Abstract
Several probiotics are known to bind aflatoxin B1 (AFB1) to their surfaces and to adhere to intestinal mucus. In this study, preincubation of two probiotic preparations with either AFB1 or mucus reduced the subsequent surface binding of mucus and AFB1, respectively, in a strain-dependent manner.
Aflatoxin B1 (AFB1) is an unavoidable food contaminant: current decontamination methods have failed to eliminate aflatoxins from crops. Novel approaches focus on preventing its absorption in the gastrointestinal tracts of animals (15) and humans (4).
Several probiotic bacteria, commonly used in food products, have been shown to bind AFB1 efficiently in vitro (2, 6, 9), but ex vivo results are controversial [3; S. Gratz, H. El-Nezami, and H. Mykkännen, Abstr. 5th Congr. Toxicol. Developing Countries, Guilin, China, abstr. C26, Toxicology 191(Suppl.):50, 2003]. These differences may be caused by intestinal mucus, since several intestinal and probiotic bacteria have been shown to adhere to mucus (8). Additionally, in vitro studies have focused on bacterial binding sites involved in mucus adhesion (8, 12, 13, 14, 16, 19) and AFB1 adsorption to the bacterial surface (2, 3, 5, 6, 9). Therefore, the present study investigated whether AFB1 and intestinal mucus influence each other's binding by surface structures of two probiotic preparations.
Lactobacillus rhamnosus GG (hereafter referred to as GG) is a potent binder for AFB1 in vitro (1, 5) and ex vivo (3) and for mucus (8, 12). A mixture of L. rhamnosus LC-705 and Propionibacterium freudenreichii subsp. shermanii JS (LC-705 plus JS), a biopreservative (Bioprofit), showed moderate AFB1 binding in chicks [Gratz et al., Toxicology 191(Suppl.):50, 2003] and was also used in a human study (4). Both were obtained from Valio, Ltd. (Helsinki, Finland), as lyophilized powder and stored at −80°C. Bacterial counts were determined with a flow cytometer as described previously (1). Lyophilized bacteria (1, 2.5, or 5 × 1010 CFU) were suspended in 1 ml of phosphate-buffered saline (PBS), incubated for 30 min at 37°C, and centrifuged (7 min, 3,000 × g, and 4°C) prior to use. Crystalline AFB1 (product no. A 6636; Sigma, St. Louis, Mo.) was dissolved in methanol and stored at −20°C. Aqueous AFB1 solutions were prepared in 10 mM PBS (pH 7.4) (2). Intestinal mucus was collected from three porcine colon samples kindly provided by the University of Kuopio Animal Centre. Mucus was removed and prepared as described previously (12), and protein content was determined with a protein assay kit (Bio-Rad, Espoo, Finland).
Interference in surface binding was assessed in two assays, either by allowing bacteria to bind AFB1 first and subsequently incubating them with mucus or by allowing bacteria to bind mucus first and then incubating them with AFB1. In the first assay, the bacterial pellet was suspended in aqueous AFB1 solutions of various concentrations (0.32 to 64.1 μM) and incubated (30 min and 37°C) to allow AFB1 binding to the bacterial surface. After centrifugation, the supernatant was analyzed for free AFB1 by reverse-phase high-performance liquid chromatography as described previously (1). The pelleted complex of bacteria and AFB1 was resuspended in intestinal mucus with different protein concentrations (0.1 to 10 mg of protein per milliliter) and incubated at 37°C for 1 h. Following centrifugation, the supernatant was analyzed for free mucus proteins. As controls, bacteria without AFB1 preincubation were included in the mucus binding assay. The stability of this bacterium-mucus complex was tested by washing the solution with 0.5 ml of Milli-Q water, centrifuging it, and measuring mucus proteins released in the supernatant. To estimate the impact of heat treatment on bacterial mucus binding ability, boiled (1 h in PBS) bacteria were included in the assay. In the second assay, bacteria were incubated with mucus and centrifuged, and the bacterium-mucus complex was resuspended in AFB1 solutions as described above. In order to rule out the possibility that AFB1 binds to the mucus glycoproteins instead of binding to the bacterial surface, AFB1 and mucus were mixed, incubated (1 h at 37°C), and centrifuged, and the amounts of free mucus and free AFB1 were determined. All data are expressed as means of results of four replicates and were analyzed by Student's t test. P values of <0.05 were considered significant.
