Abstract
Observations in enrichment cultures of ferric iron-reducing bacteria indicated that ferrihydrite was reduced to ferrous iron minerals via sulfur cycling with sulfide as the reductant. Ferric iron reduction via sulfur cycling was investigated in more detail with Sulfurospirillum deleyianum, which can utilize sulfur or thiosulfate as an electron acceptor. In the presence of cysteine (0.5 or 2 mM) as the sole sulfur source, no (microbial) reduction of ferrihydrite or ferric citrate was observed, indicating that S. deleyianum is unable to use ferric iron as an immediate electron acceptor. However, with thiosulfate at a low concentration (0.05 mM), growth with ferrihydrite (6 mM) was possible and sulfur was cycled up to 60 times. Also, spatially distant ferrihydrite in agar cultures was reduced via diffusible sulfur species. Due to the low concentrations of thiosulfate, S. deleyianum produced only small amounts of sulfide. Obviously, sulfide delivered electrons to ferrihydrite with no or only little precipitation of black iron sulfides. Ferrous iron and oxidized sulfur species were produced instead, and the latter served again as the electron acceptor. These oxidized sulfur species have not yet been identified. However, sulfate and sulfite cannot be major products of ferrihydrite-dependent sulfide oxidation, since neither compound can serve as an electron acceptor for S. deleyianum. Instead, sulfur (elemental S or polysulfides) and/or thiosulfate as oxidized products could complete a sulfur cycle-mediated reduction of ferrihydrite.
Microorganisms have been recognized as important catalysts for many geochemical transformation reactions. In complex natural systems, transformation reactions of one element often drive transformations of other elements. One important example of such complex geochemical interactions are the reactions between iron and sulfur species and the numerous compounds they can form; in addition, their transformations have further impact on the transformation or mobilization of other elements such as manganese, phosphorus, trace elements, or heavy metals (12).
In particular, in marine habitats which are rich in sulfate (28 mM in seawater), the formation of iron mono- and disulfides is taken as an indicator of the activity of sulfate-reducing bacteria, since it is known that they can reduce iron indirectly by producing sulfide, which reduces ferric iron oxides chemically. The sulfidation of ferric iron oxides initiates a complex reaction sequence that results in the formation of various iron monosulfides and iron disulfides including pyrite, which is the most abundant stable iron sulfide (10, 12, 15). In contrast, the production of siderite (ferrous iron carbonate) or vivianite (ferrous iron phosphate) from ferric iron oxides is attributed exclusively to the direct enzymatic reduction by ferric iron-reducing bacteria (6).
Since the complex process of sulfidation of ferric iron oxides is surface controlled, it is not surprising that the nature of the products depends on the type of ferric iron oxide used, its surface area, and the concentrations of iron and sulfide. Despite the importance of sulfidation of ferric iron oxides, the products of sulfide oxidation were determined experimentally only in a few studies which focused on the kinetics and mechanisms of the process. Depending on the concentrations of the reactants, iron sulfides are not necessarily the (major) products. In studies on the sulfidation of hematite (α-Fe2O3), sulfate, thiosulfate, and traces of sulfite were detected as products but were not quantified in detail (8). In contrast, sulfidation experiments with goethite (α-FeOOH) produced elemental sulfur as the main product (∼86%) of sulfide oxidation; the production of sulfur was explained as a consequence of the structure of goethite that is thought to favor a two-electron exchange (14). Unfortunately, sulfidation reactions with ferrihydrite, an important, poorly crystallized iron oxide, were investigated in only one study with gaseous hydrogen sulfide. In this study, the only products under anoxic conditions were iron monosulfides (∼67%) and elemental sulfur (∼33%) (5). Since the protonation of surface absorption sites on ferric iron oxides plays an important role in aqueous systems, it has yet to be determined how much reactions in aqueous systems have in common with gas-solid reactions (7). In addition, it is likely that in more complex environments and in laboratory culture media other compounds such as carbonates or phosphates influence and further complicate the reactions between sulfide and iron.
