Abstract
Aims
Atherosclerosis is a chronic inflammatory disease occurring within the artery wall. A crucial step in atherogenesis is the infiltration and retention of monocytes into the subendothelial space of large arteries induced by chemokines and growth factors. Angiopoietin-1 (Ang-1) regulates angiogenesis and reduces vascular permeability and has also been reported to promote monocyte migration in vitro. We investigated the role of Ang-1 in atherosclerosis-prone apolipoprotein-E (Apo-E) knockout mouse.
Methods and results
Apo-E knockout (Apo-E-/-) mice fed a western or normal chow diet received a single iv injection of adenovirus encoding Ang-1 or control vector. Adenovirus-mediated systemic expression of Ang-1 induced a significant increase in early atherosclerotic lesion size and monocyte/macrophage accumulation compared with control animals receiving empty vector. Ang-1 significantly increased plasma MCP-1 and VEGF levels as measured by ELISA. FACS analysis showed that Ang-1 selectively increased inflammatory Gr1+ monocytes in the circulation, while the cell-surface expression of CD11b, which mediates monocyte emigration, was significantly reduced.
Conclusions
Ang-1 specifically increases circulating Gr1+ inflammatory monocytes and increases monocyte/macrophage retention in atherosclerotic plaques, thereby contributing to development of atherosclerosis.
Keywords: Angiopoietin-1 , Atherosclerosis , Monocytes
1. Introduction
Atherosclerosis is a chronic inflammatory disease of the artery wall.1–3 Monocyte-derived macrophages participate in a maladaptive, non-resolving inflammatory response that expands the subendothelial layer due to the accumulation of cells, lipid and matrix.4 Signals such as monocyte chemoattractant protein-1 (MCP-1) increase monocyte recruitment into atherogenic foci,5,6 in particular, Ly6Chigh(Gr1+) inflammatory monocytes that gives rise to macrophages in atheromata.7
Angiopoietin-1 (Ang-1) and its cognate receptor Tie2 are well-established regulators of vascular development and angiogenesis.8–10 Ang-1 plays a crucial role in endothelial cell survival, vessel wall remodelling and mural cell recruitment.11 Overexpression of Ang-1 dramatically blocks increases in vascular permeability induced by VEGF,12,13 suggesting that it has protective properties in the microvasculature.14 However, long-term Ang-1 expression using adeno-associated virus failed to protect against the development of rat cardiac allograft arteriosclerosis.15 Jeansson et al.8 recently reported that Ang-1 is not necessary for normal steady-state physiological processes in the adult, being expendable in the blood vasculature from E13.5 onwards,10 but when combined with injury, Ang-1 deficiency results in accelerated angiogenesis and fibrosis.10 Long et al.14 have reported that Ang-1 therapy accompanied pro-fibrotic and inflammatory effects in folic acid-induced tubular necrosis and in a murine model of acute renal injury.16 More importantly, Ang-1 is expressed at a higher level in atherosclerotic lesions obtained from endarterectomy of the carotid artery compared to healthy controls.17 Furthermore, Ang-1 stimulates TNF-α, a key cytokine that modulates the inflammatory process of atherosclerosis.18 expression in peripheral blood mononuclear cells,19 Indeed, we and others have demonstrated that Ang-1 stimulates monocyte20 and neutrophil21 migration, both of which are the critical players in atherosclerosis,22,23 implicating Ang-1 as a potential player in monocyte recruitment and retention mechanisms especially in a high-lipid environment.
Based on these observations, we hypothesized that Ang-1 plays a role in the progression of atherosclerosis in a hypercholesterolaemic environment through its effects on inflammatory monocytes. In this study, we demonstrate that high circulating Ang-1 levels promote a pro-atherogenic phenotype by specifically increasing Gr1+ inflammatory monocytes and elevating the circulating levels of pro-remodelling cytokines, VEGF and MCP-1 in ApoE-/- mice. Furthermore, Ang-1-induced monocyte/macrophage retention in atherosclerotic plaques was accompanied by decreased cell-surface expression of CD11b on circulating monocytes.
2. Methods
2.1 Adenoviruses
Recombinant adenovirus encoding human Ang-1* (AdAng-1) was provided by Regeneron Pharmaceuticals (Tarrytown, New York, USA), propagated in Human embryonic kidney cells 293 (HEK293), purified on CsCl gradients, titered, and stored at −80 °C in 4% sucrose buffer. Control, empty adenovirus (AdEV) was generated as described previously.22
2.2 Adenovirus-mediated expression of Ang-1 in ApoE-/- mice
All procedures conformed to the recommendations of the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication, 8th Edition, 2011) and also in accordance with Directive 2010/63/EU of the European Parliament and with the UK Home Office Animal (Scientific Procedures) Act 1986. All procedures passed local ethical review. Eight- to nine-week-old male ApoE-/- mice on a C57BL/6J background (B6.129P2-apoEtm1Unc, SN: 002052; Jackson Labs, Maine, USA) were maintained with a 12-hour light/dark cycle and had free access to food and water. To investigate the effects of Ang-1 on atherosclerosis under high-fat environment, the mice were fed a western-style diet (TD 88137; Harlan Teklad, South Easton, MA, USA) for 1 week and then divided into two groups (n = 12 per group) and injected via tail vein with 5 × 109 pfu of AdAng-1 or control empty virus (AdEV) diluted into 100 μL PBS. These mice were maintained on a western diet for another 4 weeks. In another sets of experiments, mice fed a normal chow diet (n = 5 in each group in each experiment). These mice were injected with 5 × 109 pfu of AdAng-1 or control empty virus (AdEV) diluted into 100 μL PBS through tail vain. The mice were euthanized 4 weeks after virus delivery. Briefly, cardiac puncture was performed with 2.3% isoflurane inhalation, and blood was collected in EDTA tube. Mice were injected with Pentabarbitone (Euthatal, 270 mg/kg, ip injection) to euthanize and then perfuse-fixed with 1% paraformaldehyde. Heart and proximal aortae were harvested. Serial 5 µm frozen sections of the aortae were prepared as described previously.24 For the evaluation of atherosclerotic lesions, nine sections were taken at 40 µm intervals, stained with oil red O, counterstained with Mayer’s hematoxylin, and the lesions were quantified using NIH Image software (v. 1.62). The mean area in nine sections was determined for each animal. Up to three sections from each animal were immunostained for monocytes/macrophages using a monoclonal rat anti-mouse monocyte/macrophage antibody (MOMA-2, BD PharMingen) and quantification of MOMA-2-positive area was performed. The mean MOMA-2-positive area was determined for each animal. The aortic arches were available from some animals and Sudan IV en face staining was performed for analysis of lipid accumulation, as described elsewhere.25 Blood was taken at 3 and 10 days after virus injection by tail bleed and at 28 days by cardiac puncture.
