Abstract
Accidental or intentional exposures to parathion, an organophosphorus (OP) pesticide, can cause severe poisoning in humans. Parathion toxicity is dependent on its metabolism by the cytochrome P450 (CYP) system to paraoxon (diethyl 4-nitrophenyl phosphate), a highly poisonous nerve agent and potent inhibitor of acetylcholinesterase (AChE). We have been investigating inhibitors of CYP-mediated bioactivation of OPs as a method of preventing or reversing progressive parathion toxicity. It is well recognized that NADPH–cytochrome P450 reductase, an enzyme required for the transfer of electrons to CYPs, mediates chemical redox cycling. In this process, the enzyme diverts electrons from CYPs to support chemical redox cycling, which results in inhibition of CYP-mediated biotransformation. Using menadione as the redox-cycling chemical, we discovered that this enzymatic reaction blocks metabolic activation of parathion in rat and human liver microsomes and in recombinant CYPs important to parathion metabolism, including CYP1A2, CYP2B6, and CYP3A4. Administration of menadione to rats reduces metabolism of parathion, as well as parathion-induced inhibition of brain cholinesterase activity. This resulted in inhibition of parathion neurotoxicity. Menadione has relatively low toxicity and is approved by the FDA for other indications. Its ability to block parathion metabolism makes it an attractive therapeutic candidate to mitigate parathion-induced neurotoxicity.
Keywords: redox cycling, parathion, paraoxon, menadione, organophosphorus pesticides
Introduction
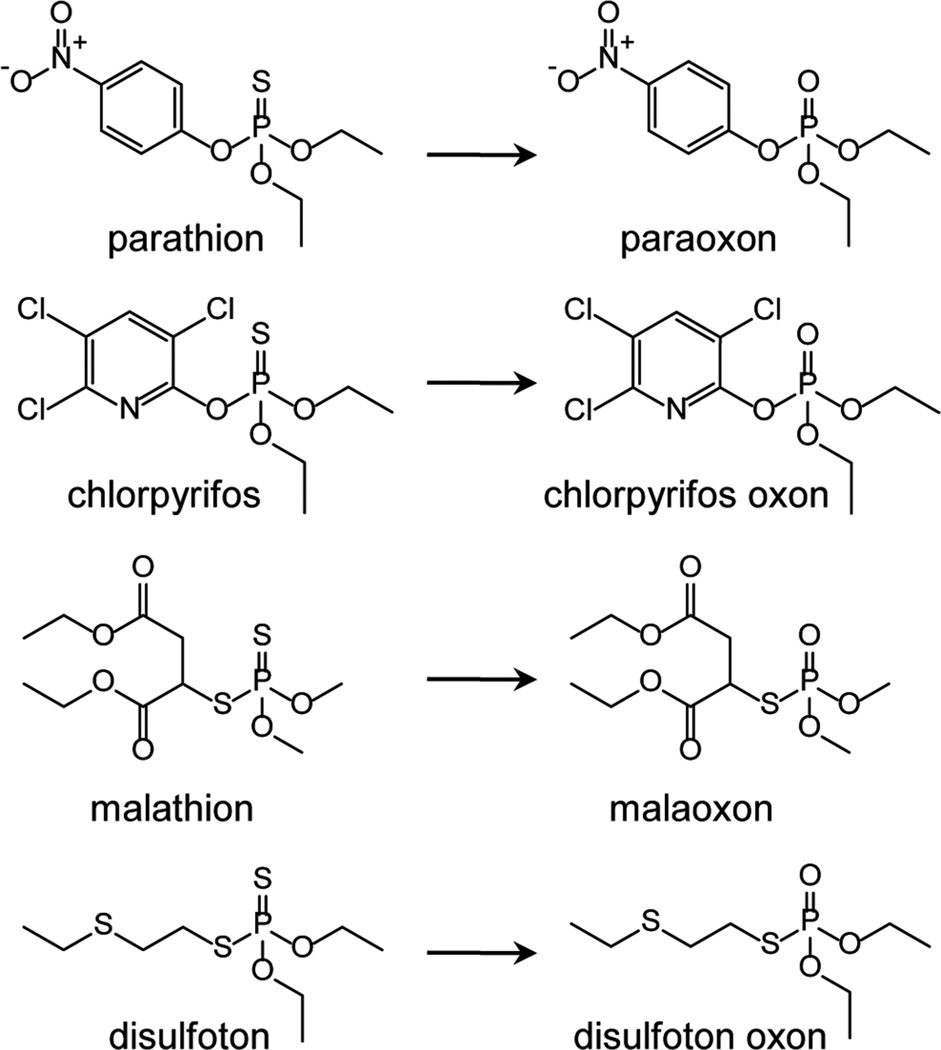
Organophosphates (OPs) are widely used as pesticides, as they are inhibitors of many serine esterases, notably including acetylcholinesterase (AChE).1,2 Exposure of humans to OPs, either as a result of accidental or intentional exposures, can result in severe neurotoxicity.1–3 The most common organophosphates are modified esters of phosphoric acid. Phosphotriesters, which contain a phosphoryl (P=O bond) group, are direct inhibitors of AChE, while phosphorothioate pesticides, which contain a thiophosphoryl (P=S bond) group, are indirect inhibitors of AChE and require metabolic activation to became biologically active oxons.3 This latter class of cholinesterase inhibitors includes a variety of OP pesticides, such as parathion, chlorpyrifos, malathion, and disulfoton, each of which becomes more toxic than the parent compound following metabolic activation by the cytochrome P450 (CYP) system (see Fig. 1 for structures). The former class of OPs includes traditional nerve warfare agents, such as tabun, sarin, soman, VX, and oxono metabolites of phosphorothioate pesticides. As a result of inhibiting AChE, these agents cause an accumulation of acetylcholine in synapses, a process that can cause overstimulation of muscarinic and nicotinic cholinergic receptors.1,3 This can lead to a “cholinergic crisis,” leading to tremors, convulsions, and increased secretions.1,3
