Abstract
Aims
Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy (ARVD/C) is often associated with desmosomal mutations. Recent studies suggest an interaction between the desmosome and sodium channel protein Nav1.5. We aimed to determine the prevalence and biophysical properties of mutations in SCN5A (the gene encoding Nav1.5) in ARVD/C.
Methods and results
We performed whole-exome sequencing in six ARVD/C patients (33% male, 38.2 ± 12.1 years) without a desmosomal mutation. We found a rare missense variant (p.Arg1898His; R1898H) in SCN5A in one patient. We generated induced pluripotent stem cell-derived cardiomyocytes (hIPSC-CMs) from the patient’s peripheral blood mononuclear cells. The variant was then corrected (R1898R) using Clustered Regularly Interspaced Short Palindromic Repeats/Cas9 technology, allowing us to study the impact of the R1898H substitution in the same cellular background. Whole-cell patch clamping revealed a 36% reduction in peak sodium current (P = 0.002); super-resolution fluorescence microscopy showed reduced abundance of NaV1.5 (P = 0.005) and N-Cadherin (P = 0.026) clusters at the intercalated disc. Subsequently, we sequenced SCN5A in an additional 281 ARVD/C patients (60% male, 34.8 ± 13.7 years, 52% desmosomal mutation-carriers). Five (1.8%) subjects harboured a putatively pathogenic SCN5A variant (p.Tyr416Cys, p.Leu729del, p.Arg1623Ter, p.Ser1787Asn, and p.Val2016Met). SCN5A variants were associated with prolonged QRS duration (119 ± 15 vs. 94 ± 14 ms, P < 0.01) and all SCN5A variant carriers had major structural abnormalities on cardiac imaging.
Conclusions
Almost 2% of ARVD/C patients harbour rare SCN5A variants. For one of these variants, we demonstrated reduced sodium current, Nav1.5 and N-Cadherin clusters at junctional sites. This suggests that Nav1.5 is in a functional complex with adhesion molecules, and reveals potential non-canonical mechanisms by which Nav1.5 dysfunction causes cardiomyopathy.
Keywords: Arrhythmogenic right ventricular cardiomyopathy , SCN5A , Genetics , Cardiomyopathy , Ion channel electrophysiology
1. Introduction
The cardiac intercalated disc hosts a molecular network that integrates intercellular adhesion, electrical coupling, and excitability at the site of contact between cardiomyocytes. Disruption of this complex undermines the integrity of the myocardium, as demonstrated by various disease states associated with intercalated disc remodelling. One of these diseases is Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy (ARVD/C), an inherited disorder characterized by ventricular arrhythmias and fibrofatty replacement of predominantly the right ventricular (RV) myocardium. Mutations in genes encoding the cardiac desmosome are identified in the majority of affected patients,1 although the genetic basis extends beyond desmosomal proteins.2–4 Of particular interest in this regard is Nav1.5, the pore-forming subunit of the voltage-gated cardiac sodium channel. Early studies showed that the desmosomal protein plakophilin-2 (PKP2) co-precipitates with Nav1.5, and that loss of PKP2 expression alters the amplitude and kinetics of the sodium current (INa).3,5 Furthermore, mutations in PKP2 have been associated with a sodium channelopathy phenotype,6 while decreased immunoreactive Nav1.5 protein has been detected in the majority of human ARVD/C heart samples.7 These observations indicate a close functional association between Nav1.5 and mechanical junction proteins. The concept is further validated by observations that Nav1.5 co-precipitates with the adherens junction protein N-Cadherin,5 and by super-resolution microscopy studies demonstrating the presence of ‘adhesion/excitability’ nodes formed by aggregates of Nav1.5 and N-Cadherin.8 Likewise, we postulated that mutations in the voltage-gated sodium channel complex may be part of the molecular substrate underlying ARVD/C.
2. Methods
2.1 Study population and molecular genetic screening
We first screened the entire exome for mutations among a ‘discovery cohort’ of six unrelated Caucasian patients with a clinical diagnosis of ARVD/C according to the 2010 Task Force Criteria9 and without mutations in the ARVD/C-associated desmosomal genes (PKP2, DSC2, DSG2, DSP, and JUP). All individuals underwent whole-exome sequencing using the Illumina HiSeq2000 platform. We used the human reference genome GRCh37/hg19 for mapping.10 Detailed genetic screening methodology for the discovery cohort is described in the Supplementary material online.
We subsequently analysed SCN5A in a multicentre transatlantic ‘validation cohort’ of 281 unrelated patients who had a clinical diagnosis of ARVD/C according to the 2010 Task Force Criteria9 and who underwent sequencing for the ARVD/C-associated desmosomal genes (PKP2, DSC2, DSG2, DSP, and JUP). Patients were divided into those with a pathogenic desmosomal mutation (n = 146) and those without a pathogenic desmosomal mutation (n = 135). Pathogenicity of desmosomal mutations was determined as done previously.11 Most participants were Caucasian (n = 276); four were Asian and one was African American. Detailed genetic screening methodology for the validation cohort is described in the Supplementary material online.
Potentially causal variants were identified using standard filtering criteria. Variants were excluded if they had a minor allele frequency (MAF) >1% in the Exome Aggregation Consortium, Cambridge, MA, Exome Variant Server (release ESP6500SI-V2), or 1000 Genomes Project, and/or if they were present in dbSNP 137. We also excluded all variants with an SIFT score >0.03 and/or a Polyphen2 score of <0.90. Nucleic acid deviations were compared with the reference sequence for SCN5A (NM_198056.2). All mutations were confirmed by Sanger sequencing. The study complies with the Declaration of Helsinki and a locally appointed ethics committee approved the research protocol. Informed consent was obtained from all participants.
2.2 Generation of induced pluripotent stem cell-derived cardiomyocytes
We assessed the cellular and molecular phenotype of the SCN5A mutation identified in our discovery cohort [p.Arg1898His (c.5693G > A)] in induced pluripotent stem cell-derived cardiomyocytes (IPSC-CMs) that were derived from the patient’s peripheral blood mononuclear cells. Detailed methodology is described in the Supplementary material online. T-cells-enriched peripheral blood mononuclear cells from the patient and two normal controls were used for human IPSC derivation using standard techniques (CytoTune-iPS 2.0 Sendai Reprogramming Kit, Invitrogen, Carlsbad CA, USA). Human IPSC clones were picked, cultured, and expanded on the mouse embryonic fibroblasts while following the manufacturer’s instructions.
