Abstract
Background and objectives
AKI is a serious complication after cardiac surgery. Although high urinary concentrations of the tubular protein uromodulin, a marker of tubular health, are associated with less AKI in animal models, its relationship in humans is unknown.
Design, setting, participants, & measurements
A post hoc analysis of a prospective cohort study of 218 adults undergoing on–pump cardiac surgery between 2004 and 2011 was conducted. Multivariable logistic and linear regression analyses were used to evaluate the associations of preoperative urinary uromodulin-to-creatinine ratio with postoperative AKI (defined as a rise in serum creatinine of >0.3 mg/dl or >1.5 times baseline); severe AKI (doubling of creatinine or need for dialysis) and peak postoperative serum creatinine over the first 72 hours.
Results
Mean age was 68 years, 27% were women, 95% were white, and the median uromodulin-to-creatinine ratio was 10.0 μg/g. AKI developed in 64 (29%) patients. Lower urinary uromodulin-to-creatinine ratio was associated with higher odds for AKI (odds ratio, 1.49 per 1-SD lower uromodulin; 95% confidence interval, 1.04 to 2.13), which was marginally attenuated after multivariable adjustment (odds ratio, 1.43; 95% confidence interval, 0.99 to 2.07). The lowest uromodulin-to-creatinine ratio quartile was also associated with higher odds for AKI relative to the highest quartile (odds ratio, 2.94; 95% confidence interval, 1.19 to 7.26), which was slightly attenuated after multivariable adjustment (odds ratio, 2.43; 95% confidence interval, 0.91 to 6.48). A uromodulin-to-creatinine ratio below the median was associated with higher adjusted odds for severe AKI, although this did not reach statistical significance (odds ratio, 4.03; 95% confidence interval, 0.87 to 18.70). Each 1-SD lower uromodulin-to-creatinine ratio was associated with a higher adjusted mean peak serum creatinine (0.07 mg/dl per SD; 95% confidence interval, 0.02 to 0.13).
Conclusions
Lower uromodulin-to-creatinine ratio is associated with higher odds of AKI and higher peak serum creatinine after cardiac surgery. Additional studies are needed to confirm these preliminary results.
Keywords: uromodulin; acute kidney injury; cardiac surgery; tubular function; adult; Animals; Cardiac Surgical Procedures; Cohort Studies; creatinine; Female; Humans; Kidney Function Tests; Models, Animal; Odds Ratio; Postoperative Period; Prospective Studies; Regression Analysis; renal dialysis; Uromodulin
Introduction
AKI is a serious complication of on–pump cardiac surgery (1,2) and it is largely believed that the culprit lesion is acute tubular necrosis (3). Current preoperative assessment of kidney function is limited to measures of the glomerular axis, namely eGFR and urinary albumin-to-creatinine ratio (ACR). Although a number of tubular injury biomarkers have been evaluated to diagnose early AKI in the postoperative setting (4), preoperative tubular function assessment is not part of routine care.
Uromodulin is a 95-kD glycoprotein synthesized by the thick ascending limb of the loop of Henle and early distal convoluted tubule (5). It is the most abundant urinary protein in healthy adults (20–70 mg/d). In vitro studies suggest that uromodulin binds pathogenic bacteria, prevents stone formation, and assists in excretion of uric acid (6–8). Urinary uromodulin positively correlates with eGFR, volume status, and tubular function in the general population (9,10). We have previously shown that higher urinary concentrations of uromodulin are associated with a lower risk of decline in eGFR and mortality (11).
Data suggest that Umod gene knockout mice lacking uromodulin are more susceptible to ischemia-reperfusion kidney injury (12) and more likely to experience tubular inflammation and necrosis, especially in the S3 segment of the proximal tubule. This is also the most common site of tubular injury in humans. To our knowledge, no studies have evaluated whether urinary uromodulin concentrations are associated with risk of AKI in humans. In this study, we evaluated whether urinary uromodulin associates with the risk of AKI in adults undergoing on–pump cardiac surgery.
Materials and Methods
Design and Participants
We performed a post hoc analysis of a cohort of patients undergoing on–pump cardiac surgery at three tertiary care academic centers (Tufts Medical Center, St. Elizabeth’s Medical Center, and University of Massachusetts Medical Center, Worcester, MA) between 2004 and 2011. The aim of the parent study was to evaluate genetic risk factors for AKI after cardiac surgery (13,14). Consecutive adults (≥18 years of age) scheduled to undergo on–pump cardiac surgery were eligible for enrollment. Written informed consent was obtained from study participants or next of kin. The institutional review board of each participating center approved the study protocol.
Data and Sample Collection
Urine samples were freshly collected before surgery, kept on ice, and centrifuged within 30 minutes to remove insoluble elements. The supernatant was treated with a protease inhibitor cocktail tablet (Complete Mini; Roche Diagnostics, Indianapolis, IN) to prevent biomarker degradation by urinary proteases. Urinary aliquots were stored at −80°C and not thawed before this study.
