Abstract
Cryptosporidium parvum is a waterborne pathogen that poses potential risk to drinking water consumers. The detection of Cryptosporidium oocysts, its transmissive stage, is used in the latest U.S. Environmental Protection Agency method 1622, which utilizes organic fluorophores such as fluorescein isothiocyanate (FITC) to label the oocysts by conjugation with anti-Cryptosporidium sp. monoclonal antibody (MAb). However, FITC exhibits low resistance to photodegradation. This property will inevitably limit the detection accuracy after a short period of continuous illumination. In view of this, the use of inorganic fluorophores, such as quantum dot (QD), which has a high photobleaching threshold, in place of the organic fluorophores could potentially enhance oocyst detection. In this study, QD605-streptavidin together with biotinylated MAb was used for C. parvum oocyst detection. The C. parvum oocyst detection sensitivity increased when the QD605-streptavidin concentration was increased from 5 to 15 nM and eventually leveled off at a saturation concentration of 20 nM and above. The minimum QD605-streptavidin saturation concentration for detecting up to 4,495 ± 501 oocysts (mean ± standard deviation) was determined to be 20 nM. The difference in the enumeration between 20 nM QD605-streptavidin with biotinylated MAb and FITC-MAb was insignificant (P > 0.126) when various C. parvum oocyst concentrations were used. The QD605 was highly photostable while the FITC intensity decreased to 19.5% ± 5.6% of its initial intensity after 5 min of continuous illumination. The QD605-based technique was also shown to be sensitive for oocyst detection in reservoir water. This observation showed that the QD method developed in this study was able to provide a sensitive technique for detecting C. parvum oocysts with the advantage of having a high photobleaching threshold.
Cryptosporidium parvum has been recognized widely as a cause of waterborne diseases since the 1993 Milwaukee cryptosporidiosis outbreak incident which infected 403,000 people and caused 100 deaths (4). Its transmissive stage, the oocyst, is a frequent inhabitant in raw water sources of potable water (7). C. parvum has a long survival time in water and has a low infectious dose. In addition, C. parvum is resistant to conventional water treatment processes, including chemical disinfection, which causes this pathogenic organism to be a potential risk for drinking water consumers. Thus, detection of C. parvum oocysts in raw water sources is important for the control of this pathogen in drinking water supplies. Among the various methods developed for the detection of C. parvum, method 1622 established by the U.S. Environmental Protection Agency (EPA) under the Safe Drinking Water Act Amendments of 1996 is the most updated and widely used method (9). This method uses the labeling of the oocyst with fluorescent antibody. The fluorescent dye used in EPA method 1622 is an organic fluorophore that exhibits low resistance to photodegradation (1). This property will inevitably limit the detection accuracy after a short period of continuous illumination. The development of novel inorganic fluorophores such as luminescence colloidal semiconductor nanocrystals, more commonly known as quantum dots (QDs), is potentially able to overcome some of the limitations encountered with organic fluorophores. QDs are made from nanometer-scale crystals of semiconductor material, such as CdSe, which has been coated with an additional semiconductor shell, such as ZnS, to improve the optical properties of the material. This core-shell material is further coated with a polymer shell that allows the materials to be conjugated to biological molecules and to retain their optical properties (6). The photoluminescence from these QDs can be detected at concentrations comparable to organic dyes by conventional florescence methods (5). Brighter signals have been reported in experiments with QDs than in experiments with organic dyes due to greater adsorption of the excitation light (10) and the high photobleaching threshold in QDs (3).
QD bioconjugates have been successfully applied to the detection of the breast cancer marker Her2 on fixed cells (11), long-term imaging of live cells (3), and intracellular targets such as microtubules, actin filaments, nuclei, and mitochondria (11). The successful application of QDs in medical research suggested that there is a potential merit to the usage of QD bioconjugates in waterborne pathogen monitoring, as it could be a promising technology for enhancing pathogen detection sensitivity.
