Abstract
Genomic analysis of the hyperthermophilic archaeon Pyrococcus furiosus revealed the presence of an open reading frame (ORF PF1939) similar to the enzymes in glycoside hydrolase family 13. This amylolytic enzyme, designated PFTA (Pyrococcus furiosus thermostable amylase), was cloned and expressed in Escherichia coli. The recombinant PFTA was extremely thermostable, with an optimum temperature of 90°C. The substrate specificity of PFTA suggests that it possesses characteristics of both α-amylase and cyclodextrin-hydrolyzing enzyme. Like typical α-amylases, PFTA hydrolyzed maltooligosaccharides and starch to produce mainly maltotriose and maltotetraose. However, it could also attack and degrade pullulan and β-cyclodextrin, which are resistant to α-amylase, to primarily produce panose and maltoheptaose, respectively. Furthermore, acarbose, a potent α-amylase inhibitor, was drastically degraded by PFTA, as is typical of cyclodextrin-hydrolyzing enzymes. These results confirm that PFTA possesses novel catalytic properties characteristic of both α-amylase and cyclodextrin-hydrolyzing enzyme.
The recent advent of genomic research has produced vast amounts of sequence information for many different taxa of Bacteria, Eukarya, and Archaea, now collected in databases such as GenBank; the full genome sequences of more than 140 different microorganisms have been completed. With a generally applicable combination of conventional genetic engineering and genomic research techniques, many extremely thermostable enzymes are being developed for biotechnological purposes. The genome sequences of some hyperthermophilic microorganisms, such as Thermotoga maritima, Pyrococcus furiosus, and Sulfolobus solfataricus, are of considerable biotechnological interest because they encode many highly heat-stable enzymes that are active under conditions previously regarded as incompatible with biological materials (20, 25). Amylolytic enzymes are of great significance in many industrial processes, including those for foods, textiles, and detergents. Many hyperthermophilic microorganisms possess starch-hydrolyzing enzymes, such as α-amylase, α-glucosidase, pullulanase, and cyclodextrinase, in their genomes even though they live in environments where starch is rare (23).
Analysis of the full genome of P. furiosus, a hyperthermophilic archaeon, has revealed that this microorganism has several amylolytic enzymes. An amylopullulanase and two distinct α-amylase genes of P. furiosus were identified and expressed in E. coli. These enzymes can hydrolyze a wide variety of substrates, such as soluble starch, amylose, amylopectin, glycogen, and oligosaccharides. However, α-amylase does not hydrolyze pullulan and cyclodextrin, whereas amylopullulanase can degrade pullulan (7, 8).
The multiple sequence alignment of amylolytic enzymes from P. furiosus revealed some interesting features of the gene (PF1939) homologous with those for various cyclodextrin-hydrolyzing enzymes such as cyclodextrinase, maltogenic amylase, and neopullulanase in glycoside hydrolase family 13. Therefore, in this study, the gene corresponding to a putative amylolytic enzyme of P. furiosus (referred to PFTA hereafter) was cloned and expressed in E. coli. In addition, the recombinant enzyme was purified and its enzymatic characteristics were examined and compared with those of α-amylase and cyclodextrin-hydrolyzing enzyme. Herein, we provide evidence that the putative amylolytic enzyme of P. furiosus is a novel amylase, possessing characteristics of both cyclodextrin-hydrolyzing enzyme and α-amylase.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
E. coli MC1061 [F− araD139 recA13 (araABC-leu)7696 galU galK lacX74 rpsL thi hsdR2 mcrB] and BL21(DE3) (Novagen Inc., Madison, Wis.) were used as hosts for gene cloning and expression, respectively. Plasmid pET-22b(+) (Novagen) was used as a cloning and expression vector. E. coli cultures were grown at 37°C either on Luria-Bertani (LB) agar containing 1% (wt/vol) Bacto-tryptone, 0.5% (wt/vol) yeast extract, and 1% (wt/vol) NaCl, or in LB broth supplemented with ampicillin (100 μg/ml) or chloramphenicol (34 μg/ml) if needed.
General DNA manipulation, DNA sequencing, and computer analysis.