In the present study, we demonstrate that Sulfurospirillum deleyianum, which is known as a sulfur-reducing bacterium and is unable to reduce ferric iron directly, can grow by reduction of ferrihydrite via sulfur cycling with no or with only little precipitation of iron sulfides. For efficient electron cycling, a small sulfur pool was crucial. This sulfur-dependent ferrihydrite reduction may be of importance in freshwater habitats and in transition zones where the pool of biologically available sulfur is small.
MATERIALS AND METHODS
Sources of bacteria.
Samples for enrichment cultures were collected from ditches and littoral sediment of Lake Constance, near the city of Constance, Germany. S. deleyianum (DSM 6946T) was obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany.
Medium composition and growth conditions.
Techniques for preparation of media and cultivation of bacteria under anoxic conditions have been described elsewhere (22). In the present study, a defined, bicarbonate-buffered freshwater medium was used which contained (per liter of distilled water) 0.3 g of NH4Cl, 0.025 g of MgSO4 · 7H2O, 0.4 g of MgCl2 · 6H2O, 0.6 g of KH2PO4, and 0.1 g of CaCl2 · 2H2O. After autoclaving and cooling under an atmosphere of 80% N2-20% CO2, 30 ml of NaHCO3 solution (1 M, autoclaved under CO2), vitamins, a nonchelated mixture of trace elements, and a selenite and tungstate solution were added (22). The pH was adjusted to 7.0.
Enrichment cultures were supplied with ferrihydrite (5 to 10 mM) as an electron acceptor; a mixture of acetate (5 to 10 mM), propionate (1 to 2 mM), and butyrate (1 to 2 mM) as possible electron donors and carbon sources; and ferrous chloride (2 mM) as a reducing agent.
Cultures of S. deleyianum were supplied with formate (10 mM) as the electron donor and acetate (5 mM) as the carbon source. Since S. deleyianum is unable to assimilate sulfate, either l-cysteine, l-cystine, or thiosulfate was added as the sulfur source. In many experiments, cysteine (2 mM) was also used as a reducing agent. All experiments were inoculated from cultures that had been grown with 20 mM fumarate and 0.5 mM cysteine.
Cultures were incubated at 28°C in the dark. For cultures growing with iron, tubes were incubated horizontally and shaken every other day to allow for homogeneous distribution of bacteria and iron minerals.
Agar dilution series were prepared as described by Widdel and Bak (22). For experiments with enrichment cultures, they were supplied with acetate, propionate, butyrate, and biologically produced ferrihydrite (10 mM). Agar dilutions inoculated with S. deleyianum were supplied with formate, acetate, cysteine, and either biologically produced ferrihydrite (10 mM) or fumarate (5 mM) as the electron acceptor; thiosulfate (0.05 mM) was added to some of the ferrihydrite-containing agar dilutions.
Microbial and synthetic production of ferrihydrite.
The electron acceptor 2-line ferrihydrite was generated by allowing a lithotrophic, nitrate-reducing enrichment culture to oxidize ferrous iron (18, 19). The produced ferrihydrite was washed five times with a 10-fold volume of distilled water to remove medium components. The resulting ferrihydrite suspension was deoxygenated by stirring under N2 and repeated flushing of the headspace in a tightly sealed flask. The suspension was autoclaved and stored in the same tightly sealed flask under N2. Biologically produced ferrihydrite was used only in the agar dilution series. Presumably due to its small particle size (1 to 2 nm) (19), it mixes better with the agar than synthetically produced ferrihydrite (8 to 20 nm) (19). Synthetic ferrihydrite was produced as described by Lovley and Phillips (11). The suspension of synthetically produced ferrihydrite was deoxygenated, sterilized, and stored in the same manner as described above for the biologically produced ferrihydrite.
Analytical methods.
Ferrous iron was quantified photometrically at 562 nm after reaction with ferrozine (17). Immediately before sampling, cultures were agitated to disperse iron precipitates homogeneously. Samples were taken with anoxic syringes and were immediately acidified by 10-fold dilution in 1 M HCl. The concentration of ferric iron was determined in the same way after reduction with 0.28 M hydroxyl ammonium chloride; the ferrous iron concentration determined before reduction was subtracted.
Sulfide concentrations were determined colorimetrically by methylene blue formation reaction in a microassay (1).
