Abstract
Aims
Thrombospondin-1 (TSP1) is a ligand for CD47 and TSP1−/− mice are protected from pulmonary hypertension (PH). We hypothesized the TSP1–CD47 axis is upregulated in human PH and promotes pulmonary arterial vasculopathy.
Methods and results
We analyzed the molecular signature and functional response of lung tissue and distal pulmonary arteries (PAs) from individuals with (n = 23) and without (n = 16) PH. Compared with controls, lungs and distal PAs from PH patients showed induction of TSP1–CD47 and endothelin-1/endothelin A receptor (ET-1/ETA) protein and mRNA. In control PAs, treatment with exogenous TSP1 inhibited vasodilation and potentiated vasoconstriction to ET-1. Treatment of diseased PAs from PH patients with a CD47 blocking antibody improved sensitivity to vasodilators. Hypoxic wild type (WT) mice developed PH and displayed upregulation of pulmonary TSP1, CD47, and ET-1/ETA concurrent with down regulation of the transcription factor cell homolog of the v-myc oncogene (cMyc). In contrast, PH was attenuated in hypoxic CD47−/− mice while pulmonary TSP1 and ET-1/ETA were unchanged and cMyc was overexpressed. In CD47−/− pulmonary endothelial cells cMyc was increased and ET-1 decreased. In CD47+/+ cells, forced induction of cMyc suppressed ET-1 transcript, whereas suppression of cMyc increased ET-1 signaling. Furthermore, disrupting TSP1–CD47 signaling in pulmonary smooth muscle cells abrogated ET-1-stimulated hypertrophy. Finally, a CD47 antibody given 2 weeks after monocrotaline challenge in rats upregulated pulmonary cMyc and improved aberrations in PH-associated cardiopulmonary parameters.
Conclusions
In pre-clinical models of PH CD47 targets cMyc to increase ET-1 signaling. In clinical PH TSP1–CD47 is upregulated, and in both, contributes to pulmonary arterial vasculopathy and dysfunction.
Keywords: Clinical pulmonary hypertension, Thrombospondin-1, CD47, cMyc, ET-1
1. Introduction
Pulmonary hypertension (PH) is a chronic and rapidly progressive disease with survival rates comparable to primary lung cancer.1 Hyperactive cell signaling promotes PH in animal models and in human disease, in part through unbalanced vascular remodeling of the pulmonary arterial smooth muscle compartment.2 Endothelin-1 (ET-1), via interactions with endothelin receptor A (ETA), stimulates pulmonary arterial smooth muscle cell proliferation and vasoconstriction and contributes to PH.3 Transcription factor regulator cell homolog of the v-myc oncogene (cMyc) controls genes that regulate cell growth.4,5 However, the processes that drive hyperactive ET-1 signaling, as well as the role of cMyc, in PH remain to be fully determined.
Thrombospondin-1 (TSP1) is a secreted glycoprotein that modifies cell responses through binding to matrix, growth factors and a number of cell receptors including integrins, CD36, and CD47.6–8 TSP1 is upregulated in experimental PH9 and in hypoxic pulmonary arterial smooth muscle10 and endothelial9 cells. Conversely, mice lacking TSP1 are resistant to hypoxia-mediated PH and display decreased smooth muscle cell hyperplasia in the pulmonary vasculature.9,11 TSP1 stimulates migration and proliferation of arterial smooth muscle cells12 while hypoxic TSP1−/− pulmonary arterial smooth muscle cells display a migration defect.10 We have shown that TSP1 is a high affinity ligand for receptor CD4713 and CD47 is widely expressed on both systematic and pulmonary vascular cells.14 TSP1 on binding and activation of vascular CD47 limits nitric oxide signaling15,16 and blood flow.17,18 However, it is not known through which receptor TSP1 acts to promote PH or if this has functional implications in human pulmonary vessels.
We tested the hypothesis that TSP1–CD47 signaling contributes to vasculopathy in clinical PH. Employing lungs and distal pulmonary arteries (PAs) from individuals with and without PH we show that the TSP1–CD47 signaling is induced in human PH together with activation of ET-1/ETA and that this contributes to a loss of vasodilator sensitivity and increased vasoconstrictor response in pulmonary vessels. Furthermore, a CD47 blocking antibody improved vasodilation in PAs from patients with end-stage PH. Findings in human lungs and vessels were confirmed in animal models of PH and in pulmonary vessels and vascular cells from the same. This study finds a novel role for maladaptive TSP1–CD47 signaling in clinical PH to promote vasculopathy and vascular dysfunction through inhibition of vasodilation and potentiation of vasoconstriction and provides mechanistic insight identifying the TSP1-CD47 axis as a proximate regulator of pulmonary ET-1.
2. Methods
Detailed information on the materials and methods used in this study is provided in the supplementary data.
2.1 Animal studies
All studies were performed under a protocol approved by the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC) in accordance with NIH guidelines or under a protocol approved by the Committee for Research and Ethics of the Universidad Autonoma of Madrid in accordance with Spanish and European guidelines (Directive 2010/63/EU of the European Parliament). Male C57BL/6 wild-type (WT, CD47+/+), TSP1−/− (B6.129S2-Thbs1tm1Hyn/J) and CD47−/− mice (B6.129S7-Cd47tm1Fpl/J), catalogue numbers 00664, 006141, and 003173, respectively, were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Male Sprague-Dawley rats were obtained from Harlan (Indianapolis, IN, USA).
2.2 Human tissue studies
Use of human tissues was within ongoing IRB or CORID approved protocols (970946, PRO14010265, and CORID No. 300) at the University of Pittsburgh Medical Center and conformed to the Declaration of Helsinki. Informed consent was obtained as part of the ongoing protocols. Tissue samples were characterized as non-PH or PH based on one or more of the following: diagnosis, clinical history, and cardiopulmonary findings (Table 1).
Table 1.
Demographic characteristics of human cohorts
| Non-PH | PH | |
|---|---|---|
| Data available (total n) | 4 (16) | 16 (23) |
| Diagnosis |
|
|
| Age (years) | 36.3 ± 14.8 | 58.4 ± 12.6 |
| range 20–49 | range 19–69 | |
| Gender | Three males, one female | Nine males, seven females |
| Ethnicity | N/A | 10 Caucasian, 4 African-American |
| Heart rate (bpm) | N/A | 79.5 ± 12.7 |
| mPAP (mmHg) | N/A | 40.6 ± 10.8 |
| range 22–54 | ||
| PCWP (mmHg) | N/A | 20.1 ± 15.3 |
| range 8–61 |
Human lungs explanted at the time of death (non-PH cohort) or at time of lung transplantation (PH cohort) were analyzed for changes in specific molecular pathways while distal 5th-order PAs were also assessed for vasoactivity. Patient demographic data, as available, is presented.
PH, pulmonary hypertension; IPF, idiopathic pulmonary fibrosis; COPD, chronic obstructive pulmonary disease; bpm, beats per minute; mPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; N/A, not available.
2.3 Cell cultures
The rat fibroblast cell line transfected with a conditional cMyc construct (rat-1a cMyc/ER) was kindly provided by Dr. Edward Prochownik (University of Pittsburgh). Control rat-2 fibroblasts were purchased from Sigma Aldrich (St. Louis, MO). Human pulmonary arterial smooth muscle cells (PA VSMC) and endothelial cells (hPAEC) were purchased from Lonza (Basel, Switzerland) and grown in proprietary medium. Primary murine pulmonary vascular cells were harvested from lungs of WT, TSP1−/−, and CD47−/− mice as adapted from Marelli-Berg et al.19 For some experiments, cells were weaned from serum and growth factors and subjected to hypoxia (O2 1%, 24 h) with or without CD47 blocking antibody (1 µg/ml).
2.4 Rodent models of PH
Male age matched WT, TSP1−/− and CD47−/− mice were placed in a hypoxia chamber and exposed to short-term (FiO2 10%, 24 h) or, in the case of WT and CD47−/− mice, chronic hypoxia (FiO2 10%, 3 weeks). Mice maintained in room air served as controls. As previously published,9 male Sprague-Dawley rats were treated with monocrotaline (mct, 50 mg/kg) with or without a CD47 blocking antibody (clone OX101, 0.4 µg/g body weight) administered on day 14 following mct-challenge. On day 28, animals underwent cardiopulmonary phenotyping.
2.5 Hemodynamic and ventricular weight measurements
Animals were anesthetized with a mixture of 5% isoflurane and 95% O2 titrated to effect. A 1.2F Transonic catheter (Transonic Science Inc., Ottawa, CA) was inserted into the right and left ventricles and cardiopulmonary parameters were measured with EMKA software monitoring equipment (EMKA Technologies, Falls Church, VA). Ventricular tissue was isolated by the same experienced operator and weighed.
2.6 Rodent lung histology
Lungs were flushed and then fixed with 4% paraformaldehyde under standardized inflation pressure, paraffin embedded and sectioned (4 µm), and stained for α-smooth muscle actin (Abcam, Cambridge, UK). For pulmonary vascular morphometric studies, images of peripheral arterioles (50–100 μm diameter) were captured with a Nikon Eclipse E 800 microscope.
2.7 siRNA transfection
Transfection with cMyc or control siRNA (Invitrogen, Carlsbad, CA) was performed using lipofectamine 2000 (Invitrogen) according to manufacturer instructions. Gene silencing was established by qPCR 72 h after transfection.