Figure 1.
Examples of phosphorothioate pesticides and corresponding oxon metabolites generated following CYP-mediated metabolism.
Because of the threat of toxicity resulting from exposure to OPs, there remains a considerable interest in the development and use of effective antidotes. Two drugs that are currently approved for OP poisoning are atropine and pralidoxime (2-PAM).4,5 Atropine is a competitive AChE antagonist but is limited by the fact that it only targets muscarinic AChE.4 2-PAM binds to OP-inactivated AChE and regenerates the enzyme; however, it is a charged molecule and does not efficiently cross the blood–brain barrier.6 New strategies to generate more effective drugs to treat OP intoxication have included oxime reactivators that can cross the blood–brain barrier,7,8 chemical scavengers such as cyclodextrins,9 and bioscavengers, which include enzymes that neutralize and/or detoxify OPs, such as AChE, butyrylcholinesterases, carboxylesterases, paraoxonases, and phosphotriesterases.10–13 Many of these drug candidates do not possess a broad spectrum for many OP substrates; enzymes scavengers are also difficult to produce, and many lack an efficient delivery system to target organs.14–16
An alternative approach to protect against the toxicity of indirect OP AChE inhibitors is to limit their metabolism to active compounds; this can be accomplished by increasing enzymes that detoxify OPs and inhibiting CYPs critical for their metabolism. Earlier studies using pretreatments with agents that either increased detoxification enzymes or inhibited CYP activity met with only limited success. For example, compounds that increase detoxification of OPs, such as chlorcyclizine, phenobarbital, beta-napthoflavone, and polychlorinated biphenyls reduce the toxicity of the OP and parathion in animal models; however, several days of pretreatments are required.17,18 Compounds that directly inhibit CYPs, such as cimetidine (1-cyano-2-methyl-3-[2-[(5-methyl-1H-imidazol-4-yl)methylsulfanyl]ethyl]guanidine), a H2 histamine receptor blocker, unexpectedly enhanced oxon formation from parathion, as well as parathion toxicity.19,20 Piperonyl butoxide, a pesticide that also inhibits CYP activity, was found to reduce the lethality of parathion in mice.21 However, in rats, it had no effect on parathion-induced inhibition of brain cholinesterase.22 Whereas nonselective CYP antagonists, including SKF525A (2-diethylaminoethyl 2,2-diphenylpentanoate) and ketoconazole, were found to inhibit OP metabolism in rodents, their ability to suppress OP intoxication was limited, possibly due to the fact that they also inhibit degradation of the biologically active OP oxons.23,24 Because of deficits in the ability of different inhibitors of OP metabolism to prevent or reverse toxicity, none have been approved by the Food and Drug Administration for use in humans suffering from OP intoxication.
