Abstract
Aims
Myxomatous valve disease (MVD) is the most common aetiology of primary mitral regurgitation. Recent studies suggest that defects in heart valve development can lead to heart valve disease in adults. Wnt/β-catenin signalling is active during heart valve development and has been reported in human MVD. The consequences of increased Wnt/β-catenin signalling due to Axin2 deficiency in postnatal valve remodelling and pathogenesis of MVD were determined.
Methods and results
To investigate the role of Wnt/β-catenin signalling, we analysed heart valves from mice deficient in Axin2 (KO), a negative regulator of Wnt/β-catenin signalling. Axin2 KO mice display enlarged mitral and aortic valves (AoV) after birth with increased Wnt/β-catenin signalling and cell proliferation, whereas Sox9 expression and collagen deposition are decreased. At 2 months in Axin2 KO mice, the valve extracellular matrix (ECM) is stratified but distal AoV leaflets remain thickened and develop aortic insufficiency. Progressive myxomatous degeneration is apparent at 4 months with extensive ECM remodelling and focal aggrecan-rich areas, along with increased BMP signalling. Infiltration of inflammatory cells is also observed in Axin2 KO AoV prior to ECM remodelling. Overall, these features are consistent with the progression of human MVD. Finally, Axin2 expression is decreased and Wnt/β-catenin signalling is increased in myxomatous mitral valves in a murine model of Marfan syndrome, supporting the importance of Wnt/β-catenin signalling in the development of MVD.
Conclusions
Altogether, these data indicate that Axin2 limits Wnt/β-catenin signalling after birth and allows proper heart valve maturation. Moreover, dysregulation of Wnt/β-catenin signalling resulting from loss of Axin2 leads to progressive MVD.
Keywords: Axin2 • Heart valve development • Myxomatous valve disease • ECM remodelling • Wnt/β-catenin signalling
1. Introduction
Myxomatous heart valves are characterized by thickening of leaflets leading to abnormal leaflet displacement, incomplete closure of the valve, and ultimately regurgitation. The mitral valve is the most affected heart valve and myxomatous valve disease is the most common aetiology of primary mitral regurgitation.1 Late stage human myxomatous diseased valves exhibit increased accumulation of glycosaminoglycans (GAG) accompanied by disorganization of collagen fibres,2 increased cellularity3 and induction of TGF-β and BMP signalling.2,3 Chondrogenic regulatory pathways, including expression of the transcription factor Sox9 and aggrecan,3,4 also are increased supporting the idea that MVD mimics aspects of chondrogenesis.5 In addition, infiltration of inflammatory cells has been reported but their contribution to MVD is unknown.2 Nevertheless, molecular pathways and cellular origins involved in the onset and progression of MVD are incompletely understood. Therefore, therapeutic targets are not yet available and surgery is still the only treatment.
Recent genetic studies indicate that congenital heart valve defects leading to defective leaflet remodelling precede MVD diagnosed much later in life6–9 when heart valve insufficiency occurs. Before birth, heart valve development starts with endothelial–mesenchymal transformation (EMT) of the endocardium to form mesenchymal cushions. During late gestation and after birth, the GAG-rich endocardial cushions undergo ECM remodelling and transform into stratified leaflets.10 Along with ECM stratification and compaction, cell proliferation decreases during heart valve maturation.10 Still, molecular pathways regulating heart valve maturation are far from being elucidated but are of critical importance, as defects in ECM organization and remodelling are common features of adult valve disease.
Axin2 is a downstream target of Wnt/β-catenin signalling and also is an inhibitor of the pathway as part of a protein complex promoting β-catenin degradation.11 In the developing skeleton, loss of Axin2 accelerates chondrocyte maturation by increasing Wnt/β-catenin signalling.12 Before birth, Wnt/β-catenin signalling is active in VICs13 and contributes to valve remodelling and proliferation in endocardial cushions.14 Interestingly, β-catenin is required for adult heart valve homeostasis4 and Wnt/β-catenin signalling is activated in human MVD.15 However, the requirements for Axin2/Wnt/β-catenin signalling in postnatal valve remodelling or progression of MVD have not been determined previously.
Using Axin2 knockout (KO) mice (Axin2LacZ/LacZ), we investigated the role of Axin2/Wnt/β-catenin signalling in postnatal heart valve morphogenesis and adult disease progression. Axin2 is expressed throughout the valve leaflets and inhibits Wnt/β-catenin signalling and cell proliferation after birth. Axin2-deficiency leads to decreased fibrillar collagen deposition and Sox9 expression with progressive development of myxomatous features and aortic insufficiency in adult mice over the course of 4 months. Thus, Axin2 limits Wnt/β-catenin signalling in postnatal heart valve maturation, and loss of Axin2 contributes to MVD progression in adulthood.
2. Methods
Detailed Materials and Methods can be found in the Supplemental Material online.
2.1 Mice
All mouse experiments conform to NIH guidelines (Guide for the care and use of laboratory animals) and were performed with protocols approved by the Institutional Animal Care and Use Committee at Cincinnati Children’s Research Foundation. Axin2LacZ/+ mice, Fbn1C1039G/+ mice and TCF/Lef:H2B-GFP transgenic mice were previously described.11,16,17 To obtain littermate cohorts of Axin2 mice or Fbn1C1039G/+ mice with TCF/Lef: H2B-GFP reporter, TCF/Lef:H2B-GFP mice were crossed with Axin2LacZ/+ mice or Fbn1C1039G/+ mice. Mice were sacrificed under isoflurane inhalation followed by cervical dislocation at the specified time point for each experiment. Male and female mice were used together for all analyses. Mice were maintained in mixed B6.129 strain background. Embryonic litters were collected at E14.5, with E0.5 determined by evidence of a vaginal plug.
2.2 Mouse echocardiography
Two-dimensional, M-mode and Doppler transthoracic echocardiography was performed on 2-month-old mice under anesthesia induced with inhaled isoflurane 0.8–1.5%. At least 12 mice per genotype were analysed.
2.3 Histology, morphometric analysis and immunofluorescence labelling
Murine tissues were harvested from foetal, neonatal, juvenile and adult mice at indicated time-points and processed for either paraffin embedding or cryo-preservation. Standard protocols were used for staining and immunohistochemistry. The integrity of collagen network was determined with picrosirius red polarization microscopy to quantify the ratio between thick closely packed mature collagen fibres as orange-red birefringence and the loosely packed less cross-linked and immature collagen fibres as yellow-green birefringence.18 Morphometric and immunofluorescence analyses were performed at the thickest part of aortic valve leaflet or mitral valve leaflet.
2.4 RNA isolation and real-time quantitative PCR (qPCR)
Aortic valve tissues were extracted using NucleoSpin RNA/Protein extraction kit (Macherey-Nagel) following manufacturer’s instructions. Primer sequences are listed in Table S1, see Supplementary material online.