2.3 Aortic ring culture
Male ApoE-/- mice (8–10 weeks old) were euthanized and bled out. Thoracic aortas were removed into a Petri dish filled with cold sterile PBS and aortas were mechanically cleaned of surrounding fat tissue. Using a surgical blade, aortas were evenly cut into 1 mm rings, which were transferred to fresh DMEM medium supplemented with 1% fetal calf serum (FCS), penicillin and streptomycin. Aortic rings were stimulated with Ang-1 (400 ng/mL; R&D Systems, Abingdon, UK) for 24 hours at 37 °C. Some of rings were pre-incubated with Tie2 blocking peptide26 (NLLMAAS, 100 µM; Peptide Protein Research Ltd, Hampshire, UK) for 30 min prior to stimulation with Ang-1. Each condition was performed in duplicates. Commercial ELISA as described below measured the levels of MCP-1 and VEGF in the conditioned medium.
2.4 ELISA assay
Whole blood was collected into EDTA-containing tubes. ELISA was used to measure plasma levels of Ang-1, MCP-1 and VEGF (R&D Systems).
2.5 Immunohistochemistry
Immunohistochemistry was performed on serial frozen sections. Primary rat anti-mouse antibodies to monocyte/macrophage (MOMA-2), and CD31 were from BD Pharmingen. Isotype-matched non-binding immunoglobulin was used as a negative control. Binding of secondary antibodies (Vector Laboratories, Peterborough, UK) was detected with Vectastain ABC reagent (Vector Laboratories, Peterborough, UK) and DAB substrate kits (Dako, Cheadle, UK). Cells were counterstained with Mayer’s hematoxylin.
2.6 Flow cytometry
Whole-blood samples (15 μL) were washed with cold PBS before incubating for 20 min on ice with directly conjugated antibodies: anti-Ly6C-APC (eBioscience, Cheshire, UK), anti-CD11b (eBioscience, Cheshire, UK) and anti-Ly6G (Gr1)-FITC (eBioscience, Cheshire, UK). Monocytes were gated according to CD11b expression and side scatter and then further separated by Gr1 and Ly6C staining. Two monocyte subsets were identified as SSClowCD11b+ Gr1 + Ly6Chigh and SSClowCD11b+ Gr1-Ly6Clow monocytes, whereas neutrophils were identified as SSChighCD11b+ Gr1highLy6Cinte (Figure 3A and B) using FACSCalibur. Combination of anti-CD11b, anti-Gr1 and anti-Tie2-PE (eBioscience, Cheshire, UK) were used to stain cells to analyse Tie2-positive monocyte population.
Figure 3.
Ang-1 increases circulating numbers of Gr1+ monocytes. Whole-blood samples were stained with CD11b, Gr1 and Ly6C antibodies and analysed by flow cytometry. (A) CD11b-positive cells were gated (R1) according to CD11b expression and side scatter. R1 cells were further divided into SSClowCD11b+Gr1-Ly6Clow monocytes (R2) and SSClowCD11b+Gr1+Ly6Chigh monocytes (R3) and SSChighCD11b+ Gr1highLy6Cinte neutrophils (R4). (B) 10 days after virus administration, the proportions of Gr1- and Gr1+ moncoytes in each treatment group was calculated as the percentage of total white blood cells. (C) To correct for variability among individual experiments, the Gr1+/Gr1- ratio was calculated for each treatment group. (D) The correlation between MOMA-2-positive areas and Gr1+ and Gr1- monocytes on day 10 after AdAng-1 administration was analysed. Pooled data are presented from three separate independent experiments (n = 4–5 per experiment). **P<0.01.
2.7 Measurement of plasma Ang-1
Blood was collected from mice at days 0, 3, 7, 14, 21, and 28 after injection of AdEV (n = 3) or AdAng-1 (n = 3), mixed with EDTA. Plasma levels of Ang-2 were determined a using specific ELISA following the manufacturer’s instructions (R&D Systems, MN, USA).
2.8 Confocal immunofluorescence staining
Tissue sections were stained with rat monoclonal anti-Ly6C (IgG2c; eBioscience, Paisley, UK) followed by Alexa Fluor 568-conjugated anti-rat IgG (Invitrogen, Paisley, UK). Then counter stained with anti-CD11b-FITC (eBioscience, Cheshire, UK) followed by goat anti-FITC-conjugated with Alexa Fluor488 (Invitrogen, Paisley, UK). Sections were washed with PBS, coverslip mounted and imaged on a Leica SP5C inverted confocal laser scanning microscope.