The cytochrome P450 system and chemical redox cycling
CYPs play essential roles in the biotransformation of a wide variety of endogenous and exogenous compounds, including OPs.25,26 Biotransformation is dependent on the transfer of reducing equivalents from NADPH to the CYPs, a process mediated by the flavin adenine dinucleotide (FAD) and the flavin mononucleotide (FMN)–containing enzyme NADPH–cytochrome P450 reductase.26 This reaction involves an interflavin electron transfer between stable semiquinone FAD/FMN intermediates in NADPH–cytochrome P450 reductase followed by a sequential two-electron transfer to CYP heme. Of interest is the fact that NADPH–cytochrome P450 reductase also mediates a process known as chemical redox cycling.27 In this NADPH-dependent process, redox-active compounds are enzymatically reduced to radical anions, which are unstable. Readily reducing molecular oxygen, these radicals generate superoxide anion and, in the process, regenerate the parent compound, which is then free, once again, to be reduced by NADPH–cytochrome P450 reductase. As this process continually recurs, utilizes, a disproportionate amount of reducing equivalents and oxygen, generates reactive oxygen species, and does not appear to be metabolically relevant for cell function, it has been referred to as “futile metabolism” or “futile redox cycling.”27,28 Superoxide anions formed through this cycle can be converted to hydrogen peroxide via superoxide dismutase.28 While hydrogen peroxide can be detoxified by enzymes, such as catalase, glutathione peroxidase, and the peroxiredoxins, in the presence of transition metals, hydrogen peroxide can also form highly toxic hydroxyl radicals, which can cause cell damage.29 Superoxide anions can also react with nitric oxide, forming peroxynitrite, a reactive nitrogen intermediate that can induce nitrosative stress.30
In addition to generating reactive oxygen species, which can lead to oxidative and nitrosative stress, chemical redox cycling has the potential to deplete cells of NADPH and oxygen, compromising metabolic reactions requiring these co-substrates.31 Low oxygen tension can also stabilize radical anions; their reactivity with cellular macromolecules, including DNA, lipids, and proteins, can also lead to toxicity. Potential damage will depend on localized concentrations of the redox-cycling chemical, length of time of exposure to these agents, activity of redox-cycling enzymes, and the ability of cells and tissue to compensate by increasing metabolic activity under low oxygen tension and regenerating NADPH. Under normal homeostatic conditions, it would be expected that levels of NADPH would not be compromised and that diffusion of oxygen into cells and tissues would be sufficient to overcome losses during redox cycling. There are a number of bioreductive anticancer drugs that generate radical species during redox cycling.32 It is thought that the long half-life of these radicals at low oxygen tensions in tumors increases their reactivity and results in increased antitumor activity.32 Conversely, redox cycling under aerobic conditions and the generation of reactive oxygen species are thought to be important in the antitumor and/or antimicrobial action of a number of chemotherapeutic agents, including nitrofurantoin, mitomycin C, and the anthracycline antibiotics.33–35