2.3 Whole-cell patch clamping and three-dimensional super-resolution fluorescence microscopy
Whole-cell INa recordings were conducted using standard protocols that are described in the Supplementary material online. In addition, we performed two-colour (Nav1.5 and N-Cadherin) three-dimensional imaging by direct stochastic optical reconstruction microscopy. Our spatial resolution in the X–Y plane was ∼20 nm. Detailed methods are described in Leo-Macias et al.8; a brief description is provided in the Supplementary material online.
2.4 Genetic correction of the SCN5A mutation in human IPSC-CMs
The p.Arg1898His mutation in patient-specific IPSC-CMs was corrected using Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) with CRISPR-associated (Cas9) system, following a recently published protocol.12 Successful correction of the p.Arg1898His mutation was confirmed by direct sequencing (see Supplementary material online, Figure S1), and off-target effects generated by CRISPR-Cas9 were evaluated to confirm that the rescued phenotype is a direct result of the genetic correction (see Supplementary material online, Table S1). Successfully corrected cell clones were selected for cardiac differentiation. Detailed methodology is described in the Supplementary material online.
2.5 Generation of HEK293 cells
HEK293 cells, derived from human embryonic kidney cells, were used to complement IPSC-CMs experiments. DNA plasmids containing cDNA for human SCN5A or mutant SCN5A-c.5693A were transfected into HEK293 cells using Lipofectamine 2000 reagent (Life Technologies, Carlsbad, CA, USA). Patch clamping was performed 48 h after transfection. Expression of the transfectant was confirmed by western blots for Nav1.5 using a polyclonal Nav1.5 antibody (Sigma, St. Louis, MO, USA). Please see the Supplementary material online for more details.
2.6 Statistical analysis
Continuous data were presented as mean ± standard deviation or median (inter-quartile range) and categorical variables as numbers (percentages). Continuous variables were compared using the independent Student’s t-test or Mann–Whitney U test for comparison between two groups and analysis of variance or Kruskall–Wallis test for comparison between three groups. Post hoc tests were performed by Bonferroni correction to account for multiple comparisons. Categorical data were compared using χ2 test or Fisher’s exact test. Analyses were performed using STATA 13.1 (Stata Corp, College Station, TX, USA). A P-value <0.05 was considered statistically significant.
3. Results
3.1 Discovery cohort
3.1.1 Molecular genetic screening
We performed whole-exome sequencing in six unrelated ARVD/C patients [2 (33%) male, age at presentation 38.2 ± 12.1 years] who did not harbour a desmosomal mutation. A heterozygous SCN5A missense variant (c.5693G > A) was identified in a female patient, indicating the substitution of a highly conserved arginine to histidine at position 1898 (p.Arg1898His). Detailed information of the variant is shown in Table 1. The variant was rare, with an MAF of 0.007% (present in 8/12,0768 alleles reported in the Exome Aggregation Consortium, http://exac.broadinstitute.org, accessed 30 July 2016). It localized to a highly conserved region of the C-terminus of Nav1.5 (Genomic Evolutionary Rate Profiling score 4.86). The variant was predicted pathogenic by SIFT (score 0.03) and Polyphen2 (score 0.960). Supplementary material online, Table S2 includes other variants identified on whole-exome sequencing in this individual, with the factors that led to prioritizing them below SCN5A.
Table 1.
Summary of SCN5A rare variants associated with ARVD/C
| Amino acid change | cDNA change | Exon | Type | Location | MAF (%) ExACa | MAF (%) EVSa | Polyphen2 score | Polyphen2 prediction | SIFT score | SIFT prediction | Conservation (GERP) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Discovery cohort | ||||||||||||
| p.Arg1898His | c.5693G>A | 28 | Missense | C-terminus | 0.007 | 0.0158 | 0.960 | Probably damaging | 0.03 | Damaging | 4.86 | – |
| Validation cohort | ||||||||||||
| p.Tyr416Cys | c.1247A>G | 10 | Missense | IL I–II | 0.008 | 0.008 | 1.0 | Probably damaging | 0 | Damaging | 5.54 | 31 |
| p.Leu729del | c.2184–2186 del | 14 | In-frame deletion | DII S1 | – | – | – | – | – | – | – | – |
| p.Arg1623Ter | c.4867C>T | 28 | Stop codon | DIV S4 voltage sensor | – | – | – | – | – | – | – | – |
| p.Ser1787Asn | c.5360G>A | 28 | Missense | C-terminus | 0.0604 | 0.0692 | 0.976 | Probably damaging | 0 | Damaging | 4.82 | 22, 25–27b |
| p.Val2016Met | c.6048G>A | 28 | Missense | C-terminus | 0.0037 | – | 0.999 | Probably damaging | 0 | Damaging | 3.59 | 28, 29 |
ARVD/C, arrhythmogenic right ventricular dysplasia/cardiomyopathy; DII, Domain II; EVS, exome variant server; ExAc, Exome Aggregation Consortium; GERP, Genomic Evolutionary Rate Profiling; IL, interdomain linker; MAF, minor allele frequency; S1, transmembrane spanning segment 1.
aAccessed 30 July 2016.
3.1.2 Clinical evaluation
The patient presented at age 29 years with ventricular tachycardia (VT) of left bundle branch block (LBBB) inferior axis morphology after playing soccer. Figure 1 shows a summary of her clinical evaluation. ARVD/C diagnosis was made based on T-wave inversions in V1-2 (minor TFC), RV dyskinesia with RV end-diastolic volume 128 mL/m2 (major TFC), and LBBB VT (minor TFC). The PR (145 ms), QRS (90 ms), and QTc (375 ms) intervals were within normal limits. The patient did not have a history of spontaneous ST-segment elevation in the right precordial leads; a pharmacological sodium blocker challenge was not performed. Family members were not amenable to genetic testing.
Figure 1.