2.8 Protein expression
Tissue lysates were subjected to Western analysis. Protein was resolved by SDS–PAGE and transferred onto nitrocellulose or PVDF membranes. Blots were probed with primary antibody to the respective proteins and were visualized after incubation in secondary antibody on an Odyssey Imaging System (Licor, Lincoln, NE).
2.9 Real-time PCR
Total RNA was extracted using Qiagen RNeasy® Mini Kits (Qiagen, Hilden, Germany) with on-column DNase digestion. RNA was reverse-transcribed using Superscript III First Strand Synthesis Supermix (Invitrogen). cDNA was amplified using Platinum® PCR SuperMix-UDG (Invitrogen). Details regarding primers and cycle conditions are provided in the supplementary material online.
2.10 ELISA
TSP1 and ET-1 ELISAs (R&D, Minneapolis, MN; Cusabio, Wuhan, China, respectively) were performed according to the manufacturer instructions.
2.11 Immunofluorescence
Distal PAs in OCT were sectioned (5 µm); cells were grown on 35-mm plates. Samples were fixed in ethanol, blocked with 5% goat serum or 1% bovine serum albumin in PBS, incubated with relevant primary antibody overnight, followed by washing with PBS, addition of secondary antibody and DAPI staining. Samples were fixed with 4% paraformaldehyde and coverslipped with Gelvatol mounting medium. Fluorescent images were captured with a Nikon Eclipse E 800 microscope. Antibody details are provided in the supplementary material online.
2.12 2-Pin pulmonary artery myography
Myography of PAs was performed as previously published20,21 with minor modifications. Rat main or human 5th-order PAs were mounted on myograph pins (Danish Myo Technology, Atlanta, GA). Vessels were brought to an optimal resting tension and viability was ascertained by a response to potassium chloride. Phenylephrine (PE; Sigma-Aldrich, St Louis, MO) concentration–response curves were generated. Vessels were then contracted with an EC80 of PE and vasodilation to acetylcholine (Ach) and sodium nitroprusside (SNP) tested (both Sigma Aldrich). For drug responses, vessels were pre-incubated with TSP1 (2.2 nM), ET1 (0.5 µM), or a CD47 blocking antibody (clone B6H12, 1 µg/ml) followed by a dose response curve for Ach, SNP or ET-1.
2.13 Fluid-filled pressure pulmonary artery myography
Murine PAs were isolated, cannulated and pressurized to 20 mmHg in a Danish MyoTechnology (DMT) pressure myograph and perfused with myography buffer at 37°C. TSP1 (2.2 nM) was added were noted to both the lumen and bath of the artery. Potassium chloride was added to test vessel constriction and acetylcholine to test endothelial function. Arteries were treated with log doses of Ach, SNP, and ET-1. The maximum vessel diameter was measured by incubating the artery in calcium-free myography buffer supplemented with ethylene glycol tetraacetic acid and SNP. Quantification of artery diameter was performed using DMT vessel acquisition software and data are expressed as the percentage of initial diameter.
2.14 Statistics
Results are presented as mean ± SD. Data were analyzed for two group comparisons by Student’s t-test (parametric data) and by Mann–Whitney U-test (non-parametric data); for multiple group comparisons by ANOVA using Sidak’s multiple comparisons test; and for myography using two-way ordinary ANOVA employing GraphPad Prism software (GraphPad Software Inc., La Jolla, CA). P < 0.05 was considered statistically significant. Biased-reduced logistic regression analysis was performed where indicated using R version 3.2.2 (https://www.R-project.org/, 11 October 2016)22 and the R package brglm 0.5.9 (http://www.ucl.ac.uk/∼ucakiko/software.html, 11 October 2016).23
3. Results
3.1 TSP1 and CD47 are upregulated in human PH and promote PH-related arterial vasculopathy and dysfunction
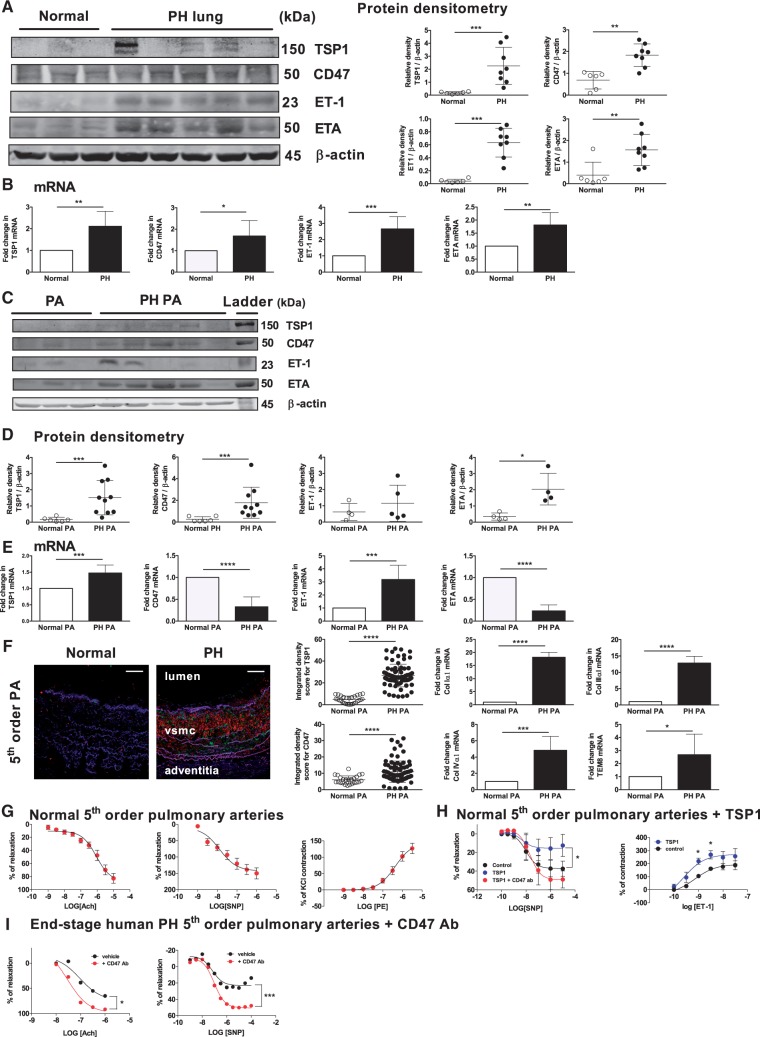
Some studies have indicated plasma TSP1 levels positively correlate with the degree of human vascular disease.24,25 Nonetheless, the exact role of TSP1 and its receptor CD47 in human PH is unknown. In individuals with end-stage PH (n = 23, Table 1), both TSP1 and CD47 protein and mRNA were elevated in lung parenchyma compared to controls without overt lung disease (non-PH, n = 16, Table 1) (Figure 1A and B). More specifically, in distal 5th-order PAs from end-stage PH lungs, TSP1, CD47 protein, and TSP1 mRNA levels were upregulated compared with control non-PH vessels (Figure 1C–E) whereas CD47 mRNA levels were decreased (Figure 1E). Immunofluorescent staining of distal 5th-order PAs from individuals with PH confirmed widespread TSP1 and CD47 expression throughout the vessel wall including the smooth muscle cell layer and adventitia concurrent with significant tissue remodeling (Figure 1F). In contrast, in control non-PH vessels TSP1 was undetectable and CD47 minimally expressed (Figure 1F). Bias-reduced logistic regression analysis26 of this cohort (see Table 1 for demographics) determined statistically significant positive relationships (P < 0.05) between both parenchymal and pulmonary arterial TSP1 protein expression and PH, and to some extent pulmonary arterial CD47 protein expression and PH (P = 0.058) (Supplementary material online, Figure 1).
Figure 1.
TSP1 and CD47 are upregulated in human PH and contribute to PH-related arterial vasculopathy and dysfunction. (A) Western analysis of lysates from parenchyma and (C) 5th-order PAs from normal (non-PH) and PH human lungs was performed. Representative blots are demonstrated. Densitometry analysis of (A) parenchyma and (D) vessels is presented as mean ± SD (n = 4–8 normal and 5–15 PH samples). *P < 0.05, **P < 0.01, ***P < 0.001. (B) mRNA expression of TSP1, CD47, cMyc, ET-1, and ETA in parenchyma and (E) 5th-order PAs from normal (non-PH) and PH human lungs. Representative data from n = 5 normal and n = 8 PH lungs is presented. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (F) Immunofluorescent staining of 5th-order PA vessels from normal and PH lungs. TSP1 and CD47 are stained red and green, respectively. Original magnification ×10; scale bar 200 μm. Blinded quantitative analysis of intensity of staining was performed and presented as mean ± SD, n = 5–7 patients per group; for each tissue section the hpf was divided into three to six approximately equal quadrants and fluorescence calculated via the integrated density function found in ImageJ. ****P < 0.0001. mRNA expression of collagen matrix genes in n = 5–8 normal and n = 6–12 PH lungs. Distal 5th-order PAs were dissected from normal (non-PH) human and end-stage PH lungs and mounted on a dual pin myography. (G) Analysis of vasodilation or vasoconstriction of non-PH vessels to the indicated doses of acetylcholine (Ach), sodium nitroprusside (SNP), and phenylephrine (PE) is presented. Results are the mean ± SD of n = 4 vessels per treatment group. (H) Myography results of distal 5th-order PA from normal human lungs to a log dose of SNP ± TSP1 (2.2 nM) ± CD47 antibody (clone B6H12, 1 µg/ml) or ET-1 ± TSP1 (2.2 nM). Results are the mean ± SD of n = 3–5 vessels per treatment group, *P < 0.05. (I) Myography results of distal 5th-order PAs from end-stage PH lungs to a log dose of Ach and SNP ± CD47 antibody (clone B6H12, 1 µg/ml). As absolute changes in pressure varied in each instance, representative graphs from n = 2–5 separate experiments in each group are presented. *P < 0.05, ***P < 0.001.