2.9 Statistical analysis
Results are expressed as the mean ± SEM. Comparisons between two groups were performed using unpaired Student’s t-tests, and comparisons among multiple groups were performed using One-way Analysis of variance (ANOVA). Statistical analyses were performed using Prism 7.0 (GraphPad Software, Inc., La Jolla, CA, USA). P values <0.05 were considered statistically significant.
3. Results
3.1. Ang-1 enhances atherosclerotic plaque formation in ApoE-/- mice fed a western diet
To investigate the effect of Ang-1 on atherosclerosis development, ApoE-/- mice fed a western diet for 1 week were given AdAng-1 or control AdEV injection and maintained on a western diet for a further 4 weeks. It has been demonstrated that iv delivery of adenovirus leads to a widespread distribution of vector with the highest level of expression in the liver.27 The level of Ang-1 in plasma peaked 3 days after injection and remained elevated for >10 days (Supplementary material online, Figure S1). No significant differences in body weight or circulating lipid profiles were detected between these groups (Supplementary material online, Table S1). Four weeks after systemic adenovirus administration, atherosclerotic lesions were analysed in aortas sections using oil red O staining (Figure 1A). Systemic overexpression of Ang-1 significantly increased the mean atherosclerotic lesion size compared with AdEV-infected ApoE-/- mice (0.687 ± 0.071 mm2 vs. 0.437 ± 0.045 mm2; P < 0.01; Figure 1B). Uninfected ApoE-/- mice had lesions similar in size to the AdEV-infected group (0.547 ± 0.05 mm2, data not shown). In addition, with macrophages being a primary cell type contributing to development of the atherosclerotic lesion, we examined the effect Ang-1 on monocyte/macrophage accumulation in atherosclerotic lesion using monocyte/macrophage marker MOMA-2. As expected, Ang-1 induced a significantly increased monocyte/macrophage accumulation in lipid lesions compared with control animals receiving empty vector (0.15 ± 0.015 mm2 vs. 0.09 ± 0.021 mm2; P < 0.05; Figure 1C). Endothelium staining using CD31 antibody showed no significant vascularization in aortas (Figure 1A)
Figure 1.
Ang-1 enhances atherosclerotic plaque formation in ApoE-/- mice fed a Western diet. ApoE-/- mice fed a Western diet for 4 weeks were infected systemically with adenoviruses encoding Ang1 (AdAng-1) or a control virus (AdEV). The mice were euthanized 4 weeks after adenovirus treatment and effects on early to intermediate atherosclerotic lesions were investigated. (A) Representative sections stained with oil red O (top panel) and immunohistochemical staining of monocytes/macrophages and endothelium with the MOMA-2 monoclonal antibody (middle panel) and CD31 antibody (bottom panel) respectively in AdEV or AdAng1-treated ApoE-/- mice. Original magnification, ×40 (Oil red), ×100 (MOMA-2). Bars, 100 µm. (B) Atherosclerotic lesion area was quantified by oil red O staining of lesions from serial aortic sections using ImageJ image analysis software. Nine serial sections at 40 µm intervals were used from each animal for analysis. Results show the mean from nine cross-sectional lesion size (mm2) for each animal and the line indicates median value per treatment of mice. *P < 0.01. (C) Similarly, five mice were randomly picked from each treatment group, and tissue sections from each animal were analysed for MOMA-2-positive lesion area. *P<0.05.
3.2. Ang-1 promotes atherosclerotic plaque formation in ApoE-/- mice fed a normal chow diet
In order to further confirm the effects of Ang1 in atherosclerosis development without the confounding effects of western diet on inflammation in apoE-/- mice, AdAng-1 or control AdEV were injected in apoE-/- mice fed a normal chow diet and en face analyses of Sudan IV-stained areas in thoracic aortas were quantified 4 weeks after treatment. Ang-1 significantly increased plaque area compared with AdEV-treated ApoE-/- mice (3.90 ± 0.195 mm2 vs. 0.73 ± 0.169 mm2; P < 0.01; Figure B). Oil red O-positive atherosclerotic area and MOMA-2-positive area were also analysed as before (Figure D). As expected, mice fed a normal chow diet developed much smaller plaques compared with mice on a western diet. However, the plaques were again significantly larger in the AdAng-1-treated group compared to the AdEV-treated group (0.20 ± 0.034 mm2 vs. 0.11 ± 0.024 mm2; P < 0.05; Figure 2C). Similarly, the accumulation of MOMA-2-positive cells, which co-localized with oil red O staining in the aortic root (Figure 1A), was also increased in the AdAng-1-treated group (0.03 ± 0.003 mm2 vs. 0.02 ± 0.004 mm2; P < 0.01; Figure 2D)
Figure 2.
Ang-1 promotes early atherosclerotic plaque formation in ApoE-/- mice fed a normal chow diet. ApoE-/- mice fed a normal chow diet were infected systemically with adenoviruses encoding Ang-1 (AdAng-1), or control virus (AdEV). The mice were euthanized after 4 weeks of adenovirus treatment and effects on early to intermediate atherosclerotic lesions was investigated. (A) Representative picture of Sudan IV en face (top panel) (n = 4 per group). (B) Sudan IV en face staining was quantified from the beginning of aortic arch to the left common carotid artery using image analysis software and expressed as the percentage lesion area in each vessel. **P < 0.001. (C) Oil red O-positive atherosclerotic areas were calculated by using ImageJ image analysis software (n = 12–15 per group). Atherosclerotic plaques were quantified as described in the legend to Figure 1. *P<0.05. (D) Similarly, three sections from each animal were analysed for MOMA-2-positive lesion size (mm2) (n = 12–15 per group). *P<0.05. Original magnification ×2.5 (Sudan IV en face); bars, 1 mm (Sudan IV).