Genetic and clinical evaluation of the 29-year-old female ARVD/C patient harbouring an SCN5A mutation (p.Arg1898His) identified through whole-exome sequencing. (A) Whole-exome sequencing revealed a heterozygous G>A missense mutation at position 5693. This variant located to a highly conserved region of the sodium channel (B) in the C-terminal domain (CTD) (C). 12-lead electrocardiography showed T-wave inversions in V1–2 with normal conduction intervals (PR 145 ms; QRS 90 ms; QTc 375 ms) (D) and Holter monitoring revealed 217 PVCs/24 h (E). End-diastolic (F1) and end-systolic (F2) cardiac magnetic resonance images showed an enlarged right ventricle (end-diastolic volume 128 mL/m2) and dyskinesia in the RV outflow tract (arrow, F2). Please also note biatrial enlargement (right atrium > left atrium), which is a common finding in ARVD/C cases.30 Topology of Nav1.5 obtained with kind permission of Oxford University Press (original manuscript Cardiovasc Res 2007;76:381–9).
3.1.3 Functional characterization: biophysical effect on sodium channel
The consequence of the p.Arg1898His mutation on the biophysical and structural properties of Nav1.5 was studied in IPSC-CM from the patient’s peripheral blood mononuclear cells. Gene editing (CRISPR-Cas9) was used to generate a parallel hIPSC-CM line from the same patient, one in which the histidine in position 1898 was reversed back to arginine (p.Arg1898His reversed to p.Arg1898Arg). Using whole-cell patch clamp, we demonstrated that the peak INa density of cells carrying p.Arg1898His was 36% reduced when compared with those expressing the wild-type amino acid (p.Arg1898Arg) in the same position (P = 0.002; see Figure 2A). To document that the change in current amplitude is robust and independent of cell-type, we examined the properties of the sodium current generated by p.Arg1898Arg or p.Arg1898His in an exogenous cell system. For this purpose, we transiently transfected HEK293 cells with cDNA coding for SCN5A. Similar to the IPSC-CMs, the current density generated by a construct containing the SCN5A p.Arg1898His mutation was significantly reduced compared with wild-type SCN5A (p.Arg1898Arg) (P = 0.002; see Figure 2B). Both in the case of the hIPSC-CMs and in the HEK cells, the steady-state voltage dependence of inactivation was not affected by the mutation (Figure 2C and D).
Figure 2.
Biophysical effect of the SCN5A mutation on sodium current properties. (A) I–V relationship of INa in hIPSC-CMs from the ARVD/C patient (red) and cells from the same patient after gene-editing (black). INa,peak −70.3 ± 6.7 (p.Arg1898His) vs. −109.4 ± 8.2 pA/pF (p.Arg1898Arg), equivalent to a 36% [100 − (−70.3/−109.4*100)] reduction in peak sodium current, P = 0.002 (Student’s t-test). (B) I–V relationship of INa in HEK cells transiently transfected with the SCN5A mutant (red) and wild-type (black). INa,peak −27.7 ± 2.7 (mutant) vs. −42.7 ± 3.4 pA/pF (wild-type), P = 0.002 (Student’s t-test). (C) Voltage dependence of steady-state inactivation in IPSC-CMs from the ARVD/C patient (red) and cells from the same patient after gene-editing (black). V1/2,inactivation −70.9 ± 1.9 mV (p.Arg1898His) vs. −68.7 ± 0.9 mV (p.Arg1898Arg), P > 0.05 (Student’s t-test). (D) Voltage dependence of steady-state inactivation in HEK cells transiently transfected with the SCN5A mutant (red) and wild-type (black). V1/2,inactivation −89.0 ± 1.3 mV (mutant) vs. −90.0 ± 1.2 mV (wild-type), P > 0.05 (Student’s t-test).
3.1.4 Effect of mutation on intercalated disc organization
We examined the effects of the p.Arg1898His SCN5A mutation on intercalated disc organization by three-dimensional super-resolution fluorescence microscopy (3D-SRFM) (Figure 3). Cells used for 3D-SRFM were obtained and processed in parallel to those used for the patch clamp experiments. We focused our analysis on cells that showed a striated pattern of α-actinin staining. Data acquisition was limited to areas of cell–cell contact positive for N-Cadherin staining (Figure 3A). The same two cell populations characterized in terms of INa density (Figure 2) were studied. As shown in Figure 3B, the density of protein clusters of both NaV1.5 (P = 0.0054) and N-Cadherin (P = 0.026) was larger in gene-edited cells, whereas cluster size was not dependent on whether arginine or histidine occupied position 1898 of Nav1.5 (P = 0.126 for Nav1.5 and P = 0.536 for N-Cadherin; Figure 3C and D). Overall, these results suggest that the p.Arg1898His SCN5A mutation associates with reduced average peak sodium current density and reduced density of Nav1.5 and N-Cadherin clusters at the site of cell contact.
Figure 3.
Effect of the SCN5A mutation on intercalated disc organization using super-resolution fluorescence microscopy. (A) 3D-SRFM image of IPSC-CMs stained for NaV1.5 (green) and N-Cadherin (magenta). Top left panel shows a region of cell–cell contact. Inset images at the bottom show the interaction of the proteins at different Y-axis angle rotation. (B–D) 3D-SRFM cluster analysis of IPSC-CMs from ARVD/C patient cells (p.Arg1898His, R1898H) and gene-edited cells (p.Arg1898Arg, R1898R). (B) Cluster density of both Nav1.5 (Green) and N-Cadherin (Magenta) was significantly higher in gene-edited cells [4.27 × 10−6 ± 5.05 × 10−7 (p.Arg1898His) vs. 8.80 × 10−6 ± 1.25 × 10−6 (p.Arg1898Arg) for Nav1.5, P = 0.0054; 18.03 × 10−6 ± 4.07 × 10−6 (p.Arg1898His) vs. 42.16 × 10−6 ± 8.47 × 10−6 (p.Arg1898Arg) for N-Cadherin, P = 0.026]. (C) Average Nav1.5 cluster size was similar in both cell lines [406,386 ± 42,637 nm3 (p.Arg1898His) vs. 315,359 ± 20,321 nm3 (p.Arg1898Arg), P = 0.126]. (D) Average N-Cadherin cluster size was similar in both cell lines [116,893 ± 3901 nm3 (p.Arg1898His) vs. 103,217 ± 1365 nm3 (p.Arg1898Arg) P = 0.536]. p.Arg1898Arg, n= 12 images; 44480 Nav1.5 clusters and 8268 N-Cadherin clusters. p.Arg1898His, n= 10 images; 9566 Nav1.5 clusters and 44480 N-Cadherin clusters. All data are reported and analysed at the level of clusters. *P < 0.05, **P = 0.005 (statistics performed using Student’s t-test).