Exposure Variable
Uromodulin was assayed in urine using a commercially available ELISA kit (MD Bioproducts, St. Paul, MN). The colorimetric sandwich immunoassay uses a polyclonal antibody against human uromodulin as the capture antibody and a biotinylated polyclonal antibody against human uromodulin as the detection antibody. Absorbance was read on a Bio-Rad Benchmark Plus Plate Reader (Bio-Rad, Hercules, CA) at 450 nm, with correction at 620 nm. Uromodulin concentration was calculated from a standard curve generated with a four–parameter logistic regression. Data for each run were calculated from a set of eight standards (created by serial dilution) using a cubic regression curve fit. Curve R values below 0.9 were rejected, and the assay was repeated. The correlation coefficient for the assay run was 1.00. The interassay coefficient of variation was 5.02%. The minimum detectable concentration as reported by the manufacturer was 0.75 ng/ml. No measured values in our cohort were below the detectable limit. To account for urine dilution, uromodulin was indexed to urinary creatinine, and the resulting uromodulin-to-creatinine ratio was expressed as micrograms per gram (15).
Outcome Variable
Serum creatinine was assayed using the modified Jaffe method. We defined AKI as a rise in serum creatinine of at least >0.3 mg/dl or >1.5 times the preoperative value during the first 72 hours after surgery as previously reported (16–18). This definition differs slightly from the Kidney Disease Improving Global Outcomes (KDIGO) criteria, requiring the serum creatinine rise to occur within 48 hours (19). Given the possibility of hemodilution associated with cardiac surgery, we anticipated an early postoperative decline in serum creatinine concentration in some patients and a delay in achieving the KDIGO criterion threshold for diagnosing AKI over the first 48 hours (20,21). We, therefore, adopted a 72-hour window for the rise in the serum creatinine. Severe AKI was defined as at least the doubling of the serum creatinine or the need for RRT. Urine output was only collected at 24-hour intervals, and thus precluded its use in identifying patients with AKI (19). Peak serum creatinine was defined as the highest value recorded over the first 72 hours after surgery.
Covariates
Patient characteristics included age, sex, history of diabetes (including the use of at least one oral antidiabetic drug or insulin), heart failure as defined by the Society of Thoracic Surgeons (22), left ventricular ejection fraction assessed by echocardiography, chronic obstructive pulmonary disease (COPD), peripheral vascular disease (PVD), preoperative eGFR calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (23), and urine ACR. Surgical parameters of interest included type of surgery (coronary artery bypass graft surgery, valvular surgery, or both) and elective versus emergent surgery (i.e., immediately after cardiac catheterization). To account for the possibility of sample degradation over time, we also adjusted for storage time in our final models.
Statistical Analyses
We used descriptive statistics to compare the characteristics of the cohort by quartiles of uromodulin-to-creatinine ratio. Continuous variables were described as means (with SDs) or medians (with 25th and 75th percentiles) as appropriate. Categorical variables were expressed as frequencies (with percentages). Normally distributed continuous variables were compared using ANOVA, and non–normally distributed variables were evaluated using the Kruskal–Wallis test. Categorical variables were compared using the chi-squared test and the Fisher exact test as appropriate.
To evaluate the association of preoperative uromodulin-to-creatinine ratio with the development of postoperative AKI, we used univariate and multivariable logistic regression analyses. We first included demographic variables (age and sex; model 1), and then, diabetes, heart failure, left ventricular ejection fraction, eGFR, type and elective nature of the cardiac surgery, and urine ACR were added (model 2) (24–27). The effect estimates are displayed as odds ratios (ORs; per 1 SD lower or across quartiles) with the corresponding 95% confidence intervals (95% CIs). Given that the majority of patients were white, we accounted for the quasicomplete separation seen in our logistic models using the penalized likelihood method proposed by Firth (28). We evaluated the interaction between CKD status (defined as eGFR<60 ml/min per 1.73 m2) and the need for nonelective surgery with uromodulin-to-creatinine ratio with AKI. In a sensitivity analysis, we adjusted for additional confounders, including COPD, PVD, prior radiocontrast use, cardiopulmonary bypass time, and urine sample storage time. In additional sensitivity analyses, we also evaluated the association between raw (not indexed to creatinine) uromodulin concentration and AKI.
We used restricted cubic splines in the rms package in R (29) to explore the functional relationship between the uromodulin-to-creatinine ratio and peak serum creatinine. We used the default number and location of knots along the distribution of uromodulin-to-creatinine ratio quantiles 5%, 35%, 65%, and 95% with the following values of uromodulin: 2.11, 6.03, 17.32, and 44.77 μg/g, respectively. Multivariable linear regression analyses were then used to examine the association of the preoperative uromodulin-to-creatinine ratio with peak postoperative serum creatinine after adjustment for the same confounders. Among patients who developed AKI, we compared the mean (SD) serum creatinine values across quartiles at different time points (preoperative, 24, 48, and 72 hours postoperatively) using trend P values, which were calculated using the P value from the Spearman rank correlation of the ordered quartiles and serum creatinine. All analyses were performed using SAS, version 9.3 (SAS Institute Inc., Cary, NC), and a two–sided P value <0.05 was considered statistically significant.
Results
Of the 274 patients enrolled in the original study, urine samples were available for 254 participants. After excluding patients with missing urine creatinine (n=36), our final sample included 218 patients. Other than eGFR, which was higher (78 versus 72 ml/min per 1.73 m2), there were no statistically significant differences in the baseline characteristics between the excluded and included cohorts (Supplemental Table 1). The mean age was 68 years, 27% were women, 95% were white, and the mean (SD) eGFR was 71.9 (19.3) ml/min per 1.73 m2. The median urinary uromodulin-to-creatinine ratio was 10.0 μg/g (interquartile range, 4.2–22.8). Except for higher proportions of women in the third and fourth quartiles, there were no differences in the patient characteristics across quartiles (P>0.05 for all) (Table 1).
Table 1.