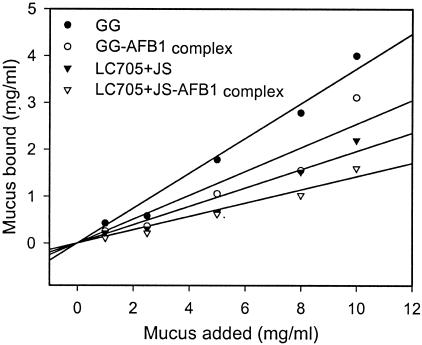
Both GG and LC-705 plus JS were able to remove mucus from the incubation mixture (Fig. 1). This could be caused by bacterial degradation of mucus, since killing the bacteria with heat disabled mucus binding (data not shown). However, although lactobacilli (10) and propionibacteria (18) possess proteolytic activity, they do not degrade intestinal mucus (17). Therefore, surface binding of intestinal mucus must be at least partly involved in mucus removal, since aqueous washing released 30.7% ± 3.9% of bound mucus from LC-705 plus JS and 67.1% ± 1.1% from GG. This finding suggests that intestinal mucus is reversibly bound to the bacterial surface and that the unrecoverable part of mucus may be bound more tightly, hindering the release by aqueous washing. Perhaps more-rigorous washing or sonication could result in greater release of bound mucus. The lower level of mucus binding by LC-705 plus JS is not surprising, since both LC-705 and JS have poor adhesion ability to intestinal mucus and do not influence each other's adhesion capacity in vitro (13, 19), whereas GG was found to adhere well (8, 11, 12, 14, 19). Furthermore, the mixture LC-705 plus JS also binds AFB1 less efficiently in vitro than GG does (S. Gratz et al., unpublished data). After preincubation with AFB1, subsequent mucus binding by both probiotics was reduced (Fig. 1), confirming results of Kankaanpää and coworkers (7), who found that AFB1 binding by GG reduced its subsequent adhesion to Caco-2 cells.
FIG. 1.
Effect of AFB1 preincubation (3.2 μM) on mucus binding at different mucus concentrations (1 to 10 mg/ml) by L. rhamnosus GG (GG) and a mixture of L. rhamnosus LC-705 and P. freudenreichii subsp. shermanii JS (LC705+JS). R2 values were calculated from original replicates as follows: 0.8399 for GG, 0.8447 for GG-AFB1, 0.9013 for LC-705 plus JS, and 0.8184 for LC-705 plus JS-AFB1.
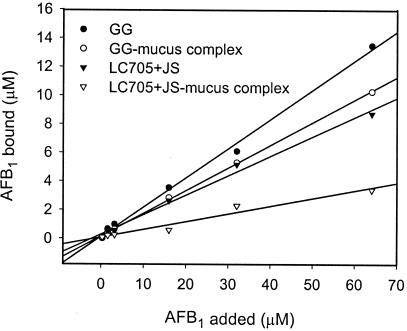
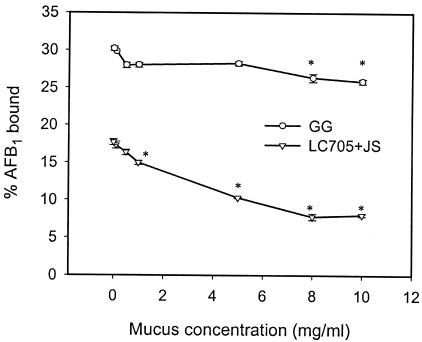
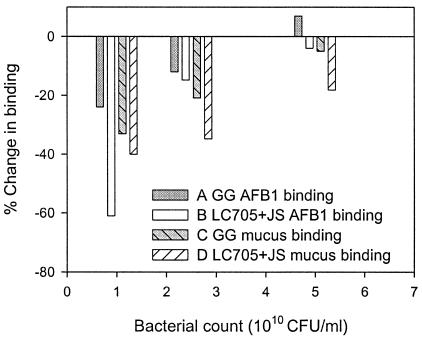
Binding capacities of both probiotics for mucus and AFB1 were not saturated, as indicated by linear slopes in Fig. 1 and 2. AFB1 binding by the bacterium-mucus complex must occur to the bacteria rather than to mucus structures, since incubating AFB1 and mucus alone did not remove any of the free AFB1 from the incubation mixture (data not shown). At the highest AFB1 concentration used (Fig. 2), mucus preincubation significantly (P < 0.01) reduced AFB1 binding by 23.8% for GG and 61.2% for LC-705 plus JS, although the mixture is less capable of binding mucus initially. These strain differences may be explained by the fact that LC-705 plus JS binds mucus more tightly (smaller recovery by washing), possibly interfering more with AFB1 binding than was the case with GG. After preincubation with mucus with different protein concentrations, AFB1 binding by LC-705 plus JS was significantly reduced by low mucus concentrations (1 mg/ml), while for GG, significant reduction was achieved only at mucus concentrations above 8 mg/ml (Fig. 3). The reductive effect of mucus and AFB1 on each other's binding was strongest at low bacterial counts but decreased with increasing numbers of CFU (Fig. 4). Especially at the low numbers of CFU, AFB1 binding by LC-705 plus JS was strongly impaired. This result suggests that the probiotic mixture is less capable of binding AFB1 in the presence of mucus and is more susceptible to interfering factors in the intestinal tract, which may explain poorer results in AFB1 binding in animals [Gratz et al., Toxicology 191(Suppl.):50, 2003].