3.2 Validation cohort
To validate the presence of SCN5A variants in ARVD/C patients, we performed sequencing of SCN5A in a multicentre validation cohort of 281 unrelated ARVD/C patients [169 (60%) male, age at presentation 34.8 ± 13.7 years]. Approximately half (n = 135, 48%) of subjects did not harbour a mutation in any of the ARVD/C-associated desmosomal genes; the remainder (n = 146, 52%) carried a pathogenic desmosomal mutation (77% PKP2, see Supplementary material online, Table S3).
We identified a putatively pathogenic heterozygous SCN5A variant in 5/281 (1.8%) patients. Prevalence of SCN5A variants was 2.2% (n = 3/135) in those without a desmosomal mutation compared with 1.4% (n = 2/146) in those with a desmosomal mutation. A summary of SCN5A variants is shown in Table 1. All variants were rare (MAF ≤ 0.0692%; 4/5 variants with an MAF <0.05%) or novel, occurred in highly conserved region of the sodium channel, and were predicted pathogenic by SIFT and Polyphen2.
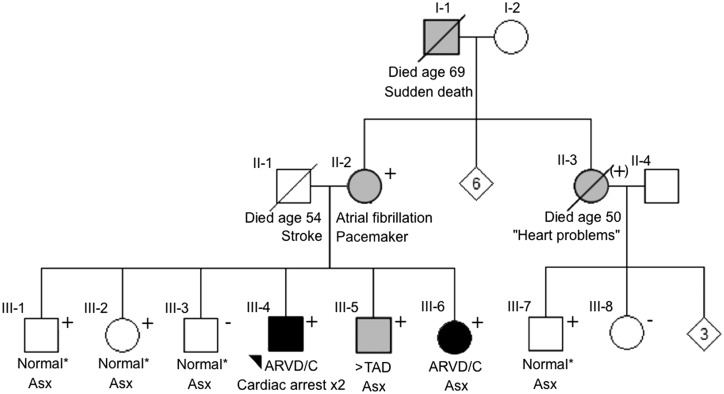
Table 2 shows detailed clinical data of the five SCN5A mutation carriers. All patients fulfilled ARVD/C diagnosis according to the 2010 diagnostic Task Force Criteria;9 of note, they all had major structural abnormalities on cardiac imaging. One SCN5A mutation carrier (p.Tyr416Cys) also harboured a variant of uncertain significance in the LMNA gene [p.Arg190Gln (c.569G > A)] (see Table 2 for details). Among SCN5A variant carriers, mean QRS duration was prolonged (119 ± 15 ms), whereas PR and QTc intervals were within normal limits (168 ± 19 and 416 ± 34 ms, respectively). None of the subjects had spontaneous ST-segment elevation. One SCN5A mutation carrier (p.Leu729del) underwent pharmacological sodium channel blocker challenge, which failed to unmask the electrocardiographic pattern of Brugada syndrome at peak intravenous ajmaline dose 1 mg/kg. As shown in Figure 4, an ARVD/C family history was observed in one SCN5A mutation carrier, in whom the variant (p.Leu729del) co-segregated with the phenotype with reduced penetrance. The other variant carriers did not have a family history of ARVD/C, although cardiac screening was limited.
Table 2.
Clinical phenotype of five SCN5A variant carriers in the validation cohort
| p.Tyr416Cys | p.Leu729del | p.Arg1623Ter | p.Ser1787Asn | p.Val2016Met | |
|---|---|---|---|---|---|
| Age at presentation (years) | 52 | 24 | 23 | 32 | 37 |
| Sex | Female | Male | Male | Male | Male |
| Ancestry | German/Italian | Dutch | Northern European | Ashkenazi Jewish | Dutch |
| Presentation | Palpitations | Sudden cardiac arrest | Sudden cardiac arrest | Palpitations | Ventricular tachycardia |
| Genetics | |||||
| Desmosomal mutation | –a | – | PKP2 IVS10-1G>C | – | PKP2 Trp676Ter |
| Task force criteria fulfilment | |||||
| Repolarization | V1–4 in presence CRBBB | TWI V1–3 | TWI V1–3 | TWI V1–3 | TWI V1–2 |
| Depolarization | None | Prolonged TAD | Prolonged TAD | Late potentials on SAECG | Prolonged TAD |
| Arrhythmia | LBI VT, 217 PVCs/24 h | LBS VT | LBI VT, 2297 PVCs/24 h | LBS VT, 1179 PVCs/24 h | LBS VT |
| Structural | Major | Major | Major | Major | Major |
| Family history | No | Yes | No | No | No |
| Conduction intervals | |||||
| PR (ms) | 180 | 186 | 140 | 174 | 158 |
| QRS (ms) | 138 | 126 | 118 | 96 | 116 |
| QTc (ms) | 448 | 367 | 410 | 447 | 406 |
| Clinical course | |||||
| Sustained VT/VF | No | Yes | Yes | Yes | Yes |
| Heart failure | No | No | Yes | No | No |
| Death | No | No | No | No | No |
CRBBB, complete right bundle branch block; ECG, electrocardiogram; LBI, left bundle inferior; LBS, left bundle superior; VF, ventricular fibrillation; VT, ventricular tachycardia; PKP2, Plakophilin-2; PVC, premature ventricular complex; SAECG, signal-averaged ECG; TAD, terminal activation duration; TWI, T-wave inversion.
aThe SCN5A p.Tyr416Cys carrier also carried a variant in LMNA [p.Arg190Gln (c.569G > A)]. Although SIFT and Polyphen2 classify this LMNA variant as damaging (score 0 and 1, respectively), and ClinVar reports on this variant as pathogenic, the literature on this variant is conflicting. Several sources classify the variant as likely polymorphism, given an UMD-predictor score 59 (probable polymorphism; http://www.umd.be/LMNA/4DACTION/WV/1705, accessed 22 July 2015), MutationAssessor score 2.76 (medium impact; http://mutationassessor.org/, accessed 22 July 2015), PANTHER score 0.675 (http://pantherdb.org/, accessed 22 July 2015), and SNP&GO classification as neutral (http://snps.biofold.org/snps-and-go/pages/method.html, accessed 22 July 2015). In addition, codon 190 seems to be a hotspot for variation, as other reports have described R190Q with reduced penetrance or with other mutations (Cenni et al., J Med Genet 2005;42:214–20; Maraldi et al., Eur J Histochem 2006;50:1–8); and co-segregation data were absent in two probands with R190Q as well as another LMNA mutation [Garcia-Pavia, Eur Heart J (abstract) http://eurheartj.oxfordjournals.org/content/ehj/34/suppl_1/P4234.full.pdf].