We reported that animals lacking CD47 had lower expression of pulmonary ET-1.20 Together with new data herein these findings suggested the hypothesis that TSP1–CD47 signaling regulates pulmonary ET-1. ET-1 and ETA protein and transcript were upregulated in the pulmonary parenchyma in PH compared with non-PH samples, while in 5th-order PAs ETA, but not ET-1 protein and ET-1 mRNA were significantly increased and ETA mRNA levels decreased (Figure 1A–E). Accumulation of pulmonary extracellular matrix is noted in clinical PH27,28 and TSP1 promotes experimental pulmonary29 and human fibrosis.30 Interestingly, transcript levels of ColIα1, ColIIIα1, and ColIVα1 and TEM8 (a stimulator of vascular cell–collagen interactions31) were also elevated in PH vessels compared to non-PH vessels (Figure 1F).
It was not known if the findings of PH-associated upregulation of TSP1 and CD47 had any functional consequences in the human pulmonary vasculature. To test this we studied distal 5th-order PAs from human PH and non-PH lungs. Vessels from control non-PH lungs were sensitive to acetylcholine (Ach, an activator of endothelium that increases nitric oxide production)- and sodium nitroprusside (SNP, a nitric oxide pro-drug)-stimulated vasodilation and phenylephrine (PE)-stimulated vasoconstriction (Figure 1G). In loss-of-function experiments, treatment of non-PH control PAs with TSP1 (2.2 nM, a concentration we reported occurs in the blood of individuals with vascular dysfunction24) decreased SNP-stimulated vasodilation (EC50 1.04 × 10−9 versus 8.47 × 10−9, respectively, Table 2). In gain-of-function experiments, pre-treatment with a CD47 antagonist antibody (clone B6H12, 1 µg/ml) that disrupts TSP1–CD47 signaling,13 limited the inhibitory effect of TSP1 and improved SNP-stimulated vasodilation (EC50 8.47 × 10−9 versus 1.24 × 10−8, respectively, Table 2) (Figure 1H). Furthermore, treatment of control non-PH PAs with TSP1 (2.2 nM) enhanced ET-1-mediated vasoconstriction (Figure 1H). Diseased vessels from human PH lungs, which we now find have widespread overexpression of TSP1 and CD47 (see Figure 1F), were significantly less sensitive to SNP- (EC50 8.98 × 10−9, Table 2), although not Ach- (EC50 6.2 × 10−8, Table 2) (Figure 1I), mediated vasodilation as compared with non-PH control vessels (compare with Figure 1G). Importantly from a therapeutic perspective, treating diseased PAs from individuals with end-stage PH with the CD47 antagonist antibody improved both Ach- and SNP-mediated vasodilation (EC50 2.01 × 10−8 and 3.9 × 10−9, respectively, Table 2) (Figure 1I).
Table 2.
Human lung 5th-order pulmonary arterial EC50 analysis
| Non-PH PA–SNP response | |||
|---|---|---|---|
| LogEC50 | EC50 | 95% CI | |
| Untreated | −7.98 | 1.04 × 10−9 | 3.14 × 10−9–3.47 × 10−8 |
| TSP1 | −8.072 | 8.47 × 10−9 | 4.3 × 10−10–1.68 × 10−7 |
| TSP1+αCD47 | −7.906 | 1.24 × 10−8 | 4.38 × 10−10–3.51 × 10−7 |
|
Non-PH PA–Ach response | |||
| LogEC50 | EC50 | 95% CI | |
| Untreated | −6.89 | 1.3 × 10−7 | 2.48 × 10−9–6.81 × 10−6 |
| TSP1 | −7.822 | 1.5 × 10−8 | 0–6.4 × 10−14 |
|
PH PA–SNP response | |||
| LogEC50 | EC50 | 95% CI | |
| Untreated | −8.047 | 8.98 × 10−9 | 9.0 × 10−10–8.94 × 10−8 |
| +αCD47 | −8.409 | 3.9 × 10−9 | 1.05 × 10−10–1.45 × 10−7 |
|
PH PA–Ach response | |||
| LogEC50 | EC50 | 95% CI | |
| Untreated | −7.208 | 6.2 × 10−8 | 1.5 × 10−10–2.56 × 10−5 |
| +αCD47 | −7.696 | 2.01 × 10−8 | 2.91 × 10−11–1.4 × 10−5 |
From n = 5 independent experiments, P < 0.001 by ANOVA.
Untreated versus TSP1: P < 0.0001.
TSP1 versus TSP1 + CD47Ab: P = 0.0663.
Untreated versus TSP1 + CD47Ab: P = 0.1648.
From n = 2 independent experiments, P < 0.01 by ANOVA.
From n = 5 independent experiments, P < 0.0001 by ANOVA.
Untreated versus CD47Ab: P < 0.0001.
From n = 2 independent experiments.
Untreated versus CD47Ab: P < 0.05.
3.2 Absence of CD47 protects from hypoxia-mediated PH
To further test the importance of CD47 in transducing pulmonary TSP1 signaling, we employed a rodent model of PH. Twelve-week-old male WT (CD47+/+) and CD47−/− mice were challenged with 3 weeks of hypoxia (FiO2 10%) followed by cardiopulmonary phenotyping. Hypoxic WT mice displayed increased RV maximum systolic pressure (RV max SP), RV-free wall weight and Fulton index compared with hypoxic CD47−/− mice (Figure 2A). RV max SP is recognized as a surrogate for pulmonary artery systolic pressure32 and is preferred when catheter advancement into the main PA can be problematic in the hypertrophied murine heart. RV end-diastolic pressure, as an indicator of RV dysfunction,33 was significantly elevated in hypoxic WT mice and was not significantly elevated in hypoxic CD47−/− animals but did not reach significance (Figure 2A), whereas RV ejection fraction and contractility index were equivalent between strains and treatment groups (Figure 2B). WT mice experienced a loss of body weight in response to chronic hypoxia that was not found in CD47−/− animals (Figure 2C), while tibia length was not different between strains or treatment groups (Figure 2C). Importantly, pulmonary arterial thickening, as a maker of PH-related vascular remodeling, was significantly greater in lungs from hypoxic WT compared with CD47−/− mice (Figure 2D and E).
Figure 2.
Absence of CD47 protects from hypoxia-mediated PH. Male age-matched C57BL/6 WT (CD47+/+) and CD47−/− mice were exposed to normoxia (Nx) or hypoxia (Hx, FiO2 10%, 3 weeks), and underwent (A, B) analysis of the indicated cardiopulmonary parameters and determination of the RV free wall weight and Fulton index. (C) Determination of the change in total body weight and tibia length of WT and CD47−/− mice under normoxia and hypoxia. Data shown are mean ± SD, n = 4–6 animals per group, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (D) Representative lung sections prepared from each treatment group were stained for α-smooth muscle actin (brown) and blinded quantitative analysis of vascular wall thickness was performed on vessels between 50 and 100 μm in cross-sectional diameter. Wall thickness was measured at four equally separate points around the circumference of each vessel. Scale bars 50 μm; original magnification ×10 (D) and ×20 (E). Quantification is presented as mean ± SD, n = 4–5 tissue sections per treatment group, ****P < 0.0001.
3.3 TSP1-CD47 signaling is required for hypoxia-mediated induction of pulmonary ET-1/ETA
To interrogate the kinetics of TSP1–CD47 signaling in our PH model, we exposed WT, TSP1−/−, and CD47−/− mice to short-term hypoxia (FiO2 10%, 24 h). Pulmonary TSP1 protein and mRNA, but not CD47 mRNA, were rapidly increased in hypoxic WT lungs (Figure 3A), indicating that pulmonary induction of TSP1–CD47 signaling precedes development of PH-related vascular remodeling and cardio-pulmonary dysfunction.
Figure 3.
CD47 is required for hypoxia-mediated induction of pulmonary ET-1/ETA. (A) Age-matched male WT (CD47+/+), TSP1−/− and CD47−/− mice were challenged with normoxia (Nx) or short-term hypoxia (Hx) (10% FiO2, 24 h). Lung tissue lysates from WT and TSP1−/− mice (n = 3–5 per group) were prepared and protein separated via SDS–PAGE electrophoresis. Densitometry is presented as the mean ratio of target protein to α-tubulin ± SD; **P < 0.01, ***P < 0.001, ****P < 0.0001. Whole lung mRNA expression of TSP1 and CD47 from Nx or acutely Hx WT, TSP1−/−, and CD47−/− mice. Data from n = 3–5 mice per group are presented as mean ± SD, **P < 0.01. (B) Age-matched male WT and CD47−/− mice were challenged with Nx or chronic Hx (10% FiO2, 3 weeks). Lung tissue homogenates were prepared and protein separated by SDS–PAGE. Representative western immunoblots from treatment groups (n = 5–12 per group) for indicated proteins are presented. Densitometry is presented as the mean ratio of target protein to β-actin ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ET-1 levels in lung homogenates from WT and CD47−/− mice under Nx or chronic Hx were measured by ELISA. Data are presented as mean ± SD from n = 4–8 samples per group, ***P < 0.001, ****P < 0.0001. (D) mRNA expression of TSP1, CD47, cMyc, ET-1, ETA, and ETB in whole lung homogenates. Data from n = 6–8 mice per group are presented as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (E) Platelet poor plasma TSP1 levels from WT and CD47−/− mice under Nx or chronic Hx. Data are presented as the mean ± SD from n = 5–9 mice per group, *P < 0.05, **P < 0.01. (F) Myography results of proximal PA vessels from age-matched male WT mice treated with TSP1 (2.2 nM) and the indicated concentrations of acetylcholine (Ach), sodium nitroprusside (SNP), or endothelin-1 (ET-1). Results are the mean ± SD of n = 3–4 vessels per treatment, #P = 0.05, *P < 0.05, ***P < 0.001.