Chemical redox cycling by NADPH–cytochrome P450 reductase inhibits CYP activity and OP metabolism
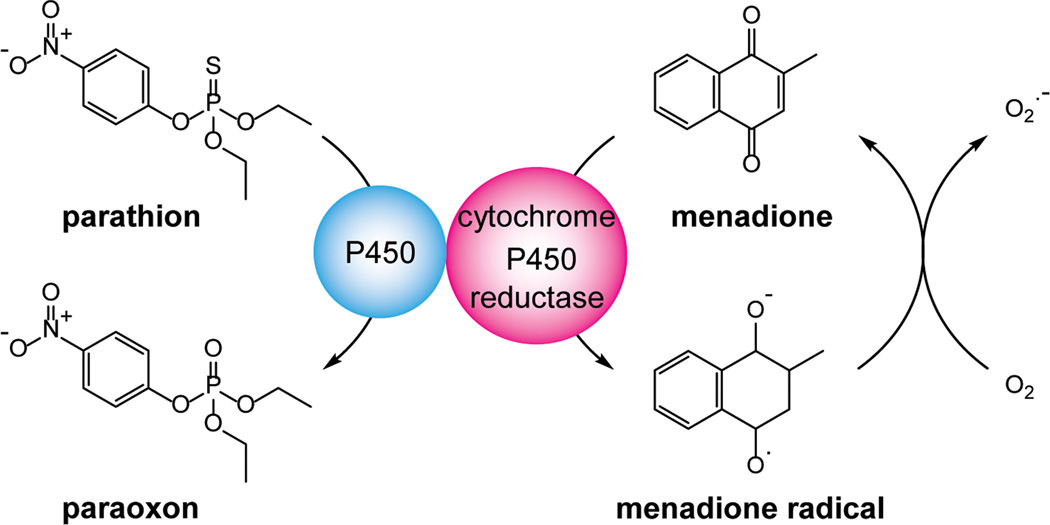
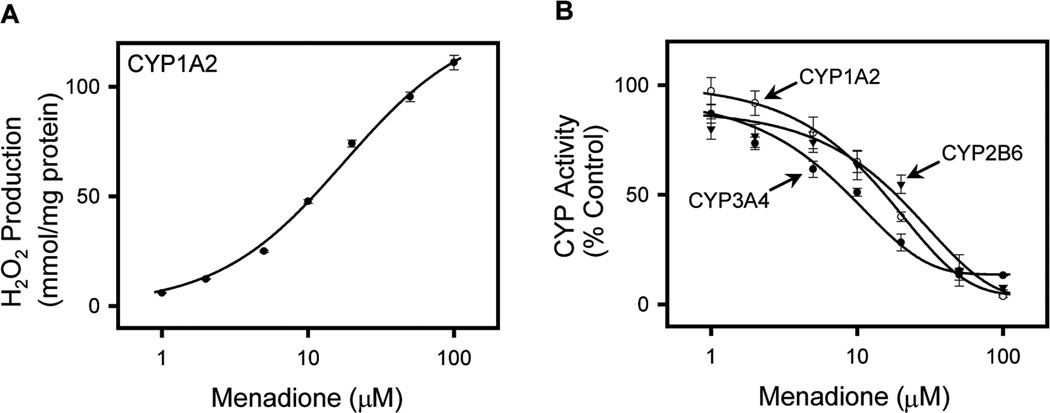
Our laboratory has been characterizing the redox-cycling activity of the p-quinone menadione (methyl-1,4-naphthoquinone), also known as vitamin K3.36 In the presence of NADPH–cytochrome P450 reductase, menadione readily redox cycles, generating superoxide anion and hydrogen peroxide. Due to its relatively low activation energy, this reaction preferentially diverts electrons from their supply to CYPs, resulting in inhibition of CYP-mediated biotransformation reactions (see Fig. 2 for a model of the effects of menadione on CYP-mediated metabolism). It is not specific for an individual CYP. Thus, using recombinant enzymes, we found that menadione inhibits multiple human P450 isoforms from different subfamilies, including CYP1A1, CYP1A2, CYP1B1, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4, CYP3A5, and CYP 3A7 (see Fig. 3 for an example of chemical redox cycling and the inhibition of CYP activity).36 These data are consistent with earlier studies showing that menadione can inhibit CYP-mediated aniline-p-hydroxylation and aminopyrine-N-demethylation in rat liver microsomes.37
Figure 2.
Redox cycling of menadione suppresses parathion metabolism by the P450 system. NADPH–cytochrome P450 reductase catalyzes the one-electron reduction of menadione, generating a semiquinone radical. Under aerobic conditions, these radicals react rapidly with molecular oxygen to form superoxide anion, regenerating menadione in the process. Redox cycling diverts electrons from CYP-mediated parathion metabolism. This inhibits formation of paraoxon and suppresses parathion toxicity.
Figure 3.
Menadione redox cycling inhibits CYP enzyme activities. (A) CYP1A2 mediates menadione redox cycling as measured by the formation of hydrogen peroxide (H2O2). H2O2 formation was assayed using the Amplex Red assay and is presented as mmol/min/mg protein. (B) Menadione causes a concentration-dependent inhibition of the activities of the major CYPs that mediate parathion metabolism. CYP1A2, CYP2B6, and CYP3A4 are recombinant human CYP enzyme preparations prepared from insect cells coexpressing human NADPH–cytochrome P450 reductase. Substrates used to measure CYP1A2, CYP2B6, and CYP3A4 activity were 7-ethoxyresorufin, 7-ethoxymethyloxy-3-cyanocoumarin, and dibenzylfluorescein, respectively. The data points shown the mean ± SE, n = 3. Summary of data from Ref. 36.