Figure 4.
Pedigree of the p.Leu729del mutation carrier reveals co-segregation of the variant with the ARVD/C phenotype with reduced penetrance. The proband (III-4) is indicated with an arrowhead. The proband’s mother (II-2) had atrial fibrillation and harboured the p.Leu729del variant. Among five siblings of the proband, four harboured the SCN5A variant, one of whom (III-6) was diagnosed with ARVD/C at age 34. Two siblings (III-1 and III-2) with the SCN5A variant were clinically unaffected at age 46 and 45. The 44-year-old male sibling without the SCN5A variant had a normal cardiac evaluation (III-3). The proband’s grandfather (I-1) had sudden death at 69 years of age, but DNA was not available for testing. An aunt of the proband (II-3) was reported to have heart problems and was an obligate carrier of the mutation. Symbols: square: male, circle: female, +: SCN5A mutation carrier, (+): obligate SCN5A mutation carrier, solid: clinical ARVD/C diagnosis, grey: clinical symptoms and/or borderline ARVD/C diagnosis, empty: negative phenotype for ARVD/C. *No abnormalities on comprehensive cardiac evaluation including 12-lead electrocardiogram, Holter monitoring, and cardiac imaging. ARVD/C, arrhythmogenic right ventricular dysplasia/cardiomyopathy; ASx, asymptomatic; >TAD, prolonged terminal activation duration.
3.3 Comparison of demographics and clinical phenotype by genotype
We compared the demographic and phenotypic characteristics of ARVD/C patients with an SCN5A variant with those with and without a desmosomal mutation. As shown in the Supplementary material online, Table S4, QRS duration was significantly prolonged in SCN5A variant carriers compared with all other ARVD/C subjects (119 ± 15 ms in SCN5A variant carriers vs. 94 ± 14 ms in others, P < 0.01). There were no significant differences in PR or QTc duration, or in any domain of the Task Force Criteria between the groups.
4. Discussion
This study represents the largest cohort of ARVD/C patients to date investigated for SCN5A mutations. Using hIPSC-CMs from an ARVD/C patient, we first defined the functional (patch clamp) and structural (3D-SRFM) effects caused by a single SCN5A mutation. Using gene editing, we were able to demonstrate that an amino acid substitution in Nav1.5 not only has the (somewhat expected) repercussion of changing sodium current amplitude but also causes a structural deficit in the organization of a protein directly relevant to cell-adhesion (N-Cadherin). We subsequently screened 281 ARVD/C patients for SCN5A mutations, and showed that rare variants in SCN5A are present in approximately 2% of ARVD/C patients. Overall, these results support the notion that Nav1.5 is in a functional complex with cell adhesion molecules, and reveal potential non-canonical mechanisms by which Nav1.5 dysfunction may contribute to cardiomyopathy.
4.1 SCN5A mutations and Nav1.5 function
4.1.1 Canonical Nav1.5 function
Functional analyses were performed in an IPSC-CM cell model containing the SCN5A mutation (p.Arg1898His) observed in our discovery cohort. Super-resolution images show a normal cluster size, but reduced cluster density for Nav1.5 at the intercalated disc, indicating that Nav1.5 clusters are less abundant at the membrane. This suggests that the total INa that can potentially be generated is less, which is indeed confirmed by our whole-cell patch clamping results. Of note, the change in current amplitude was robust, and independent of cell type, as it was reproduced in an exogenous expression system.
The p.Arg1898His mutation in our ARVD/C patient localizes to the Nav1.5 C-terminal domain (CTD), a highly conserved region of the sodium channel that regulates channel function through many auxiliary proteins including Fibroblast Growth Factor 13 (FGF13; also known as fibroblast growth factor homologous factor 2) and calmodulin (CaM).13 As shown in Figure 5, the mutated residue in our patient, p.Arg1898, sits at the hinge between the Nav CTD and the IQ domain helix, where it interacts with the side chain of p.Glu1901 in the Nav CTD and forms a cation- interaction with the side chain of p.Tyr98 in FGF13. p.Tyr98 in FGF13 also interacts with p.Lys95 in CaM. Interestingly, sequence alignments indicate that p.Arg1898 in Nav1.5 is homologous to p.Arg1902 in Nav1.2, which, when mutated to Cys, has been associated with familial autism.14 In Nav1.2, the p.Arg1902Cys mutation leads to reduced binding of Ca2+/CaM and thereby to abnormal Ca2+-dependent regulation.14,15 It is reasonable to think that a mutation of the equivalent residue in Nav1.5, p.Arg1898, affects the same set of interactions, and therefore plays a similar role in the development of cardiac disease.
Figure 5.
Crystal Structure of the Nav1.5 C-Terminal Domain (CTD) (Green), FGF13 (Magenta), and ApoCaM (Cyan) complex. (A) Crystal structure of the Nav1.5 CTD in cartoon representation. Annotations for all its six helices as well as for its domains containing the IQ and EFL motifs are shown. (B) Important residues for the interaction between the three molecules (Nav1.5 CTD, FGF13, and ApoCaM) are shown in stick representation. The mutated residue in our patient (p.Arg1898 in Nav1.5) is the only site in the ternary complex that unites all three molecules. p.Arg1898 interacts with the side chain of p.Glu1901 one turn below along the IQ domain helix of Nav1.5 and also forms a cation- interaction with the side chain of p.Tyr98 in FGF13. p.Glu1901 in the Nav1.5 CTD and p.Tyr98 in FGF13 also interact with p.Lys95 within the third CaM EF-hand in the ApoCaM C-lobe through hydrogen bonds. ApoCaM, apocalmodulin; FGF13, fibroblast growth factor 13; PDB accession code 4DCK (Wang et al. 2014).15
4.1.2 Non-canonical Nav1.5 function
Although it is not surprising that a Nav1.5 mutation causes sodium channel dysfunction, it is well recognized that SCN5A mutations may also lead to cardiac structural abnormalities.16,17 However, the mechanism by which SCN5A mutations cause cardiomyopathy remains unknown. We recently described the presence of an adhesion/excitability node in cardiac myocytes.8 In this prior work, we showed that (i) the adherens junction protein N-Cadherin serves as an attractor for Nav1.5 clusters, (ii) the Nav1.5 in these clusters are major determinants of the cardiac sodium current, and (iii) clustering of Nav1.5 facilitates its regulation by molecular partners. In this study, we confirm and expand prior data by showing that Nav1.5 is in a functional complex with cell adhesion molecules, and that a primary Nav1.5 defect may affect N-Cadherin biology resulting in reduced size and density of N-Cadherin clusters at the intercalated disc. These results are particularly interesting in the context of prior studies showing that Nav1.5 is delivered to the membrane via the microtubule network,18 and that microtubules at the intercalated disc anchor at N-Cadherin-rich sites.6,19 Whether Nav1.5 interacts directly with N-Cadherin, or whether this interaction is mediated through unknown molecular partners remains currently unknown. Further investigation is warranted to establish the role of molecules at the Nav1.5 CTD in orchestrating the interaction between mechanical and electrical junctions.