The genomic DNA of P. furiosus DSM 3638 was kindly provided by A. M. Grunden of North Carolina State University (Raleigh, N.C.). In order to obtain the recombinant enzyme, the pfta gene was amplified with PCR with the Pfu DNA polymerase (Roche Molecular Biochemicals, Mannheim, Germany) and the genomic DNA of P. furiosus as a template. The pfta gene-specific oligonucleotide primers flanking the 5′ and 3′ gene ends were designed from the known pfta sequence. The forward primer (PFTA-Fnd, 5′-CAATTGCTACACATATGTATAAGCTCG-3′) and the reverse primer (PFTA-Rxo, 5′-GCGGATGGGTACTCGAGGAGGCACCACCT-3′) contained NdeI and XhoI restriction sites, respectively (italic). Amplification was performed at an annealing temperature of 55°C. The DNA fragment (2 kb) amplified by PCR was digested with NdeI and XhoI and ligated into the expression vector pET-22b(+) to finally create pETPFTA-6h. In the final construction, the 3′ end of the pfta gene product was extended with eight additional amino acid residues (Leu, Glu, and six His residues). The nucleotide sequence of the PCR-generated gene was determined with the BigDye terminator cycle sequencing kit for the ABI 377 Prism (Perkin-Elmer, Norwalk, Conn.). The other genetic manipulations were performed as described by Sambrook et al. (24). The initial similarity search was carried out with the BLAST program (1). Detailed analyses of the DNA and the deduced amino acids of various genes were performed with the DNASIS, PROSIS (v7.0, Hitachi Software, Tokyo, Japan), and CLUSTAL programs (27).
Purification of the recombinant PFTA enzyme.
The E. coli BL21(DE3) transformant harboring pETPFTA-6h was grown in LB broth supplemented with 100 μg of ampicillin per ml at 37°C until the optical density at 600 nm reached 0.6 and then induced with 1 mM isopropylthiogalactopyranoside (IPTG) for 3 h. The cells were harvested by centrifugation (7,000 × g for 30 min at 4°C) and resuspended in lysis buffer [50 mM Tris-HCl buffer (pH 7.0) containing 300 mM NaCl, and 10 mM imidazole]. An extract of the E. coli transformant was obtained after sonication (4 × 5 min, output control 4, 50% duty cycle; VC-600, Sonics & Materials, Newtown, Conn.) followed by heat treatment at 70°C for 20 min to eliminate endogenous heat-labile proteins of E. coli. After centrifugation (10,000 × g for 30 min at 4°C), the supernatant was collected, and the recombinant PFTA was purified with nickel-nitrilotriacetic acid (Ni-NTA) affinity column chromatography (Qiagen, Hilden, Germany) (Table 1).
TABLE 1.
Steps in purification of the recombinant PFTAa
| Purification step | Total vol (ml) | Total activity (U) | Total protein (mg) | Sp act (U/mg)b | Yield (%) | Purifi- cation (-fold) |
|---|---|---|---|---|---|---|
| Cell extraction | 150 | 3,904 | 723 | 5.4 | 100 | 1 |
| Heat treatment | 140 | 3,389 | 128 | 27.5 | 87 | 5.1 |
| Ni-NTA affinity chromatography | 9.5 | 1,623 | 9.2 | 176.4 | 42 | 32.6 |
The starting materials were 10.2 g of IPTG-induced wet cells (1.5-liter cultures).
One unit of enzyme activity was defined as the amount of enzyme that split 1 μmol equivalent of glycosidic bonds in the substrate (β-cyclodextrin) in 1 min under standard assay conditions.
The molecular mass of the recombinant PFTA was estimated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) with a 10% (wt/vol) gel. Activity staining of PFTA was performed on the gel. The gel was soaked into 0.5% (wt/vol) Triton X-100 solution to remove SDS for 30 min with gentle agitation. After washing the gel twice, it was incubated in 1% (wt/vol) soluble starch in 50 mM sodium acetate buffer (pH 4.5) at 85°C for 30 min. The gel was then washed with distilled water and finally stained with iodine solution [50% (vol/vol) methanol, 1% (wt/vol) iodine, and 10% (wt/vol) potassium iodide] for 5 min at room temperature. The appearance of a clear zone on a dark-blue background was associated with PFTA activity. The protein concentration was determined according to the Bradford method (4), with bovine serum albumin as a standard.
Enzyme assay.
The activity of PFTA was assayed at 90°C in 50 mM sodium acetate buffer (pH 4.5) with 3,5-dinitrosalicylic acid, as described by Miller (18). The reaction mixture was composed of 150 μl of 1% (wt/vol) β-cyclodextrin solution as a substrate and 150 μl of enzyme solution. The mixture was incubated at 90°C for 10 min to facilitate the enzymatic reaction, and then the reaction was terminated by quenching on ice. One unit of enzyme activity was defined as the amount of enzyme that split 1 μmol equivalent of glycosidic bonds in the substrate in 1 min under the reaction conditions.
Effects of pH and temperature on the activity and stability of PFTA.