QD conjugates with biotinylated immunoglobulin Gs can be formed by using streptavidin via streptavidin-biotin binding, which is highly specific and forms very stable streptavidin-biotin binding phenomena (2). In view of the advantages of using QDs in the biolabeling of biological samples, this study aimed to develop a specific, sensitive, and photostable detection method for C. parvum oocysts by using luminescence QD-streptavidin together with biotinylated anti-Cryptosporidium sp. monoclonal antibody (MAb).
MATERIALS AND METHODS
Source of oocyst suspension.
C. parvum oocysts were obtained from Waterborne, Inc. (New Orleans, La.). These oocysts were isolated from infected calves and are referred to as the Iowa strain. The feces of experimentally infected calves were collected and clarified by using sucrose and Percoll density gradient centrifugation after initial extraction of the feces with diethyl ether. Purified oocysts were stored in a solution containing phosphate-buffered saline (PBS) supplemented with 1,000 U of penicillin per ml and 1,000 μg of streptomycin per ml at 4°C. Oocysts which were inactivated in 5% formalin were used in this study. The age of the oocysts used in this study was less than 2 months.
Fixation of oocysts on glass well microscope slides.
The oocysts were fixed on the glass well microscope slides (Waterborne) by evaporation to dryness in an incubator at 42°C. Following this, 50 μl of absolute ethanol was applied to each well containing the dried sample and allowed to air dry until the methanol had evaporated.
Enumeration of oocyst stock suspensions with FITC-MAb conjugate.
C. parvum oocysts in suspension were enumerated by placing five replicates of fixed 10-μl aliquots on a glass well microscope and incubating them with fluorescein isothiocyanate (FITC)-MAb conjugate (Waterborne) in a humidified chamber protected from light for 90 min. The slides were then rinsed with sterile PBS (pH 7.4). The slides were then allowed to dry in a desiccator protected from lights for 1 h. No-Fade mounting medium (Waterborne) was used to mount the slides, and the slides were examined with an epifluorescence microscope as described in EPA method 1622 (9).
Enumeration of oocyst stock suspensions with QD605-streptavidin and biotinylated MAb.
C. parvum oocysts in suspension were enumerated by placing five replicates of fixed 10-μl aliquots on glass well microscope slides and incubating them with biotinylated MAb (Waterborne) in a humidified chamber protected from light for 90 min. The slides were then rinsed with sterile PBS (pH 7.4). The preparation was subsequently stained with QD605-streptavidin (Quantum Dot Corporation, Hayward, Calif.) and incubated in the humidified chamber protected from light for 30 min. After incubation, the slides were rinsed with sterile PBS and dried in the desiccator protected from lights for 1 h. The slides were then mounted with 90% glycerol in PBS and examined with an epifluorescence microscope.
Epifluorescence microscopy.
An Olympus BX51 fluorescence microscope fitted with a 100-W mercury lamp was used in this study. A blue filter block (excitation wavelength, 490 nm; emission wavelength, 510 nm) was used to detect FITC-labeled C. parvum oocysts while a barrier filter block (excitation wavelength, 546 nm; emission wavelength, 590 nm) was used to visualize QD605-labeled C. parvum oocysts. The examination and enumeration of oocysts were carried out at a magnification of ×200. Images were taken at magnification of ×400 with the MetaMorph imaging system (Universal Imaging Corporation, Downingtown, Pa.).
Determination of optimum QD605-streptavidin concentration.
QD605-streptavidin with concentrations ranging from 5 to 25 nM was used for the detection of 10-μl aliquots of purified oocysts from the stock. QD605-streptavidin was diluted to a desired concentration by using the QD incubation buffer (Quantum Dot Corporation). An enumeration from the detection with FITC-MAb conjugate (Waterborne) in accordance with EPA method 1622 was used for comparison with the detection by QD605-streptavidin. The number of oocysts detected was used to determine the sensitivity of the detection methods.