For quantification of thiol groups, a photometric assay was used which was based on Ellmann's reagent (9); concentrations were calculated with l-cysteine as the standard.
Protein contents were determined with Coomassie brilliant blue G-250 (4) and bovine serum albumin fraction V as the calibration standard.
RESULTS AND DISCUSSION
Considerations on ferrihydrite concentrations.
In most studies on microbial ferrihydrite reduction in batch cultures, elevated concentrations of ferrihydrite (typically 100 mM) were used. Such elevated ferrihydrite concentrations impart a dark brown coloration to the medium. The typical end product of microbial ferrihydrite reduction under these conditions is magnetite, a black iron oxide. Hence, the color of the medium changes from dark brown to black. Possible other iron mineral phases formed either transiently or as end product(s) may therefore be masked and overlooked. The reason for magnetite formation under these conditions has still not been definitively established. Since even slightly alkaline conditions favor formation of magnetite, a rise of pH in batch cultures may play a key role (3). Also, accumulation of rather high amounts of ferrous iron in batch cultures may favor magnetite formation. However, magnetite is not necessarily the end product of bacterial ferrihydrite reduction: when low concentrations of ferrihydrite (5 to 10 mM) are used, ferrihydrite can be reduced completely to the ferrous state. This was demonstrated, for example, with ferrihydrite of biological origin and different Geobacter species, but it also applies to synthetically produced ferrihydrite (19; K. L. Straub, unpublished data). Medium containing 5 to 10 mM ferrihydrite is orange-brown in color. Concomitant with complete reduction of ferrihydrite, the color of the iron precipitates changes because ferrous iron forms precipitates presumably with carbonate and phosphate, which are white in this range of concentrations.
Enrichment cultures for ferric iron-reducing bacteria.
Six enrichment cultures for ferric iron-reducing bacteria were started with inocula from ditches and littoral sediment of Lake Constance, close to the city of Constance. A mixture of acetate, propionate, and butyrate was added to the medium as electron donors and/or carbon sources, and ferrihydrite (5 to 10 mM) was supplied as the electron acceptor. To minimize the growth of sulfate-reducing bacteria, which were most likely present in the sample material (2), only 0.05 mM sulfate was included in the medium as a sulfur source. Complete reduction of ferrihydrite was observed in all enrichment cultures, and they were transferred repeatedly at intervals of 1 to 2 weeks. Even after repeated transfers, the whitish ferrous minerals that formed during ferrihydrite reduction were accompanied in all enrichment cultures by a small amount of black precipitate giving the ferrous iron precipitates a gray color. These ferrous iron precipitates disintegrated immediately in 1 M HCl into ferrous iron and sulfide, indicating that they contained ferrous sulfides mixed with siderite and vivianite rather than magnetite.
A possible production of electron-shuttling compounds or iron chelators was checked in these enrichment cultures with ferrihydrite embedded in 1% agar (20). When agar dilution cultures with ferrihydrite were inoculated from the fifth transfers, agar in the lower dilutions completely turned in color from orange-brown to whitish gray. In higher dilutions, round white zones appeared in the orange-brown agar. The color change from orange-brown to whitish gray or white indicated that ferrihydrite was completely reduced. Again, the gray color of the agar disappeared within minutes if pieces of this agar were smashed and mixed with 1 M HCl; this indicated again the presence of low concentrations of ferrous sulfides rather than magnetite. This could be explained if sulfate-reducing bacteria were enriched that reduced ferrihydrite by producing and excreting electron-shuttling compounds. A possible alternative explanation for the origin of the whitish gray or white precipitates is a mechanism involving ferrihydrite reduction via sulfur cycling by sulfate-reducing bacteria.
To explore a possible reduction of ferrihydrite to siderite and vivianite via sulfur cycling with sulfide as the reductant in a defined culture, experiments with S. deleyianum were started. Routinely, experiments in this study were inoculated from cultures of S. deleyianum that had been grown with fumarate (20 mM) as the sole substrate and cysteine (0.5 mM) as the sulfur source.
Sulfur sources and sulfur-containing electron acceptors for S. deleyianum.