4.2 Prevalence and clinical implications of SCN5A mutations in ARVD/C
A previous study described SCN5A variants in 1 of 12 Chinese ARVD/C patients.20 However, clinical data in this study were sparse and analyses were not corroborated by functional data. Our study shows that approximately 2% of ARVD/C cases harbour putatively pathogenic mutations in SCN5A. This yield is similar to the historical yield of 1–3% of other rare genes associated with ARVD/C.1 One patient had an additional variant of uncertain significance in LMNA (Table 2). A pedigree can be found in the Supplementary material online, Figure S2. Her 33-year-old son had presyncope and palpitations, with frequent ventricular extrasystoles and borderline RV dimensions (RV end-diastolic volume 97.0 mL/m2). He also harboured the SCN5A, but not the LMNA, variant. Although we cannot exclude the possibility that the LMNA variant contributes to this proband’s phenotype, this highlights the well-established finding of multiple mutations in a subset of ARVD/C patients.1,21
As per study design, all individuals fulfilled diagnostic TFC for ARVD/C. In addition, each proband with an SCN5A mutation fulfilled major structural TFC for ARVD/C, further supporting the evidence that Nav1.5 plays a role in cardiac structural integrity. Interestingly, QRS duration was significantly prolonged in SCN5A variant carriers compared with all other subjects, suggestive of a loss-of-function effect of Nav1.5. Future studies are necessary to determine whether specific management recommendations should be made for ARVD/C patients with SCN5A variants. As in sodium channelopathies, avoidance of sodium channel blocking agents may prove to be prudent.
4.3 Implications for understanding of SCN5A pathophysiology
SCN5A mutations have been associated with a variety of clinical phenotypes including Brugada Syndrome,22 Long QT Syndrome,23 progressive cardiac conduction disease,23 dilated cardiomyopathy,16 and now also ARVD/C. Based on our results, we speculate that Nav1.5 not only forms ion-selective pores but also is a multifunctional protein in a functional adhesion/excitability complex with mechanical junctions. Depending on the protein interaction affected, genetic changes in Nav1.5 may thereby cause a predominantly structural phenotype (e.g. dilated cardiomyopathy), predominantly electrical phenotype (e.g. Brugada Syndrome, Long QT syndrome), or mixed electrical and structural phenotype (e.g. ARVD/C). As such, the affected protein interaction, more than the exact gene mutation, may determine the phenotype. Although further investigation is warranted to establish the role of auxiliary proteins in orchestrating the interaction between mechanical and electrical junctions at the intercalated disc, these data provide an additional explanation for the pathophysiological mechanism by which SCN5A mutations may result in cardiomyopathy.
4.4 Limitations
Recent studies have indicated that SCN5A has a 2–5% background rate of rare non-synonymous variants,24 which complicates the interpretation of our results. Although the functional analyses (p.Arg1898His) and co-segregation data (p.Leu729del) in two of our patients support a deleterious, disease-causing effect, we were not able to obtain functional or segregation data for the other patients, However, all these mutations are radical or have previously been associated with disease.22,25–29 Although all individuals in our study underwent genetic testing for the desmosomal genes, one-third [n = 93/281 (33%)] did not undergo whole-exome or large-panel-targeted sequencing, and mutations in genes infrequently associated with ARVD/C cannot be excluded in these individuals. Given the differences in screening methods, the finding of SCN5A variants in our validation cohort should be regarded as ‘proof of principle’ rather than definite evidence of pathogenicity in a single-gene Mendelian fashion.
IPSC-CMs are not fully mature cardiomyocytes: although these cells develop a contractile apparatus, the sarcomeres are disorganized and oriented in multiple directions. Also, IPSC-CMs do not have a proper mature intercalated disc structure connecting adjacent cells. As such, we focused our analysis on protein clusters in the immediate vicinity of the intercellular junction. In addition, it is important to note that statistical analyses on our IPSC-CMs and HEK293 cells are based on only one mutation. Although our study provides a possible explanation for the pathophysiological mechanism underlying structural changes in patients with an SCN5A mutation, it is important to recognize that many cardiac diseases associated with SCN5A do not exhibit structural abnormalities.
5. Conclusions
The results of our study show that putatively pathogenic SCN5A variants occur in approximately 2% of ARVD/C patients. SCN5A variants are associated with a significantly prolonged QRS duration. In one of our study subjects, we confirm a loss of Nav1.5 function in two experimental systems. More importantly, we reveal reduced abundance of Nav1.5 as well as N-Cadherin clusters at the intercalated disc, suggesting that Nav1.5 is in a functional adhesion/excitability complex with mechanical junctions. Furthermore, our studies demonstrate the utility of the CRISPR/Cas9 technology in the phenotypical characterization of hIPSC-CMs containing suspect pathogenic substitutions. Overall, our data provide a novel, alternative explanation as to the pathophysiological mechanisms by which SCN5A mutations may cause a cardiomyopathic phenotype.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Acknowledgement
We are grateful to the ARVD/C patients and families who have made this work possible.