Verification of C. parvum oocyst detection with QD conjugate with antibody.
The sensitivity of detecting QD605-streptavidin with biotinylated MAb stained oocysts was quantified on aliquots prepared from a series of dilutions with sterile PBS (pH 7.4) obtained from the C. parvum oocyst stock, namely, 5×, 50×, and 100×. The enumeration from the detection with FITC-MAb conjugate (Waterborne) was used for comparison. The sensitivity of QD605-streptavidin detection on environmental samples was also tested by spiking the C. parvum oocyst stock at the 100× dilution with reservoir water.
Photostability of FITC-MAb conjugate and QD605-streptavidin with biotinylated MAb.
Both FITC-labeled and QD605-labeled oocysts were continuously illuminated for 5 min with an Olympus BX51 fluorescence microscope fitted with a 100-W mercury lamp. The images were captured with a cooled charge-coupled device camera SPOT-RT slider (Diagnostic Instruments, Sterling Heights, Mich.) at every 15-s interval, and the intensity was analyzed with the MetaMorph imaging system (Universal Imaging Corporation).
RESULTS
Effect of QD605-streptavidin concentrations on C. parvum oocyst detection sensitivity.
The C. parvum oocysts from the 10-μl aliquot stock were enumerated with FITC-MAb conjugate and QD605-streptavidin with biotinylated MAb. The numbers of C. parvum oocysts enumerated by the mentioned methods are summarized in Fig. 1. The number of FITC-MAb conjugate-stained oocysts detected from the 10-μl aliquot stock was 4,334 ± 778 (mean ± standard deviation) (n = 5).
FIG. 1.
Enumeration of C. parvum oocysts by use of FITC-MAb conjugates and various concentrations of QD605-streptavidin with biotinylated MAb (n = 5).
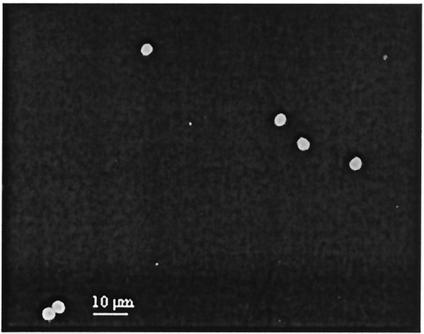
It is noted from Fig. 1 that increasing the QD605-streptavidin concentration from 5 to 20 nM had resulted in an increased number of C. parvum oocysts being detected. The t test indicated that the detection sensitivities associated with 5, 10, and 15 nM QD605-streptavidin were significantly lower than the detection sensitivity achieved with the FITC-MAb conjugate (P < 0.041). However, the detection sensitivities achieved with 20 and 25 nM QD605-streptavidin were similar to that achieved with theFITC-MAb conjugate (P > 0.517, as determined by a t test). There were no significant differences in the number of C. parvum oocysts detected with 20 and 25 nM QD605-streptavidin (P = 0.642, as determined by a t test). Thus, 20 nM QD605-streptavidin would be the minimum concentration required to provide similar sensitivity of oocyst detection as that attainable with FITC-MAb conjugate. The number of oocysts detected with 20 nM QD605-streptavidin with biotinylated MAb was 4,495 ± 501 (n = 5). Figures 2 and 3 show epifluorescence images of FITC-labeled C. parvum oocysts and QD605-labeled C. parvum oocysts with 20 nM QD605-streptavidin, respectively.
FIG. 2.
Epifluorescence image of FITC-labeled C. parvum oocysts.
FIG. 3.
Epifluorescence image of QD605-labeled C. parvum oocysts with 20 nM QD605-streptavidin.
Detection of various C. parvum oocyst concentrations with optimum concentration of QD605-streptavidin with biotinylated MAb.