S. deleyianum had been isolated as a sulfur-reducing bacterium unable to dissimilate or assimilate sulfate (16, 23). Because it was important for understanding the role of sulfur cycling in bacterial reduction of ferrihydrite, the known abilities of S. deleyianum to use certain sulfur sources and sulfur-containing electron acceptors were retested. In accordance with earlier studies, S. deleyianum was able to grow on fumarate with cysteine, sulfide, or thiosulfate, but not with sulfate, as the sulfur source (16, 23). In addition, growth was also observed in the presence of cystine as the sole sulfur source. As described earlier, S. deleyianum grew well with sulfur or thiosulfate as the electron acceptor but not with sulfate; sulfur and thiosulfate were reduced to sulfide (16, 23). If supplied as an electron acceptor, cystine was reduced but only at very low rates. In contrast to earlier observations, we found no assimilation or dissimilation of sulfite. In toxicity tests with fumarate as the sole substrate and cysteine as the sulfur source, S. deleyianum showed good growth in the presence of up to 1 mM sulfite, but no growth was observed in the presence of 5 or 10 mM sulfite. No growth was observed with sulfite as the sole sulfur source in experiments with 0.05, 0.1, or 1 mM sulfite from freshly prepared stock solutions. No growth was observed with either 0.5 or 1 mM sulfite as an electron acceptor. These observations indicate that the S. deleyianum culture had lost its capacity to assimilate or dissimilate sulfite.
Growth experiments with S. deleyianum and ferrihydrite.
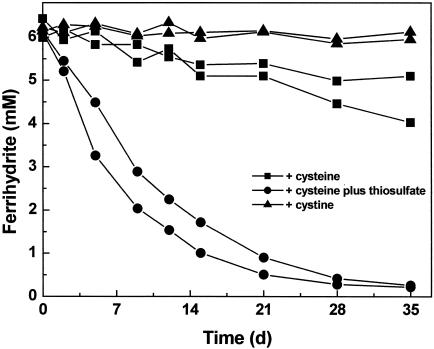
We determined in an initial experiment whether S. deleyianum could reduce ferrihydrite directly. In growth experiments with ferrihydrite as an electron acceptor and cystine (0.5 mg ml−1) as a sulfur source, no reduction of ferrihydrite was observed within 5 weeks (Fig. 1). As already mentioned, S. deleyianum can use cystine as an electron acceptor. Because we observed no ferrihydrite reduction upon addition of cystine, we ruled out the possibility that cystine (together with cysteine) can act as an efficient electron shuttle in the reduction of ferrihydrite by S. deleyianum.
FIG. 1.
Ferrihydrite reduction in growth experiments with S. deleyianum supplied with acetate, formate, ferrihydrite, and 0.01% inoculum. One of the following was added: cysteine (2 mM), cysteine (2 mM) plus thiosulfate (0.05 mM), or cystine (0.5 mg ml−1). The inoculum had been grown with fumarate as the substrate and cysteine as the sulfur source. Means of two determinations are shown for duplicate experiments.
In exploring the possibility of ferrihydrite reduction by sulfur cycling, we added thiosulfate at various concentrations. We also varied the size of the inoculum. When thiosulfate concentrations exceeded 0.05 mM and the inoculum size was greater than 0.01%, we observed precipitation of black ferrous sulfides. This formation of ferrous sulfides depleted the pool of free sulfur. However, more ferrihydrite was reduced than could be recovered as ferrous iron, suggesting that sulfur had cycled between iron and bacterial cells about three to six times. The nature of the produced ferrous sulfides was not further investigated. Rickard had previously identified a complex mixture of both iron monosulfides (mackinawite, pyrrhotite, and greigite) and iron disulfides (pyrite and marcasite) in cultures of Desulfovibrio desulfuricans, which were grown with sulfate as an electron acceptor in the presence of ferrous or ferric iron (15).