2.5 Statistics
Mann–Whitney U tests were used to determine the significance of observed differences between the medians using PRISM6 software package (GraphPad). Data are reported as dot plots and interquartile ranges, where each dot represents a single mouse or biological sample and the horizontal bar indicates the median. A P value < 0.05 was considered significant.
3. Results
3.1 Axin2 is expressed in heart valves and loss of Axin2 results in delayed heart valve maturation
To evaluate the expression and function of Axin2 in heart valves, we used Axin2LacZ/+ reporter mice in which β-galactosidase expression is under the control of the endogenous Axin2 locus while abolishing Axin2 gene transcription.11 X-gal staining reveals that Axin2LacZ is expressed in aortic valve (AoV) (see Supplementary material online, Figure S1A) and mitral valve (MV) (see Supplementary material online, Figure S1B) leaflets after birth at postnatal day 0 (P0). Axin2LacZ is detected in valve endothelial cells (VECs) on the fibrosa side, whereas its expression is not observed in VECs on the ventricularis side exposed to blood flow (see Supplementary material online, Figure S1A′ and B′). Axin2LacZ is also expressed in VICs and is preferentially detected distally in the AoV and MV leaflets (see Supplementary material online, Figure S1A′ and B′). Thus, VECs and VICs express Axin2LacZ postnatally.
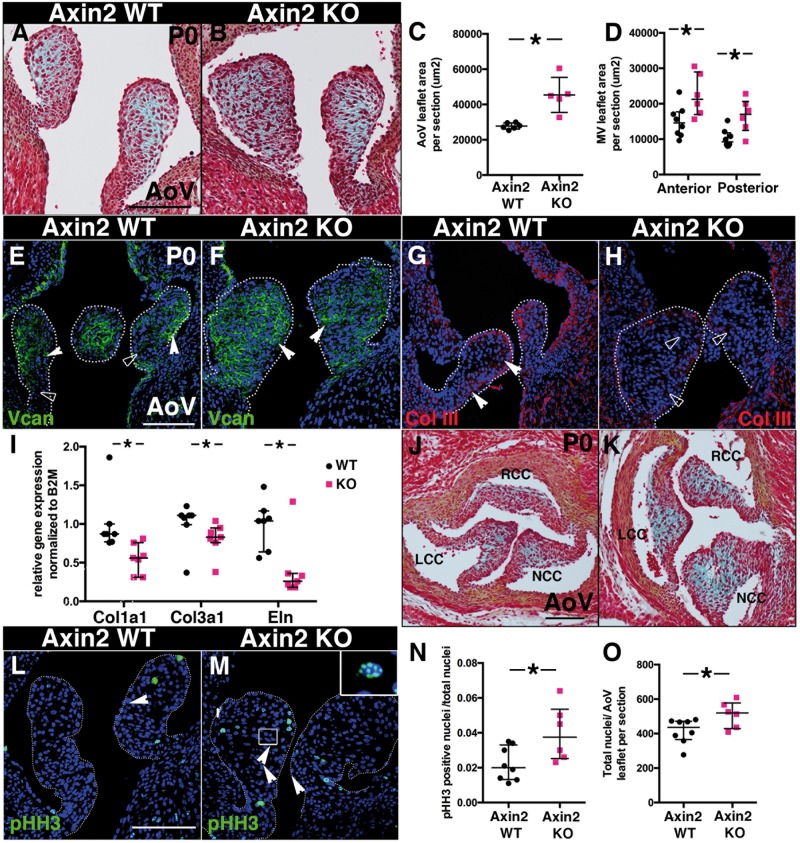
To investigate the role of Axin2 in heart valve development and postnatal remodelling, we analysed the homozygous Axin2LacZ/LacZ knockout mutants (KO).19 At E14.5, Axin2LacZ is expressed in AoV leaflets, as confirmed by X-gal staining in Axin2LacZ/+ reporter mice (see Supplementary material online, Figure S1C and C′), but loss of Axin2 does not significantly alter the overall ECM composition and morphology of AoV (see Supplementary material online, Figure S1D–F). However, at P0, AoV leaflets are enlarged and composed predominantly of GAGs, as indicated by alcian blue staining (Figure K). Thus, loss of Axin2 leads to abnormal postnatal ECM remodelling evident in increased proteoglycan expression and decreased fibrillar collagen and elastin expression.
During normal postnatal heart valve remodelling, VIC proliferation decreases.10 To determine if Axin2 KO VICs go through normal postnatal cell cycle arrest, we assessed cell proliferation with pHH3 immunofluorescence (Figure M). The percent of pHH3-positive cells was determined at P0 and increased cell proliferation was detected in Axin2 KO AoV (Figure 1N). In Axin2 KO AoV, the total number of cells is increased at P0 (Figure 1O), however, cell numbers were not significantly altered in E14.5 Axin2 KO AoV (see Supplementary material online, Figure S1G). Like Axin2 expression, the majority of pHH3-positive cells are found in the distal part of Axin2 KO AoV leaflets. Therefore, loss of Axin2 leads to increased VIC proliferation after birth.
Figure 1.
Axin2 KO mice display immature thickened aortic valves (AoV) and mitral valves (MV). (A and B) Representative Movat’s Pentachrome staining performed on P0 heart sections from Axin2 WT and KO mice. (C) AoV leaflet area per section was measured (WT n = 6, KO n = 5). (D) Anterior and posterior MV leaflet areas per section were measured (WT n = 8 and KO n = 6). (E and F) Representative versican (vcan) staining performed on Axin2 WT and KO P0 heart sections. (G and H) Representative collagen type III (col III) staining performed on P0 heart sections from Axin2 WT and Axin2 KO mice. (I) Collagen 1a1, collagen 3a1 and elastin expression were measured by qPCR in P0 Axin2 KO AoV, normalized to B2M and compared to Axin2 WT (WT n = 7, KO n = 7). (J and K) Representative Movat’s Pentachrome staining performed on cross sections of P0 hearts from Axin2 WT and KO mice (RCC, LCC and NCC for right, left and non-coronary cusps). (L and M) Representative pHH3 staining of P0 heart sections from Axin2 WT and Axin2 KO mice. Inset displays nuclear pHH3 staining. (N) The number of pHH3-positive nuclei/total nuclei was quantified in AoV leaflet sections (WT n = 8, KO n = 6). (O) The total number of nuclei per AoV leaflets section was counted (WT n = 8 and KO n = 6). Data are reported as dot plots and interquartile ranges, where each dot represents a mouse (C, D and N) or biological sample (I) and the horizontal bar indicates the median. *P < 0.05 determined with Mann–Whitney U test. Scale bar =100 μm.