Both ET-1 and cMyc are important regulator of cell growth,34,35 and while cMyc is known to regulate other genes, a role for cMyc in PH has not been previously suggested. We hypothesized that TSP1–CD47 signaling dysregulates cMyc and thus ET-1 to promote PH. To test this we again challenged mice with chronic hypoxia (FiO2 10%, 3 weeks) and assessed protein and mRNA levels of relevant genes. Lungs from chronically hypoxic WT mice displayed increased TSP1, CD47, ET-1, and decreased cMyc protein expression, and increased TSP1, CD47, ET-1, and ETA mRNA, and decreased ETB mRNA compared with lungs from normoxic WT mice (Figure 3B–D and Supplementary material online, Figure 2). cMyc mRNA levels were low in normoxic WT lungs and although they trended lower with hypoxia the change was not significant (Figure 3D). In contrast, TSP1, ET-1, ETA, and ETB protein and TSP1, ET-1, and ETA, but not ETB, mRNA remained unchanged in lungs from chronically hypoxic CD47−/− mice compared with normoxic controls (Figure 3D). Also, cMyc protein was significantly elevated in lungs from CD47−/− mice compared with normoxic WT animals and remained elevated in the face of chronic hypoxia (Figure 3B and C). It was not clear if changes in ET-1 protein and mRNA mirrored changes in active (soluble) ET-1 protein. By ELISA, soluble ET-1 levels were significantly increased in homogenates of hypoxic WT lungs, while in lungs from CD47−/− mice soluble ET-1 protein levels were increased to a less degree in response to chronic hypoxia (Figure 3C). Conversely, soluble ET-1 protein levels were comparable in lungs from normoxic WT and CD47−/− mice (Figure 3C).
Platelets are activated in and contribute to PH.36,37 They are also a source of TSP1 in the circulation.38 It is not known if circulating TSP1 is elevated in PH. We analyzed platelet-poor plasma obtained from normoxic and chronically hypoxic mice. Plasma TSP1 levels were elevated in hypoxic WT mice, while in CD47−/− animals, plasma TSP1 levels were lower compared with WT under normoxia and did not increase in response to chronic hypoxia (Figure 3E).
Having determined that TSP1, via CD47, promotes acute vascular dysfunction in human PAs, it was not clear if this effect would pertain in rodent vessels. We employed ex vivo myography to test the responses of murine WT PAs. Exogenous TSP1 (2.2 nM) inhibited endothelial and smooth muscle cell-mediated vasodilation in murine PAs (Figure 3F). The ability of TSP1 to acutely limit NO-mediated vasodilation in murine and human pulmonary arterial segments suggested that perhaps TSP1 potentiated vasoconstrictor activity. Interestingly, exogenous TSP1 increased the baseline tone of murine PAs while not significantly enhancing ET-1-mediated vasoconstriction (Figure 3F) implying TSP1 may have inherent vasoconstrictor activity.
3.4 TSP1, via CD47, suppresses cMyc to increase ET-1/ETA signaling in pulmonary vascular cells
To better assess the molecular mechanism behind our findings in human and murine lungs and PAs, we undertook cell culture experiments. In gain-of-function studies we employed a fibroblast cell line (rat-1a fibroblasts) transfected with an estrogen receptor (ER)-dependent cMyc construct.39 These cells express increased nuclear cMyc40 and increased growth compared with cells carrying a non-functional cMyc construct.39 Immunofluorescent imaging of rat-1a fibroblasts expressing the c-Myc/ER fusion protein confirmed significant nuclear localization of cMyc (Figure 4A and Supplementary material online, Figure 3A). Densitometry analysis of Western blots (not shown) of cultured cells demonstrated increased total cMyc protein expression in rat-1a fibroblasts compared to control rat fibroblasts (0.416 ± 0.059 versus 0.812 ± 0.080, cMyc/β-actin ratio of n = 4 replicates in two separate experiments; P > 0.05 rat-1a versus control rat fibroblasts). Cells treated with 4-OHT showed a decrease in ET-1 and ETA transcript levels while CD47 expression was unchanged (Figure 4A). To determine the genetic priority of CD47, we cultured pulmonary endothelial cells from WT and CD47−/− mice. First, we confirmed the phenotype of our cell cultures using immunofluorescent staining for the endothelial proteins von Willebrand Factor (vWF) and CD31 (Supplementary material online, Figure 3B). CD47−/− pulmonary endothelial cells displayed increased cMyc and decreased ET-1 mRNA levels compared with WT cells (Figure 4B), whereas ETA mRNA did not differ significantly between cell types. ET-1 production by pulmonary arterial endothelial cells is increased in PH.41 We exposed human pulmonary arterial endothelial cells (hPAEC) to hypoxia (FiO2 1%, 24 h), which in these cells we reported increases TSP1 production,9 in the presence or absence of a CD47 antagonist antibody (clone B6H12, 1 μg/ml). hPAEC exposed to short-term hypoxia demonstrated induction of CD47, ET-1, and ETA transcript levels, and increased active (soluble) ET-1 protein, as determined via ELISA of cell culture supernatants (Figure 4C). Treatment of hPAEC with the CD47 antagonist antibody blocked hypoxia-mediated increases in ET-1 and ETA transcript and soluble ET-1 protein (Figure 4C), but not CD47 mRNA. Our finding of decreased CD47 mRNA in the face of CD47 protein accumulation in human PH PAs (see Figure 1C–E) suggested that changes in the rate of protein degradation contribute to CD47 accumulation in PH. hPAEC were challenged with normoxia or hypoxia (FiO2 1%) ± the protein synthesis inhibitor cycloheximide (100 μg/ml) for 24 h. Short-term hypoxia resulted in increased TSP1 and CD47 protein in hPAEC compared with normoxia, whereas treatment with cycloheximide resulted in decreased TSP1 and CD47 protein under both normoxia and hypoxia, suggesting that hypoxia-mediated accumulation of TSP1 and CD47 is independent of changes in degradation/stability (Supplementary material online, Figure 3C).
Figure 4.
TSP1, via CD47, suppresses cMyc to increase ET-1/ETA signaling in pulmonary vascular cells. (A) Representative immunofluorescence image of cultured rat-1a Myc/ER fibroblast cells expressing the c-Myc/ER fusion protein display nuclear c-Myc (green), DAPI (blue), phalloidin (red); scale bar 50 μm, original magnification ×63. Rat-1a fibroblasts were treated with 4-hydroxytamoxifen at the designated concentrations for 12 h. mRNA expression of CD47, ET-1, and ETA was determined. Data from n = 4 experiments with each run in triplicate are presented as mean ± SD, ****P < 0.0001. (B) Murine pulmonary endothelial cells were isolated from WT and CD47−/− mice. mRNA expression for CD47, cMyc, and ET-1 was determined. Data from n = 6 independent experiments are presented as mean ± SD, *P < 0.05, **P < 0.01, ****P < 0.0001. (C) Human pulmonary arterial endothelial cells (hPAEC) (passage 3–6) were cultured to 80% confluence then treated with normoxia (Nx), hypoxia (Hx, FiO2 1%), or hypoxia + CD47 blocking antibody (1 μg/ml) for 24 h. mRNA expression of CD47, ET-1, and ETA was determined. ET-1 level in cell culture supernatants was measured by ELISA. Representative data from four experiments are presented as mean ± SD; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (D) hPAEC were transfected with control (CTRL) or cMyc siRNA for 72 h and transcript levels of CD47, cMyc, ET-1, and ETA measured. ET-1 level in cell culture supernatants was measured by ELISA. Data are represented as mean ± SD from n = 5 experiments; **P < 0.01, ***P < 0.001, ****P < 0.0001. (E) Human pulmonary vascular smooth muscle cells (hPVSMC) were subjected to Nx or Hx (FiO2 1%, 24 h) and protein expression for TSP1 and cMyc determined. Representative blots are shown and data from n = 4 independent experiments are presented as mean ± SD, *P < 0.05. (F) hPVSMC were subjected to Nx or Hx (FiO2 1%, 24, 48, and 72 h) and mRNA transcript levels determined for TSP1, CD47, and ETA. Data from n = 4–5 experiments are presented as mean ± SD, *P < 0.05 Nx versus Hx at indicated time point. (G) Pulmonary VSMC from WT, TSP1−/−, and CD47−/− mice were isolated and subjected to Nx or Hx (FiO2 1%, 24 h). Cell lysate was prepared and protein and mRNA analysis performed. Representative western immunoblots for the indicated proteins are shown. Densitometry from n = 4 independent experiments for cMyc and n = 4–5 independent experiments for TSP1 is presented as the mean ratio of target protein to tubulin ± SD, *P < 0.05, **P < 0.01, ****P < 0.0001. mRNA data are presented as the mean ratio of target protein to the housekeeping gene (± SD), *P < 0.05 Nx versus Hx. (H) hPVMSC were incubated with ET-1 (1 μM) with or without a CD47 blocking antibody (clone B6H12, 1 μg/ml) for 24 h. Cell size was analyzed by FACS. Data from n = 3–5 independent experiments are presented as mean ± SD, *P < 0.05, **P < 0.01.