With low concentrations of thiosulfate (0.05 mM) and a small inoculum size (0.01%), ferrihydrite was reduced only partially, but no or only little ferrous sulfides were precipitated. The experimental conditions were improved by adding a reducing agent such as cysteine (2 mM), ascorbate (2 mM), or dithiothreitol (1 mM). The addition of a reducing agent probably helped to trap traces of oxygen and protected the small amounts of sulfide from oxidation by oxygen, which may have leaked into the tubes, for example, during sampling. Oxidation of sulfide with oxygen to sulfite or sulfate would decrease the pool of sulfur compounds available for S. deleyianum. Since cysteine can also serve as a sulfur source for S. deleyianum, we chose it as a reducing agent for the majority of our experiments. In the presence of both 0.05 mM thiosulfate and 2 mM cysteine, the complete reduction of ferrihydrite (5 to 6 mM) was observed in growth experiments within 3 to 4 weeks (Fig. 1; see also Fig. 3 and 4). Thus, in these growth experiments sulfur was cycled up to 60 times. In control experiments supplied solely with cysteine (2 mM), only a small amount of ferrihydrite was reduced, which can be attributed to a chemical reaction of cysteine with ferrihydrite (Fig. 1). S. deleyianum actually grew by sulfur cycle-mediated reduction of ferrihydrite as described above: the bacterium was successfully transferred five times consecutively, with an inoculum size of 0.01% each time, and approximately 12 μg of protein per ml was detected in each of these transfers.
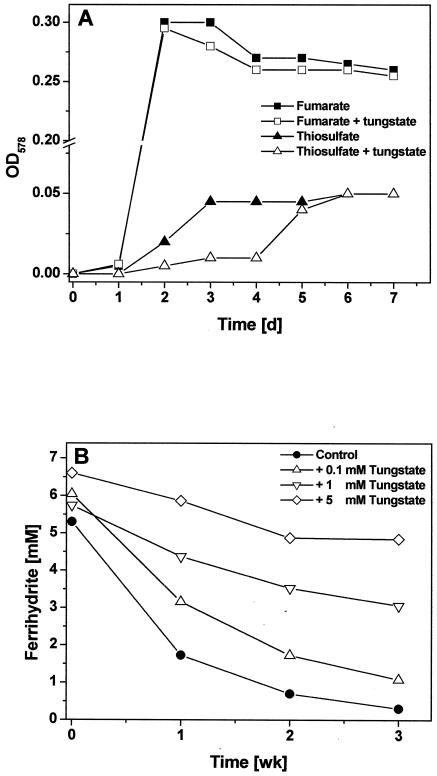
FIG. 3.
Growth experiments with S. deleyianum supplied with acetate, formate, cysteine, and 0.01% inoculum. (A) Growth with fumarate (20 mM) or thiosulfate (2 mM) as electron acceptor in the absence or presence of tungstate (5 mM). Data from representative cultures are shown. (B) Ferrihydrite reduction in growth experiments supplied with ferrihydrite and thiosulfate. Except for the control experiment, different concentrations of tungstate were added. Means of duplicate determinations are shown for representative cultures.
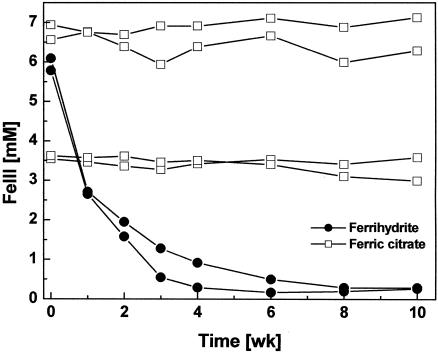
FIG. 4.
Ferric iron reduction in growth experiments with S. deleyianum supplied with acetate, formate, cysteine, and 0.01% inoculum. Either ferrihydrite or ferric citrate was added as the electron acceptor; cultures with ferrihydrite were also supplied with thiosulfate (0.05 mM). Means of two determinations are shown for duplicate experiments.
Our growth experiments with S. deleyianum showed that small amounts of an appropriate sulfur source are sufficient to catalyze indirect reduction of ferrihydrite via sulfur cycling. Therefore, it is important to consider the culture conditions and the size of an inoculum when testing a strain for its ability to reduce ferric iron oxides.
Test for reduction of spatially distant ferrihydrite.