Aliquots prepared from C. parvum oocyst stock and a series of dilutions from the stock, namely 5×, 50×, and 100× dilutions with sterile PBS (pH 7.4), were used to determine the sensitivity of 20 nM QD605-streptavidin with biotinylated MAb. Table 1 shows the number of C. parvum oocysts detected at different stock dilutions with FITC-MAb conjugate and 20 nM QD605-streptavidin with biotinylated MAb. The results indicated that the difference in the number of oocysts detected in the different stock dilutions with the two different staining methods were not significantly different (P > 0.126, as determine by a t test). The sensitivity of 20 nM QD605-streptavidin with biotinylated MAb on an environmental sample was also tested by diluting the stock oocysts to 100× with reservoir water. The number of oocysts detected in the spiked reservoir water was 43 ± 5 (n = 5). This result was not significantly different from the detection sensitivity of the same oocyst stock dilution with PBS (P = 0.233, as determined by a t test).
TABLE 1.
Various dilutions of C. parvum oocyst stocks enumerated with FITC-MAb conjugates and 20 nM QD605-streptavidin with biotinylated MAb
| Dilution | No. of oocysts detected witha:
|
|
|---|---|---|
| FITC-MAb | 20 nM QD605-streptavidin with biotinylated MAb | |
| None (stock solution) | 4,334 ± 778 | 4,495 ± 501 |
| 5× | 772 ± 36 | 772 ± 22 |
| 50× | 107 ± 8 | 100 ± 4 |
| 100× | 45 ± 6 | 48 ± 7 |
Results are given as means ± standard deviations (n = 5).
Photostability comparison between FITC-MAb conjugate and QD605-streptavidin with biotinylated MAb.
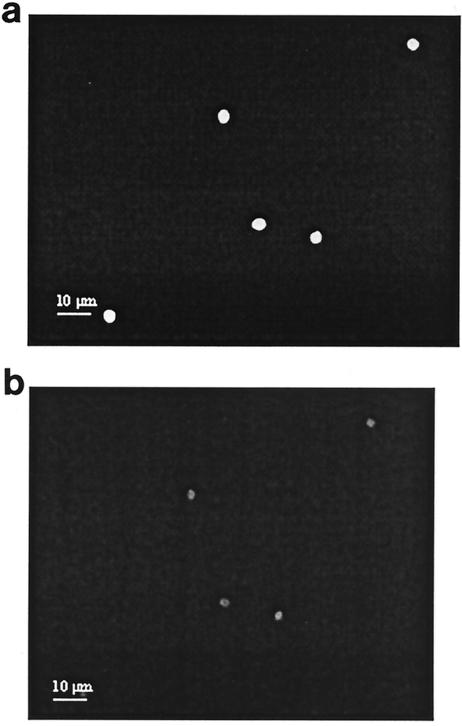
The main advantage of QDs over organic fluorescence labels is photostability. However, the observed brightness of QDs could differ for QDs that are conjugated and used in staining experiments (3). Figure 4 summarized the normalized fluorescence intensity of FITC-labeled and QD605-labeled oocysts. It is noted from Fig. 5a that an FITC-labeled oocyst exhibited bright signals at the beginning of the illumination period. However, the signals weaken significantly after 5 min of illumination (Fig. 5b), which corresponded to a residual intensity of 19.5% ± 5.6% (n = 3) of its initial intensity. In contrast, it is observed from Fig. 6 that there were insignificant deteriorations in the QD605-labeled oocyst signals from the beginning (Fig. 6a) to the end of the photobleaching test (Fig. 6b). That is, the QD605-labeled oocysts were highly photostable even after 5 min of continuous illumination.
FIG. 4.
Photostability comparison between FITC-labeled and QD605-labeled oocysts (n = 3).
FIG. 5.
Fluorescence image of FITC-labeled C. parvum at initial illumination (a) and after 5 min of continuous illumination (b).
FIG. 6.