3.2 Wnt/β-catenin signalling is increased in Axin2 KO AoV mesenchyme while Sox9 expression is decreased
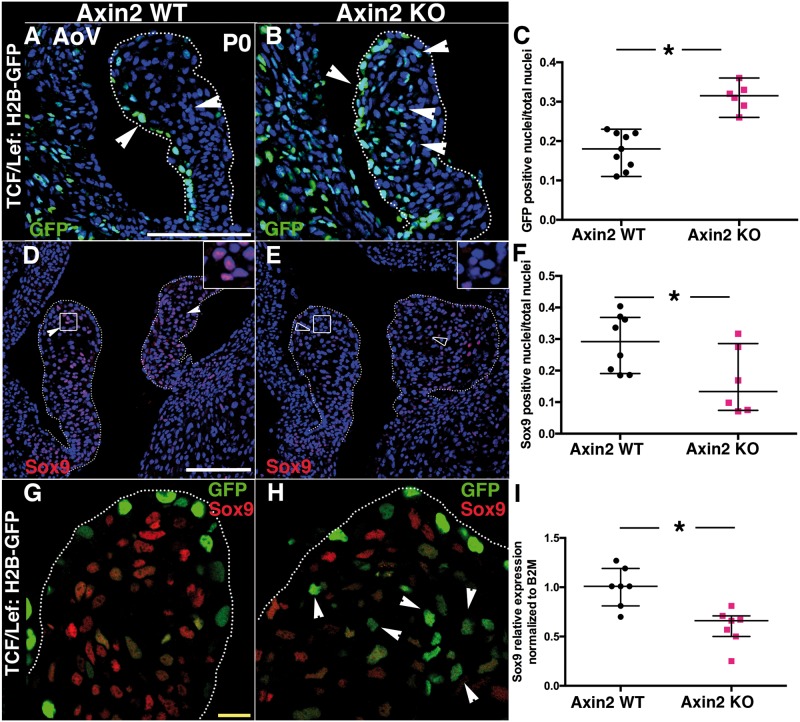
To test whether the absence of Axin2 stimulates Wnt/β-catenin signalling in AoV, we generated Axin2 KO that carry one copy of TCF/Lef:H2B-GFP, a transgenic reporter of canonical Wnt/β-catenin signalling mediated by TCF/LEF transcriptional activity.16 At P0, Wnt/β-catenin signalling, indicated by GFP staining, is detectable in many WT AoV VECs localized to the fibrosa side, but only in a few VICs (Figure 2A). However, TCF/Lef:H2B-GFP expression is markedly activated in VICs when Axin2 is absent (Figure 2B). Interestingly, Wnt/β-catenin signalling is mostly activated at the tip of the leaflet (Figure 2B), which overlaps with Axin2 expression (see Supplementary material online, Figure S1A). Quantification of GFP-positive cells in Axin2 KO AoV confirms the increased Wnt/β-catenin signalling (Figure 2C).
Figure 2.
Increased Wnt/β-catenin signalling in P0 Axin2 KO mice correlates with decreased Sox9 expression. (A and B) Wnt/β-catenin signalling was detected at P0 with GFP staining in TCF/Lef:H2B-GFP/Axin2 WT and TCF/Lef:H2B-GFP/Axin2 KO mice. (C) The number of GFP-positive cells/total nuclei was quantified (WT n = 9, KO n = 6). (D and E) Representative Sox9 immunofluorescence of P0 heart sections from Axin2 KO and WT mice. (Solid arrowheads indicate Sox9 expression). (F) The number of Sox9-positive cells/total nuclei was quantified in AoV leaflets section (WT n = 8, KO n = 6). (G and H) Co-staining of Sox9 and GFP in TCF/Lef:H2B-GFP/Axin2 WT and TCF/Lef:H2B-GFP/Axin2 KO). (I) Sox9 expression was measured by qPCR in P0 Axin2 KO AoV, normalized to B2M and compared to Axin2 WT set to 1 (WT n = 7, KO n = 7). Data are reported as dot plots and interquartile ranges, where each dot represents a mouse (C and F) or biological sample (I) and the horizontal bar indicates the median. *P < 0.05 determined with Mann–Whitney U test. Scale bar =100 μm. Yellow scale bar =10 μm.
Expression of Sox9, a transcription factor essential for ECM maturation during heart valve development and antagonized by Wnt/β-catenin signalling during chondrogenesis, was measured.7,20,21 In WT, nuclear Sox9 is widely expressed throughout valve leaflets (Figure F), and the majority of cells with Wnt/β-catenin signalling in Axin2 KO AoV do not express Sox9 (Figure 2H). Finally, Sox9 mRNA expression is significantly decreased in Axin2 KO AoV (Figure 2I). Thus, loss of Axin2 leads to increased Wnt/β-catenin signalling and reduced Sox9 expression during heart valve maturation after birth.
3.3 Adult Axin2 KO mice display AoV thickening and insufficiency
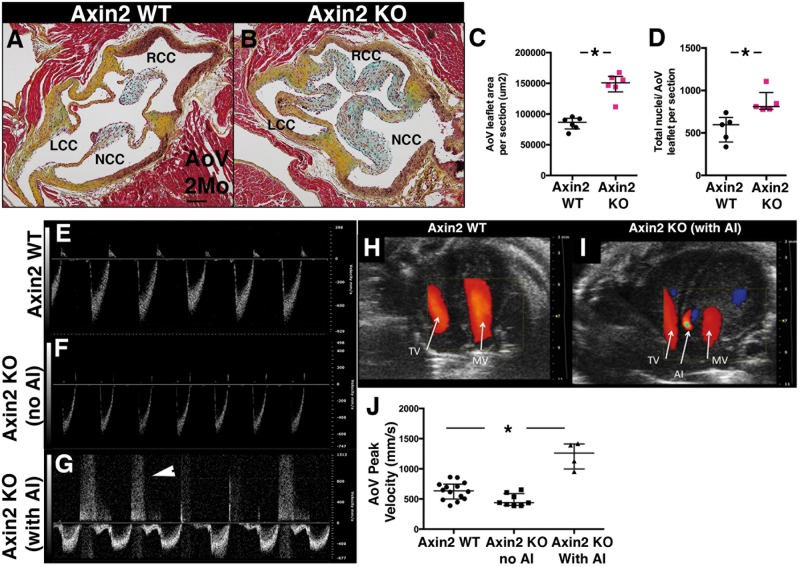
The consequences of loss of Axin2 on adult heart valve structure and function were examined in 2-month-old Axin2 KO mice compared to WT. In contrast to P0, Wnt/β-catenin signalling, indicated by TCF/Lef:H2B-GFP (see Supplementary material online, Figure S2A and B), is not changed in 2-month-old adult Axin2 KO mice (see Supplementary material online, Figure S2C). However, adult Axin2 KO mice display thickened AoV (see Supplementary material online, Figures S2B vs. S2A and 3C) with enlargement of the three AoV cusps (Figure 3B vs. 3A and see Supplementary material online, Figure S6B) and increased cell numbers (Figure 3D). As revealed by pentachrome staining (yellow stain in Figure S2E, see Supplementary material online) and collagen type III immunofluorescence (see Supplementary material online, Figure S2G), collagen is expressed in the tip of Axin2 KO AoV whereas collagen is barely detected in the tip of Axin2 WT AoV (see Supplementary material online, Figure S2D and empty arrowhead in Figure S2F). Similarly, versican is expressed and accumulates in Axin2 KO AoV (see Supplementary material online, Figure S2I vs. S2H). Therefore, lack of Axin2 gives rise to immature AoV that remain thickened with disorganized ECM into adulthood.