FIG. 2.
Effect of preincubation of mucus (10 mg/ml) on AFB1 binding at different AFB1 concentrations (0.32 to 64.1 μM) by L. rhamnosus GG (LGG) and the mixture of LC-705 and P. freudenreichii subsp. shermanii JS (PJS). R2 values were calculated from original replicates as follows: 0.9965 for GG, 0.9892 for GG-mucus, 0.9386 for LC-705 plus JS, and 0.9599 for LC-705 plus JS-mucus.
FIG. 3.
Effect of mucus preincubation at different concentrations (0 to 10 mg/ml) on AFB1 binding (percentage of the level obtained with the 3.2 μM concentration) by GG and the mixture of LC-705 plus JS. * indicates a significant (P < 0.05) difference compared to values for controls without mucus preincubation (0 mg/ml).
FIG. 4.
Changes in levels of binding of AFB1 (3.2 μM) by L. rhamnosus GG (GG) (A) and the mixture of LC-705 and P. freudenreichii subsp. shermanii JS (LC705+JS) (B) due to preincubation of mucus (10 mg/ml) at different numbers of CFU (1, 2.5 and 5 × 1010 CFU) and changes in levels of mucus binding by GG (C) and the LC-705-JS mixture (D) due to AFB1 preincubation at different bacterial concentrations.
Despite the influence of AFB1 and mucus on each other's binding, similar binding sites are unlikely to be involved, since heat-treated bacteria lost their ability to bind intestinal mucus, whereas AFB1 binding was previously found to be enhanced by heat treatment (2, 9). Tuomola and coworkers (19) also showed that heat treatment disturbs mucus adhesion by GG and LC-705 and concluded that proteins must be involved in the binding of mucus, whereas carbohydrates are known to bind AFB1 (5). Other mechanisms, such as steric hindrance, may cause interference in AFB1 and mucus binding by bacteria.
Nevertheless, these findings are important, since probiotics adhering to the intestinal wall are less likely to bind and consequently accumulate AFB1 in the host. On the other hand, probiotics with AFB1 bound to their surfaces are less likely to adhere to the intestinal wall and prolong exposure to dietary AFB1. Hence, specific probiotics may be potent and safe means to reduce absorption and increase excretion of dietary AFB1 from the body.
Acknowledgments
This study was supported by The Academy of Finland (grant 1574).