3.3. Ang-1 increases proportion of circulating Gr1+/Ly6Chigh monocytes
It is widely accepted that bone marrow-derived circulating monocytes play an important role in atherosclerosis28 and that different subsets of monocytes commit for specific functions while still in the circulation.29 Particularly, Ly6Chigh(Gr1+) inflammatory monocytes give rise to macrophages in atheromata.7 To investigate the role of Ang-1 on the dynamics of monocyte turnover/recruitment, peripheral monocytes in ApoE-/- mice fed a normal chow diet were evaluated at different time points after AdAng-1 treatment (3, 10 and 28 days). Monocytes were gated according CD11b+ and SSC (Figure 3A R1) and two monocyte subsets were identified as SSClowCD11b + Gr1 + Ly6Chigh (Figure 3A R3) and SSClowCD11b + Gr1-Ly6Clow monocytes (Figure 3A R2), which correspond to inflammatory and residential subsets, respectively.30 A third CD11b+ cells population with high SSC, low Ly6C are neutrophils (Figure 3A R4).7,30 The proportion of circulating Gr1+ monocytes in AdAng-1-treated mice was significantly increased at day 10 compared with AdEV-treated mice (4.95 ± 0.503 vs. 2.93 ± 0.163; P < 0.01), whereas Gr1- monocytes remained unchanged (Figure 3B). Interestingly, neutrophil proportion was also increased (9.53 ± 3.92 vs. 13.74 ± 4.04; P < 0.05). Significant increase in the ratio of Gr1+/Gr1- monocytes (Figure 3C) was positively correlated with plaque size (Figure 3D), indicating the importance of monocyte subsets balance in disease progression. At day 3 and day 28, the proportions of Gr1- and Gr1+ monocytes showed no differences between AdEV and AdAng-1 treated groups (data not shown). Furthermore, immunostaining of aorta obtained from ApoE-/- mice received AdAng-1 treatment revealed that Ly6C-positive cells localized in the plaques (Figure 4) in line with the current understanding that this group of cells is likely to account for the observed accumulation of monocytes/macrophages in the plaques.
Figure 4.

Ang-1 induces Ly6C + CD11b+ monocytes accumulation in ApoE-/- mice fed a normal chow diet. Representative immunofluorescence staining of Ly6C and CD11b monoclonal antibodies in AdAng1-treated ApoE-/- mice. Red: Ly6C, green: CD11b, blue: 4’,6-diamidino-2-phenylindole (DAPI). Original magnification, ×40 (left), ×80 (right). White arrow is the border line between arterial wall and plaque judging by out layer of muscle filament. Yellow arrow is double staining mononuclear cell.
It is believed that inflammatory monocytes derived from bone marrow play a crucial role in atherogenesis. Recently, spleen has been identified as a site for storage and rapid deployment of monocytes, which contribute to atherosclerosis development.31 Interestingly, preliminary study showed that proportion of bone marrow Ly6Chigh monocytes was reduced following AdAng-1 treatment, whereas splenic Ly6Chigh monocytes remained unchanged (data not shown), suggesting that Ly6Chigh inflammatory monocytes are likely mobilized from bone marrow in response to Ang-1.
3.4 Ang-1 increases plasma levels of VEGF and MCP-1 in ApoE-/- mice fed a normal chow diet
Chemokines such as MCP-1 is crucial for monocyte mobilization from bone marrow.32 To test whether overexpression of Ang-1 alters circulating cytokine/chemokine profiles, we analysed the plasma concentrations of VEGF and MCP-1 by ELISA. Ang-1 significantly increased both plasma VEGF (Figure 5A) and MCP-1 (Figure 5B) levels on day 3 and 10 after administration of viruses. Notably, the increased level of MCP-1 persisted even on day 28 when plasma Ang-1 concentrations had subsided (Supplementary material online, Figure S1). Furthermore, the plasma level of VEGF significantly correlated with expression of MCP-1 on day 10 in AdAng-1-treated but not in AdEV-treated animals (Supplementary material online, Figure S2).
Figure 5.
Ang-1 increases plasma levels of VEGF and MCP-1 ApoE-/- mice fed a normal chow diet. Whole blood was collected at 3 and 10 and 28 days after AdEV or AdAng-1 administration and plasma was isolated. ELISA was used to measure the level of VEGF (A) and MCP-1 (B). Pooled data are presented from four independent experiments (n = 17–19 per group). **P < 0.01.
3.5 Ang-1 induces release of VEGF and MCP-1 from aortic rings
Next, we performed aortic ring cultures to investigate the origin of VEGF and MCP-1 upon stimulation with Ang-1. Ang-1 significantly increased release of both VEGF and MCP-1 from aortic rings, and these effects were blocked by pre-incubation with the Tie2 peptide (Figure 6), suggesting that Ang-1 induces vascular cell expression of VEGF and MCP-1, thereby creating a pro-remodelling environment in large vessels in which atherosclerotic plaques form. In addition, expression of VEGF and MCP-1 was significantly correlated with one another in these aortic rings (Supplementary material online, Figure S3).
Figure 6.
Ang-1 induces the VEGF and MCP-1 release in conditioned medium from aortic ring. Thoratic artery from apoE-/- mouse was cut into 1 mm segments and aortic rings were stimulated with Ang-1 (400 ng/mL) with or without pre-incubation with Tie2 blocking peptide (NLLMAAS, 100 µM) for 30 min. Medium containing 0.01% DMSO was served as vehicle control. The levels of VEGF (A) and MCP-1 (B) in conditioned medium were measured by ELISA assay. Pooled data are presented from four independent experiments performed in duplicates.