Figure 3.
Aortic valve regurgitation occurs in a subset of 2-month-old Axin2 KO mice. (A and B) Representative Movat’s pentachrome staining performed on cross sections of 2-month-old Axin2 WT and KO heart sections (RCC, LCC and NCC for right, left and non-coronary cusps). (C) AoV area per section was measured (WT n = 6 and KO n = 6). (D) The total number of nuclei per AoV leaflets section was counted (WT n = 5 and KO n = 5). (E and F) Representative pulsed wave Doppler tracings of Axin2 WT (E) and KO (F) mice show normal systolic and diastolic flow patterns across the aortic valve. (G) Representative pulsed wave Doppler tracing of Axin2 KO with holodiastolic flow reversal (arrowhead), indicative of aortic insufficiency (AI) (H). (I) Representative color Doppler images obtained in the apical 4-chamber view. (H) Diastolic inflow in WT mouse across the tricuspid and mitral valves is shown as a red signal. (I) Diastolic inflow is abnormal in an Axin2 KO mouse. The transducer was angled slightly anteriorly to demonstrate the left ventricular outflow track and the red jet of diastolic aortic insufficiency (AI) is indicated. TV, tricuspid valve; MV, mitral valve; AI, aortic insufficiency. (J) Hemodynamic evaluation in Axin2 KO mice with AI compared to Axin2 KO without AI and Axin2 WT mice. Measurements were performed on WT n = 14 and KO n = 12. Data are reported as dot plots and interquartile ranges, where each dot represents a mouse and the horizontal bar indicates the median. *P < 0.05 determined with Mann–Whitney U test. Scale bar =100 μm.
Thickening of heart valves may impair their function, as MVD leads to heart valve insufficiency. Therefore, left side heart valve function was assessed by echocardiography. Pulsed wave Doppler across AoV indicates heterogeneity of dysfunction in Axin2 KO mice. Indeed, 4/12 of 2-month-old Axin2 KO mice display retrograde diastolic flow across the AoV (arrowhead in Figure 3G), whereas the other Axin2 KO mice show a pulsed wave Doppler (Figure 3F) similar to Axin2 WT mice (Figure 3E). The abnormal pulsed wave Doppler is typical of aortic insufficiency (AI), which is detected by colour Doppler during diastole (Figure 3I vs. 3H). Based on colour Doppler, Axin2 KO mice were divided in two groups (Axin2 KO with and Axin2 KO without AI) and peak transvalvular velocities were compared. The 4 Axin2 KO mice with AI had both significantly higher peak velocities (Figure 3J) and colour Doppler signal aliasing of antegrade aortic flow (Figure 3I). By comparison, Axin2 KO mice without AI had peak velocities indistinguishable from WT mice, and neither of these latter two groups had aliasing of the antegrade colour Doppler signal. Two of the four Axin2 KO mice with AI also demonstrated doming of the aortic valve leaflets, a finding not present in Axin2 KO mice without AI nor in any WT mice. Aortic valve leaflet prolapse was not observed in any of the animals and no difference was found in aortic dimensions (see Supplementary material online, Figure S3A–D). Although histology demonstrated thickened mitral valves, no mitral valve insufficiency, prolapse, abnormal leaflet excursion or flow abnormalities (see Supplementary material online, Figure S3F–H) were observed in Axin2 KO mice. Altogether, these results indicate that loss of Axin2 leads to thickened AoV in adult mice with AoV regurgitation in a subset of animals.
3.4 Axin2 KO AoV exhibit progressive myxomatous valve disease
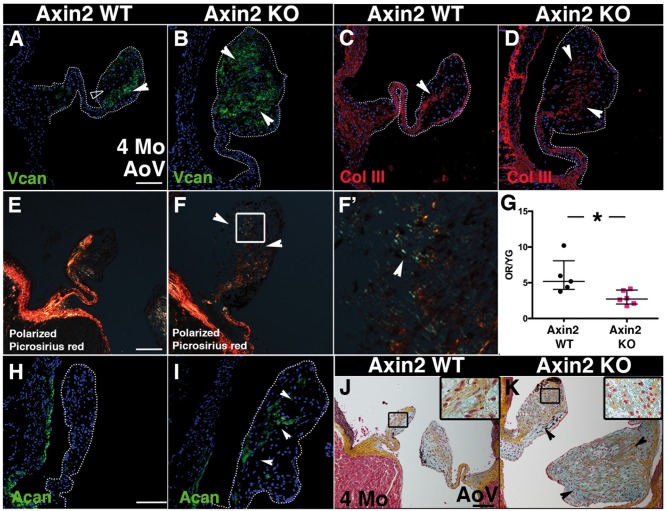
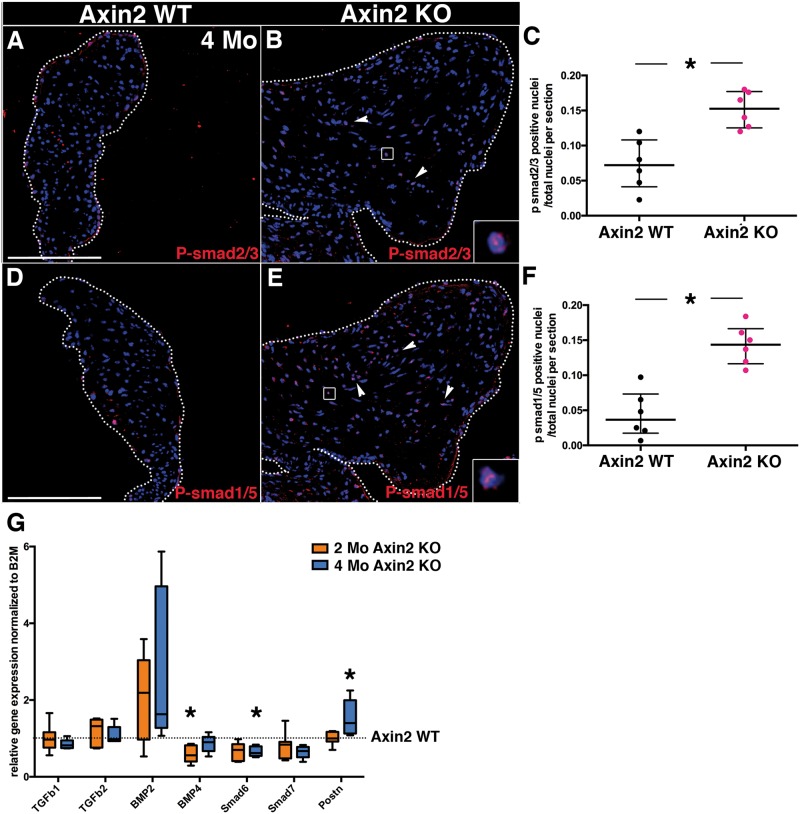
The progression of MVD was examined in older Axin2 KO mice at 4 months. At 4 months, the ECM abnormalities are even more striking than in 2-month-old Axin2 KO AoV (Figure K) and overlaps with versican expression (Figure C). Therefore, collagen fibre maturity was assessed by picrosirius red staining of AoV analysed under polarized light (Figure F). The ratio of orange-red (OR) birefringence, indicative of tightly packed and organized collagen fibres, relative to yellow-green (YG) loosely packed and less cross-linked collagen fibres (4G) indicates that the collagen network in Axin2 KO AoV is disorganized (Figure 4F′). Along with the extensive ECM remodelling at 4 months, expression of aggrecan is detected in the remodelled area of Axin2 KO leaflets (Figure 4I), while its presence is barely detectable in Axin2 WT (Figure 4H). Aggrecan expression was not detected in 2-month-old Axin2 KO AoV. Thus, Axin2 KO mice exhibit more severe abnormalities in ECM organization and valve morphology at 4 months vs. 2 months, indicative of myxomatous disease progression.