Characteristics of the total cohort according to urinary uromodulin-to-creatinine ratio quartiles
| Characteristics | Total, n=218 (0.8–93.7), μg/g | Quartile 1, n=54 (0.8–4.1), μg/g | Quartile 2, n=55 (4.2–9.9), μg/g | Quartile 3, n=55 (10.0–22.8), μg/g | Quartile 4, n=54 (22.9–93.7), μg/g | Trend P Value |
|---|---|---|---|---|---|---|
| Patient | ||||||
| Uromodulin/Cr, μg/ga | 10.0 (4.2, 22.8) | 2.7 (2.2, 3.4) | 6.7 (5.7, 7.9) | 15.3 (11.6, 20.1) | 33.3 (27.4, 41.0) | |
| Men, n (%) | 159 (72.9) | 43 (79.6) | 45 (81.8) | 37 (67.3) | 34 (63.0) | 0.02 |
| Age, yr | 68.1±11.3 | 68.6±11.2 | 65.8±12.5 | 69.9±9.1 | 68.1±11.9 | 0.65 |
| White, n (%) | 207 (95.0) | 51 (94.4) | 52 (94.6) | 53 (96.4) | 51 (94.4) | 0.89 |
| Diabetes, n (%) | 69 (31.7) | 15 (27.8) | 21 (38.2) | 18 (32.7) | 15 (27.8) | 0.84 |
| Hypertension, n (%) | 190 (87.2) | 48 (88.9) | 51 (92.7) | 47 (85.5) | 44 (81.5) | 0.15 |
| Heart failure, n (%) | 75 (34.4) | 25 (46.3) | 17 (30.9) | 17 (30.9) | 16 (29.6) | 0.08 |
| PVD, n (%) | 40 (18.4) | 6 (11.1) | 13 (23.6) | 13 (23.6) | 8 (14.8) | 0.64 |
| COPD, n (%) | 69 (31.7) | 14 (25.9) | 20 (36.4) | 22 (40.0) | 13 (24.1) | 0.95 |
| eGFR, ml/min per 1.73 m2 | 71.9±19.3 | 71.5±19.5 | 73.2±23.2 | 67.4±15.6 | 75.4±17.8 | 0.53 |
| Serum creatinine, mg/dl | 1.1±0.3 | 1.1±0.3 | 1.1±0.4 | 1.1±0.2 | 1.0±0.2 | 0.12 |
| Urine ACR, mg/ga | 16.6 (6.2, 46.5) | 16.7 (7.1, 30.7) | 15.1 (5.4, 98.1) | 15.8 (5.9, 36.2) | 18.6 (7.8, 52.7) | 0.48 |
| LVEF, % | 51.1±14.5 | 50.8±16.2 | 50.1±15.9 | 50.8±13.1 | 52.8±12.6 | 0.87 |
| Preoperative radiocontrast, n (%) | 150 (68.8) | 39 (72.2) | 34 (61.8) | 41 (74.6) | 36 (66.7) | 0.90 |
| Sample storage time, yr | 8.4±2.1 | 8.4±1.8 | 8.5±2.1 | 8.4±2.2 | 8.4±2.4 | 0.57 |
| Surgery | ||||||
| Valve only, n (%) | 99 (45.4) | 26 (48.2) | 22 (40.0) | 27 (49.1) | 24 (44.4) | 0.95 |
| CPB perfusion time, min | 115.8±47.5 | 116.3±41.2 | 117.8±52.1 | 115.7±43.2 | 113.3±53.6 | 0.60 |
| Nonelective surgery, n (%) | 142 (65.1) | 33 (61.1) | 35 (63.6) | 37 (67.3) | 37 (68.5) | 0.37 |
All values are expressed as n (%) or mean±SD unless noted otherwise. Uromodulin/Cr, uromodulin-to-creatinine ratio; PVD, peripheral vascular disease; COPD, chronic obstructive pulmonary disease; ACR, albumin-to-creatinine ratio; LVEF, left ventricular ejection fraction; CPB, cardiopulmonary bypass.
Median (25th, 75th percentiles).
Of the 218 patients in our study cohort, AKI developed in 64 (29%). Of these 64 patients, 20 (37%) were in the first quartile of uromodulin-to-creatinine ratio, 18 (33%) were in the second quartile, 17 (31%) were in the third quartile, and nine (17%) were in the fourth quartile (trend P=0.02) (Table 2). Patients developing AKI were older, had a greater prevalence of heart failure, and had lower eGFR and lower uromodulin-to-creatinine ratio (Supplemental Table 2). They were also more likely to have cardiac valve surgery and longer cardiopulmonary bypass time. In unadjusted models, lower uromodulin-to-creatinine ratio was associated with higher odds for AKI (OR, 1.49 per 1 SD lower; 95% CI, 1.04 to 2.13; P=0.03), which remained significant after adjustment for model 1 variables (OR, 1.53; 95% CI, 1.06 to 2.20; P=0.02). After further adjustment (model 2), the association between uromodulin-to-creatinine ratio and AKI was marginally attenuated (OR, 1.43; 95% CI, 0.99 to 2.07; P=0.06) (Table 2). A sensitivity analysis that included further adjustment for COPD, PVD, prior radiocontrast use, bypass perfusion time, and urine sample storage time did not significantly alter the results (OR, 1.43; 95% CI, 0.99 to 2.08; P=0.06). Compared with patients in the fourth uromodulin-to-creatinine ratio quartile, those in the first quartile had a threefold higher odds for AKI after adjustment for model 1 variables (OR, 2.98; 95% CI, 1.19 to 7.46; P=0.02) (Table 2), although this was modestly attenuated and failed to reach statistical significance after adjusting for model 2 variables (OR, 2.43; 95% CI, 0.91 to 6.48; P=0.06). Only eight patients developed severe AKI, with three requiring dialysis. Of these, seven were in the first and second uromodulin-to-creatinine ratio quartiles, and none were in the fourth quartile. Although each 1-SD lower uromodulin-to-creatinine ratio was associated with two times greater adjusted odds for severe AKI or need for dialysis in adjusted models, this did not reach statistical significance (OR, 2.03; 95% CI, 0.80 to 5.17; P=0.14). Because of few severe AKI events, we compared the odds of AKI in persons with above– and below–median uromodulin-to-creatinine ratio. After adjusting for variable in model 2, compared with persons with uromodulin-to-creatinine levels above the median, those with levels below the median had a four times greater odds of severe AKI, although this was not statistically significant (OR, 4.03; 95% CI, 0.87 to 18.70; P=0.08). We found no significant interaction between nature of surgery (P=0.87) or CKD status (P=0.50) and uromodulin-to-creatinine ratio with AKI.