Funding
This work was supported by the Dutch Heart Foundation (2015T058 to A.S.J.M.t.R.); the Interuniversity Cardiology Institute of the Netherlands (project 06901); the Netherlands Cardiovascular Research Initiative of the Dutch Heart Foundation, Dutch Federation of University Medical Centres, the Netherlands Organization for Health Research and Development and the Royal Netherlands Academy of Sciences (CVON 2012-10 PREDICT to M.P.v.d.B., A.A.M.W., C.R.B., and J.P.V.T.); the American Heart Association (SDG 14SDG15850014 to M.C. and 15POST25550087 to E.A.-P.); Post-doctoral Fellowship to E.A.-P.; the National Institutes of Health (U54HG006542 and UL1TR001079, R01HL116906 to L.M., UL1RR025780 to L.M., R01HL69071 to L.M., K23JL067915 to M.R.G.T., R01HL109209 to M.R.G.T., HL106632-04 to M.D., and GM57691-17 to M.D.); the CRTrieste and Generali Assicurazioni Foundations (to G.S.); and the Children’s Cardiomyopathy Foundation (to M.D.). The Johns Hopkins ARVD/C Program is supported by the Bogle Foundation, the Healing Hearts Foundation, the Campanella family, the Patrick J. Harrison Family, Dr Francis P. Chiaramonte Private Foundation, the Peter French Memorial Foundation, the Wilmerding Endowments, the Dr Satish, Rupal, and Robin Shah ARVD Fund at Johns Hopkins, the St Jude Medical Foundation, and Medtronic Inc.
Conflict of interest: none declared.
References
- 1.Groeneweg JA, Bhonsale A, James CA, Te Riele AS, Dooijes D, Tichnell C, Murray B, Wiesfeld AC, Sawant AC, Kassamali B, Atsma DE, Volders PG, de Groot NM, de Boer K, Zimmerman SL, Kamel IR, van der Heijden JF, Russell SD, Cramer MJ, Tedford RJ, Doevendans PA, van Veen TA, Tandri H, Wilde AA, Judge DP, van Tintelen JP, Hauer RN, Calkins H. Clinical presentation, long-term follow-up, and outcomes of 1001 arrhythmogenic right ventricular dysplasia/cardiomyopathy patients and family members. Circ Cardiovasc Genet 2015;8:437–446. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan SR, Gard JJ, Protonotarios N, Tsatsopoulou A, Spiliopoulou C, Anastasakis A, Squarcioni CP, McKenna WJ, Thiene G, Basso C, Brousse N, Fontaine G, Saffitz JE. Remodeling of myocyte gap junctions in arrhythmogenic right ventricular cardiomyopathy due to a deletion in plakoglobin (naxos disease). Heart Rhythm 2004;1:3–11. [DOI] [PubMed] [Google Scholar]
- 3.Cerrone M, Noorman M, Lin X, Chkourko H, Liang FX, van der Nagel R, Hund T, Birchmeier W, Mohler P, van Veen TA, van Rijen HV, Delmar M. Sodium current deficit and arrhythmogenesis in a murine model of plakophilin-2 haploinsufficiency. Cardiovasc Res 2012;95:460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noorman M, Hakim S, Asimaki A, Vreeker A, van Rijen HV, van der Heyden MA, de Jonge N, de Weger RA, Hauer RN, Saffitz JE, van Veen TA. Reduced plakoglobin immunoreactivity in arrhythmogenic cardiomyopathy: methodological considerations. Cardiovasc Pathol 2013;22:314–318. [DOI] [PubMed] [Google Scholar]
- 5.Sato PY, Coombs W, Lin X, Nekrasova O, Green KJ, Isom LL, Taffet SM, Delmar M. Interactions between ankyrin-g, plakophilin-2, and connexin43 at the cardiac intercalated disc. Circ Res 2011;109:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerrone M, Lin X, Zhang M, Agullo-Pascual E, Pfenniger A, Chkourko Gusky H, Novelli V, Kim C, Tirasawadichai T, Judge DP, Rothenberg E, Chen HS, Napolitano C, Priori SG, Delmar M. Missense mutations in plakophilin-2 cause sodium current deficit and associate with a brugada syndrome phenotype. Circulation 2014;129:1092–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noorman M, Hakim S, Kessler E, Groeneweg JA, Cox MG, Asimaki A, van Rijen HV, van Stuijvenberg L, Chkourko H, van der Heyden MA, Vos MA, de Jonge N, van der Smagt JJ, Dooijes D, Vink A, de Weger RA, Varro A, de Bakker JM, Saffitz JE, Hund TJ, Mohler PJ, Delmar M, Hauer RN, van Veen TA. Remodeling of the cardiac sodium channel, connexin43, and plakoglobin at the intercalated disk in patients with arrhythmogenic cardiomyopathy. Heart Rhythm 2013;10:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leo-Macias A, Agullo-Pascual E, Sanchez-Alonso JL, Keegan S, Lin X, Arcos T, Feng Xia L, Korchev YE, Gorelik J, Fenyo D, Rothenberg E, Rothenberg E, Delmar M. Nanoscale visualization of functional adhesion/excitability nodes at the intercalated disc. Nat Commun 2016;7:10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Eur Heart J 2010;31:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.UCSC Genome Browser. Accessible via https://genome.ucsc.edu (30 July 2016, date last accessed).