In loss-of-function experiments, hPAEC were treated with cMyc targeting or control siRNA (CTRL). qPCR demonstrated ∼40% transcript suppression in cells treated with either 50 or 100 pmol of cMyc siRNA (Figure 4D) indicating that a maximal effect was obtained with 50 pmol of targeting siRNA. As predicted, treatment of hPAEC with cMyc, but not control siRNA, resulted in increased ET-1 and ETA mRNA levels and active (soluble) ET-1 protein (Figure 4D). CD47 mRNA levels trended upward in cMyc siRNA-treated cells but this did not reach significance (Figure 4D). While these data support a role for TSP1–CD47 proximate to cMyc-mediated regulation of ET-1, it is not clear if cMyc is necessary for this or if CD47 alters ET-1 independent of cMyc. To test this we treated normoxic hPAEC with cMyc or control siRNA for 72 h ± a CD47 antibody (clone B6H12, 1 μg/ml) for 24 h and assessed changes in ET-1 mRNA. As predicted, treatment of hPAEC with cMyc siRNA significantly suppressed cMyc transcript compared with control siRNA treated cells and this was associated with a significant increase in ET-1 mRNA (Supplementary material online, Figure 3D). Interestingly, in cMyc-deficient cells additional treatment with a CD47 blocking antibody tended to decrease ET-1 mRNA compared with control siRNA treated cells, although results did not reach significance (Supplementary material online, Figure 3D).
Endothelial cell-derived ET-1 stimulates PA VSMC hypertrophy.42 To test for possible auto- and/or paracrine signaling we assessed changes in relevant signaling intermediates in PA VSMC. Exposing human PA VSMC to short-term hypoxia (O2 1%, 24 h) resulted in increased TSP1 and decreased cMyc protein expression (Figure 4E). At the transcriptional level, TSP1 increased following 72 h of hypoxia suggesting possible hypoxia-sensitive effects on TSP1 protein uptake and degradation. CD47 mRNA levels showed a transient decline at 24 h of hypoxia that recovered by 48 h, while ETA mRNA levels increased by 72 h (Figure 4F). To assess the genetic priory of these signaling mediators we cultured PA VSMC from WT, TSP1−/−, and CD47−/− mice and exposed the cells to short-term hypoxia (O2 1%, 24 h). Immunofluorescent staining of murine PA VSMC cultures for myosin heavy chain (green) and dual color staining for SM22 alpha (red) and smooth muscle actin (green) validated culture purity (Supplementary material online, Figure 3E). Similarly, increased prolyl hydroxylase 3 (PHD3) mRNA levels confirmed a robust hypoxic response in the cells (Figure 4G). TSP1 protein expression was elevated in hypoxic WT PA VSMC, whereas in CD47−/− cells hypoxia-mediated induction of TSP1 protein was absent (Figure 4G). Conversely, cMyc protein expression was increased in both normoxic and hypoxic TSP1−/− and normoxic CD47−/− PA VSMC compared with WT cells (Figure 4G). cMyc transcript levels tended to rise in normoxic and short-term hypoxic CD47−/− but not TSP1−/− or WT cells, although these changes did not reach statistical significance (Supplementary material online, Figure 3F). Furthermore, treatment of normoxic human PA VSMC with exogenous ET-1 protein stimulated cell hypertrophy, as quantified by FACS, and this was abrogated by pre-treating cells with the CD47 antagonist antibody (clone B6H12, 1 μg/ml) (Figure 4H).
3.5 RV TSP1–CD47 signaling is increased in experimental PH
In the setting of PH, concordant signaling between the lung and RV has been proposed.43 However, it is unknown whether in the RV if TSP1 and CD47 are induced in PH. Western immunoblot analysis found increased expression of TSP1 and CD47 in RV samples from chronically hypoxic WT mice (Figure 5A), although here too hypoxia-mediated induction of TSP1 was absent in RV samples from CD47−/− mice (Figure 5A). At the transcriptional level, TSP1 and CD47 mRNA were decreased and ET-1/ETA mRNA levels unchanged in WT RV samples from hypoxic mice compared with samples from normoxic WT mice (Figure 5B). TSP1 mRNA levels were lower in RV samples from normoxic and hypoxic CD47−/− mice compared with normoxic WT samples, while CD47 mRNA levels were lower in RV samples from CD47−/− mice under normoxia and hypoxia compared with WT (Figure 5B). This is in distinction to changes in TSP1 and CD47 mRNA found in hypoxic lungs (see Figure 3D). However, paralleling results from lung samples, cMyc transcript was upregulated in both normoxia and after chronic hypoxia in CD47−/− compared with WT RV samples (Figure 5B). In clinical PH, RV heart failure is associated with increased RV matrix accumulation.44,45 Interestingly, collagen expression was comparable between WT and CD47−/− RV samples following chronic hypoxia (Figure 5C). This is in distinction to our previous finding that following chronic cardiac stress induced by partial transverse aortic constriction left ventricle samples from CD47−/− mice displayed less fibrosis compared with samples from WT mice.46
Figure 5.
RV TSP1–CD47 signaling is increased in experimental PH. WT and CD47−/− mice were challenged with Nx or chronic Hx (FiO2 10%, 3 weeks). (A) Homogenates of right ventricle (RV) were prepared and protein separated by SDS–PAGE. Representative western immunoblots from treatment groups (n = 3–6 per group) for indicated proteins are presented. Densitometry is presented as the mean ratio of target protein to β-actin ± SD, *P < 0.05, **P < 0.01. (B) mRNA expression of TSP1, CD47, cMyc, ET-1, and ETA in RV. Data from n = 3–6 mice per group are presented as mean ± SD, ***P < 0.001, ****P < 0.0001. (C) Immunofluorescent staining of RV from WT and CD47−/− mice subjected to Nx or chronic Hx. Type I collagen and myocytes are stained green and red, respectively. Scale bars 50 μm, original magnification ×40. Blinded quantitative analysis of fibrosis (total length × intensity of matrix/total surface area of myocytes) was performed and presented as mean ± SD, n = 5–7 tissue sections from three different mice per treatment group. One-way ANOVA with Tukey post hoc test was performed for statistical analysis.
3.6 Disrupting TSP1–CD47 signaling induces pulmonary cMyc and mitigates some cardiopulmonary effects in established PH
Animal and rodent and human cell culture experiments place TSP1 and CD47 upstream of cMyc-ET-1/ETA in promoting PH and vascular dysfunction. To further examine the generality of this signaling cascade, we assessed rat PAs ex vivo via myography. Similar to results in human and murine pulmonary vessels, rat PAs treated with TSP1 (2.2 nM) displayed an enhanced vasoconstrictor response (Figure 6A), while treatment with TSP1 or with a TSP1-derived peptide that selectively activates CD47 (peptide 7N3, 10 μM) inhibited Ach- and SNP-mediated vasodilation, respectively (Figure 6A).
Figure 6.
Disrupting TSP1–CD47 signaling induces pulmonary cMyc and mitigates some cardiopulmonary effects in established PH. (A) Dual-pin myography results of proximal PA vessels from male Sprague-Dawley rats treated with the indicated concentrations of KCl (100 nM), Ach and SNP ± TSP1 (2.2 nM), SNP ± a CD47 specific peptide (7N3, 10 μM). Results are the mean ± SD of n = 4 vessels per treatment group, *P < 0.05, ***P < 0.001. (B) Sprague-Dawley rats (CD47+/+) were treated with monocrotaline (MCT, 50 mg/kg). Two weeks post-MCT animals were treated with a single dose of CD47 antagonist antibody (clone OX101, 0.4 µg/g body weight), n = 3–5 per group. Representative western immunoblots (B) for the indicated proteins are presented. Lung tissue lysate was prepared and (C) protein and (D) mRNA analysis of TSP1, CD47, cMyc, ET-1, and ETA performed. Densitometry is presented as the mean ratio of target protein to β-actin ± SD, *P < 0.05, **P < 0.01. (E) Analysis of the RV and LV-free wall weight and Fulton index from the indicated treatment groups. Data shown are mean ± SD, n = 4–5 animals per group, *P < 0.05, **P < 0.01, ****P < 0.0001. (F) Analysis of the indicated cardiac parameters via open chest Millar catheter. Data shown are mean ± SD, n = 4–5 animals per group, **P < 0.01.