Figure 4.
4-month-old Axin2 KO AoV display myxomatous valve disease features. (A and B) Representative versican staining performed on heart sections from 4-month-old Axin2 WT and KO mice. (C and D) Representative collagen type III staining performed on heart sections from 4-month-old Axin2 WT and KO mice. (E and F) Representation picrosirius red staining under polarized light (F′ higher magnification). (G) The ratio of orange red (OR) per yellow green (YG) under polarized light was quantified (WT n = 5 and KO n = 6). (H and I) Representative aggrecan immunofluorescence of heart sections from 4-month-old Axin2 WT and KO mice (WT n = 6 and KO n = 6). (J and K) Representative Movat’s Pentachrome staining performed on P0 heart sections from Axin2 WT and KO mice. Data are reported as dot plots and interquartile ranges, where each dot represents a mouse and the horizontal bar indicates the median. *P < 0.05 determined with Mann–Whitney U test. Scale bar =100 μm.
TGF-β2 and BMP3 signalling pathways linked to MVD were examined in Axin2 KO mice at 2 and 4 months of age. Immunofluorescence for phosphorylation of smad2/3 was used as an indicator of TGF-β signalling, and phosphorylation of smad1/5/8 was used to assess BMP signalling in the pathogenesis of myxomatous Axin2 KO AoV. Interestingly, while neither phosphorylation of smad2/3 (see Supplementary material online, Figure S4C) nor smad1/5/8 (see Supplementary material online, Figure S4E) is altered in 2-month-old Axin2 KO mice, both display increased phosphorylation in 4-month-old Axin2 KO mice. Indeed, nuclear phosphorylation of smad2/3 is significantly increased in the VICs of Axin2 KO AoV (Figure C), but is limited to VECs in WT AoV (Figure F), while only few cells display p-smad1/5 in WT (Figure 5D). Gene expression of TGF-β family ligands (TGF-β1, TGF-β2, BMP2, BMP4) as well as BMP and TGF-β inhibitors (smad6 and smad7, respectively) along with periostin (postn), is not significantly altered in 2-month-old AoV, with the exception of decreased BMP4 expression (Figure 5G). Interestingly, while ligand mRNA expression is unchanged, smad6 is significantly decreased and postn increased in 4-month-old Axin2 KO AoV (Figure 5G), indicative of potential activation of BMP signalling in 4-month-old Axin2 KO mice. Therefore, loss of Axin2 ultimately leads to increased TGF-β and BMP signalling in myxomatous valves with severe ECM abnormalities at 4 months.
Figure 5.
Phosphorylation of smad2/3 and smad1/5 is increased in 4-month-old Axin2-deficient AoV leaflets. (A and B) Representative p-smad2/3 immunofluorescence performed on heart sections from 4-month-old Axin2 WT and KO mice. (C) The number of p-smad2/3-positive cells/total nuclei was quantified in AoV leaflets (WT n = 6 and KO n = 6). (D and E) Representative p-smad1/5 immunofluorescence performed on heart sections from 4-month-old Axin2 WT and KO mice. (inset indicate positive nuclei). (F) The number of p-smad1/5-positive cells/total nuclei was quantified in AoV leaflets (WT n = 6 and KO n = 6). Data are reported as dot plots and interquartile ranges, where each dot represents a mouse and the horizontal bar indicates the median. (G) TGF-β1, TGF-β2, BMP2, BMP4, smad6, smad7 and periostin (postn) expression were measured by qPCR in 2-month-old (WT n = 7, KO n = 7) and 4-month-old (WT n = 6, KO n = 5) Axin2 KO AoV, normalized to B2M and compared to Axin2 WT (set as 1, dotted line). Data are represented as box and whiskers. *P < 0.05 determined with Mann–Whitney U test. Scale bar =100 μm.
Increased Wnt/β-catenin signalling has been associated with AoV calcification.5 Thus, AoV calcification was evaluated in 1-year-old Axin2 KO mice by alizarin red staining. Calcification (see Supplementary material online, Figure S5C) is not detected in Axin2 KO or WT mice (see Supplementary material online, Figure S5B vs. S5A) at 1 year. It was noted that black deposits (see Supplementary material online, Figure S6B) accumulate in Axin2 KO AoV with progression of disease (see Supplementary material online, Figure S6B vs. S6A). The black cells are evident in unstained specimens and thus are not calcification. Prussian blue staining was used to test the possibility that these black deposits might be hemosiderin-laden macrophages. However, the absence of blue staining reveals that these accumulations are not iron (see Supplementary material online, Figure S6C–E). The black deposits are most likely melanin, as melanocytes populate heart valves,22 and mitf and dct melanocytic markers are significantly increased in 2-month-old Axin2 KO AoV (see Supplementary material online, Figure S6F). Therefore, Axin2 KO mice exhibit features of progressive MVD, but do not become calcified over time.