For determining the optimal pH of PFTA, the relative activities of PFTA against β-cyclodextrin in various pHs ranging from 3 to 8 were examined. The buffers used were as follows: 50 mM sodium citrate buffer (pH 3.0 to 4.0); 50 mM sodium acetate buffer (pH 4.0 to 6.0); 50 mM sodium phosphate buffer (pH 6.0 to 7.5); and 50 mM Tris-HCl buffer (pH 7.5 to 8.0). To examine the pH stability, the enzyme (0.1 mg/ml) was incubated in various pH solutions made with 0.1 M Britton-Robinson buffer (22). After incubation at 37°C for 24 h, the remaining activity was measured at the optimal temperature under the standard conditions described above. The optimal temperature of PFTA activity was determined in 50 mM sodium acetate buffer (pH 4.5) in a range from 55 to 110°C. The thermostability of the purified enzyme, which was dissolved in 50 mM sodium acetate buffer (pH 5.0), was analyzed by incubating the enzyme solution (0.1 mg/ml) at different temperatures (85, 90, 95, and 100°C), from which aliquots were taken at various time points and placed immediately in an ice-water bath. The residual β-cyclodextrin-hydrolyzing activities of the aliquots were measured at the optimal temperature condition.
Differential scanning calorimetry was carried out on a Nano-Cal differential scanning calorimeter (Calorimetry Sciences Corp., Provo, Utah), operating over a temperature range of 25 to 125°C. The sample cell was pressurized to 3.0 atm to prevent boiling at temperatures of >100°C. Purified enzyme was dialyzed against 50 mM sodium phosphate buffer (pH 7.0) and concentrated to 1 mg/ml in 50 mM sodium phosphate buffer (pH 7.0) with a Microcon filter (Millipore Co.). The equilibrated enzyme was scanned at a rate of 1.0°C/min. The enzyme scan was corrected with a buffer-buffer baseline.
Hydrolytic patterns of PFTA.
In order to examine the hydrolytic pattern of PFTA, 0.5 ml of purified PFTA was incubated with 0.5 ml of 1.0% (wt/vol) substrate in 50 mM sodium acetate buffer (pH 4.5) at 90°C for various lengths of time. The substrates used were β-cyclodextrin, maltooligosaccharides (maltotriose to maltoheptaose), p-nitrophenyl-α-d-maltopentaoside, pullulan, soluble starch, and acarbose. Acarbose was obtained from Bayer (Leverkusen, Germany). The reaction products were analyzed with thin-layer chromatography on Whatman K5F silica gel plates (Whatman, Maidstone, United Kingdom) with isopropyl alcohol-ethyl acetate-water (3:1:1, vol/vol/vol) as the solvent system. After irrigating twice, the thin-layer chromatography plate was dried and visualized by dipping it into a solution containing 0.3% (wt/vol) N-(1-naphthyl)-ethylenediamine and 5% (vol/vol) H2SO4 in methanol and then heating it for 10 min at 110°C (23).
Kinetic parameters of PFTA.
Kinetic parameters of PFTA for cyclodextrins, soluble starch, maltotriose, and acarbose were determined. The sample (50 μl) from the reaction mixture containing PFTA and substrate in 50 mM sodium acetate buffer (pH 4.5) at 90°C were taken at intervals of 90 s, and then the reaction was immediately stopped by adding an equal volume of 0.1 M HCl on ice. After neutralizing by adding an equal volume of 0.1 N NaOH, the amount of glucose released from acarbose or maltotriose was assayed by the glucose oxidase-peroxidase method (19). The amount of reducing sugars produced from cyclodextrins (α-, β-, and γ-cyclodextrin) or soluble starch (average molecular weight, 10,000) was measured by the copper-bicinchoninate method (9). Kinetic data were transformed to Lineweaver-Burk plots with the SigmaPlot program (version 5.0; SPSS Inc., Chicago, Ill.). The Km values were calculated from the slopes of the curves, and the catalytic turnover values (kcat) were calculated by dividing the maximal reaction velocities by the total amount of enzyme in the reaction mixture.
RESULTS
Comparison of PFTA with various amylolytic enzymes.
It was expected that the open reading frame of PFTA (1,938 bp) would encode a single polypeptide of 645 amino acids with an estimated molecular mass of 76,084 Da. The analysis of the PFTA gene showed that it was highly homologous with various cyclodextrin-hydrolyzing enzymes such as maltogenic amylase, cyclodextrinase, and neopullulanase (21). The predicted amino acid sequence of PFTA shared 55% identity with that of cyclodextrinase from Thermococcus sp. strain B1001 (10), 24% with that of neopullulanase from Bacillus stearothermophilus (15), and 24% with that of maltogenic amylase from Thermus sp. strain IM6501 (12). However, it exhibited unexpectedly low sequence homology with other amylolytic enzymes, including α-glucosidase, α-amylase, and pullulanase.