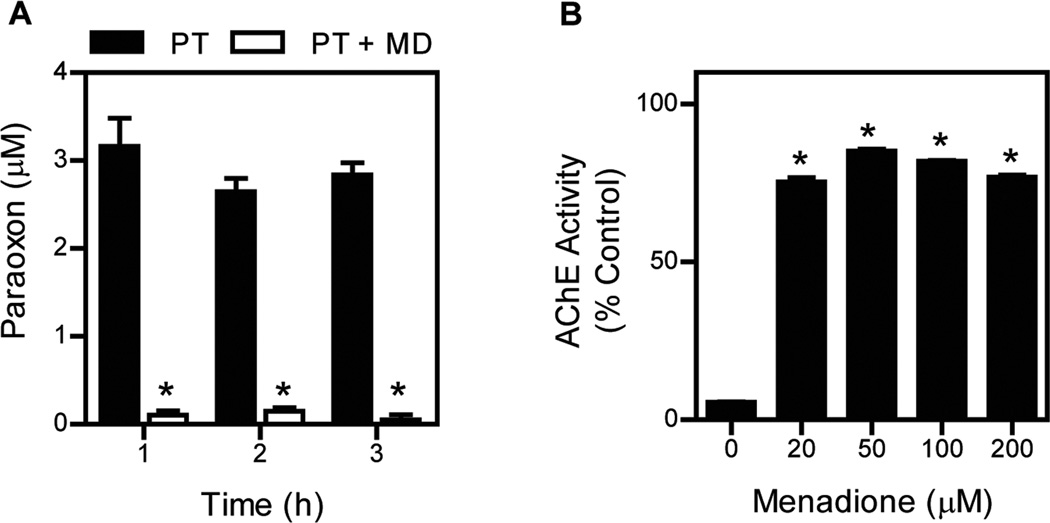
As CYP activity is required for OP metabolism, we tested the ability of menadione to inhibit metabolic activation of an OP to its oxon. For these studies we used parathion (O,O-diethyl O-(4-nitrophenyl) phosphorothioate), an important agricultural OP pesticide that is considered a high-priority chemical threat. The toxicity of parathion is dependent on CYP-mediated conversion to paraoxon (diethyl 4-nitrophenyl phosphate), a highly active inhibitor of cholinesterases.36 Enzymes important in metabolizing parathion include CYP1A2, CYP2B6, and CYP3A4;38 menadione was found to inhibit metabolism of parathion to paraoxon by each of these enzymes. Moreover, menadione was also found to inhibit parathion metabolism in human and rat liver microsomes (see Fig. 4 showing that menadione inhibits parathion metabolism).36 In these studies, we also confirmed that menadione inhibited CYP-mediated metabolism of parathion to paraoxon by demonstrating that it prevented inhibition of AChE. Thus, human liver microsome–mediated metabolism of parathion to paraoxon led to the inhibition of AChE, which was blocked by menadione (see Fig. 4, showing that menadione inhibits parathion metabolism and AChE inhibition induced by parathion and its metabolites).
Figure 4.
Menadione redox cycling inhibits parathion metabolism. (A) Human liver microsomes were treated with 20 µM parathion (PT) in the absence and presence of 50 µM menadione. At the indicated times, reactions were assayed for paraoxon. Data are mean ± SE (n= 3). Asterisks show that the formation of paraoxon in the presence of menadione was significantly different (P < 0.05) from that in the absence of menadione. (B) The ability of menadione to reverse the inhibitory effects of parathion on AChE activity in reactions with human liver microsomes. Reactions containing human liver microsomes were supplemented with AChE. Incubations with parathion inhibited AChE activity, and this was reversed by menadione. The figures show that menadione redox cycling readily inhibited paraoxon formation. Each point is the mean ± SE (n = 3). Asterisks show that the inhibition of AChE in the presence of menadione was significantly different (p < 0.05) from that in the absence of menadione. Summary of data from Ref. 36.
Menadione inhibits parathion intoxication in rats
Since it blocks parathion metabolism in rodents, menadione would be expected to inhibit signs of parathion intoxication, and indeed this was found to be the case. Treatment of rats with parathion (8 mg/kg, oral gavage) was found to induce neurotoxicity, which included a significant startle response, tremulous jaw movements, tremors, and convulsions.36 Each of these signs of toxicity was significantly diminished in rats treated with menadione (40 mg/kg, intraperitoneal) 30 min after parathion exposure. As expected, metabolism of parathion to paraoxon in the rats was also inhibited, as menadione caused a significant increase in levels of brain and serum parathion. Moreover, inhibition of cholinesterase activity in the forebrain and hindbrain, an indication of paraoxon formation in rats treated with parathion, was blocked by menadione (Table 1).36 These data demonstrate that menadione is effective in inhibiting parathion metabolism in vivo.