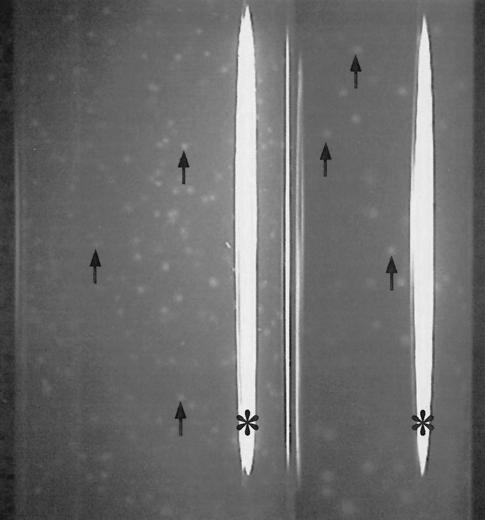
To check whether ferrihydrite that was not in direct contact with S. deleyianum cells could be reduced by diffusible reduced sulfur species, we prepared dilutions of S. deleyianum in agar medium containing biologically produced ferrihydrite (20). After approximately 10 days of incubation, ferrihydrite reduction was observed only when thiosulfate (0.05 mM) was added to the medium. At lower dilutions, the ferrihydrite-containing agar changed color completely from orange-brown to whitish gray, whereas white zones were formed at higher dilutions (Fig. 2). The color change indicated a complete reduction of the agar-embedded ferrihydrite. The white zones at higher dilutions were initially tiny but eventually grew to a size of up to 0.5 cm in diameter after incubation for 6 to 10 weeks. Because of precipitation of ferrous iron minerals, the zones in the agar were white and opaque. Therefore, the size of a colony inside such a white zone could not be determined. When agar dilutions were prepared with 5 mM fumarate as an electron acceptor, S. deleyianum formed very tiny, discrete colonies, indicating that cells of S. deleyianum were too big to swim through pores of medium solidified with 1% agar. Unfortunately, precise ratios between concentrations of electron acceptors and sizes of colonies cannot be given because, unlike ferrihydrite, fumarate can diffuse through the agar (20).
FIG. 2.
Tubes from agar dilutions inoculated with S. deleyianum after 3 weeks of incubation. The medium was solidified with 1% agar and contained acetate, formate, biologically produced ferrihydrite, cysteine, and thiosulfate (0.05 mM). Arrows point to white zones, which indicate ferrihydrite reduction. Note, white stripes (*) originate from flash light reflections.
The similarity between agar dilutions inoculated with S. deleyianum and identical ones inoculated with the ferrihydrite-reducing enrichment cultures described above was striking. It suggested that in some enrichment cultures ferrihydrite reduction may indeed proceed via sulfur cycling. However, the system with ferrihydrite-containing agar does not allow a distinction between sulfur cycle-mediated ferrihydrite reduction and alternative electron transfer processes involving production and excretion of electron-shuttling or iron-chelating compounds. These questions can be addressed only with pure cultures isolated from these enrichment cultures.
Inhibition by tungstate and molybdate.
Tungstate and molybdate are known to inhibit sulfate reduction by sulfate-reducing bacteria; they have been shown to act as antimetabolites of sulfate (for examples, see references 13 and 21). Since they also bear some stereochemical similarity to thiosulfate, tungstate or molybdate may also act as a competitive inhibitor for enzymes involved in the utilization of thiosulfate. We first determined whether tungstate was toxic to S. deleyianum. We found the organism to grow well in the presence of up to 20 mM tungstate if fumarate was supplied as an electron acceptor and cysteine was supplied as a sulfur source. In experiments with thiosulfate as an electron acceptor and cysteine as a sulfur source, the addition of tungstate slowed the reduction of thiosulfate considerably (Fig. 3A). In growth experiments with ferrihydrite, tungstate also impaired the reduction of ferrihydrite (initiated by thiosulfate reduction), but the inhibition was incomplete (Fig. 3B). These results further indicate that S. deleyianum reduces ferrihydrite only indirectly and that thiosulfate may play a role in the observed sulfur cycling. Results from similar experiments with molybdate were not conclusive, since the addition of molybdate (5 to 20 mM) impaired the growth of S. deleyianum no matter whether fumarate or thiosulfate was supplied as the electron acceptor in the presence of cysteine as the sulfur source (data not shown).