Table 2.
Association of urinary uromodulin-to-creatinine ratio and postoperative AKI
| Urinary Uromodulin-to-Creatinine Ratio | No. of Events (%) | Unadjusted OR (95% CI) | Model 1 OR (95% CI) | Model 2 OR (95% CI) |
|---|---|---|---|---|
| AKI | ||||
| Per 1 SDa ↓ | 64 (29.4) | 1.49 (1.04 to 2.13), P=0.03 | 1.53 (1.06 to 2.20), P=0.02 | 1.43 (0.99 to 2.07), P=0.06 |
| Quartile 1 (0.8–4.1), μg/g | 20 (37.0) | 2.94 (1.19 to 7.26) | 2.98 (1.19 to 7.46) | 2.43 (0.91 to 6.48) |
| Quartile 2 (4.2–9.9), μg/g | 18 (32.7) | 2.43 (0.98 to 6.05) | 2.70 (1.06 to 6.86) | 2.21 (0.81 to 6.01) |
| Quartile 3 (10.0–22.8), μg/g | 17 (30.9) | 2.24 (0.89 to 5.59) | 2.10 (0.84 to 5.26) | 1.69 (0.64 to 4.48) |
| Quartile 4 (22.9–93.7), μg/g | 9 (16.7) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Trend P value | 0.02 | 0.02 | 0.06 | |
| Severe AKI | ||||
| Per 1 SDa ↓ | 8 (3.7) | 3.15 (0.71 to 13.96), P=0.13 | 2.68 (0.85 to 8.42), P=0.09 | 2.03 (0.80 to 5.17), P=0.14 |
| Below versus above medianb | 7 (87.5) | 7.41 (0.90 to 61.3), P=0.06 | 6.67 (1.22 to 36.30), P=0.03 | 4.03 (0.87 to 18.70), P=0.08 |
Model 1: adjusted for age, sex, and race. Model 2: model 1 plus eGFR, diabetes, heart failure, left ventricular ejection fraction, elective versus nonelective surgery, valvular versus other cardiac surgery, and urine albumin-to-creatinine ratio. OR, odds ratio; 95% CI, 95% confidence interval.
1 SD=14.8 μg/g.
There was a total of eight events of severe AKI, of which seven (85%) occurred in the first two quartiles (below the median).
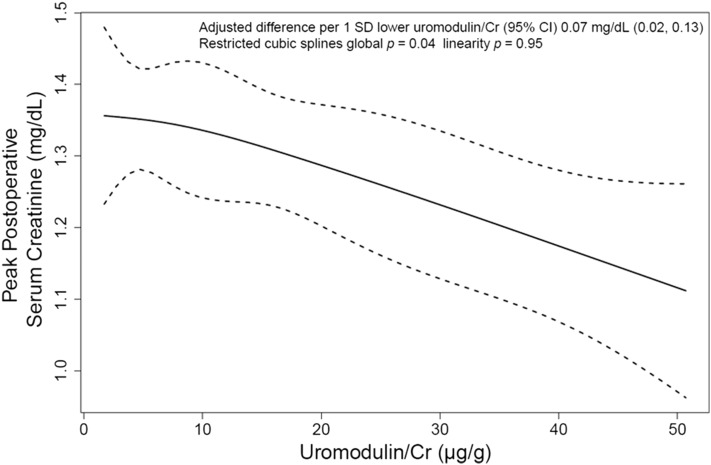
Lower levels of uromodulin-to-creatinine ratio were associated with a higher peak postoperative serum creatinine after multivariable adjustment (Figure 1). Each 1-SD lower uromodulin-to-creatinine ratio was associated with a 0.07-mg/dl (95% CI, 0.02 to 0.13; P=0.01) higher peak serum creatinine (Table 3).
Figure 1.
Higher uromodulin-to-creatinine ratio is associated with lower peak serum creatinine in patients undergoing cardiac surgery. Restricted cubic spline depicting the relationship of preoperative uromodulin concentration and postoperative peak serum creatinine adjusted for age, sex, race, diabetes, heart failure, left ventricular ejection fraction, eGFR, type and elective nature of the cardiac surgery, and urine albumin-to-creatinine ratio. The solid line represents the estimated association of urinary uromodulin-to-creatinine ratio with peak serum creatinine, and the dashed lines represent the corresponding 95% confidence interval (95% CI). We used the default number and location of knots along the distribution of uromodulin quantiles 5%, 35%, 65%, and 95% with the following values of uromodulin-to-creatinine ratio: 2.11, 6.03, 17.32, and 44.77 μg/g, respectively. The global P tests for the overall association between uromodulin concentration and postoperative peak serum creatinine, with P<0.05 suggestive of a significant association; the linearity P tests for departure from linearity in this association, with P>0.05 suggestive of a significant linear relationship. Cr, creatinine.