3.6 Ang-1 reduces cell-surface expression of CD11b on Gr1+ monocytes
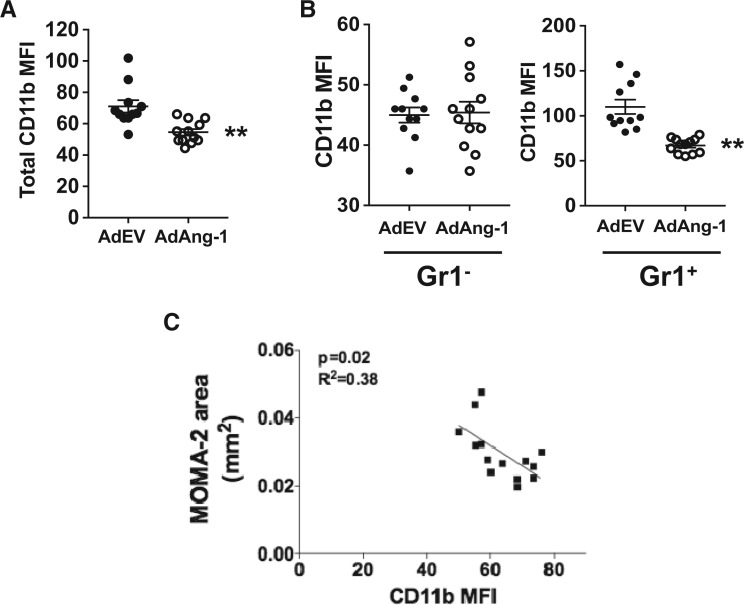
The β2-integrin heterodimer CD11b/CD18 (αMβ2, also called Mac-1) is one of the major adhesion molecules on monocytes that mediates firm adhesion to endothelial cells via intercellular adhesion molecule-1 (ICAM-1).33,34 It is required for monocyte reverse migration and may play a role in atherosclerosis development.35,36 Immunostaining of ICAM1 at the sinus did not show any difference between control virus-treated and AdAng-1-treated mice (Supplementary material online, Figure S4). However, CC chemokine receptor 2 (CCR2) expressions on circulating monocytes showed significant increase 10 days after AdAng-1 infection (data not shown), suggesting that Ang-1 may contribute to increased monocyte recruitment. To determine whether the pro-atherogenic effects of Ang-1 are associated with changes in β2-integrins on monocytes, we analysed the expression of CD11b on circulating monocytes. Mice treated with AdAng-1 have a significantly lower level of monocyte CD11b expression 10 days after AdAng-1 treatment (Figure 7A). In addition, this Ang-1-induced down-regulation of CD11b was Gr1+ monocyte specific (Figure 7B), and the down-regulation of CD11b persisted up to 28 days after virus administration, after expression of Ang-1 had declined (Supplementary material online, Figure S5). Furthermore, there was a significant negative correlation between CD11b and MOMA-2-positive lesion area in ApoE-/- mice (Figure 7C), suggesting a possible protective function of CD11b, which is inhibited by Ang-1, thereby resulting in enhanced atherosclerosis in ApoE-/- mice.
Figure 7.
Ang-1 reduces cell-surface CD11b expression on Gr1+ monocytes. Whole-blood samples were stained with anti-CD11b and anti-Gr1 antibodies, and analysed by flow cytometry as described in the legend to Figure 3. (A) CD11b expressions [median fluorescence intensity (MFI)] on circulating monocytes in control and AdAng-1 groups were compared 10 days after virus administration. (B) CD11b expression on Gr1- and Gr1+ monocytes in control and AdAng-1 groups were analysed and representative histograms were depicted. (C) The correlation between MOMA-2-positive areas and CD11b expression on Gr1+ monocytes on day 10 after AdAng-1 administration was analysed. CD11b expression was represented by MFI. Pooled data are presented from three independent experiments (n = 5 per experiment). **P<0.01.
To further investigate the mechanism by which down-regulation of CD11b may reduce monocyte transmigration, we examined the interactions of monocytes with human umbilical vein endothelial cells (HUVECs). We found that blocking CD11b function with the monoclonal antibody ICRF44 reduced monocytes reverse transmigration through HUVEC monolayers by more than two-fold (IgG control; n = 3, transmigrated 15.70% vs. ICRF44; n = 3, transmigrated 32.43%, P = 0.068) (Supplementary material online, Figure S6), suggesting that down-regulation of CD11b expression on monocytes may lead to increased monocyte retention.
In addition, we explored Tie2 expression in Gr1 + CD11b+ and Gr1-CD11b+ monocytes and found that Tie2 expression was largely restricted to Gr1+ monocytes compared to Gr1- monocytes in both blood (Supplementary material online, Figure S7, 4.000 ± 0.1941 vs. 1.973 ± 0.176; P < 0.01) and spleen (Supplementary material online, Figure S7, 10.44 ± 1.376 vs. 3.892 ± 0.3050; P < 0.01). Immunostaining of aorta obtained from ApoE-/- mice received AdAng-1 treatment revealed that Tie2-positive macrophages were present in the plaques (Supplementary material online, Figure S8), suggesting that Ang-1/Tie2 pathway on monocytes, is in part, responsible for Ang-1 pro-atherogenic effects.