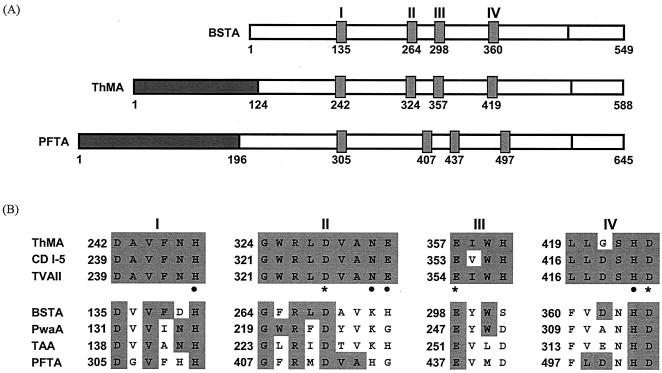
The multiple amino acid sequence alignments revealed some interesting features of PFTA. It contained an extra N-terminal domain (13), composed of 190 amino acid residues, which is absent from α-amylase. This N-terminal domain is known to exist in cyclodextrin-hydrolyzing enzymes and is involved in the oligomerization of the enzyme. However, the length of the amino acid chain in the N-terminal domain of PFTA was longer (Fig. 1A) than that of cyclodextrin-hydrolyzing enzymes. In addition, although four well-known conserved regions of glycoside hydrolase family 13 and the invariant catalytic residues were evident (5), there were some variations in amino acid composition in the conserved regions of PFTA (Fig. 1B).
FIG. 1.
Comparison of the primary structures and homologous regions of PFTA and related enzymes. (A) Schematic drawing of the primary structures and the four conserved regions of Bacillus stearothermophilus α-amylase, Thermus malogenic amylase, and PFTA. The N-terminal domains of Thermus malogenic amylase and PFTA are represented as dark boxes. Other boxes represent the four conserved regions in the amylolytic enzymes. (B) Comparison of amino acid residues in the conserved regions (I, II, III, and IV) of various amylolytic enzymes such as maltogenic amylase, cyclodextrinase, neopullulanase, and α-amylases. Invariant or highly conserved amino acids are emphasized by shaded boxes. In cyclodextrin- and pullulan-hydrolyzing enzymes, the catalytic amino acid residues are indicated by asterisks and substrate-binding amino acids are indicated by dots. BSTA, B. stearothermophilus α-amylase (1713273A); ThMA, Thermus sp. strain IM6501 maltogenic amylase (AAC15072); CD I-5, alkalophilic Bacillus sp. strain I-5 cyclodextrinase (AAA92925); TVAII, Thermoactinomyces vulgaris R-47 α-amylase II (1911217A); PwaA, Pyrococcus woesei α-amylase (AAD54338); TAA, Aspergillus oryzae α-amylase (CAA31218).
Expression of PFTA in E. coli.
At first, the expression of the pfta gene in the recombinant E. coli strain harboring pETPFTA-6h was inefficient. It has been pointed out that insufficient expression of archaeal genes in E. coli might be due to the frequent use of codons that are rarely used in E. coli, such as AGA and AGG for arginine, AUA for isoleucine, and GGA for glycine (6, 17, 28). Such rare codons could particularly cause problems when the codons are arranged in clusters or close to one another. In fact, in the pfta gene, AGA and AGG for arginine (38 out of 40 times), AUA for isoleucine (17 out of 47 times), and GGA for glycine (23 out of 44 times) are frequently used.
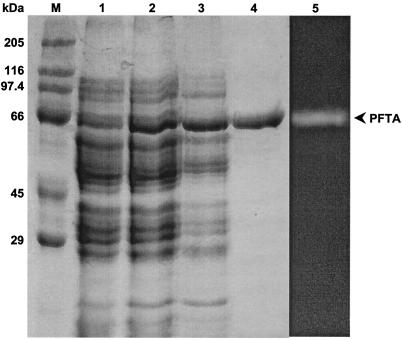
To overcome the codon bias of the pfta gene and enhance protein expression in E. coli, the pRARE plasmid (Novagen) containing extra tRNAs for six rare codons, AUA, AGG, AGA, CUA, CCC, and GGA, was introduced into the E. coli BL21(DE3) transformants harboring pETPFTA-6h. As a result, the expression of the pfta gene was enhanced, and a significant amount of the recombinant protein was obtained (Fig. 2, lanes 1 and 2). This result implies that the poor expression of the pfta gene in E. coli is mainly caused by inefficient translation due to codon bias. The recombinant PFTA was successfully purified by a convenient two-step procedure. The cell extract was heated to 70°C for 20 min to remove considerable amounts of E. coli proteins. Later, the recombinant PFTA was efficiently purified 32.6-fold by Ni-NTA affinity chromatography. In SDS-PAGE, a single band of purified 70-kDa protein appeared (Fig. 2, lane 4). However, matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF MS; Voyager-DE STR Biospectrometry Workstation, Applied Biosystems, Inc., Foster City, Calif.) spectra indicated that the enzyme had a molecular mass of 77,163 Da, which was close to the deduced molecular mass of 77,149 Da (data not shown).