Table 1.
Effects of menadione on parathion levels and esterase activities in rat tissues36
| Treatment |
||
|---|---|---|
| Parathion | Parathion + menadione | |
| Parathion levels in tissue | ||
| Liver (µg/g tissue) | 1.9 ± 0.3 | 1.9 ± 0.5 |
| Brain (µg/g tissue) | 0.1 ± 0.05 | 0.9 ± 0.3 |
| Serum (µg/mL) | 0.4 ± 0.1 | 2.4 ± 0.3 |
| CES activity (% control) in tissue | ||
| Liver | 6.4 ± 0.5 | 15.3 ± 1.5 |
| Serum | 7.0 ± 1.8 | 18.0 ± 2.1 |
| ChE activity (% control) in brain | ||
| Forebrain | 7.3 ± 1.1 | 90.0 ± 3.6 |
| Hindbrain | 15.5 ± 1.3 | 86.8 ± 2.3 |
Rats were administered parathion (8 mg/kg, oral gavage) followed 20 min later by menadione (40 mg/kg, IP). Sixty minutes after parathion treatment, rats were sacrificed. Carboxylesterase (CES) and cholinesterase (ChE) activities were determined using p-nitrophenylvalerate and acetylthiocholine as the substrates, respectively.
Summary
CYP-mediated metabolism of parathion to paraoxon is critical for its ability to interfere with cholinergic signaling and cause toxicity. By redox cycling with NADPH–cytochrome P450 reductase in an energetically favorable reaction, menadione inhibits CYP activity, blocks the formation of paraoxon from parathion, and reduces or prevents a cholinergic crisis. These data suggest that menadione may be an effective pharmacologic approach to mitigating parathion poisoning in humans, as well as poisoning by other phosphorothioate pesticides. Since menadione can inhibit other metabolic enzymes using NADPH–cytochrome P450 reductase as an electron donor, its use may be limited to short-term treatments for acute OP exposure to prevent toxicity. It should also be noted that menadione will only be effective in preventing OP metabolism and subsequent neurotoxicity, as it is not expected to reverse OP-inactivated AChE. Menadione is an FDA-approved drug for use in humans with a known acceptable safety profile. This should facilitate our ability to further develop the drug for clinical use as a countermeasure against parathion toxicity.
Acknowledgments
This work was supported by NIH Grants NS079249, NS072097, ES007148, AR055073, and ES005022.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Marrs TC. Organophosphate poisoning. Pharmacol. Ther. 1993;58:51–66. doi: 10.1016/0163-7258(93)90066-m. [DOI] [PubMed] [Google Scholar]
- 2.King AM, Aaron CK. Organophosphate and carbamate poisoning. Emerg. Med. Clin. North Am. 2015;33:133–151. doi: 10.1016/j.emc.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Jett DA, Richardson JR. Neurotoxic Pesticides. In: Dobbs MR, editor. Clinical Neurotoxicology: Syndromes, Substances, and Environments. San Diego, CA: Elsevier; 2009. pp. 491–499. [Google Scholar]
- 4.Lotti M. Treatment of acute organophosphate poisoning. Med. J. Aust. 1991;154:51–55. doi: 10.5694/j.1326-5377.1991.tb112852.x. [DOI] [PubMed] [Google Scholar]
- 5.Kuca K, Juna D, Musilek K. Structural requirements of acetylcholinesterase reactivators. Mini Rev. Med. Chem. 2006;6:269–277. doi: 10.2174/138955706776073510. [DOI] [PubMed] [Google Scholar]
- 6.de Silva HJ, Wijewickrema R, Senanayake N. Does pralidoxime affect outcome of management in acute organophosphorus poisoning? Lancet. 1992;339:1136–1138. doi: 10.1016/0140-6736(92)90733-j. [DOI] [PubMed] [Google Scholar]
- 7.Okolotowicz KJ, Dwyer M, Smith E, et al. Preclinical studies of noncharged oxime reactivators for organophosphate exposure. J. Biochem. Mol. Toxicol. 2014;28:23–31. doi: 10.1002/jbt.21519. [DOI] [PubMed] [Google Scholar]
- 8.Kalisiak J, Ralph EC, Zhang J, et al. Amidine-oximes: reactivators for organophosphate exposure. J. Med. Chem. 2011;54:3319–3330. doi: 10.1021/jm200054r. [DOI] [PubMed] [Google Scholar]