4. Discussion
The activation of endothelial cells and the recruitment of monocytes are key events in the early onset of atherosclerosis; however, these initial processes may be reversible and typically do not cause clinical consequences.1,37 It is the subsequent prolonged retention of monocytes/macrophages in the intimal space and their uptake of oxidized LDL to form foam cells that constitutes the major cellular component in early atherosclerotic lesion development.38 The major finding of this study is that systemic overexpression of Ang-1 accelerates atherosclerosis development in ApoE-/- mice. It has been shown that levels of Ang-1, but not of Ang-2, are significantly increased in conditioned medium from cultured atherosclerotic arteries compared to healthy arteries.17 It has also been shown that Tie2 expression is increased in atherosclerotic arteries compared to healthy arteries. These findings support our results and suggest that the Ang-1/Tie2 system has a significant role in the development of atherosclerosis. Although Ang-1 has been shown to have anti-inflammatory properties in endothelial cells, our present study clearly demonstrates pro-remodelling effects of Ang-1 on monocytes that translate into increased atherosclerosis in the context of elevated cholesterol levels in ApoE-/- mice. The mechanisms by which Ang-1 promotes atherosclerosis appear to involve (i) Ang-1-induced pro-remodelling cytokine release; (ii) Ang-1 increases the proportion of circulating inflammatory monocytes and (iii) Ang-1-mediated reduction in CD11b expression on monocytes, resulting in increased monocyte/macrophage retention in atherosclerotic plaques.
In the present study, plasma levels of VEGF and MCP-1, both of which promote atherosclerosis in mice,39,40 are increased following systemic overexpression of Ang-1. Ang-1 increased VEGF and MCP-1 release via Tie2 from aortic rings suggesting that the inflammatory effects of Ang-1 are evident in large arteries, in which atherosclerotic plaques form. Taken together our data suggest that Ang-1/Tie2 pathway participates in the inflammatory process under these conditions. VEGF is a potent regulator of vascular permeability.41 It not only stimulates endothelial cell proliferation but also upregulates other pro-remodelling cytokines release from endothelial cells, such as MCP-1.42 Ang-1-induced increased levels of VEGF and MCP-1 are positively correlated with each other in both in vivo and in vitro systems, suggesting Ang-1 can create a positive pro-remodelling cytokine feedback loop in large arteries, which resulted in sustained inflammation even after systemic Ang-1 levels dropped to baseline 14 days after AdAng-1 injection (Supplementary material online, Figure S1).
Hypercholesterolemia induces monocytosis and monocytes accumulation in the plaques.43 Recently, the importance of neutrophil in early atherosclerosis development has been increasingly recognized.44 Doring et al.43 have demonstrated that the mechanism of neutrophil-driven atherosclerosis is mediated through neutrophil granule protein cathelicidin-induced inflammatory monocytes recruitment,45 suggesting that bone marrow-derived monocytes are crucial in atherosclerosis development. Hypercholesterolemia is known to induce selective expansion of Gr1+/Ly6Chigh monocytes in ApoE–/– mice, and these cells preferentially adhere to activated endothelium, infiltrate the arterial wall and develop into atherosclerotic macrophages.7 Ang-1 overexpression leads to a reduction in the prevalence of Gr1+/Ly6Chigh inflammatory monocytes in bone marrow but an increase in circulation in AdAng-1-treated animals, suggesting that Ang-1 may trigger monocyte mobilization from bone marrow most likely through up-regulation of MCP-1.32,40,46–49 The imbalance of monocyte subsets in circulation induced by Ang-1 overexpression is associated with increased atheroma (Figure 3D). In addition, we show for the first time that Gr1+/Ly6Chigh not Gr1-/Ly6Clow monocytes express Tie-2 in ApoE-/- mice (Supplementary material online, Figure S7). Until recently, Tie-2 was thought to be restricted to endothelial cells. However, De Palma et al.48 identified a subset of Tie-2-positive monocytes that promote angiogenesis in experimental tumour model.50 Recently, studies have revealed that functions of Tie-2-expressing monocytes (TEMs) may not be restricted to angiogenesis and immunosuppression. TEMs are involved in inflammatory process.51,52 Our findings suggest that Ang-1/Tie2 is, in part, responsible for migration/recruitment of Gr1+/CCR2+ monocytes in addition to MCP-1. Furthermore, the presence of Ly6C + cells within atherosclerotic plaques (Figure 4) provides further evidence that this group of cells is likely to account for the observed accumulation of monocytes/macrophages, which contributed to Ang-1-induced atherosclerosis development.
Adhesion molecules participating in monocyte–endothelial cell interactions are known to play a critical role in atherogenesis.35 In this study, we observed that overexpression of Ang-1 increased oil red-positive lesion size and it is associated with down-regulation of CD11b expression on circulating Gr1+ monocytes. It has become increasingly clear that the dynamic trafficking of monocyte-derived cells within atherosclerotic lesions is closely linked to disease progression.53 Prolonged retention of monocytes/macrophages in the intimal space1,2 or reduced rate of mononuclear cell emigration (i.e. reverse migration) from lesions53 is crucial for atherosclerosis development. Reverse migration is a physiological feature of human mononuclear phagocytes54 and that this process is dependent on both ICAM-1 and CD18 (integrin β2).54 Since CD11b forms functional heterodimer complex with CD18, down-regulation of CD11b expression is likely to affect monocyte reverse migration and as a consequence would favour monocyte retention and progression of atherosclerosis. Our assumption is supported by in vitro study showing that blockade of CD11b function leads to reduced monocyte reverse transmigration through endothelial cells and inverse correlation between CD11b expression and plaque monocyte/macrophage accumulation (Figure 7C). Merched et al.53 showed that β2 integrin deficiency accelerates early atherosclerosis in LDLR−/− mice, suggesting that CD11b may play a dynamic role in the development of atherosclerosis. Interestingly, CD11b/CD18 activation has been proven to be able to inhibit macrophage lipid uptake, and CD11b-deficient peritoneal macrophages have up-regulated level of CD36 expression, and lipid accumulation compare to wild-type controls,54 suggesting that Ang-1-induced down-regulation of CD11b may contribute to lipid accumulation in the lesions.