FIG. 2.
SDS-PAGE analysis of recombinant PFTA at different stages of purification. Lane M, protein size standards; lane 1, cellular proteins from crude extract (pETPFTA-6h); lane 2, cellular proteins from crude extract (pETPFTA-6h and pRARE); lane 3, proteins after heat treatment; lane 4, purified PFTA after Ni-NTA column chromatography; lane 5, zymogram analysis with purified PFTA. PFTA was visualized in a zymogram developed by soaking the gel in 1% (wt/vol) soluble starch solution at 85°C followed by iodine solution (7). The enzyme activity was detected as a white band on the gel.
Enzymatic properties of PFTA.
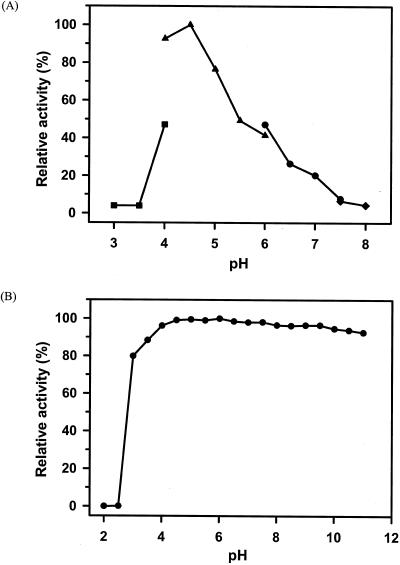
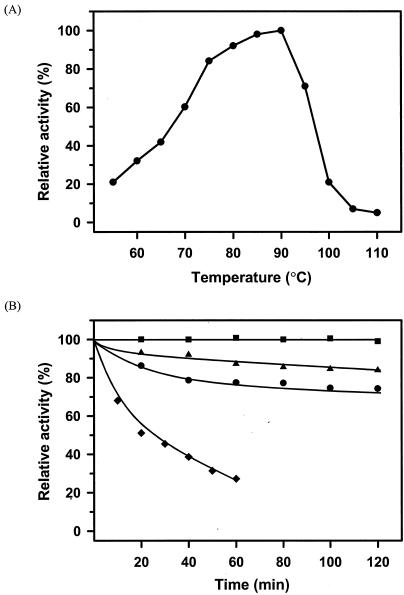
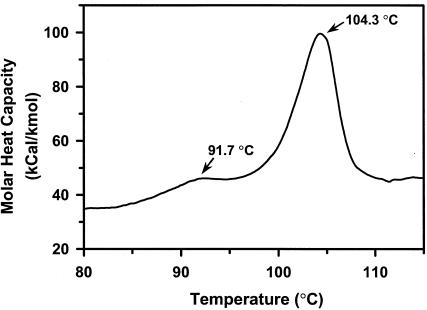
The range of pHs at which recombinant PFTA was active and stable was determined with β-cyclodextrin as a substrate. As shown in Fig. 3A, the maximum activity of PFTA was observed at pH 4.5. More than 50% of the maximum activity was retained in the range between pH 4.0 and pH 5.5. In addition, PFTA was shown to be stable in the broad range between pH 3 and pH 11 (Fig. 3B). The optimal temperature of PFTA was about 90°C (Fig. 4A). As expected, PFTA exhibited a remarkable thermal stability by retaining its full activity after 120 min of incubation at 85°C. The half-life of PFTA was determined to be 398 and 114 min at 90 and 95°C, respectively. More than 27% of the enzyme activity remained after 1 h of incubation at 100°C. Differential scanning calorimetry analysis of PFTA was performed to determine the melting temperature (Tm) of PFTA. The result showed that the Tm of PFTA was 104.3°C, confirming that this enzyme is highly thermostable (Fig. 5). In addition, there was a small endothermic peak at a lower temperature (Tm = 91.7°C), which might have been generated by a monomeric form of the enzyme. This indicated that the recombinant enzyme from P. furiosus was successfully expressed in E. coli.
FIG. 3.
Effect of pH on the activity and stability of PFTA. (A) For the determination of optimal pH, the following buffers were used: for pH 3.0 to 4.0, 50 mM sodium citrate (▪); for pH 4.0 to 6.0, 50 mM sodium acetate (▴); for pH 6.0 to 7.5, 50 mM sodium phosphate (•); and for pH 7.5 to 8.0, 50 mM Tris-HCl (♦). The values are shown as percentages of the maximum specific activity of PFTA observed at pH 4.5, which was taken as 100%. (B) To assess the pH stability of PFTA, the enzyme was incubated at the indicated pH in 0.1 M Britton-Robinson buffer and at 37°C for 24 h. The residual activity was measured at 90°C under the standard conditions of the assay. The values are shown as percentages of the original activity, which was taken as 100%.