Growth experiments with S. deleyianum and ferric citrate.
All ferrihydrite-reducing bacteria tested so far can also reduce ferric citrate, which is a soluble ferric iron complex. Growth experiments with ferric citrate at two different concentrations (3.5 and 7 mM) indicated again that S. deleyianum could not reduce ferric iron oxides directly. It did not reduce ferric citrate directly during 10 weeks of incubation, whereas in a control experiment, it reduced ferrihydrite completely within 4 weeks by sulfur cycling (Fig. 4).
Sulfur cycle-mediated reduction of ferric iron oxides.
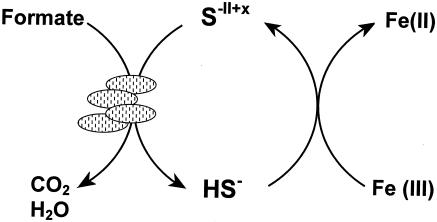
Our observations with S. deleyianum can be summarized in the model depicted in Fig. 5. The cycling was initiated with 0.05 mM thiosulfate, which S. deleyianum used as an electron acceptor for the oxidation of formate. Because of the low concentration of thiosulfate used, only small amounts of sulfide were produced. The low concentration of sulfide favored the delivery of electrons to ferrihydrite without precipitation of ferrous mono- or disulfides. Instead, ferrous iron and oxidized sulfur species were produced. Ferrous iron formed white precipitates, presumably with carbonate and/or phosphate, which were present in the medium. The oxidized sulfur species have not yet been identified. However, due to the limited metabolic capacity of S. deleyianum, sulfate and sulfite cannot be major products of sulfide oxidation by ferrihydrite. Neither sulfate nor sulfite can serve as an electron acceptor for S. deleyianum. Their accumulation would therefore quickly deplete the pool of sulfur compounds available for sulfur cycling. Instead, sulfur and/or thiosulfate as products could complete a sulfur cycle that mediates reduction of ferrihydrite.
FIG. 5.
Proposed model for sulfur cycle-mediated reduction of ferrihydrite, which was initiated with 0.05 mM thiosulfate. The oxidized sulfur species (S−II+x) have not been identified yet.
In contrast to the observations with S. deleyianum, Geobacter bremensis, Geobacter pelophilus, and Geobacter sulfurreducens reduced ferrihydrite in consecutive transfers that were prepared with medium that contained cysteine as the sole sulfur source (K. L. Straub, unpublished data).
Implications.
In our experiments with ferrihydrite-containing agar, we showed that ferrihydrite not in physical contact with bacteria was available as an electron acceptor to them because of the presence of soluble sulfur species that can act as electron shuttles between the electron donor and ferrihydrite. This transfer of electrons through water-soluble reduced carriers may be of major importance in the reduction of ferric iron oxides in natural habitats because they help to bridge distances between bacteria and ferric iron oxides, which are not evenly distributed in natural environments. In freshwater environments with a small pool of available sulfur, for example, bacteria may funnel electrons to abundant reservoirs of ferric oxides by means of sulfur cycling. The ecological importance of sulfur cycle-mediated ferric iron reduction needs to be determined in further studies.
Acknowledgments
We thank Bernhard Wehrli (Kastanienbaum, Switzerland) for helpful advice with respect to iron chemistry.
This work was financed by the European Commission through the PURE project (EVK1-CT-1999-00030).