Table 3.
Association of urinary uromodulin-to-creatinine ratio with postoperative peak serum creatinine
| Uromodulin-to-Creatinine Ratio | Mean (SD) Peak Creatinine | Difference in Mean Peak Serum Creatinine (95% CI) | Model 1 Difference in Mean Peak Serum Creatinine (95% CI) | Model 2 Difference in Mean Peak Serum Creatinine (95% CI) |
|---|---|---|---|---|
| Per 1 SDa ↓ | 1.3 (0.5) | 0.11 (0.04 to 0.18), P=0.002 | 0.10 (0.03 to 0.17), P=0.003 | 0.07 (0.02 to 0.13), P<0.01 |
| Quartile 1 (0.8–4.1), μg/g | 1.4 (0.5) | 0.27 (0.08 to 0.47) | 0.23 (0.05 to 0.42) | 0.14 (−0.01 to 0.29) |
| Quartile 2 (4.2–9.9), μg/g | 1.4 (0.7) | 0.35 (0.16 to 0.55) | 0.34 (0.16 to 0.53) | 0.23 (0.08 to 0.38) |
| Quartile 3 (10.0–22.8), μg/g | 1.3 (0.5) | 0.21 (0.02 to 0.40) | 0.17 (−0.01 to 0.36) | 0.06 (−0.09 to 0.21) |
| Quartile 4 (22.9–93.7), μg/g | 1.1 (0.3) | 0 | 0 | 0 |
| Trend P value | 0.002 | <0.01 | 0.01 |
Model 1: adjusted for age, sex, and race. Model 2: model 1 plus eGFR, diabetes, heart failure, left ventricular ejection fraction, elective versus nonelective surgery, valvular versus other cardiac surgery, and urine albumin-to-creatinine ratio. 95% CI, 95% confidence interval.
1 SD=14.8 μg/g.
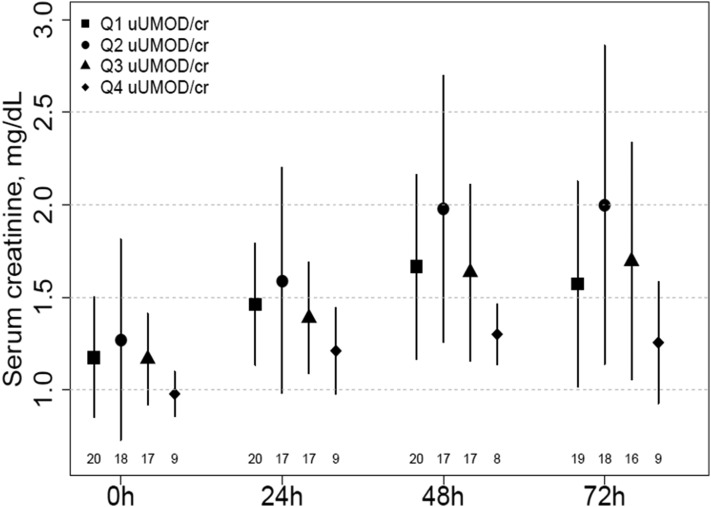
The mean peak serum creatinine values at 24, 48, and 72 hours among patients who developed AKI were lowest among persons in the highest quartile of uromodulin-to-creatinine ratio (Figure 2). At 72 hours, the mean serum creatinine in the highest quartile was at least 0.31 mg/dl lower than the mean serum creatinine in any other quartile, although this did not reach statistical significance (Supplemental Table 3).
Figure 2.
Higher uromodulin-to-creatinine ratio is associated with lesser rise of serum creatinine in patients with AKI. Figure shows the trend of mean (SD) serum creatinine values over 72 hours in patients with AKI. Each point represents the mean serum creatinine for patients in the respective uromodulin-to-creatinine ratio quartile (Q) preoperatively (0 hours) and 24, 48, and 72 hours postoperatively. The lines on either side of the point represent the SDs. The numbers at each time point indicate the numbers of patients with serum creatinine measures. uUMOD/cr, urinary uromodulin-to-creatinine ratio.
In a sensitivity analysis, the direction of the association of the raw nonindexed uromodulin was similar to the primary results in both the continuous models (OR, 1.13; 95% CI, 0.82 to 1.56; P=0.46) and the lowest versus highest quartile models (OR, 1.61; 95% CI, 0.67 to 3.90; P=0.30), but the strength of the association was weaker.
Discussion
In this study, we found that lower urinary concentrations of uromodulin are associated with higher odds for AKI in adults undergoing on–pump cardiac surgery. This association was of borderline statistical significance in multivariable models and consistent for more severe AKI, although there were too few events to make definitive conclusions. In addition, urinary uromodulin levels were inversely associated with the peak postoperative serum creatinine.