3.5 Axin2 KO mice exhibit increased inflammatory cells in AoV
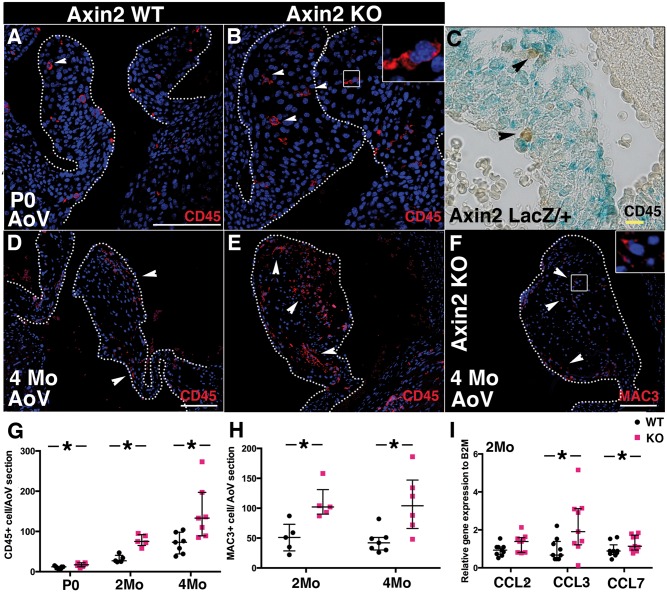
Hematopoietic (CD45+) cells have been described in normal heart valves23 while increased inflammation has been reported in MVD.2,24 In normal WT AoV, the majority of CD45+ cells are localized under the endothelium at P0 (Figure G). Loss of Axin2 is systemic and could affect migration of hematopoietic cells, however absence of colocalization between CD45 expression and X-gal staining indicates that CD45+ cells are not expressing Axin2 in AoV (Figure H). Likewise, expression of chemokines CCL3 and CCL7 is increased at 2 months during early stages of pathogenesis (Figure 6I), indicating increased inflammation in Axin2 KO AoV prior to extensive myxomatous changes. Thus, loss of Axin2 leads to increased infiltration of inflammatory cells in AoV, preceding and potentially contributing to progression of MVD.
Figure 6.
Increased inflammatory cell infiltration in Axin2 KO AoV. (A and B) Representative CD45 immunofluorescence performed on P0 heart sections from Axin2 WT and KO mice. (C) CD45 immunostaining performed on X-gal stained P0 valve section. (D and E) Representative CD45 immunofluorescence performed on heart sections from 4-month-old Axin2 WT and KO mice. (F) Representative Mac3 immunofluorescence performed on heart sections from 4-month-old Axin2 KO mice. (G) The total number of CD45+ cells in AoV leaflets was counted at P0 (WT n = 7 and KO n = 6), 2 months (WT n = 6 and KO n = 6) and 4 months (WT n = 7 and KO n = 7). (H) The total number of Mac3+ cells in AoV leaflets was counted at 2 months (WT n = 6 and KO n = 6) and 4 months (WT n = 6 and KO n = 6). (I) CCL2, CCL3 and CCL7 chemokine expression was measured by qPCR in 2-month-old Axin2 KO AoV, normalized to B2M and compared to Axin2 WT (WT n = 9 and KO n = 9). Data are reported as dot plots and interquartile ranges, where each dot represents a mouse (G and H) or biological sample (I) and the horizontal bar indicates the median. *P < 0.05 determined with Mann–Whitney U test. Scale bar =100 μm. Yellow scale bar =10 μm.
3.6 Axin2 expression is decreased and Wnt/β-catenin signalling is increased in myxomatous mitral valves of a murine model of Marfan syndrome
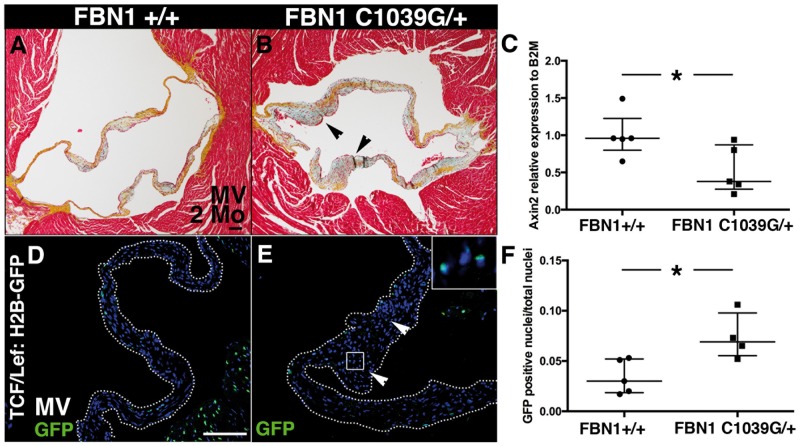
To determine if Axin2 expression is altered in an established model of myxomatous valve disease, we measured its expression in a murine model of Marfan syndrome.25 These mice, carrying a mutation in Fbn1 (C1039G), recapitulate pathogenesis of Marfan syndrome, and display thickened mitral valves by 1 week after birth with increased cell proliferation.25 Adult mice develop myxomatous mitral valves with mitral regurgitation detected at 9 months.25 At 2 months, Fbn1C1039G/+ mice exhibit thickened mitral valves with abnormal ECM remodelling (Figure F). Thus, Wnt/β-catenin signalling is activated with myxomatous valve disease in a mouse model of Marfan syndrome.
Figure 7.
Axin2 is decreased and Wnt/β-catenin signalling is increased in 2-month-old myxomatous mitral valves from Fbn1C1039G/+ Marfan syndrome mice. (A and B) Representative Movat’s pentachrome staining performed on heart sections of MV in 2-month-old FBN1C1039G/+ mice. (C) Axin2 expression was measured by qPCR in MV from 2-month-old FBN1C1039G/+ mice, relative to B2M and compared to FBN1+/+ (FBN1+/+ n = 5 and FBN1C1039G/+ n = 5). (D and E) Wnt/β-catenin signalling was detected at 2 months with GFP staining in TCF/Lef:H2B-GFP/Fbn1+/+and TCF/Lef:H2B-GFP/Fbn1C1039G/+ mice. (F) The number of GFP-positive cells/total nuclei was quantified (WT n = 5, KO n = 4). Data are reported as dot plots and interquartile ranges, where each dot represents a biological sample (C) or mouse (F) and the horizontal bar indicates the median. *P < 0.05 determined with Mann–Whitney U test. Scale bar =100 μm.
4. Discussion
Here we show that Axin2 is required to limit activation of Wnt/β-catenin signalling after birth and that loss of Axin2 leads to progressive myxomatous ECM and signalling changes in adults, typical of human myxomatous valve disease. At P0, Axin2 KO AoV exhibit thickening, increased cell proliferation, and loss of ECM maturation. At 2 months, structural changes are apparent by histopathology, and a subset of Axin2 KO develops aortic insufficiency. Finally, postnatal heart valve defects in Axin2 KO mice lead ultimately to extensive ECM remodelling, chondrogenic protein expression, and activation of BMP signalling, hallmarks of myxomatous disease, apparent at 4 months. Interestingly, increased inflammatory cell infiltration and aortic insufficiency precede myxomatous changes in Axin2 KO mice, highlighting their potential contribution to ECM remodelling in MVD. Moreover, Axin2 KO AoV do not demonstrate calcification up to 1 year of age, although Wnt/β-catenin signalling is increased after birth. Altogether, our study shows that Axin2 and inhibition of Wnt/β-catenin signalling are required for proper heart valve maturation after birth and proposes the Axin2 KO mouse as a novel murine model of myxomatous valve disease.