Fluorescence image of QD605-labeled C. parvum at initial illumination (a) and after 5 min of continuous illumination (b).
DISCUSSION
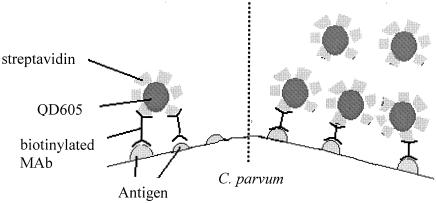
QD nanocrystals have been coupled to streptavidin directly through a carbodiimide-mediated coupling reaction. This preparation procedure yields a material with a high loading of streptavidin on the surface (i.e., typically 15 to 25 streptavidin units per QD conjugate) (3). Thus, below the QD saturation concentration, one QD is capable of bridging multiple biotinylated MAb sites (as shown in Fig. 7, left). However, if the saturation concentration of QD605-streptavidin is used, the dominant mode will be one QD conjugate per biotinylated MAb site (as shown in Fig. 7, right). The number of C. parvum oocysts detected increased with increasing concentrations of QD605-streptavidin. This was expected, since more available biotinylated MAb sites would be occupied with QD605-streptavidin. Thus, it is necessary to use the QD605-streptavidin at saturation conditions to avoid the phenomenon of cross-linking with biotinylated MAb. It is noted in this study that the saturation concentration of QD605-streptavidin was about 20 nM, above which the increase in QD605-streptavidin concentration would not further enhance the detection of oocysts. This is not surprising, as all of the available biotinylated MAb sites would be occupied by QD605-streptavidin when a higher than saturation concentration is used. Thus, the number of oocysts detected should remain constant regardless of the additional QD605-streptavidin concentration being added. A similar observation had also been reported by Goldman et al. (1) when an increasing concentration of QD-MAb 2b (an anti-staphylococcal enterotoxin antibody) was used to determine the concentration of toxins. They noted that the fluorescent signal increased with increasing concentrations of QD-MAb 2b conjugate until saturation, which occurred above 120 nM.
FIG. 7.
Schematic diagrams of various scenarios when different QD605 streptavidin concentrations are used. (Left) Low concentration; (right) saturation concentration.
The detection of various C. parvum oocyst concentrations showed that 20 nM QD605-streptavidin with biotinylated MAb has a detection sensitivity that was comparable to that of the FITC-MAb conjugate. It was also noted in this study that 20 nM QD605-streptavidin with biotinylated MAb was able to provide sensitive C. parvum oocyst detection in environmental water samples such as reservoir water. The optimum QD605-streptavidin concentration determined in this study will provide an accurate quantification of the oocysts with a detection sensitivity that was comparable to that of the EPA method 1622 for C. parvum oocysts detected in aliquots of up to 4,395 oocysts.
The comparison between the photostability of FITC-labeled and QD605-labeled oocysts indicated that QD605 has a high photobleaching threshold, as its fluorescent signal intensity remained unchanged throughout the 5-min continuous illumination period (with 90% glycerol in PBS as the antifade mounting medium). In contrast, the FITC-labeled oocysts were noted to photobleach quickly even with the protection of antifade mounting medium. A similar comparison between the photostability of QD608-streptavidin and an organic fluorescent dye was conducted by Wu et al. (11). They noted that Alexa 488-streptavidin with specimens mounted with antifade mounting medium retained only about 55% of its initial intensity while 97% of the initial intensity was detected with QD608-streptavidin at the end of 3 min of illumination. In this study, the fluorescence intensity of FITC decreased to 36.8% ± 10.4% of its initial intensity after 3 min of continuous illumination. This observation showed that FITC has a higher photobleaching rate than Alexa 488 while the QD605 used in this study was slightly more photostable than the QD608 used in Wu et al.'s study (11). Sukhanova et al. (8) reported that the QD used in the immunolabeling of p-glycoprotein, a cell membrane protein, was 4,200- and 420-fold more resistance to photobleaching than its labeling with FITC and Alexa fluor 488 (organic fluorophores), respectively. The results collectively indicated that QD is highly photostable compared with organic florescence dyes. This study demonstrated that 20 nM QD605-streptavidin with biotinylated MAb was able to provide a comparably sensitive detection method for C. parvum oocysts with the advantage of a high photobleaching threshold. In addition, this study has also shown the possible application of QD for the detection of waterborne pathogens such as C. parvum oocysts, which is of significant importance for public heath safety in terms of drinking water supplies.