FIG. 4.
Effect of temperature on the activity and stability of PFTA. (A) Activity was measured at the temperatures indicated on the plot in the standard activity assay (in a water bath for measurements up to 90°C or in a silicon oil bath for measurements between 90 and 110°C). The values are shown as percentages of the specific activity of PFTA observed at 90°C, which was taken as 100%. (B) For determination of the thermostability of PFTA, purified enzyme (0.1 mg/ml) was incubated at 85°C (▪), 90°C (▴), 95°C (•), and 100°C (♦) in 50 mM sodium acetate buffer (pH 5.0). After various time intervals as indicated on the plot, samples were withdrawn, and the residual activity was measured at 90°C under the standard conditions of the assay.
FIG. 5.
Differential scanning calorimetry of the recombinant PFTA. The recombinant PFTA was concentrated to 1 mg/ml in 50 mM sodium phosphate buffer (pH 7.0) with a Microcon filter (Millipore Corp.). The sample was scanned at temperatures from 25 to 125°C with a scan rate of 1°C/min.
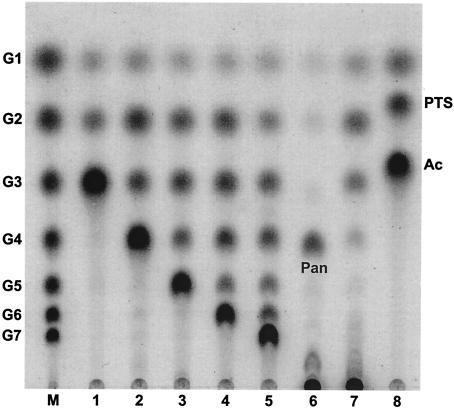
The enzymatic activity of PFTA was examined with various substrates typically used for analyzing the enzymatic activity of cyclodextrin-hydrolyzing enzymes, including soluble starch, β-cyclodextrin, maltooligosaccharides, pullulan, and acarbose (Fig. 6). PFTA hydrolyzed maltooligosaccharides and soluble starch, typical substrates for α-amylase, to produce maltose, maltotriose, and maltotetraose (Fig. 6, lanes 1 to 4, 7). The hydrolysis pattern of PFTA toward these substrates was different from that of cyclodextrin-hydrolyzing enzyme, which predominantly produces maltose from the same substrates. In addition, PFTA could attack pullulan and β-cyclodextrin, which are recalcitrant to degradation by α-amylase. Thin-layer chromatography analysis showed that PFTA hydrolyzed pullulan to panose and β-cyclodextrin to various maltooligosaccharides (G1 to G7), with maltoheptaose as a major product (Fig. 6, lanes 5 and 6). Acarbose, a potent α-amylase inhibitor, could be degraded to acarviosine-glucose and glucose by PFTA (Fig. 6, lane 8).
FIG. 6.
Hydrolysis pattern of PFTA on various substrates. Lane M, maltooligosaccharide standards (glucose to maltoheptaose); lane 1, maltotriose; lane 2, maltotetraose; lane 3, maltopentaose; lane 4, maltohexaose; lane 5, β-cyclodextrin; lane 6, pullulan; lane 7, soluble starch; lane 8, acarbose (Ac). Pan, panose; PTS, acarviosine-glucose. PFTA was reacted with various substrates at a concentration of 0.5% (wt/vol) at 90°C for 5 h.
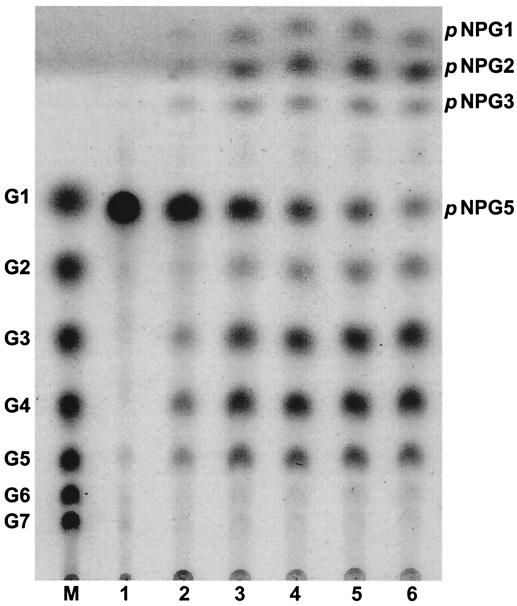
In order to understand the detailed reaction mode of PFTA, the hydrolysis pattern was investigated as a function of time with p-nitrophenyl-α-d-maltopentaoside as a substrate (Fig. 7). It was observed that p-nitrophenyl-α-d-maltopentaoside was initially degraded into maltotetraose, maltotriose, p-nitrophenyl-α-d-glucoside(pNPG1), and p-nitrophenyl-α-d-maltoside (pNPG2), suggesting that the products were mainly released from the reducing end of p-nitrophenyl-α-d-maltopentaoside. The kinetic parameters Km and kcat of PFTA were determined with α-cyclodextrin, β-cyclodextrin, γ-cyclodextrin, maltotriose, acarbose, and starch with Lineweaver-Burk plots (Table 2). The Km values of PFMA for α-cyclodextrin and β-cyclodextrin were 2.61 and 2.16 mM, respectively, whereas that for γ-cyclodextrin was about two times higher. The kcat/Km of PFTA towards maltotriose was 4.3 s−1 mM−1, suggesting that maltotriose is not a favorable substrate for PFTA. The transglycosylation activity normally found in cyclodextrin-hydrolyzing enzymes was not detected in PFTA (data not shown).