REFERENCES
- 1.Aeckersberg, F., F. Bak, and F. Widdel. 1991. Anaerobic oxidation of saturated hydrocarbons to CO2 by a new type of sulfate-reducing bacterium. Arch. Microbiol. 156:5-14. [Google Scholar]
- 2.Bak, F., and N. Pfennig. 1991. Sulfate-reducing bacteria in littoral sediment of Lake Constance. FEMS Microbiol. Ecol. 85:43-52. [Google Scholar]
- 3.Bell, P. E., A. L. Mills, and J. S. Herman. 1987. Biogeochemical conditions favoring magnetite formation during anaerobic iron reduction. Appl. Environ. Microbiol. 53:2610-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Cantrell, K. J., S. B. Yabusaki, M. H. Engelhard, A. V. Mitroshkov, and E. C. Thornton. 2003. Oxidation of H2S by iron oxides in unsaturated conditions. Environ. Sci. Technol. 37:2192-2199. [DOI] [PubMed] [Google Scholar]
- 6.Coleman, M. L., D. B. Hedrick, D. R. Lovley, D. C. White, and K. Pye. 1993. Reduction of Fe(III) in sediments by sulphate-reducing bacteria. Nature 361:436-438. [Google Scholar]
- 7.Davydov, A., K. T. Chuang, and A. R. Sanger. 1998. Mechanism of H2S oxidation by ferric oxide and hydroxide surfaces. J. Phys. Chem. B 102:4745-4752. [Google Scholar]
- 8.dos Santos Afonso, M., and W. Stumm. 1992. Reductive dissolution of iron(III) (hydr)oxides by hydrogen sulfide. Langmuir 8:1671-1675. [Google Scholar]
- 9.Lange, B., and Z. J. Vejdelek. 1980. Photometrische analyse. VCH Verlagsgesellschaft, Weinheim, Germany.
- 10.Lennie, A. R., and D. J. Vaughan. 1996. Spectroscopic studies of iron sulfide formation and phase relations at low temperatures, p. 117-131. In M. D. Dyar, C. McCammon, and M. W. Schaefer (ed.), Mineral spectroscopy: a tribute to Roger G. Burns. The Geochemical Society, St. Louis, Mo.
- 11.Lovley, D. R., and E. J. P. Phillips. 1986. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 51:683-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morse, J. W., F. J. Millero, J. C. Cornwell, and D. Rickard. 1987. The chemistry of the hydrogen sulfide and iron sulfide systems in natural waters. Earth Sci. Rev. 24:1-42. [Google Scholar]
- 13.Newport, P. J., and D. B. Nedwell. 1988. The mechanisms of inhibition of Desulfovibrio and Desulfotomaculum species by selenate and molybdate. J. Appl. Bacteriol. 65:419-423. [Google Scholar]
- 14.Pyzik, A. J., and S. E. Sommer. 2004. Sedimentary iron monosulfides: kinetics and mechanism of formation. Geochim. Cosmochim. Acta 45:687-698. [Google Scholar]
- 15.Rickard, D. T. 1969. The microbial formation of iron sulphides. Stockholm Contrib. Geol. 20:49-66. [Google Scholar]
- 16.Schumacher, W., P. M. H. Kroneck, and N. Pfennig. 1992. Comparative systematic study on “Spirillum” 5175, Campylobacter and Wolinella species. Arch. Microbiol. 158:287-293. [Google Scholar]
- 17.Stookey, L. L. 1970. Ferrozine—a new spectrophotometric reagent for iron. Anal. Chem. 42:779-781. [Google Scholar]
- 18.Straub, K. L., M. Benz, B. Schink, and F. Widdel. 1996. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl. Environ. Microbiol. 62:1458-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Straub, K. L., M. Hanzlik, and B. E. E. Buchholz-Cleven. 1998. The use of biologically produced ferrihydrite for the isolation of novel iron-reducing bacteria. Syst. Appl. Microbiol. 21:442-449. [DOI] [PubMed] [Google Scholar]
- 20.Straub, K. L., and B. Schink. 2003. Evaluation of electron-shuttling compounds in microbial ferric iron reduction. FEMS Microbiol. Lett. 220:229-233. [DOI] [PubMed] [Google Scholar]
- 21.Taylor, B. F., and R. S. Oremland. 1979. Depletion of ATP in Desulfovibrio by oxyanions of group VI elements. Curr. Microbiol. 3:101-103. [Google Scholar]
- 22.Widdel, F., and F. Bak. 1992. Gram-negative mesophilic sulfate-reducing bacteria, p. 3352-3378. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, 2nd ed. Springer, Berlin, Germany.
- 23.Wolfe, R. S., and N. Pfennig. 1977. Reduction of sulfur by Spirillum 5175 and syntrophism with Chlorobium. Appl. Environ. Microbiol. 33:427-433. [DOI] [PMC free article] [PubMed] [Google Scholar]