- 9.Estour F, Letort S, Muller S, et al. Functionalized cyclodextrins bearing an alpha nucleophile--a promising way to degrade nerve agents. Chem. Biol. Interact. 2013;203:202–207. doi: 10.1016/j.cbi.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Kovarik Z, Katalinic M, Sinko G, et al. Pseudo-catalytic scavenging: searching for a suitable reactivator of phosphorylated butyrylcholinesterase. Chem. Biol. Interact. 2010;187:167–171. doi: 10.1016/j.cbi.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Chambers JE, Chambers HW, Meek EC, et al. Novel nucleophiles enhance the human serum paraoxonase 1 (PON1)-mediated detoxication of organophosphates. Toxicol. Sci. 2015;143:46–53. doi: 10.1093/toxsci/kfu205. [DOI] [PubMed] [Google Scholar]
- 12.Ghanem E, Raushel FM. Detoxification of organophosphate nerve agents by bacterial phosphotriesterase. Toxicol. Appl. Pharmacol. 2005;207:459–470. doi: 10.1016/j.taap.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 13.Saxena A, Maxwell DM, Quinn DM, et al. Mutant acetylcholinesterases as potential detoxification agents for organophosphate poisoning. Biochem. Pharmacol. 1997;54:269–274. doi: 10.1016/s0006-2952(97)00180-9. [DOI] [PubMed] [Google Scholar]
- 14.Jokanovic M. Structure-activity relationship and efficacy of pyridinium oximes in the treatment of poisoning with organophosphorus compounds: a review of recent data. Curr. Top Med. Chem. 2012;12:1775–1789. [PubMed] [Google Scholar]
- 15.Nachon F, Brazzolotto X, Trovaslet M, et al. Progress in the development of enzyme-based nerve agent bioscavengers. Chem. Biol. Interact. 2013;206:536–544. doi: 10.1016/j.cbi.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Iyer R, Iken B, Leon A. Developments in alternative treatments for organophosphate poisoning. Toxicol. Lett. 2015;233:200–206. doi: 10.1016/j.toxlet.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Welch RM, Coon JM. Studies on the Effect of Chlorcyclizine and Other Drugs on the Toxicity of Several Organophosphate Anticholinesterases. J. Pharmacol. Exp. Ther. 1964;143:192–198. [PubMed] [Google Scholar]
- 18.Ahmad N, Brindley WA. Modification of parathion toxicity to wax moth larvae by chlorcyclizine, aminopyrine or phenobarbital. Toxicol. Appl. Pharmacol. 1969;15:433–440. doi: 10.1016/0041-008x(69)90041-6. [DOI] [PubMed] [Google Scholar]
- 19.Mourelle M, Giron E, Amezcua JL, et al. Cimetidine enhances and phenobarbital decreases parathion toxicity. J. Appl. Toxicol. 1986;6:401–404. doi: 10.1002/jat.2550060604. [DOI] [PubMed] [Google Scholar]
- 20.Agyeman AA, Sultatos LG. The actions of the H2-blocker cimetidine on the toxicity and biotransformation of the phosphorothioate insecticide parathion. Toxicology. 1998;128:207–218. doi: 10.1016/s0300-483x(98)00082-1. [DOI] [PubMed] [Google Scholar]
- 21.Mirer FE, Levine BS, Murphy SD. Parathion and methyl parathion toxicity and metabolism in piperonyl butoxide and diethyl maleate pretreated mice. Chem. Biol. Interact. 1977;17:99–112. doi: 10.1016/0009-2797(77)90075-8. [DOI] [PubMed] [Google Scholar]
- 22.Chambers JE, Chambers HW. Time course of inhibition of acetylcholinesterase and aliesterases following parathion and paraoxon exposures in rats. Toxicol. Appl. Pharmacol. 1990;103:420–429. doi: 10.1016/0041-008x(90)90315-l. [DOI] [PubMed] [Google Scholar]