To further confirm our findings, we treated rats (CD47+/+) with mct (50 mg/kg) to induce PH. Two weeks after mct-challenge, animals were treated with a CD47 antagonist antibody (clone OX10147) and 2 weeks later cardiopulmonary phenotyping was performed. Animals treated with mct alone showed upregulation of pulmonary TSP1 protein while ET-1 and ETA protein levels increased but did not reach statistical significance (Figure 6B and C). Mct treatment also resulted in significant increases in TSP1 and ETA but not ET-1 and cMyc mRNA (Figure 6D). Conversely, animals treated with mct followed by a CD47 antagonist antibody showed increased pulmonary cMyc transcript expression (Figure 6D), with protein levels trending up but not reaching significance (Figure 6B), while ETA mRNA was not significantly elevated (Figure 6D). From a therapeutic perspective, mct and CD47 antibody treated animals displayed less increase in RV-free wall weight compared with animals given mct alone. The Fulton index was elevated in rats after mct treatment and although trending lower in animals receiving mct and the CD47 antagonist antibody did not achieve significance (Figure 6E). Further, mct treatment significantly lowered the contractility index, something not seen in animals that also received a CD47 antibody (Figure 6F). Finally, heart rates were not significantly different in any treatment groups (Figure 6F).
4. Discussion
4.1 Increased TSP1–CD47 signaling is associated with pulmonary vasculopathy in clinical PH
We reported increased TSP1 protein expression in lung parenchyma from a small group of pulmonary arterial hypertension (PAH) patients.9,10 Nonetheless, it was unknown if expression in the lung parenchyma had pathophysiologic significance in the broader world of PH. We now confirm that the TSP1 is significantly induced at the level of protein and transcript in lung parenchyma but, more importantly, show widespread induction of both TSP1 and CD47 protein and mRNA in distal PAs in a large cohort of PH patients. As emphasized by ex vivo myography, the upregulation of the TSP1–CD47 axis in human PAs was of functional consequence as it was associated with vascular remodeling and a significant loss of vasodilation capacity in diseased human PAs. Conversely, adding TSP1 to healthy human PAs recapitulated the loss-of-function observed in the diseased PH vessels. Translating these results, studies in PAs from end-stage PH lungs showed that interrupting the TSP1–CD47 signaling axis with a CD47 antagonist antibody, that we have reported blocks ligand–receptor interaction,13 improved arterial sensitivity to vasodilators. Finally, logistic regression analysis found a significant positive correlation between upregulation of vascular TSP1 signaling and PH. Thus, this study identifies the TSP1–CD47 axis as a novel maladaptive mechanism that promotes human PH-associated vasculopathy and dysfunction. These data also are the first to show in real-time that therapeutic targeting of CD47 can improve the vascular response of diseased human vessels. In contrast to these data, a small cohort of familial PAH patients were found to have TSP1 mutations while cells treated with recombinant putatively mutant TSP1 protein responded less as compared to cells treated with native TSP1.48 Of note, the identified TSP1 mutations reported in this study all localized to the type I repeat domain of TSP1,48 and this region preferentially binds to cell receptor CD36.49 However, the implications of this remain to be determined given that studies indicate that CD47 is the cognate high affinity TSP1 receptor13 and that, as we reported, CD47 is necessary for TSP1 to inhibit NO signaling via CD36.16
4.2 Absence of CD47 confers protection from chronic hypoxia
TSP1 is upregulated in hypoxic mice9–11,50 and mct-treated rats51 while TSP1−/− mice are protected from PH.9,11 Extending this work we found that hypoxic CD47−/− mice had less pulmonary arterial smooth muscle cell proliferation, RV hypertrophy and elevation in RV pressure compared with WT controls. Thus in mice, absence of either the ligand (TSP1)9 or of the cognate receptor (CD47)13 provides protection from hypoxia-mediated PH. These new data, when considered along with our previous report that TSP1-CD47 signaling promoted left ventricular dysfunction,46 suggest that the TSP1–CD47 axis may have a role in both right and left heart homeostasis. Of note, mice lacking TSP1 have a greater capillary density in the heart52 and enhanced VEGF signaling,53 whereas TSP1 via inhibition of NO can alter certain proteases.54 Although not assessed in this study, increased cardiac and potentially pulmonary vascularity, VEGF signaling and/or altered protease activity, if present in CD47−/− animals, might provide a protective advantage under hypoxia-mediated PH irrespective of effects on other downstream targets.
Recently, we identified a role for TSP1 as an activator of NADPH oxidase in systemic arterial vascular smooth muscle cells55 and several studies have suggested TSP1 can enhance reactive oxygen species production to putatively contribute to PH.9,50 The role of ROS was not specifically interrogated in our experiments but could contribute to TSP1-mediated pulmonary vascular dysfunction and remodeling. Ongoing studies in rodent and human vessels and lungs will seek to address the relative contribution of TSP1 to pathologic ROS in the setting of PH.
4.3 TSP1–CD47 signaling limits cMyc to de-repress pulmonary ET-1
Previously, we noted in CD47−/− mice that cMyc expression56,57 was increased and ET-1 expression decreased.20 We now demonstrate a protective role for cMyc, via suppression of ET-1, in PH. In hypoxic WT mice, upregulated TSP1–CD47 signaling decreased pulmonary cMyc leading to elevation in ET-1/ETA and this was associated with pulmonary and RV pathology. Conversely, in the absence of TSP1–CD47, as seen in hypoxic CD47−/− mice, cMyc is upregulated, ET-1/ETA inhibited and PH attenuated. Signal transduction studies in pulmonary vascular cells confirmed that TSP1–CD47 limited cMyc, while cMyc functioned to inhibit ET-1/ETA (see signaling schematic, Figure 7). In contrast, we did not find a significant elevation in ET-1 protein levels in 5th-order PAs from human PH lungs although there was a trend in this direction. Heterogeneity in the clinical cohort is one of several possible reasons our human data did not completely mirror changes found in cell culture and in genetically similar rodents. Although ET-1 is an identified contributor to PH58 and a therapeutic target for the same,59 this is the first study to show that the matricellular protein TSP1, via CD47, is a proximate regulator of ET-1 signaling. It will be important to determine if therapeutics targeting TSP1–CD47 axis can suppresses ET-1 signaling to the same or greater degree as current ET-1 receptor blocking agents.60 In view of the beneficial effects disrupting TSP1–CD47 signaling has upon vascular NO signaling, it is attractive to speculate that a CD47 targeting agent would intersect several pathways in PH for additive therapeutic benefit. cMyc is little studied in the lung although a link with KRAS-mediated tumor survival has been proposed,61 while outside of the lung cMyc promotes proliferation of various cell types. Despite this, we found decreased vascular cell overgrowth in lungs from hypoxic CD47−/− mice that show concurrent upregulation of cMyc compared with WT. The exact reasons for this are likely multiple, although as we show herein inhibition of pro-growth ET-1 signaling is important. Of possible further relevance, CD47−/− cells have recently been reported to be resistant to stress-induced metabolic changes62 and dysregulated metabolic processes, along with glycolic shift, are believed to promote PH-associated pulmonary smooth muscle cell overgrowth63,64 suggesting a possible additional role for CD47 in dysregulating pulmonary metabolism to stimulate cellular overgrowth.
Figure 7.
TSP1–CD47 signaling limits pulmonary cMyc to upregulate ET-1/ETA and promote PH. Hypoxia-mediated induction of TSP1 in the pulmonary vasculature activates endothelial CD47 to suppress cMyc allowing upregulation of ET-1/ETA. ET-1 then targets the pulmonary arterial vascular smooth muscle cell (VSMC) compartment to promote vascular remodeling and cell hypertrophy, decreased vasodilation and increased vasoconstriction. Conversely, treatment with a CD47 antibody, that blocks TSP1 activation of CD47, limits ET-1-mediated VSMC hypertrophy and improves PA sensitivity to NO pathway activators.
4.4 Limitations of the data
This study has a number of limitations. First, use of the chronic hypoxia mouse and the monocrotaline rat models of PH could be considered a limitation. In relation to clinical PH, defined as a resting mean pulmonary artery pressure ≥25 mmHg with rarefication and remodeling of the pulmonary vasculature and eventual RV failure,65 rodent PH models do not recapitulate human disease. Specifically, animals fail to show several of the changes found in human PH lungs including plexiform vascular lesions and do not develop fatal RV heart failure, while the cardiovascular pathology corrects with removal of the stress or with therapy (reviewed in Stenmark et al.66 and Maarman et al.67). More recently, the Sugen/hypoxia rodent model has gained popularity as it captures some of the pathologic lung changes that are reminiscent of human disease and shows less recidivism and more stable disease with exposure to normoxia.68,69 Indeed, there remains ongoing debate as to the relevance of experimental rodent models for clinical PH.67,70 Nonetheless, the two rodent models employed in this study have proven useful in providing understanding into the nature of PH, and along with ex vivo functional studies of pulmonary vessels from these species, provide cross-validation of the observations from human PH lungs and confirm the importance of the TSP1–CD47 signaling mechanism in functional human PA studies. Second, as TSP1 interacts with other cell membrane receptors including, as we have shown, CD3671 and SIRP-α,21 it remains to be determined if blocking CD47 in TSP1−/− mice confers further advantages under chronic hypoxia. Further, CD47 antibody-treated rats achieved only partial correction of mct-driven cardiac changes. It is possible that extended treatment would more completely resolve pathologic changes. Finally, a recent report noted a negative association between elevated cMyc and ET-1 mRNA in pulmonary smooth muscle cells from three patients with PAH.72 Although studies herein indicate a role for cMyc as a constitutive inhibitor of pulmonary ET-1/ETA in pre-clinical PH and in rodent and human pulmonary vascular cells, this remains to be confirmed in human disease.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: J.S.I. serves as chair of the scientific advisory boards of Tioma Therapeutics, Inc. (St. Louis, MO) and Radiation Control Technologies, Inc. (Jersey City, NJ). All other authors have no conflicts of interest to report.
