed Measures for Renal Conditions, 2015. Available at: http://www.qualityforum.org/Publications/2015/12/Renal_Measures_Final_Report.aspx. Accessed June 24, 2016
- 23.Kliger A: Quality measures for dialysis: Time for a balanced scorecard. Clin J Am Soc Nephrol 11: 363–368, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mcclellan WM, Krisher JO: Collecting and using patient and treatment center data to improve care: Adequacy of hemodialysis and end-stage renal disease surveillance. Kidney Int 57: S7–S13, 2000 [Google Scholar]
- 25.Mattern WD, Krisher J, Garrett LE Jr., Hogan SL, Gunn JM, Twito C: From the facility to the network: DxBase moves on to SIMS and VISION to help track patient outcomes. Nephrol News Issues 13: 24–26, 1999 [PubMed] [Google Scholar]
- 26.Delva O, Seaton J: CROWNWeb: Producing data to measure performance. Nephrol News Issues 27: 22–25, 2013 [PubMed] [Google Scholar]
- 27.Frederick PR, Maxey NL, Clauser SB, Sugarman JR: Developing dialysis facility-specific performance measures for public reporting. Health Care Financ Rev 23: 37–50, 2002 [PMC free article] [PubMed] [Google Scholar]
- 28.Trisolini M, Zerhusen E, Bandel K, Roussel A, Frederick P, Schatell D, Harris S: Evaluation of the Dialysis Facility Compare website tool on Medicare.gov. Dial Transplant 35: 196–214, 2006 [Google Scholar]
- 29.Trisolini MG, Isenberg KL: Public reporting of patient survival (mortality) data on the dialysis facility compare web site. Dial Transplant 36: 486–499, 2007 [Google Scholar]
- 30.Layton JB, Hogan SL, Jennette CE, Kenderes B, Krisher J, Jennette JC, McClellan WM: Discrepancy between Medical Evidence Form 2728 and renal biopsy for glomerular diseases. Clin J Am Soc Nephrol 5: 2046–2052, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beaubrun AC, Kanda E, Bond TC, McClellan WM: Form CMS-2728 data versus erythropoietin claims data: Implications for quality of care studies. Ren Fail 35: 320–326, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Fischer MJ, Stroupe KT, Hynes DM, Blemur P, Sohn MW, Browning MM, Huo Z, O’Hare AM, Kaufman JS: Validation of erythropoietin use data on Medicare’s End-Stage Renal Disease Medical Evidence Report. J Rehabil Res Dev 47: 751–762, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Krishnan M, Weinhandl ED, Jackson S, Gilbertson DT, Lacson E Jr.: Comorbidity ascertainment from the ESRD Medical Evidence Report and Medicare claims around dialysis initiation: A comparison using US Renal Data System data. Am J Kidney Dis 66: 802–812, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Solid CA, Collins AJ, Ebben JP, Chen SC, Faravardeh A, Foley RN, Ishani A: Agreement of reported vascular access on the medical evidence report and on Medicare claims at hemodialysis initiation. BMC Nephrol 15: 30, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sehgal A, Leon J, Stark S: ESRD Special Study: Developing dialysis facility-specific kidney transplant referral clinical performance measures. Available at: http://www.therenalnetwork.org/qi/resources/TransTEPfinalrpt805.pdf. Accessed June 23, 2016 [Google Scholar]
- 36.Public Comment Postings: End-stage renal disease access to kidney transplantation measure development Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/CallforPublicComment.html. Accessed June 23, 2016
- 37.McClellan WM, Goldman RS: Continuous quality improvement in dialysis units: Basic tools. Adv Ren Replace Ther 8: 95–103, 2001 [DOI] [PubMed] [Google Scholar]
- 38.McClellan WM, Frankenfield DL, Frederick PR, Helgerson SD, Wish JB, Sugarman JR: Improving the care of ESRD patients: A success story. Health Care Financ Rev 24: 89–100, 2003 [PMC free article] [PubMed] [Google Scholar]
- 39.McClellan WM, Hodgin E, Pastan S, McAdams L, Soucie M: A randomized evaluation of two health care quality improvement program (HCQIP) interventions to improve the adequacy of hemodialysis care of ESRD patients: Feedback alone versus intensive intervention. J Am Soc Nephrol 15: 754–760, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Sehgal AR, Leon JB, Siminoff LA, Singer ME, Bunosky LM, Cebul RD: Improving the quality of hemodialysis treatment: A community-based randomized controlled trial to overcome patient-specific barriers. JAMA 287: 1961–1967, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Lok CE: Fistula first initiative: Advantages and pitfalls. Clin J Am Soc Nephrol 2: 1043–1053, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Vassalotti JA, Jennings WC, Beathard GA, Neumann M, Caponi S, Fox CH, Spergel LM; Fistula First Breakthrough Initiative Community Education Committee: Fistula first breakthrough initiative: Targeting catheter last in fistula first. Semin Dial 25: 303–310, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Wolfe RA, Hulbert-Shearon TE, Ashby VB, Mahadevan S, Port FK: Improvements in dialysis patient mortality are associated with improvements in urea reduction ratio and hematocrit, 1999 to 2002. Am J Kidney Dis 45: 127–135, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Sehgal AR: Impact of quality improvement efforts on race and sex disparities in hemodialysis. JAMA 289: 996–1000, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Ivers N, Jamtvedt G, Flottorp S, Young JM, Odgaard-Jensen J, French SD, O'Brien MA, Johansen M, Grimshaw J, Oxman AD: Audit and feedback: Effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev 6: CD000259, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Veer SN, Jager KJ, Nache AM, Richardson D, Hegarty J, Couchoud C, de Keizer NF, Tomson CR: Translating knowledge on best practice into improving quality of RRT care: A systematic review of implementation strategies. Kidney Int 80: 1021–1034, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Maddux FW, Nissenson AR: The evolving role of the medical director of a dialysis facility. Clin J Am Soc Nephrol 10: 326–330, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lacson E Jr., Maddux FW: Intensity of care and better outcomes among hemodialysis patients: A role for the Medical Director. Semin Dial 25: 299–302, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Soman SSYJ, Ho K: Quality improvement initiatives in kidney disease. In: Brenner and Rector’s The Kidney, 10th Ed, edited by , Skorecki K, Chertow GM, Marsden PA, Taal MW, Yu ASL, Philadelphia, Elsevier, pp 2620–2636, 2015 [Google Scholar]
- 50.Parker MG: Nephrology training in the 21st century: Toward outcomes-based education. Am J Kidney Dis 56: 132–142, 2010 [DOI] [PubMed] [Google Scholar]
- 51.1998 ESRD Core Indicators. Available at: https://archive.org/details/annualreportesrd00esrd. Accessed June 23, 2016