In conclusion, Ang-1 overexpression induces MCP-1 and VEGF in circulation and subsequently caused inflammatory monocyte mobilization, and down-regulated CD11b expression on these cells, leading to monocytes/macrophages accumulation and atherosclerotic plaque formation. Our findings provide a novel mechanism by which Ang-1 may contribute to atherosclerosis development.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Acknowledgements
A special thanks goes to Professor Hiroyuki Kuramoto, Kitasato University, Kanagawa, Japan, for providing the early guidance and support to TF. We thank Clara Yates and Professor Gerard Nash from University of Birmingham for their assistance in data acquisition of monocyte migration assay. For their continuous and generous financial support to Aston Medical School research portfolio, a special thanks goes to Sir Doug Ellis and Tim Watts of the West Midlands.
Conflict of interest: None declared.
Funding
This study was funded by grants from the British Heart Foundation (PG/06/114) and Medical Research Council (G0601295 and G0700288) to A.A., and by grants from the NIH (R01 HL70165) and Mid-Atlantic Affiliate of the American Heart Association (0355792U) to C.D.K..
References
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011;473:317–325. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol 2011;12:204–212. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Silvertown JD. Inflammation, C-reactive protein, and atherothrombosis. J Periodontol 2008;79:1544–1551. [DOI] [PubMed] [Google Scholar]
- 4.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011;145:341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 2007;117:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite Role of Angiopoietin-1, a Ligand for the TIE2 Receptor, during Embryonic Angiogenesis. Cell 1996;87:1171–1180. [DOI] [PubMed] [Google Scholar]
- 7.Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med 2006;12:235–239. [DOI] [PubMed] [Google Scholar]
- 8.Jeansson M, Gawlik A, Anderson G, Li C, Kerjaschki D, Henkelman M, Quaggin SE. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest 2011;121:2278–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witzenbichler B, Maisonpierre PC, Jones P, Yancopoulos GD, Isner JM. Chemotactic properties of angiopoietin-1 and -2, ligands for the endothelial-specific receptor tyrosine kinase Tie2. J Biol Chem 1998;273:18514–18521. [DOI] [PubMed] [Google Scholar]
- 10.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 1999;286:2511–2514. [DOI] [PubMed] [Google Scholar]
- 11.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med 2000;6:460–463. [DOI] [PubMed] [Google Scholar]
- 12.Witzenbichler B, Westermann D, Knueppel S, Schultheiss H-P, Tschope C. Protective role of angiopoietin-1 in endotoxic shock. Circulation 2005;111:97–105. [DOI] [PubMed] [Google Scholar]
- 13.Nykanen AI, Pajusola K, Krebs R, Keranen MA, Raisky O, Koskinen PK, Alitalo K, Lemstrom KB. Common protective and diverse smooth muscle cell effects of AAV-mediated angiopoietin-1 and -2 expression in rat cardiac allograft vasculopathy. Circ Res 2006;98:1373–1380. [DOI] [PubMed] [Google Scholar]
- 14.Long DA, Price KL, Ioffe E, Gannon CM, Gnudi L, White KE, Yancopoulos GD, Rudge JS, Woolf AS. Angiopoietin-1 therapy enhances fibrosis and inflammation following folic acid-induced acute renal injury. Kidney Int 2008;74:300–309. [DOI] [PubMed] [Google Scholar]
- 15.Le Dall J, Ho-Tin-Noé B, Louedec L, Meilhac O, Roncal C, Carmeliet P, Germain S, Michel J-B, Houard X. Immaturity of microvessels in haemorrhagic plaques is associated with proteolytic degradation of angiogenic factors. Cardiovasc Res 2010;85:184–193. [DOI] [PubMed] [Google Scholar]
- 16.McKellar GE, McCarey DW, Sattar N, McInnes IB. Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat Rev Cardiol 2009;6:410–417. [DOI] [PubMed] [Google Scholar]
- 17.Seok SH, Heo JI, Hwang JH, Na YR, Yun JH, Lee EH, Park JW, Cho CH. Angiopoietin-1 elicits pro-inflammatory responses in monocytes and differentiating macrophages. Mol Cells 2013;35:550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad S, Cudmore MJ, Wang K, Hewett P, Potluri R, Fujisawa T, Ahmed A. Angiopoietin-1 Induces Migration of Monocytes in a Tie-2 and Integrin-Independent Manner. Hypertension 2010;56:477–483. [DOI] [PubMed] [Google Scholar]
- 19.Sturn DH, Feistritzer C, Mosheimer BA, Djanani A, Bijuklic K, Patsch JR, Wiedermann CJ. Angiopoietin affects neutrophil migration. Microcirculation 2005;12:393–403. [DOI] [PubMed] [Google Scholar]
- 20.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med 2011;17:1410–1422. [DOI] [PubMed] [Google Scholar]
- 21.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol 2010;7:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed A, Fujisawa T, Niu XL, Ahmad S, Al-Ani B, Chudasama K, Abbas A, Potluri R, Bhandari V, Findley CM, Lam GK, Huang J, Hewett PW, Cudmore M, Kontos CD. Angiopoietin-2 confers Atheroprotection in apoE-/- mice by inhibiting LDL oxidation via nitric oxide. Circ Res 2009;104:1333–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holman RL, Mc GH, Jr., Strong JP, Geer JC. Technics for studying atherosclerotic lesions. Lab Invest 1958;7:42–47. [PubMed] [Google Scholar]
- 24.Tournaire R, Simon MP, le Noble F, Eichmann A, England P, Pouyssegur J. A short synthetic peptide inhibits signal transduction, migration and angiogenesis mediated by Tie2 receptor. EMBO reports 2004;5:262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansson GK. Inflammation, Atherosclerosis, and Coronary Artery Disease. N Engl J Med 2005;352:1685–1695. [DOI] [PubMed] [Google Scholar]