Acknowledgments
This work was supported by the National University of Singapore under research fund R-264-000-161-112.
REFERENCES
- 1.Goldman, E. R., G. P. Anderson, P. T. Tran, H. Mattoussi, P. T. Charles, and J. M. Mauro. 2002. Conjugation of luminescent quantum dots with antibodies using an engineered adaptor protein to provide new reagents for fluoroimmunoassays. Anal. Chem. 74:841-847. [DOI] [PubMed] [Google Scholar]
- 2.Goldman, E. R., E. D. Balighian, M. K. Kuno, S. Labrenz, P. T. Tran, G. P. Anderson, J. M. Mauro, and H. Mattoussi. 2002. Luminescent quantum dot-adaptor protein-antibody conjugates for use in fluoroimmunoassay. Phys. Stat. Sol. B 229:407-414. [Google Scholar]
- 3.Jaiswal, J. K., H. Mattoussi, J. M. Mauro, and S. M. Simon. 2003. Long term multiple color imaging of live cells using quantum dots bioconjugates. Nat. Biotechnol. 21:47-51. [DOI] [PubMed] [Google Scholar]
- 4.Mackenzie, W. R., N. J. Hoxie, M. E. Proctor, M. S. Gradus, K. A. Blair, D. E. Peterson, J. J. Kazmierczak, D. G. Addiss, K. R. Fax, J. B. Rose, and J. P. Davis. 1994. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N. Engl. J. Med. 331:161-167. [DOI] [PubMed] [Google Scholar]
- 5.Mattoussi, H., J. M. Mauro, E. R. Goldman, G. P. Anderson, V. C. Sundar, and M. G. Bawendi. 2000. Self-assembly of CdSe-ZnS quantum dot bioconjugates using an engineered recombinant protein. J. Am. Chem. Soc. 122:12142-12150. [Google Scholar]
- 6.Quantum Dot. 2002. Qdot 605 streptavidin conjugate user manual. Quantum Dot, Hayward, Calif.
- 7.Smith, H. V., and A. Ronald. 2001. Cryptosporidium: the analytical challenge, p. 1-43. In M. Smith and K. C. Thompson (ed.), Cryptosporidium: the analytical challenge. The Royal Society of Chemistry, Cambridge, United Kingdom.
- 8.Sukhanova, A., J. Devey, L. Venteo, H. Kaplan, M. Artemyev, V. Oleinikov, D. Klinov, M. Pluot, J. H. M. Cohen, and I. Nabiev. 2004. Biocompatible fluorescent nanocrystals for immunolabeling of membrane proteins and cells. Anal. Biochem. 324:60-67. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Environmental Protection Agency. 1999. Cryptosporidium in water by filtration, immunomagnetic separation, immunomagnetic separation, and fluorescent antibody. Publication EPA-821-R-99-001. Office of Water, U.S. Environmental Protection Agency, Washington, D.C.
- 10.Watson, A., X. Wu, and M. Bruchez. 2003. Lighting up cells with quantum dots. BioTechniques 34:296-302. [DOI] [PubMed] [Google Scholar]
- 11.Wu, X., H. Liu, J. Liu, K. N. Haley, J. A. Treadway, J. P. Larson, N. Ge, F. Peale, and M. P Bruchez. 2003. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat. Biotechnol. 21:41-46. [DOI] [PubMed] [Google Scholar]