FIG. 7.
Change of the hydrolysis product of p-nitrophenyl-α-d-maltopentaoside (pNPG5) by PFTA as a function of reaction time. Lane M, maltooligosaccharide standards (glucose to maltoheptaose); lanes 1-6, hydrolysis product of p-nitrophenyl-α-d-maltopentaoside at different reaction times (0, 0.5, 1, 1.5, 2, and 4 h, respectively). PFTA was reacted with 0.5% (wt/vol) p-nitrophenyl-α-d-maltopentaoside at 90°C.
TABLE 2.
Kinetic parameters for hydrolysis of various substratesa
| Substrate | kcat (s−1) | Km (mM) | kcat/Km (s−1 mM−1) |
|---|---|---|---|
| α-Cyclodextrin | 241 ± 6 | 2.61 ± 0.17 | 92.3 |
| β-Cyclodextrin | 196 ± 5 | 2.16 ± 0.11 | 90.7 |
| γ-Cyclodextrin | 173 ± 4 | 5.12 ± 0.42 | 33.8 |
| Maltotriose | 268 ± 8 | 62.9 ± 3.8 | 4.3 |
| Acarbose | 228 ± 6 | 11.1 ± 1.1 | 20.5 |
| Starchb | 67 ± 1 | 0.52 ± 0.02 | 128.8 |
Kinetic parameters were determined in 50 mM sodium acetate buffer (pH 4.5) at 90°C.
Km was calculated from the number-average molecular weight.
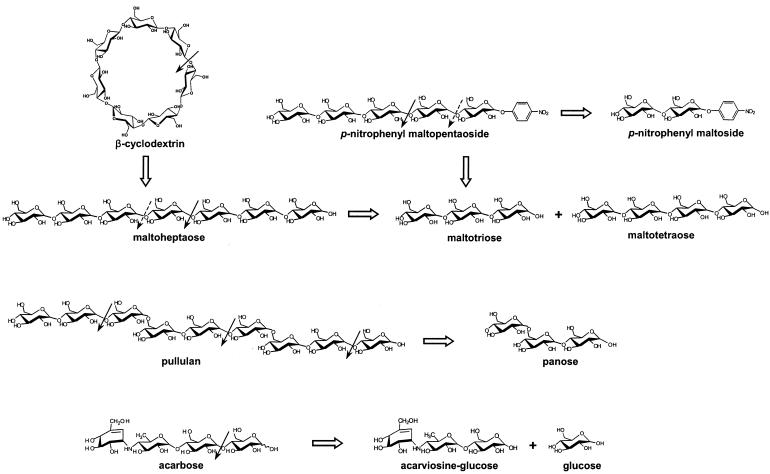
The proposed action pattern of PFTA is summarized in Fig. 8. In conclusion, PFTA showed the activities of both a cyclodextrin-hydrolyzing enzyme and an α-amylase. PFTA demonstrated the ability to hydrolyze the typical substrates of cyclodextrin-hydrolyzing enzymes without showing transglycosylation activity, but the degradation products were similar to those typical of α-amylase. This indicated that PFTA could be an intermediate enzyme located in the middle of the evolutionary process between cyclodextrin-hydrolyzing enzyme and α-amylase.
FIG. 8.
Proposed modes of action of PFTA on various substrates.
DISCUSSION
The cyclodextrin-hydrolyzing enzymes are a group of enzymes belonging to glycoside hydrolase family 13. This group of enzymes is capable of hydrolyzing cyclodextrins, pullulan, and soluble starch, in contrast to typical α-amylases, which hydrolyze only starch. The cyclodextrin-hydrolyzing enzymes include maltogenic amylase, cyclodextrinase, and neopullulanase (16). These enzymes exhibit a high level of amino acid sequence identity (40 to 60%) and broad substrate specificity for cyclodextrins, pullulan, and soluble starch. These properties distinguish this type of enzyme from other glycoside hydrolase family 13 members such as isoamylase, and pullulanase.