4.1 Axin2 limits Wnt/β-catenin signalling and promotes Sox9 expression in VICs postnatally
Increased Wnt/β-catenin signalling is apparent in Axin2 KO VICs at P0, as indicated by a TCF/LEF reporter. Although Wnt/β catenin signalling is crucial for EMT during cushion formation at the initiation of valve development,26 Axin2 is not required for that process as indicated by similar interstitial cellularity evident at E14.5 in AoV, reported here, and in E13.5 MV described in Bosada et al.14 While increased cell proliferation was observed in Axin2 KO E13.5 pulmonic valves,14 abnormalities in the developing and adult AoV in Axin2 KO mice have not been reported previously. After birth, Wnt signalling is decreased, since few VICs display active Wnt/β-catenin signalling, as indicated by TCF/Lef:H2B-GFP reporter mice. With Axin2-deficiency, Wnt/β-catenin signalling is increased in P0 VICs, predominantly in the distal tip of leaflets. Thus, Axin2 limits Wnt/β-catenin signalling in VICs after birth.
Thickened valves have been observed upon deletion of Sox9 revealing a key role for Sox9 in ECM protein regulation during heart valve development.7 Concomitant with excessive Wnt/β-catenin signalling, Sox9 expression is decreased with loss of Axin2. Likewise, Sox9 and Wnt/β-catenin signalling exhibit mutual inhibition in cartilage,20,21 while β-catenin deletion leads to increased Sox9 nuclear localization in adult heart valves.4 Thus, Axin2 limits Wnt/β-catenin signalling after birth, thereby maintaining Sox9 expression levels during heart valve maturation.
4.2 Axin2-deficiency leads to defective heart valve maturation
Signalling pathways involved in the transition from immature proliferative GAG-rich endocardial cushions to mature quiescent thin stratified valve leaflets are still largely unknown. Here, we demonstrate that loss of Axin2 maintains heart valves in an immature state after birth with increased cell proliferation, reduced collagen and elastin expression, and failure of ECM compaction. Our data revealing strong activation of Wnt/β-catenin signalling in VICs upon loss of Axin2, combined with recent study demonstrating that Wnt/β-catenin signalling supports endocardial cushion expansion and cell proliferation during development,14 indicate that sustained active Wnt/β-catenin signalling inhibits heart valve maturation after birth. Likewise, ECM compaction is not successfully achieved in Axin2 KO AoV indicating a potential link between Axin2/Wnt/β-catenin signalling and regulators of matrix compaction such as RhoGTPases.27 Therefore, Axin2 and Wnt/β-catenin signalling regulate VIC proliferation and leaflet morphogenesis during postnatal heart valve maturation.
4.3 Dysregulation of Axin2 and Wnt/β-catenin signalling at birth promote myxomatous valve disease
Here, we report that loss of Axin2 leads to immature AoV and MV leaflets after birth and ultimately to myxomatous AoV in adults. Murine models with filaminA mutation9 or deletion of cadherin 11,6 sox97 or krox208 also provide evidence that heart valve disease has its origins in defective heart valve maturation. Moreover, Axin2 expression is decreased in myxomatous mitral valves in a murine model of Marfan syndrome, while Wnt/β-catenin signalling is activated specifically in VICs located in the thickened part of diseased MV, suggesting the importance of Wnt/β-catenin signalling in the development of MVD. In future studies, assessing Wnt/β-catenin signalling in different myxomatous valve disease murine models6–9 during early stages of disease will determine if Wnt/β-catenin signalling is a suitable target to prevent MVD. Surprisingly, increased Wnt/β-catenin signalling in Axin2 KO mouse leads to MVD but not AoV calcification, previously associated with increased Wnt/β-catenin signalling.5 Nevertheless, increased Wnt/β-catenin signalling has recently been reported in microarray analyses comparing human myxomatous mitral valves to control mitral valves.15 Altogether, our study suggests that Wnt/β-catenin signalling regulates ECM remodelling after birth and contributes to heart valve disease in adults.
4.4 Increased inflammatory cell infiltration precedes myxomatous valve disease in Axin2 KO mice
Our study reveals that improper matrix composition and compaction at birth precedes heart valve dysfunction in adults. However, the process by which deficient leaflet remodelling after birth leads to myxomatous disease in adults is completely unknown. TGF-β and BMP signalling have been associated with MVD2,3 and are activated in VICs as observed in our Axin2 KO model. As TGF-β and BMP ligands are not increased in our model as opposed to human myxomatous mitral valves,2,3 the increased phosphorylation of smad2/3 and smad1/5 could be due to of decreased expression of the inhibitory smad6.28 Interestingly, thickening of heart valves has been reported in mice with smad6 loss of function29 but its expression has not been reported yet in the context of myxomatous valve disease.
Although TGF-β and BMP signalling pathways have been shown to modulate VECs, VICs and ECM in vitro, it has not yet been demonstrated that these pathways promote MVD in vivo. Our data suggest that TGF-β and BMP signalling activation occurs at later stages of disease when valve leaflets are already extensively remodelled. Thus, these pathways likely are activated in response to ECM abnormalities, functional changes, or inflammation. Therefore targeting TGF-β and BMP pathways might not be suitable to prevent the initial steps of MVD, but could slow down the progression of the disease.
Our analysis of Axin2 KO mice reveals for the first time that increased numbers of hematopoietic cells and macrophages precedes major myxomatous valvular changes evident at 4 months. Similarly, inflammation has been reported in MVD in humans2 and mice24 but the specific timing and contributions of inflammatory cells to disease progression have not been defined. The primary cause of increased inflammatory cells is still to be determined, but the absence of Axin2 expression in hematopoietic cells implies that increased inflammatory cell infiltration is the result of defective valve leaflet morphogenesis or ECM composition observed as early as P0 in Axin2 KO mice. Notably, ECM degradation and remodelling can influence immune cell infiltration and activation, as determined in studies of wound healing and fibrosis.30 In addition, increased Wnt/β-catenin signalling in VICs can induce chemokine production which ultimately will attract macrophages.31,32 Since macrophages are major effectors in tissue remodelling and fibrosis,33 our model is consistent with inflammation as a potential driver of MVD. However, future studies are needed to determine if inflammatory cells contribute to the pathogenesis of MVD or if anti-inflammatory therapy can prevent MVD during early stages of disease.
In summary, this study reveals that Axin2 and inhibition of Wnt/β-catenin signalling contribute to proper heart valve maturation. Immature heart valves deficient in Axin2 exhibit progressive MVD during adulthood. Furthermore, increased inflammation precedes and potentially drives ECM remodelling as well as TGF-β and BMP signalling in MVD. Thus,` the Axin2 KO mouse represents a novel murine model of myxomatous valve disease that will be useful for further studies of the progression of MVD and testing of potential therapeutic targets.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Acknowledgements
We thank Christine Schulte for help with echocardiography, Christina Alfieri for technical support, and Hanna Osinska for expertise in microscopy.
Funding
This work was supported by NIH R01 HL094319 (KEY) and American Association of Anatomists Postdoctoral Fellowship Award (AH).