- 23.O'Brien RD. The effect of SKF 525A (2-diethylaminoethyl 2:2-diphenylvalerate hydrochloride) on organophosphate metabolism in insects and mammals. Biochem. J. 1961;79:229–235. doi: 10.1042/bj0790229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurh E, Lee E, Lee A, et al. Effects of enzyme inducers or inhibitors on the pharmacokinetics of intravenous parathion in rats. Biopharm. Drug Dispos. 2000;21:193–204. doi: 10.1002/1099-081x(200007)21:5<193::aid-bdd229>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Isin EM, Guengerich FP. Complex reactions catalyzed by cytochrome P450 enzymes. Biochim. Biophys. Acta. 2007;1770:314–329. doi: 10.1016/j.bbagen.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Cederbaum AI. Molecular mechanisms of the microsomal mixed function oxidases and biological and pathological implications. Redox Biol. 2015;4:60–73. doi: 10.1016/j.redox.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hochstein P. Futile redox cycling: implications for oxygen radical toxicity. Fundam. Appl. Toxicol. 1983;3:215–217. doi: 10.1016/s0272-0590(83)80128-6. [DOI] [PubMed] [Google Scholar]
- 28.Parke DV, Sapota A. Chemical toxicity and reactive oxygen species. Int. J. Occup. Med. Environ. Health. 1996;9:331–340. [PubMed] [Google Scholar]
- 29.Gutteridge JM, Maidt L, Poyer L. Superoxide dismutase and Fenton chemistry. Reaction of ferric-EDTA complex and ferric-bipyridyl complex with hydrogen peroxide without the apparent formation of iron(II) Biochem. J. 1990;269:169–174. doi: 10.1042/bj2690169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jourd'heuil D, Jourd'heuil FL, Kutchukian PS, et al. Reaction of superoxide and nitric oxide with peroxynitrite. Implications for peroxynitrite-mediated oxidation reactions in vivo. J. Biol. Chem. 2001;276:28799–28805. doi: 10.1074/jbc.M102341200. [DOI] [PubMed] [Google Scholar]
- 31.Gray JP, Heck DE, Mishin V, et al. Paraquat increases cyanide-insensitive respiration in murine lung epithelial cells by activating an NAD(P)H:paraquat oxidoreductase: identification of the enzyme as thioredoxin reductase. J. Biol. Chem. 2007;282:7939–7949. doi: 10.1074/jbc.M611817200. [DOI] [PubMed] [Google Scholar]
- 32.Kovacic P, Somanathan R. Recent developments in the mechanism of anticancer agents based on electron transfer, reactive oxygen species and oxidative stress. Anticancer Agents Med. Chem. 2011;11:658–668. doi: 10.2174/187152011796817691. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Gray JP, Mishin V, et al. Role of cytochrome P450 reductase in nitrofurantoin-induced redox cycling and cytotoxicity. Free Radical Biol. Med. 2008;44:1169–1179. doi: 10.1016/j.freeradbiomed.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Gray JP, Mishin V, et al. Distinct roles of cytochrome P450 reductase in mitomycin C redox cycling and cytotoxicity. Mol. Cancer Ther. 2010;9:1852–1863. doi: 10.1158/1535-7163.MCT-09-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarasiuk J, Garnier-Suillerot A, Stefanska B, et al. The essential role of anthraquinones as substrates for NADH dehydrogenase in their redox cycling activity. Anticancer Drug Des. 1992;7:329–340. [PubMed] [Google Scholar]
- 36.Jan YH, Richardson JR, Baker AA, et al. Vitamin K3 (menadione) redox cycling inhibits cytochrome P450-mediated metabolism and inhibits parathion intoxication. Toxicol. Appl. Pharmacol. 2015;288:114–120. doi: 10.1016/j.taap.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Floreani M, Carpenedo F. Inhibition of rat liver monooxygenase activities by 2-methyl-1,4-naphthoquinone (menadione) Toxicol. Appl. Pharmacol. 1990;105:333–339. doi: 10.1016/0041-008x(90)90194-y. [DOI] [PubMed] [Google Scholar]
- 38.Foxenberg RJ, McGarrigle BP, Knaak JB, et al. Human hepatic cytochrome p450-specific metabolism of parathion and chlorpyrifos. Drug Metab. Dispos. 2007;35:189–193. doi: 10.1124/dmd.106.012427. [DOI] [PubMed] [Google Scholar]