REFERENCES
- 1.El-Nezami, H., P. Kankaanpää, S. Salminen, and J. Ahokas. 1998. Ability of dairy strains of lactic acid bacteria to bind a common food carcinogen, aflatoxin B1. Food Chem. Toxicol. 36:321-326. [DOI] [PubMed] [Google Scholar]
- 2.El-Nezami, H., P. Kankaanpää, S. Salminen, and J. Ahokas. 1998. Physicochemical alterations enhance the ability of dairy strains of lactic acid bacteria to remove aflatoxin from contaminated media. J. Food Prot. 61:466-468. [DOI] [PubMed] [Google Scholar]
- 3.El-Nezami, H., H. Mykkänen, P. Kankaanpää, S. Salminen, and J. Ahokas. 2000. Ability of Lactobacillus and Propionibacterium strains to remove aflatoxin B1 from the chicken duodenum. J. Food Prot. 63:549-552. [DOI] [PubMed] [Google Scholar]
- 4.El-Nezami, H., H. Mykkänen, P. Kankaanpää, T. Suomalainen, S. Salminen, and J. Ahokas. 2000. Ability of a mixture of Lactobacillus and Propionibacterium to influence the faecal aflatoxin content in healthy Egyptian volunteers: a pilot clinical study. Biosci. Microflora 19:41-45. [Google Scholar]
- 5.Haskard, C., C. Binnion, and J. Ahokas. 2000. Factors affecting the sequestration of aflatoxin by Lactobacillus rhamnosus strain GG. Chem. Biol. Interact. 128:39-49. [DOI] [PubMed] [Google Scholar]
- 6.Haskard, C. A., H. S. El-Nezami, P. E. Kankaanpää, S. Salminen, and J. T. Ahokas. 2001. Surface binding of aflatoxin B1 by lactic acid bacteria. Appl. Environ. Microbiol. 67:3086-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kankaanpää, P., E. Tuomola, H. El-Nezami, J. Ahokas, and S. J. Salminen. 2000. Binding of aflatoxin B1 alters the adhesion properties of Lactobacillus rhamnosus strain GG in a Caco-2 model. J. Food Prot. 63:412-414. [DOI] [PubMed] [Google Scholar]
- 8.Kirjavainen, P. V., A. C. Ouwehand, E. Isolauri, and S. J. Salminen. 1998. The ability of probiotic bacteria to bind to human intestinal mucus. FEMS Microbiol. Lett. 167:185-189. [DOI] [PubMed] [Google Scholar]
- 9.Lee, Y. K., H. El-Nezami, C. A. Haskard, S. Gratz, K. Y. Puong, S. Salminen, and H. Mykkänen. 2003. Kinetics of adsorption and desorption of aflatoxin B1 by viable and nonviable bacteria. J. Food Prot. 66:426-430. [DOI] [PubMed] [Google Scholar]
- 10.Mikelsaar, M., R. Mändar, and E. Sepp. 1998. Lactic acid microflora in the human microbial ecosystem and its development, p. 588-602. In S. Salminen and A. von Wright (ed.), Lactic acid bacteria microbiology and functional aspects, 2nd ed. Marcel Dekker, Inc., New York, N.Y.
- 11.Ouwehand, A. C., P. Niemi, and S. J. Salminen. 1999. The normal faecal microflora does not affect the adhesion of probiotic bacteria in vitro. FEMS Microbiol. Lett. 177:35-38. [DOI] [PubMed] [Google Scholar]
- 12.Ouwehand, A. C., S. Salminen, S. Tölkkö, P. Roberts, J. Ovaska, and E. Salminen. 2002. Resected human colonic tissue: new model for characterizing adhesion of lactic acid bacteria. Clin. Diagn. Lab. Immunol. 9:184-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouwehand, A. C., T. Suomalainen, S. Tölkkö, and S. Salminen. 2002. In vitro adhesion of propionic acid bacteria to human intestinal mucus. Lait 82:123-130. [Google Scholar]
- 14.Ouwehand, A. C., S. Tölkkö, J. Kulmala, S. Salminen, and E. Salminen. 2000. Adhesion of inactivated probiotic strains to intestinal mucus. Lett. Appl. Microbiol. 31:82-86. [DOI] [PubMed] [Google Scholar]
- 15.Phillips, T. D. 1999. Dietary clay in the chemoprevention of aflatoxin-induced disease. Toxicol. Sci. 52:118-126. [DOI] [PubMed] [Google Scholar]
- 16.Rojas, M., F. Ascencio, and P. L. Conway. 2002. Purification and characterization of a surface protein from Lactobacillus fermentum 104R that binds to porcine small intestinal mucus and gastric mucin. Appl. Environ. Microbiol. 68:2330-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruseler-van Embden, J. G. H., L. M. C. van Lieshout, M. J. Gosselink, and P. Marteau. 1995. Inability of Lactobacillus casei strain GG, L. acidophilus and Bifidobacterium bifidum to degrade intestinal mucus glycoproteins: clearing the way for mucosal protective therapy. Scand. J. Gastroenterol. 30:675-680. [DOI] [PubMed] [Google Scholar]
- 18.Salminen, S., and C. Grant. 1998. The potential of Propionibacterium ssp. as probiotics, p. 588-602. In S. Salminen and A. von Wright (ed.), Lactic acid bacteria microbiology and functional aspects, 2nd ed. Marcel Dekker, Inc., New York, N.Y.
- 19.Tuomola, E. M., A. C. Ouwehand, and S. J. Salminen. 2000. Chemical, physical and enzymatic pretreatments of probiotic lactobacilli alter their adhesion to human intestinal mucus glycoproteins. Int. J. Food Microbiol. 60:75-81. [DOI] [PubMed] [Google Scholar]