The incidence of AKI after cardiac surgery is as high as 30% (1–3). AKI is associated with increased hospital length of stay, in–hospital mortality, and risk of development of CKD (30). Even mild postoperative AKI may be associated with worse short– and long–term outcomes (31,32). A recent propensity–matched cohort of 833 patients undergoing cardiac surgery showed that persons who developed mild AKI had nearly eightfold greater risk of in-hospital mortality, longer intensive care and hospital stay, higher rate of neurologic complications, and greater sternal wound infection (33). Identifying persons at risk for AKI after cardiac surgery is an important step toward minimizing postoperative AKI and its complications. Assessing tubular function might help identify those at risk for AKI independent of eGFR.
Uromodulin forms a gel on the luminal surface of the thick ascending limb of the loop of Henle, preventing water permeability in this segment (6). In humans, uromodulin excretion is believed to increase gradually from birth, and concentrations remain relatively stable from the age of 4 years old to the seventh decade of life (5). Levels of uromodulin were significantly lower in our cohort than observed in healthy community–dwelling adults using the same assay (11) and also in other studies using a different ELISA (9,10) (25.7–26.0 μg/ml). Despite this, we showed better kidney outcomes among persons with higher uromodulin levels (11). Reasons for the lower uromodulin levels observed in our study are unclear, but possibilities include differences in assays and the patient characteristics. One study across diverse populations showed differences in uromodulin levels on the basis of a particular rs12917707genotype using a Luminex immunoassay (34). The patients in our study were undergoing cardiac surgery, some nonelectively, and we hypothesize that they were likely in poorer health with greater vascular disease and possibly, subclinical tubular damage, leading to lower uromodulin levels.
Umod gene knockout mice develop a greater rise in serum creatinine compared with wild-type mice (12) and display more histologic damage, with diffuse tubular necrosis affecting predominantly S3 segments of the outer medulla (12,35). Inflammation is an important contributor to the pathogenesis of AKI (36), and Umod gene knockout mice show a greater degree of neutrophil infiltration, especially around injured S3 segments, compared with wild-type mice after ischemic injury (35). During ischemia-reperfusion injury, interstitial uromodulin is associated with downregulation of inflammatory signaling in contiguous S3 proximal tubules, suggesting a role for uromodulin in a protective tubular crosstalk during AKI (35,37). Umod gene knockout mice also have impaired recovery from AKI up to 5 weeks after an ischemic injury, whereas wild-type mice experience renal recovery within 1 week of the injury (38). This suggests that the protein might be essential for the recovery from AKI.
Two small studies (n≤30) conducted in patients undergoing cardiac surgery and critically ill patients suggest that urinary uromodulin concentrations decrease after AKI (39,40). These studies did not evaluate whether lower uromodulin levels were associated with risk of AKI. To our knowledge, only two studies have assessed the relationship between uromodulin and risk of AKI. In one study of 36 liver transplant recipients, those who developed AKI had lower pretransplant urinary uromodulin concentrations compared with those who did not develop AKI (41). Concentrations of uromodulin in those who did not develop AKI were similar to those in patients not undergoing liver transplantation. In newborns admitted to an intensive care unit, low uromodulin levels were shown to be predictive of AKI (42). Our findings are consistent with the aforementioned experimental data and small observational studies in humans. We show that persons with lower preoperative urinary uromodulin concentrations have higher odds for developing AKI after cardiac surgery and higher peak serum creatinine. Studies of longer duration in patients with more severe AKI are needed to accurately assess if urinary uromodulin levels associate with faster recovery of kidney function.
Several limitations in our study should be noted. Most patients had AKI of mild severity, and therefore, our findings may not be generalizable to more severe forms of AKI. We measured uromodulin in urine specimens that were collected and stored for approximately 10 years. Storage for over 8 months, even at −80°C, may slightly decrease uromodulin levels (43). However, we do not expect a differential effect on AKI of long-term storage at this temperature. Because we did not include oligoanuria as one of the KDIGO criteria for diagnosing AKI, we may have missed some patients with AKI. However, it is unlikely that we missed any clinically important patients due to this limitation. Although urine output may have been maintained by diuretic use in our cohort, brief durations of oliguria may simply reflect insufficient volume resuscitation (44). Serum creatinine values were not systematically collected beyond 72 hours after cardiac surgery, thus limiting our ability to assess AKI later in the course of the hospitalization and also limiting the ability to assess whether uromodulin concentrations were associated with recovery from AKI. Finally, we are unable to comment on whether uromodulin concentrations change after AKI and whether serial measurement of uromodulin may be better at identifying persons at risk for nonrecovery from AKI.
Our study also has several strengths. To our knowledge, this is the first prospective study testing the hypothesis that higher urinary uromodulin, a marker of tubular health, is associated with lower postoperative risk of AKI in patients undergoing cardiac surgery. The stored urine samples in our study had never been thawed, thus minimizing the effect of freeze-thaw cycles on the measurement of uromodulin. We used a commercially available ELISA, which has been evaluated in prior studies (11,45–47). Despite relatively few events, we were able to detect an association that was of borderline significance between lower urinary uromodulin concentrations and higher odds for development of AKI. Importantly, the results were independent of baseline eGFR, which is a potential confounder (9). The higher risk of AKI with lower uromodulin concentrations is consistent with our previous work showing similar findings with progressive eGFR decline in a large cohort of community-dwelling adults (11). Our study evaluated whether uromodulin was associated with development of AKI in adults undergoing cardiac surgery with a goal of assessing kidney function through a novel axis of tubular function, but it was not to replace either eGFR or ACR. The proof of principle findings in this study are exploratory; they improve our understanding of how tubular health may be associated with AKI and highlight the need for further studies in this field.