Conflict of interest: none declared.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 2016;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 2.Geirsson A, Singh M, Ali R, Abbas H, Li W, Sanchez JA, Hashim S, Tellides G. Modulation of transforming growth factor-beta signaling and extracellular matrix production in myxomatous mitral valves by angiotensin II receptor blockers. Circulation 2012;126:S189–S197. [DOI] [PubMed] [Google Scholar]
- 3.Sainger R, Grau JB, Branchetti E, Poggio P, Seefried WF, Field BC, Acker MA, Gorman RC, Gorman JH, 3rd, Hargrove CW, 3rd, Bavaria JE, Ferrari G. Human myxomatous mitral valve prolapse: role of bone morphogenetic protein 4 in valvular interstitial cell activation. J Cell Physiol 2012;227:2595–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang M, Alfieri CM, Hulin A, Conway SJ, Yutzey KE. Loss of beta-catenin promotes chondrogenic differentiation of aortic valve interstitial cells. Arterioscler Thromb Vasc Biol 2014;34:2601–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caira FC, Stock SR, Gleason TG, McGee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, Rajamannan NM. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol 2006;47:1707–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowen CJ, Zhou J, Sung DC, Butcher JT. Cadherin-11 coordinates cellular migration and extracellular matrix remodeling during aortic valve maturation. Dev Biol 2015;407:145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lincoln J, Kist R, Scherer G, Yutzey KE. Sox9 is required for precursor cell expansion and extracellular matrix organization during mouse heart valve development. Dev Biol 2007;305:120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Odelin G, Faure E, Kober F, Maurel-Zaffran C, Theron A, Coulpier F, Guillet B, Bernard M, Avierinos JF, Charnay P, Topilko P, Zaffran S. Loss of Krox20 results in aortic valve regurgitation and impaired transcriptional activation of fibrillar collagen genes. Cardiovasc Res 2014;104:443–455. [DOI] [PubMed] [Google Scholar]
- 9.Sauls K, de Vlaming A, Harris BS, Williams K, Wessels A, Levine RA, Slaugenhaupt SA, Goodwin RL, Pavone LM, Merot J, Schott JJ, Le Tourneau T, Dix T, Jesinkey S, Feng Y, Walsh C, Zhou B, Baldwin S, Markwald RR, Norris RA. Developmental basis for filamin-A-associated myxomatous mitral valve disease. Cardiovasc Res 2012;96:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinton RB, Jr., Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res 2006;98:1431–1438. [DOI] [PubMed] [Google Scholar]
- 11.Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol 2002;22:1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dao DY, Yang X, Flick LM, Chen D, Hilton MJ, O’Keefe RJ. Axin2 regulates chondrocyte maturation and axial skeletal development. J Orthop Res 2010;28:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alfieri CM, Cheek J, Chakraborty S, Yutzey KE. Wnt signaling in heart valve development and osteogenic gene induction. Dev Biol 2010;338:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosada FM, Devasthali V, Jones KA, Stankunas K. Wnt/beta-catenin signaling enables developmental transitions during valvulogenesis. Development 2016;143:1041–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thalji NM, Hagler MA, Zhang H, Casaclang-Verzosa G, Nair AA, Suri RM, Miller JD. Nonbiased molecular screening identifies novel molecular regulators of fibrogenic and proliferative signaling in myxomatous mitral valve disease. Circ Cardiovasc Genet 2015;8:516–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrer-Vaquer A, Piliszek A, Tian G, Aho RJ, Dufort D, Hadjantonakis AK. A sensitive and bright single-cell resolution live imaging reporter of Wnt/beta-catenin signaling in the mouse. BMC Dev Biol 2010;10:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Judge DP, Biery NJ, Keene DR, Geubtner J, Myers L, Huso DL, Sakai LY, Dietz HC. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J Clin Invest 2004;114:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwanekamp JA, Lorts A, Vagnozzi RJ, Vanhoutte D, Molkentin JD. Deletion of periostin protects against atherosclerosis in mice by altering inflammation and extracellular matrix remodeling. Arterioscler Thromb Vasc Biol 2016;36:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu HM, Jerchow B, Sheu TJ, Liu B, Costantini F, Puzas JE, Birchmeier W, Hsu W. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development 2005;132:1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, de Crombrugghe B. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev 2004;18:1072–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo X, Day TF, Jiang X, Garrett-Beal L, Topol L, Yang Y. Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev 2004;18:2404–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carneiro F, Kruithof BP, Balani K, Agarwal A, Gaussin V, Kos L. Relationships between melanocytes, mechanical properties and extracellular matrix composition in mouse heart valves. J Long Term Eff Med Implants 2015;25:17–26. [DOI] [PubMed] [Google Scholar]
- 23.Visconti RP, Ebihara Y, LaRue AC, Fleming PA, McQuinn TC, Masuya M, Minamiguchi H, Markwald RR, Ogawa M, Drake CJ. An in vivo analysis of hematopoietic stem cell potential: hematopoietic origin of cardiac valve interstitial cells. Circ Res 2006;98:690–696. [DOI] [PubMed] [Google Scholar]
- 24.Sauls K, Toomer K, Williams K, Johnson AJ, Markwald RR, Hajdu Z, Norris RA. Increased infiltration of extra-cardiac cells in myxomatous valve disease. J Cardiovasc Dev Dis 2015;2:200–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, Gabrielson KL, Hausladen JM, Mecham RP, Judge DP, Dietz HC. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest 2004;114:1586–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liebner S, Cattelino A, Gallini R, Rudini N, Iurlaro M, Piccolo S, Dejana E. Beta-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J Cell Biol 2004;166:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gould RA, Yalcin HC, MacKay JL, Sauls K, Norris R, Kumar S, Butcher JT. Cyclic mechanical loading is essential for Rac1-mediated elongation and remodeling of the embryonic mitral valve. Curr Biol 2016;26:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imamura T, Takase M, Nishihara A, Oeda E, Hanai J, Kawabata M, Miyazono K. Smad6 inhibits signalling by the TGF-beta superfamily. Nature 1997;389:622–626. [DOI] [PubMed] [Google Scholar]
- 29.Galvin KM, Donovan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MA, Jr., Falb D, Huszar D. A role for smad6 in development and homeostasis of the cardiovascular system. Nat Genet 2000;24:171–174. [DOI] [PubMed] [Google Scholar]
- 30.Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol 2010;10:712–723. [DOI] [PubMed] [Google Scholar]
- 31.Yasuhara R, Irie T, Suzuki K, Sawada T, Miwa N, Sasaki A, Tsunoda Y, Nakamura S, Mishima K. The beta-catenin signaling pathway induces aggressive potential in breast cancer by up-regulating the chemokine CCL5. Exp Cell Res 2015;338:22–31. [DOI] [PubMed] [Google Scholar]
- 32.Sobel K, Tham M, Stark HJ, Stammer H, Pratzel-Wunder S, Bickenbach JR, Boukamp P. Wnt-3a-activated human fibroblasts promote human keratinocyte proliferation and matrix destruction. Int J Cancer 2015;136:2786–2798. [DOI] [PubMed] [Google Scholar]
- 33.Van Linthout S, Miteva K, Tschope C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res 2014;102:258–269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.