Funding
The non-human sample work of this study was supported by NIH grants R01 HL-108954, 1R01HL112914-01A1, 1R21EB017184-01A1 and American Heart Association (AHA) grant 11BGIA7210001 (J.S.I.); NIH grant HL-112904 and HL-128304 (A.C.S); PI13/01866 and PIE 13/00041 from ISCIII, co-funded by Fondo Europeo de Desarrollo Regional (FEDER) (M.J.C.); Post-Doctoral Award (M.S.S.); AHA 13POST14520003 award, American Society of Transplantation Basic Science Fellowship and a Joseph A. Patrick Fellowship in Transplantation (N.M.R.); and Instituto de Salud Carlos III, grant PI13/01866 and Red Cardiovascular RD12/0042/0065 (M.J.C.). Support was also provided to the hemodynamic core of the VMI by NIH grant P01 HL103455. The human sample work of this study was supported by the Institute for Transfusion Medicine, the Hemophilia Center of Western Pennsylvania and the Vascular Medicine Institute of the University of Pittsburgh School of Medicine (J.S.I.).
Supplementary Material
References
- 1.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, Levy PS, Pietra GG, Reid LM, Reeves JT, Rich S, Vreim CE, Williams GW, Wu M. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991;115:343–349. [DOI] [PubMed] [Google Scholar]
- 2.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 2008;118:2372–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shao D, Park JE, Wort SJ. The role of endothelin-1 in the pathogenesis of pulmonary arterial hypertension. Pharmacol Res 2011;63:504–511. [DOI] [PubMed] [Google Scholar]
- 4.Erol A. Deciphering the intricate regulatory mechanisms for the cellular choice between cell repair, apoptosis or senescence in response to damaging signals. Cell Signal 2011;23:1076–1081. [DOI] [PubMed] [Google Scholar]
- 5.Ho R, Chronis C, Plath K. Mechanistic insights into reprogramming to induced pluripotency. J Cell Physiol 2011;226:868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams JC, Lawler J. The thrombospondins. Cold Spring Harbor Perspect Biol 2011;3:a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts DD, Miller TW, Rogers NM, Yao M, Isenberg JS. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biol 2012;31:162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calzada MJ, Zhou L, Sipes JM, Zhang J, Krutzsch HC, Iruela-Arispe ML, Annis DS, Mosher DF, Roberts DD. Alpha4beta1 integrin mediates selective endothelial cell responses to thrombospondins 1 and 2 in vitro and modulates angiogenesis in vivo. Circ Res 2004;94:462–470. [DOI] [PubMed] [Google Scholar]
- 9.Bauer PM, Bauer EM, Rogers NM, Yao M, Feijoo-Cuaresma M, Pilewski JM, Champion HC, Zuckerbraun BS, Calzada MJ, Isenberg JS. Activated CD47 promotes pulmonary arterial hypertension through targeting caveolin-1. Cardiovasc Res 2012;93:682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labrousse-Arias D, Castillo-Gonzalez R, Rogers NM, Torres-Capelli M, Barreira B, Aragones J, Cogolludo A, Isenberg JS, Calzada MJ. HIF-2alpha-mediated induction of pulmonary thrombospondin-1 contributes to hypoxia-driven vascular remodeling and vasoconstriction. Cardiovasc Res 2016;109:115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ochoa CD, Yu L, Al-Ansari E, Hales CA, Quinn DA. Thrombospondin-1 null mice are resistant to hypoxia-induced pulmonary hypertension. J Cardiothorac Surg 2010;5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helkin A, Maier KG, Gahtan V. Thrombospondin-1, -2 and -5 have differential effects on vascular smooth muscle cell physiology. Biochem Biophys Res Commun 2015;464:1022–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isenberg JS, Annis DS, Pendrak ML, Ptaszynska M, Frazier WA, Mosher DF, Roberts DD. Differential interactions of thrombospondin-1, -2, and -4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem 2009;284:1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isenberg JS, Roberts DD, Frazier WA. CD47: a new target in cardiovascular therapy. Arterioscler Thromb Vasc Biol 2008;28:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci U S A 2005;102:13141–13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem 2006;281:26069–26080. [DOI] [PubMed] [Google Scholar]
- 17.Isenberg JS, Hyodo F, Matsumoto K, Romeo MJ, Abu-Asab M, Tsokos M, Kuppusamy P, Wink DA, Krishna MC, Roberts DD. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood 2007;109:1945–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isenberg JS, Romeo MJ, Abu-Asab M, Tsokos M, Oldenborg A, Pappan L, Wink DA, Frazier WA, Roberts DD. Increasing survival of ischemic tissue by targeting CD47. Circ Res 2007;100:712–720. [DOI] [PubMed] [Google Scholar]
- 19.Marelli-Berg FM, Peek E, Lidington EA, Stauss HJ, Lechler RI. Isolation of endothelial cells from murine tissue. J Immunol Methods 2000;244:205–215. [DOI] [PubMed] [Google Scholar]
- 20.Bauer EM, Qin Y, Miller TW, Bandle RW, Csanyi G, Pagano PJ, Bauer PM, Schnermann J, Roberts DD, Isenberg JS. Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc Res 2010;88:471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao M, Rogers NM, Csanyi G, Rodriguez AI, Ross MA, St Croix C, Knupp H, Novelli EM, Thomson AW, Pagano PJ, Isenberg JS. Thrombospondin-1 activation of signal-regulatory protein-alpha stimulates reactive oxygen species production and promotes renal ischemia reperfusion injury. J Am Soc Nephrol 2014;25:1171–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2015, Vienna. [Google Scholar]
- 23.Ioannis Kosmidis. brglm: Bias reduction in binomial-response 2013, Generalized Linear Models.
- 24.Novelli EM, Kato GJ, Ragni MV, Zhang Y, Hildesheim ME, Nouraie M, Barge S, Meyer MP, Hassett AC, Gordeuk VR, Gladwin MT, Isenberg JS. Plasma thrombospondin-1 is increased during acute sickle cell vaso-occlusive events and associated with acute chest syndrome, hydroxyurea therapy, and lower hemolytic rates. Am J Hematol 2012;87:326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smadja DM, d'Audigier C, Bieche I, Evrard S, Mauge L, Dias JV, Labreuche J, Laurendeau I, Marsac B, Dizier B, Wagner-Ballon O, Boisson-Vidal C, Morandi V, Duong-Van-Huyen JP, Bruneval P, Dignat-George F, Emmerich J, Gaussem P. Thrombospondin-1 is a plasmatic marker of peripheral arterial disease that modulates endothelial progenitor cell angiogenic properties. Arterioscler Thromb Vasc Biol 2011;31:551–559. [DOI] [PubMed] [Google Scholar]
- 26.Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med 2002;21:2409–2419. [DOI] [PubMed] [Google Scholar]
- 27.Rabinovitch M. Pathobiology of pulmonary hypertension. Extracellular matrix. Clin Chest Med 2001;22:433–449. [DOI] [PubMed] [Google Scholar]
- 28.Schreier D, Hacker T, Song G, Chesler N. The role of collagen synthesis in ventricular and vascular adaptation to hypoxic pulmonary hypertension. J Biomech Eng 2013;135:021018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azuma A, Li YJ, Abe S, Usuki J, Matsuda K, Henmi S, Miyauchi Y, Ueda K, Izawa A, Sone S, Hashimoto S, Kudoh S. Interferon-{beta} inhibits bleomycin-induced lung fibrosis by decreasing transforming growth factor-{beta} and thrombospondin. Am J Respir Cell Mol Biol 2005;32:93–98. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Leask A, Abraham DJ, Kennedy L, Shi-Wen X, Denton CP, Black CM, Verjee LS, Eastwood M. Thrombospondin 1 is a key mediator of transforming growth factor beta-mediated cell contractility in systemic sclerosis via a mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK)-dependent mechanism. Fibrogenesis Ttissue Repair 2011;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hotchkiss KA, Basile CM, Spring SC, Bonuccelli G, Lisanti MP, Terman BI. TEM8 expression stimulates endothelial cell adhesion and migration by regulating cell-matrix interactions on collagen. Exp Cell Res 2005;305:133–144. [DOI] [PubMed] [Google Scholar]
- 32.Barst RJ, McGoon M, Torbicki A, Sitbon O, Krowka MJ, Olschewski H, Gaine S. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2004;43:40S–47S. [DOI] [PubMed] [Google Scholar]
- 33.Chemla D, Castelain V, Herve P, Lecarpentier Y, Brimioulle S. Haemodynamic evaluation of pulmonary hypertension. Eur Respir J 2002;20:1314–1331. [DOI] [PubMed] [Google Scholar]
- 34.Grandori C, Cowley SM, James LP, Eisenman RN. The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu Rev Cell Dev Biol 2000;16:653–699. [DOI] [PubMed] [Google Scholar]
- 35.Galie N, Manes A, Branzi A. The endothelin system in pulmonary arterial hypertension. Cardiovasc Res 2004;61:227–237. [DOI] [PubMed] [Google Scholar]