- 11.Bhonsale A, Groeneweg JA, James CA, Dooijes D, Tichnell C, Jongbloed JD, Murray B, Te Riele AS, van den Berg MP, Bikker H, Atsma DE, de Groot NM, Houweling AC, van der Heijden JF, Russell SD, Doevendans PA, van Veen TA, Tandri H, Wilde AA, Judge DP, van Tintelen JP, Calkins H, Hauer RN. Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated mutation carriers. Eur Heart J 2015;36:847–855. [DOI] [PubMed] [Google Scholar]
- 12.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the crispr-cas9 system. Nat Protoc 2013;8:2281–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Hennessey JA, Kirkton RD, Wang C, Graham V, Puranam RS, Rosenberg PB, Bursac N, Pitt GS. Fibroblast growth factor homologous factor 13 regulates Na+ channels and conduction velocity in murine hearts. Circ Res 2011;109:775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss LA, Escayg A, Kearney JA, Trudeau M, MacDonald BT, Mori M, Reichert J, Buxbaum JD, Meisler MH. Sodium channels scn1a, scn2a and scn3a in familial autism. Mol Psychiatry 2003;8:186–194. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Chung BC, Yan H, Wang HG, Lee SY, Pitt GS. Structural analyses of Ca(2)(+)/cam interaction with Nav channel c-termini reveal mechanisms of calcium-dependent regulation. Nat Commun 2014;5:4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson TM, Keating MT. Mapping a cardiomyopathy locus to chromosome 3p22-p25. J Clin Invest 1996;97:528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olson TM, Michels VV, Ballew JD, Reyna SP, Karst ML, Herron KJ, Horton SC, Rodeheffer RJ, Anderson JL. Sodium channel mutations and susceptibility to heart failure and atrial fibrillation. JAMA 2005;293:447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casini S, Tan HL, Demirayak I, Remme CA, Amin AS, Scicluna BP, Chatyan H, Ruijter JM, Bezzina CR, van Ginneken AC, Veldkamp MW. Tubulin polymerization modifies cardiac sodium channel expression and gating. Cardiovasc Res 2010;85:691–700. [DOI] [PubMed] [Google Scholar]
- 19.Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell 2007;128:547–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu J, Hu J, Dai X, Cao Q, Xiong Q, Liu X, Liu X, Shen Y, Chen Q, Hua W, Hong K. Scn5a mutation in Chinese patients with arrhythmogenic right ventricular dysplasia. Herz 2014;39:271–275. [DOI] [PubMed] [Google Scholar]
- 21.Rigato I, Bauce B, Rampazzo A, Zorzi A, Pilichou K, Mazzotti E, Migliore F, Marra MP, Lorenzon A, De Bortoli M, Calore M, Nava A, Daliento L, Gregori D, Iliceto S, Thiene G, Basso C, Corrado D. Compound and digenic heterozygosity predicts lifetime arrhythmic outcome and sudden cardiac death in desmosomal gene-related arrhythmogenic right ventricular cardiomyopathy. Circ Cardiovasc Genet 2013;6:533–542. [DOI] [PubMed] [Google Scholar]
- 22.Kapplinger JD, Tester DJ, Alders M, Benito B, Berthet M, Brugada J, Brugada P, Fressart V, Guerchicoff A, Harris-Kerr C, Kamakura S, Kyndt F, Koopmann TT, Miyamoto Y, Pfeiffer R, Pollevick GD, Probst V, Zumhagen S, Vatta M, Towbin JA, Shimizu W, Schulze-Bahr E, Antzelevitch C, Salisbury BA, Guicheney P, Wilde AA, Brugada R, Schott JJ, Ackerman MJ. An international compendium of mutations in the scn5a-encoded cardiac sodium channel in patients referred for brugada syndrome genetic testing. Heart Rhythm 2010;7:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Postema PG, Van den Berg M, Van Tintelen JP, Van den Heuvel F, Grundeken M, Hofman N, Van der Roest WP, Nannenberg EA, Krapels IP, Bezzina CR, Wilde A. Founder mutations in the Netherlands: Scn5a 1795insd, the first described arrhythmia overlap syndrome and one of the largest and best characterised families worldwide. Neth Heart J 2009;17:422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Scouarnec S, Karakachoff M, Gourraud JB, Lindenbaum P, Bonnaud S, Portero V, Duboscq-Bidot L, Daumy X, Simonet F, Teusan R, Baron E, Violleau J, Persyn E, Bellanger L, Barc J, Chatel S, Martins R, Mabo P, Sacher F, Haissaguerre M, Kyndt F, Schmitt S, Bezieau S, Le Marec H, Dina C, Schott JJ, Probst V, Redon R. Testing the burden of rare variation in arrhythmia-susceptibility genes provides new insights into molecular diagnosis for brugada syndrome. Hum Mol Genet 2015;24:2757–2763. [DOI] [PubMed] [Google Scholar]
- 25.Ackerman MJ, Splawski I, Makielski JC, Tester DJ, Will ML, Timothy KW, Keating MT, Jones G, Chadha M, Burrow CR, Stephens JC, Xu C, Judson R, Curran ME. Spectrum and prevalence of cardiac sodium channel variants among Black, White, Asian, and Hispanic individuals: implications for arrhythmogenic susceptibility and brugada/long qt syndrome genetic testing. Heart Rhythm 2004;1:600–607. [DOI] [PubMed] [Google Scholar]
- 26.Hu RM, Tan BH, Tester DJ, Song C, He Y, Dovat S, Peterson BZ, Ackerman MJ, Makielski JC. Arrhythmogenic biophysical phenotype for scn5a mutation s1787n depends upon splice variant background and intracellular acidosis. PLoS One 2015;10:e0124921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Splawski I, Shen J, Timothy KW, Lehmann MH, Priori S, Robinson JL, Moss AJ, Schwartz PJ, Towbin JA, Vincent GM, Keating MT. Spectrum of mutations in long-qt syndrome genes. Kvlqt1, herg, scn5a, kcne1, and kcne2. Circulation 2000;102:1178–1185. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Makiyama T, Wuriyanghai Y, Ohno S, Sasaki K, Hayano M, Harita T, Nishiuchi S, Yuta Y, Ueyama T, Shimizu A, Horie M, Kimura T. Cardiac sodium channel mutation associated with epinephrine-induced qt prolongation and sinus node dysfunction. Heart Rhythm 2016;13:289–298. [DOI] [PubMed] [Google Scholar]
- 29.Shy D, Gillet L, Ogrodnik J, Albesa M, Verkerk AO, Wolswinkel R, Rougier JS, Barc J, Essers MC, Syam N, Marsman RF, van Mil AM, Rotman S, Redon R, Bezzina CR, Remme CA, Abriel H. Pdz domain-binding motif regulates cardiomyocyte compartment-specific nav1.5 channel expression and function. Circulation 2014;130:147–160. [DOI] [PubMed] [Google Scholar]
- 30.Saguner AM, Ganahl S, Kraus A, Baldinger SH, Medeiros-Domingo A, Saguner AR, Mueller-Burri SA, Wolber T, Haegeli LM, Krasniqi N, Tanner FC, Steffel J, Brunckhorst C, Duru F. Clinical role of atrial arrhythmias in patients with arrhythmogenic right ventricular dysplasia. Circ J 2014;78:2854–2861. [DOI] [PubMed] [Google Scholar]
- 31.Nagase S, Kusano KF, Morita H, Nishii N, Banba K, Watanabe A, Hiramatsu S, Nakamura K, Sakuragi S, Ohe T. Longer repolarization in the epicardium at the right ventricular outflow tract causes type 1 electrocardiogram in patients with brugada syndrome. J Am Coll Cardiol 2008;51:1154–1161. [DOI] [PubMed] [Google Scholar]