In conclusion, among adults undergoing on–pump cardiac surgery, low uromodulin-to-creatinine ratio was associated with higher odds of AKI, although this was no longer significant in fully adjusted analyses. Large studies are needed to confirm these results and evaluate whether uromodulin can be used to identify high-risk patients and target therapies to prevent AKI.
Disclosures
None.
Supplementary Material
Acknowledgments
The parent study was supported by a grant from the Norman Coplon Research Program of Satellite Healthcare, Inc. (to B.L.J.) and grant 0535367N from the American Heart Association (to O.L.). P.S.G. was supported by National Institutes of Health (NIH) training grant 5T32DK007777 and the Tufts Medical Center Division of Nephrology Driscoll Fund. P.D. was supported by grant P50 DK096418 from the NIH.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02520316/-/DCSupplemental.
References
- 1.Conlon PJ, Stafford-Smith M, White WD, Newman MF, King S, Winn MP, Landolfo K: Acute renal failure following cardiac surgery. Nephrol Dial Transplant 14: 1158–1162, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J: Independent association between acute renal failure and mortality following cardiac surgery. Am J Med 104: 343–348, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Rosner MH, Okusa MD: Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 1: 19–32, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Swaminathan M, Garg AX; TRIBE-AKI Consortium : Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 22: 1748–1757, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lhotta K: Uromodulin and chronic kidney disease. Kidney Blood Press Res 33: 393–398, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Vyletal P, Bleyer AJ, Kmoch S: Uromodulin biology and pathophysiology--an update. Kidney Blood Press Res 33: 456–475, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Pak J, Pu Y, Zhang ZT, Hasty DL, Wu XR: Tamm-Horsfall protein binds to type 1 fimbriated Escherichia coli and prevents E. coli from binding to uroplakin Ia and Ib receptors. J Biol Chem 276: 9924–9930, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Glauser A, Hochreiter W, Jaeger P, Hess B: Determinants of urinary excretion of Tamm-Horsfall protein in non-selected kidney stone formers and healthy subjects. Nephrol Dial Transplant 15: 1580–1587, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Pruijm M, Ponte B, Ackermann D, Paccaud F, Guessous I, Ehret G, Pechère-Bertschi A, Vogt B, Mohaupt MG, Martin PY, Youhanna SC, Nägele N, Vollenweider P, Waeber G, Burnier M, Devuyst O, Bochud M: Associations of urinary uromodulin with clinical characteristics and markers of tubular function in the general population. Clin J Am Soc Nephrol 11: 70–80, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troyanov S, Delmas-Frenette C, Bollée G, Youhanna S, Bruat V, Awadalla P, Devuyst O, Madore F: Clinical, genetic, and urinary factors associated with uromodulin excretion. Clin J Am Soc Nephrol 11: 62–69, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garimella PS, Biggs ML, Katz R, Ix JH, Bennett MR, Devarajan P, Kestenbaum BR, Siscovick DS, Jensen MK, Shlipak MG, Chaves PH, Sarnak MJ: Urinary uromodulin, kidney function, and cardiovascular disease in elderly adults. Kidney Int 88: 1126–1134, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Achkar TM, Wu XR, Rauchman M, McCracken R, Kiefer S, Dagher PC: Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am J Physiol Renal Physiol 295: F534–F544, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Susantitaphong P, Perianayagam MC, Tighiouart H, Liangos O, Bonventre JV, Jaber BL: Tumor necrosis factor alpha promoter polymorphism and severity of acute kidney injury. Nephron Clin Pract 123: 67–73, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perianayagam MC, Tighiouart H, Liangos O, Kouznetsov D, Wald R, Rao F, O’Connor DT, Jaber BL: Polymorphisms in the myeloperoxidase gene locus are associated with acute kidney injury-related outcomes. Kidney Int 82: 909–919, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMahon GM, Waikar SS: Biomarkers in nephrology: Core curriculum 2013. Am J Kidney Dis 62: 165–178, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liangos O, Kolyada A, Tighiouart H, Perianayagam MC, Wald R, Jaber BL: Interleukin-8 and acute kidney injury following cardiopulmonary bypass: A prospective cohort study. Nephron Clin Pract 113: c148–c154, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liangos O, Tighiouart H, Perianayagam MC, Kolyada A, Han WK, Wald R, Bonventre JV, Jaber BL: Comparative analysis of urinary biomarkers for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers 14: 423–431, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wald R, Liangos O, Perianayagam MC, Kolyada A, Herget-Rosenthal S, Mazer CD, Jaber BL: Plasma cystatin C and acute kidney injury after cardiopulmonary bypass. Clin J Am Soc Nephrol 5: 1373–1379, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.KDIGO AKI Work Group: KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 124–138, 2012 [Google Scholar]