- 36.Lopes AA, Maeda NY, Almeida A, Jaeger R, Ebaid M, Chamone DF. Circulating platelet aggregates indicative of in vivo platelet activation in pulmonary hypertension. Angiology 1993;44:701–706. [DOI] [PubMed] [Google Scholar]
- 37.Schulman LL, Grossman BA, Owen J. Platelet activation and fibrinopeptide formation in pulmonary hypertension. Chest 1993;104:1690–1693. [DOI] [PubMed] [Google Scholar]
- 38.Disdier M, Legrand C, Bouillot C, Dubernard V, Pidard D, Nurden AT. Quantitation of platelet fibrinogen and thrombospondin in Glanzmann's thrombasthenia by electroimmunoassay. Throm Res 1989;53:521–533. [DOI] [PubMed] [Google Scholar]
- 39.Eilers M, Picard D, Yamamoto KR, Bishop JM. Chimaeras of myc oncoprotein and steroid receptors cause hormone-dependent transformation of cells. Nature 1989;340:66–68. [DOI] [PubMed] [Google Scholar]
- 40.Patel JH, McMahon SB. Targeting of Miz-1 is essential for Myc-mediated apoptosis. J Biol Chem 2006;281:3283–3289. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi H, Soma S, Muramatsu M, Oka M, Ienaga H, Fukuchi Y. Discrepant distribution of big endothelin (ET)-1 and ET receptors in the pulmonary artery. Eur Respir J 2001;18:5–14. [DOI] [PubMed] [Google Scholar]
- 42.Marciniak SJ, Plumpton C, Barker PJ, Huskisson NS, Davenport AP. Localization of immunoreactive endothelin and proendothelin in the human lung. Pulm Pharmaco 1992;5:175–182. [DOI] [PubMed] [Google Scholar]
- 43.Grignola JC, Gines F, Bia D, Armentano R. Improved right ventricular-vascular coupling during active pulmonary hypertension. Int J Cardiol 2007;115:171–182. [DOI] [PubMed] [Google Scholar]
- 44.Overbeek MJ, Mouchaers KT, Niessen HM, Hadi AM, Kupreishvili K, Boonstra A, Voskuyl AE, Belien JA, Smit EF, Dijkmans BC, Vonk-Noordegraaf A, Grunberg K. Characteristics of interstitial fibrosis and inflammatory cell infiltration in right ventricles of systemic sclerosis-associated pulmonary arterial hypertension. Int J Rheumatol 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Redout EM, van der Toorn A, Zuidwijk MJ, van de Kolk CW, van Echteld CJ, Musters RJ, van Hardeveld C, Paulus WJ, Simonides WS. Antioxidant treatment attenuates pulmonary arterial hypertension-induced heart failure. Am J Physiol Heart Circ Physiol 2010;298:H1038–H1047. [DOI] [PubMed] [Google Scholar]
- 46.Sharifi-Sanjani M, Shoushtari AH, Quiroz M, Baust J, Sestito SF, Mosher M, Ross M, McTiernan CF, St Croix CM, Bilonick RA, Champion HC, Isenberg JS. Cardiac CD47 drives left ventricular heart failure through Ca2+-CaMKII-regulated induction of HDAC3. J Am Heart Assoc 2014;3:e000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maxhimer JB, Shih HB, Isenberg JS, Miller TW, Roberts DD. Thrombospondin-1/CD47 blockade following ischemia-reperfusion injury is tissue protective. Plast Reconstr Surg 2009;124:1880–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maloney JP, Stearman RS, Bull TM, Calabrese DW, Tripp-Addison ML, Wick MJ, Broeckel U, Robbins IM, Wheeler LA, Cogan JD, Loyd JE. Loss-of-function thrombospondin-1 mutations in familial pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2012;302:L541–L554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Armstrong LC, Bornstein P. Thrombospondins 1 and 2 function as inhibitors of angiogenesis. Matrix Biol 2003;22:63–71. [DOI] [PubMed] [Google Scholar]
- 50.Green DE, Kang BY, Murphy TC, Hart CM. Peroxisome proliferator-activated receptor gamma (PPARgamma) regulates thrombospondin-1 and Nox4 expression in hypoxia-induced human pulmonary artery smooth muscle cell proliferation. Pulm Circ 2012;2:483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang YJ, Zheng XW, Yang GL, Cheng DY, Zhang P. The expression of thrombospondin-1 in serum and pulmonary arterioles of hypoxic pulmonary hypertension rats. Sichuan da xue xue bao Yi xue ban = J Sichuan Univ Med Sci Ed 2012;43:839–842. [PubMed] [Google Scholar]
- 52.Malek MH, Olfert IM. Global deletion of thrombospondin-1 increases cardiac and skeletal muscle capillarity and exercise capacity in mice. Exp Physiol 2009;94:749–760. [DOI] [PubMed] [Google Scholar]
- 53.Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, Roberts DD. Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47. J Biol Chem 2010;285:38923–38932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ridnour LA, Windhausen AN, Isenberg JS, Yeung N, Thomas DD, Vitek MP, Roberts DD, Wink DA. Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and -independent pathways. Proc Natl Acad Sci U S A 2007;104:16898–16903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Csanyi G, Yao M, Rodriguez AI, Al Ghouleh I, Sharifi-Sanjani M, Frazziano G, Huang X, Kelley EE, Isenberg JS, Pagano PJ. Thrombospondin-1 regulates blood flow via CD47 receptor-mediated activation of NADPH oxidase 1. Arterioscler Thromb Vasc Biol 2012;32:2966–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaur S, Soto-Pantoja DR, Stein EV, Liu C, Elkahloun AG, Pendrak ML, Nicolae A, Singh SP, Nie Z, Levens D, Isenberg JS, Roberts DD. Thrombospondin-1 signaling through CD47 inhibits self-renewal by regulating c-Myc and other stem cell transcription factors. Sci Rep 2013;3:1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rogers NM, Zhang ZJ, Wang JJ, Thomson AW, Isenberg JS. CD47 regulates renal tubular epithelial cell self-renewal and proliferation following renal ischemia reperfusion. Kidney Int 2016;90:334–347. [DOI] [PubMed] [Google Scholar]
- 58.Chester AH, Yacoub MH. The role of endothelin-1 in pulmonary arterial hypertension. Glob Cardiol Sci Pract 2014;2014:62–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miyagawa K, Emoto N. Current state of endothelin receptor antagonism in hypertension and pulmonary hypertension. Ther Advan Cardiovasc Dis 2014;8:202–216. [DOI] [PubMed] [Google Scholar]
- 60.Nasser SA, El-Mas MM. Endothelin ETA receptor antagonism in cardiovascular disease. Eur J Pharmacol 2014;737:210–213. [DOI] [PubMed] [Google Scholar]
- 61.Wu C, Wang S, Xu C, Tyler A, Li X, Andersson C, Oji Y, Sugiyama H, Chen Y, Li A. WT1 enhances proliferation and impedes apoptosis in KRAS mutant NSCLC via targeting cMyc. Cell Physiol Biochem 2015;35:647–662. [DOI] [PubMed] [Google Scholar]
- 62.Miller TW, Soto-Pantoja DR, Schwartz AL, Sipes JM, DeGraff WG, Ridnour LA, Wink DA, Roberts DD. CD47 globally regulates metabolic pathways that control resistance to ionizing radiation. J Biol Chem 2015;290:24858–24874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rafikov R, Sun X, Rafikova O, Louise Meadows M, Desai AA, Khalpey Z, Yuan JX, Fineman JR, Black SM. Complex I dysfunction underlies the glycolytic switch in pulmonary hypertensive smooth muscle cells. Redox Biol 2015;6:278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tuder RM, Davis LA, Graham BB. Targeting energetic metabolism: a new frontier in the pathogenesis and treatment of pulmonary hypertension. Am J Respir Crit Care Med 2012;185:260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Badesch DB, Champion HC, Sanchez MA, Hoeper MM, Loyd JE, Manes A, McGoon M, Naeije R, Olschewski H, Oudiz RJ, Torbicki A. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:S55–S66. [DOI] [PubMed] [Google Scholar]
- 66.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 2009;297:L1013–L1032. [DOI] [PubMed] [Google Scholar]
- 67.Maarman G, Lecour S, Butrous G, Thienemann F, Sliwa K. A comprehensive review: the evolution of animal models in pulmonary hypertension research; are we there yet? Pulm Circ 2013;3:739–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 2010;121:2747–2754. [DOI] [PubMed] [Google Scholar]
- 69.Ciuclan L, Bonneau O, Hussey M, Duggan N, Holmes AM, Good R, Stringer R, Jones P, Morrell NW, Jarai G, Walker C, Westwick J, Thomas M. A novel murine model of severe pulmonary arterial hypertension. Am J Respir Crit Care Med 2011;184:1171–1182. [DOI] [PubMed] [Google Scholar]
- 70.Lawrie A. A report on the use of animal models and phenotyping methods in pulmonary hypertension research. Pulm Circ 2014;4:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Isenberg JS, Jia Y, Fukuyama J, Switzer CH, Wink DA, Roberts DD. Thrombospondin-1 inhibits nitric oxide signaling via CD36 by inhibiting myristic acid uptake. J Biol Chem 2007;282:15404–15415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu J, Wilson J, Taylor L, Polgar P. DNA microarray and signal transduction analysis in pulmonary artery smooth muscle cells from heritable and idiopathic pulmonary arterial hypertension subjects. J Cell Biochem 2015;116:386–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.