- 26.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest 2007;117:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood M, Perrotte P, Onishi E, Harper ME, Dinney C, Pagliaro L, Wilson DR. Biodistribution of an adenoviral vector carrying the luciferase reporter gene following intravesical or intravenous administration to a mouse. Cancer Gene Ther 1999; 6(4):367–372. [DOI] [PubMed] [Google Scholar]
- 28.Gautier EL, Jakubzick C, Randolph GJ. Regulation of the migration and survival of monocyte subsets by chemokine receptors and its relevance to atherosclerosis. Arterioscler Thromb Vasc Biol 2009;29:1412–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation 2012;125:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest 2007;117:902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shang X-Z, Issekutz AC. Contribution of CD11a/CD18, CD11b/CD18, ICAM-1 (CD54) and −2 (CD102) to human monocyte migration through endothelium and connective tissue fibroblast barriers. Eur J Immunol 1998;28:1970–1979. [DOI] [PubMed] [Google Scholar]
- 32.von Andrian UH, Chambers JD, McEvoy LM, Bargatze RF, Arfors KE, Butcher EC. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci USA 1991;88:7538–7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nageh MF, Sandberg ET, Marotti KR, Lin AH, Melchior EP, Bullard DC, Beaudet AL. Deficiency of Inflammatory Cell Adhesion Molecules Protects Against Atherosclerosis in Mice. Arterioscler Thromb Vasc Biol 1997;17:1517–1520. [DOI] [PubMed] [Google Scholar]
- 34.Collins RG, Velji R, Guevara NV, Hicks MJ, Chan L, Beaudet AL. P-Selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J Exp Med 2000;191:189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Libby P. Inflammation in atherosclerosis. Nature 2002;420:868–874. [DOI] [PubMed] [Google Scholar]
- 36.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell 2001;104:503–516. [DOI] [PubMed] [Google Scholar]
- 37.Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, Dake MD. Vascular endothelial growth factor enhances atherosclerotic plaque progression . Nat Med 2001;7:425–429. [DOI] [PubMed] [Google Scholar]
- 38.Aiello RJ, Bourassa PA, Lindsey S, Weng W, Natoli E, Rollins BJ, Milos PM. Monocyte chemoattractant protein-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 1999;19:1518–1525. [DOI] [PubMed] [Google Scholar]
- 39.Yeo KT, Wang HH, Nagy JA, Sioussat TM, Ledbetter SR, Hoogewerf AJ, Zhou Y, Masse EM, Senger DR, Dvorak HF, et al. Vascular permeability factor (vascular endothelial growth factor) in guinea pig and human tumor and inflammatory effusions. Cancer Res 1993;53:2912–2918. [PubMed] [Google Scholar]
- 40.Marumo T, Schini-Kerth VB, Busse R. Vascular endothelial growth factor activates nuclear factor-kappaB and induces monocyte chemoattractant protein-1 in bovine retinal endothelial cells. Diabetes 1999;48:1131–1137. [DOI] [PubMed] [Google Scholar]
- 41.Soehnlein O, Drechsler M, Hristov M, Weber C. Functional alterations of myeloid cell subsets in hyperlipidaemia: relevance for atherosclerosis. J Cell Mol Med 2009;13:4293–4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ Res 2012;110:875–888. [DOI] [PubMed] [Google Scholar]
- 43.Doring Y, Drechsler M, Wantha S, Kemmerich K, Lievens D, Vijayan S, Gallo RL, Weber C, Soehnlein O. Lack of neutrophil-derived CRAMP reduces atherosclerosis in mice. Circ Res 2012;110:1052–1056. [DOI] [PubMed] [Google Scholar]
- 44.Lu M, Perez VL, Ma N, Miyamoto K, Peng HB, Liao JK, Adamis AP. VEGF Increases Retinal Vascular ICAM-1 Expression In Vivo. Invest Ophthalmol Vis Sci 1999;40:1808–1812. [PubMed] [Google Scholar]
- 45.Clauss M, Weich H, Breier G, Knies U, Rockl W, Waltenberger J, Risau W. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Bio Chem 1996;271:17629–17634. [DOI] [PubMed] [Google Scholar]
- 46.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2-/- mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 1998;394:894–897. [DOI] [PubMed] [Google Scholar]
- 47.Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, Rollins BJ, Charo IF. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest 1999;103:773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 2005;8:211–226. [DOI] [PubMed] [Google Scholar]
- 49.Hamilton JA, Tak PP. The dynamics of macrophage lineage populations in inflammatory and autoimmune diseases. Arthritis Rheum 2009;60:1210–1221. [DOI] [PubMed] [Google Scholar]
- 50.Garcia S, Krausz S, Ambarus CA, Fernandez BM, Hartkamp LM, van Es IE, Hamann J, Baeten DL, Tak PP, Reedquist KA. Tie2 signaling cooperates with TNF to promote the pro-inflammatory activation of human macrophages independently of macrophage functional phenotype. PLoS One 2014;9:e82088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A 2004;101:11779–11784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Randolph GJ, Furie MB. Mononuclear phagocytes egress from an in vitro model of the vascular wall by migrating across endothelium in the basal to apical direction: role of intercellular adhesion molecule 1 and the CD11/CD18 integrins. J Exp Med 1996;183:451–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merched A, Tollefson K, Chan L. β2 integrins modulate the initiation and progression of atherosclerosis in low-density lipoprotein receptor knockout mice. Cardiovasc Res 2010;85:853–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yakubenko VP, Bhattacharjee A, Pluskota E, Cathcart MK. alphaMbeta(2) integrin activation prevents alternative activation of human and murine macrophages and impedes foam cell formation. Circ Res 2011;108:544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]