- 20.Swaminathan M, Phillips-Bute BG, Conlon PJ, Smith PK, Newman MF, Stafford-Smith M: The association of lowest hematocrit during cardiopulmonary bypass with acute renal injury after coronary artery bypass surgery. Ann Thorac Surg 76: 784–791, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M: Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: A prospective cohort study. J Am Soc Nephrol 15: 1597–1605, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Mehta RH, Grab JD, O’Brien SM, Bridges CR, Gammie JS, Haan CK, Ferguson TB, Peterson ED; Society of Thoracic Surgeons National Cardiac Surgery Database Investigators : Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation 114: 2208–2216, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Raffi HS, Bates JM Jr., Laszik Z, Kumar S: Tamm-horsfall protein protects against urinary tract infection by proteus mirabilis. J Urol 181: 2332–2338, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Coca SG, Jammalamadaka D, Sint K, Thiessen Philbrook H, Shlipak MG, Zappitelli M, Devarajan P, Hashim S, Garg AX, Parikh CR; Translational Research Investigating Biomarker Endpoints in Acute Kidney Injury Consortium : Preoperative proteinuria predicts acute kidney injury in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg 143: 495–502, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wijeysundera DN, Karkouti K, Dupuis JY, Rao V, Chan CT, Granton JT, Beattie WS: Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. JAMA 297: 1801–1809, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP: A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol 16: 162–168, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Firth D: Bias reduction of maximum likelihood estimates. Biometrika 80: 27–38, 1993 [Google Scholar]
- 29.R Core Team: R: A Language and Environment for Statistical Computing, Vienna, Austria, R Foundation for Statistical Computing, 2013
- 30.Loef BG, Epema AH, Smilde TD, Henning RH, Ebels T, Navis G, Stegeman CA: Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol 16: 195–200, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Choi JS, Kim YA, Kim MJ, Kang YU, Kim CS, Bae EH, Ma SK, Ahn YK, Jeong MH, Kim SW: Relation between transient or persistent acute kidney injury and long-term mortality in patients with myocardial infarction. Am J Cardiol 112: 41–45, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Zhu M, Li Y, Xia Q, Wang S, Qiu Y, Che M, Dai H, Qian J, Ni Z, Axelsson J, Yan Y: Strong impact of acute kidney injury on survival after liver transplantation. Transplant Proc 42: 3634–3638, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Elmistekawy E, McDonald B, Hudson C, Ruel M, Mesana T, Chan V, Boodhwani M: Clinical impact of mild acute kidney injury after cardiac surgery. Ann Thorac Surg 98: 815–822, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Olden M, Corre T, Hayward C, Toniolo D, Ulivi S, Gasparini P, Pistis G, Hwang SJ, Bergmann S, Campbell H, Cocca M, Gandin I, Girotto G, Glaudemans B, Hastie ND, Loffing J, Polasek O, Rampoldi L, Rudan I, Sala C, Traglia M, Vollenweider P, Vuckovic D, Youhanna S, Weber J, Wright AF, Kutalik Z, Bochud M, Fox CS, Devuyst O: Common variants in UMOD associate with urinary uromodulin levels: A meta-analysis. J Am Soc Nephrol 25: 1869–1882, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Achkar TM, McCracken R, Rauchman M, Heitmeier MR, Al-Aly Z, Dagher PC, Wu XR: Tamm-Horsfall protein-deficient thick ascending limbs promote injury to neighboring S3 segments in an MIP-2-dependent mechanism. Am J Physiol Renal Physiol 300: F999–F1007, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonventre JV, Zuk A: Ischemic acute renal failure: An inflammatory disease? Kidney Int 66: 480–485, 2004 [DOI] [PubMed] [Google Scholar]
- 37.El-Achkar TM, Wu XR: Uromodulin in kidney injury: An instigator, bystander, or protector? Am J Kidney Dis 59: 452–461, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Achkar TM, McCracken R, Liu Y, Heitmeier MR, Bourgeois S, Ryerse J, Wu XR: Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am J Physiol Renal Physiol 304: F1066–F1075, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dehne MG, Boldt J, Heise D, Sablotzki A, Hempelmann G: Tamm-Horsfall protein, alpha-1- and beta-2-microglobulin as kidney function markers in heart surgery. Anaesthesist 44: 545–551, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Dehne MG, Sablotzki A, Mühling J, Papke G, Kuntzsch U, Hempelmann G: Acute kidney failure. Non-invasive diagnosis of acute kidney failure in operative intensive care patients. Anaesthesist 47: 193–201, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Romero MC, Zanaro N, González L, Trigo P, Imventarza O, Nesse A: Tamm-Horsfall protein excretion to predict the onset of renal insufficiency. Clin Biochem 35: 65–68, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Askenazi DJ, Koralkar R, Hundley HE, Montesanti A, Parwar P, Sonjara S, Ambalavanan N: Urine biomarkers predict acute kidney injury in newborns. J Pediatr 161: 270–275.e1, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Youhanna S, Weber J, Beaujean V, Glaudemans B, Sobek J, Devuyst O: Determination of uromodulin in human urine: Influence of storage and processing. Nephrol Dial Transplant 29: 136–145, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Fliser D, Laville M, Covic A, Fouque D, Vanholder R, Juillard L, Van Biesen W; Ad-hoc working group of ERBP : A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: Part 1: Definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant 27: 4263–4272, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlatzer D, Maahs DM, Chance MR, Dazard JE, Li X, Hazlett F, Rewers M, Snell-Bergeon JK: Novel urinary protein biomarkers predicting the development of microalbuminuria and renal function decline in type 1 diabetes. Diabetes Care 35: 549–555, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reznichenko A, van Dijk MC, van der Heide JH, Bakker SJ, Seelen M, Navis G: Uromodulin in renal transplant recipients: Elevated urinary levels and bimodal association with graft failure. Am J Nephrol 34: 445–451, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Reznichenko A, Böger CA, Snieder H, van den Born J, de Borst MH, Damman J, van Dijk MC, van Goor H, Hepkema BG, Hillebrands JL, Leuvenink HG, Niesing J, Bakker SJ, Seelen M, Navis G; REGaTTA (REnal GeneTics TrAnsplantation) Groningen group : UMOD as a susceptibility gene for end-stage renal disease. BMC Med Genet 13: 78, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.