In many aspects, PFTA resembles cyclodextrin-hydrolyzing enzymes: PFTA showed a broad range of substrate specificity toward cyclodextrins, pullulan, and soluble starch, it could also degrade acarbose, a strong α-amylase inhibitor, and PFTA possesses the N-terminal domain which exists in cyclodextrin-hydrolyzing enzymes but not in α-amylase. However, in terms of catalytic action, PFTA was quite different from cyclodextrin-hydrolyzing enzymes. Unlike cyclodextrin-hydrolyzing enzymes, which are known to produce mainly maltose from β-cyclodextrin and soluble starch, PFTA liberated various small maltooligosaccharides (G1 to G7) from those substrates. The overall comparisons of cyclodextrin-hydrolyzing enzyme, α-amylase, and PFTA are listed in Table 3.
TABLE 3.
Overall comparisons of cyclodextrin-hydrolyzing enzyme, PFTA, and α-amylasea
| ThMA | PFTA | BSTA | |
|---|---|---|---|
| Action mode | Maltose from reducing end | Random | Random |
| Major product(s) from: | |||
| Maltooligosaccharide | G2 | G3, G4 | G2≈G5 |
| Pullulan | Panose | Panose | N.D. |
| β-Cyclodextrin | G2 | G7 | N.D. |
| Acarbose | PTS | PTS | N.D. |
| Length of N-terminal domain (amino acids) | 124 | 196 | N.A. |
| Oligomeric state | Dimer | Dimer | Monomer |
| Optimal temp (°C) | 60 | 90 | 80 |
| Optimal pH | 6.0 | 4.5 | 5.5 |
ThMA, maltogenic amylase from Thermus sp. strain IM6501; PFTA, thermostable amylase from P. furiosus, BSTA, thermostable α-amylase from B. stearothermophilus (29). N.D., not detected; N.A., not available.
In addition, PFTA was not capable of performing the transglycosylation reaction, which cyclodextrin-hydrolyzing enzymes typically can perform. Kim et al. reported that Glu332 in Thermus malogenic amylase played an important role in the formation and accumulation of transfer products by modulating the relative rates of hydrolysis and transglycosylation (2, 14). In PFTA, Gly415 was located in the position corresponding to Glu332 in Thermus malogenic amylase. This might explain why transglycosylation activity was not observed for this enzyme. Also, the amino acid compositions in the four conserved regions in PFTA were relatively different from those in cyclodextrin-hydrolyzing enzymes and rather close to those of typical α-amylases (Fig. 1). Possibly, not only the invariant amino acid in the catalytic domain but also the amino acid composition adjacent to the preserved amino acid in each of the four conserved regions are related to the catalytic pattern of PFTA.
Superposition of the three-dimensional structure of Thermus malogenic amylase (11) and Bacillus stearothermophilus α-amylase (26) revealed that the (β/α)8-barrel and the C-terminal domains were conserved, whereas the N-terminal domain (≈124 amino acids) of Thermus malogenic amylase was not found in B. stearothermophilus α-amylase. PFTA has an N-terminal domain of about 190 amino acids, which is significantly longer than that of Thermus malogenic amylase. Moreover, the two N-terminal sequences show very low sequence similarity to each other. Deletion and swapping of this N-terminal region are under investigation to elucidate the role of the N-terminal domain in PFTA.
Acknowledgments
This study was partially supported by the Korean Ministry of Science and Technology through the 21st Century Frontier R&D Programs in Microbial Genomics & Applications Center (grant no. MG02-0301-004-2-2-0). We acknowledge the financial support of the Brain Korea 21 Project in the form of scholarships to S.-J. Yang.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Baek, J. S., M. J. Kim, H. J. Cha, H. S. Lee, D. Li, J. W. Kim, Y. R. Kim, T. W. Moon, and K. H. Park. 2003. Enhanced transglycosylation activity of Thermus maltogenic amylase in acetone solution. Food Sci. Biotechnol. 12:639-643. [Google Scholar]
- 3.Bibel, M., C. Brettl, U. Gosslar, G. Kriegshäuser, and W. Liebl. 1998. Isolation and analysis of genes for amylolytic enzymes of the hyperthermophilic bacterium Thermotoga matitima. FEMS Microbiol. Lett. 158:9-15. [DOI] [PubMed] [Google Scholar]
- 4.Bradford, M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Cha, H. J., H. G. Yoon, Y. W. Kim, H. S. Lee, J. W. Kim, K. S. Kweon, B. H. Oh, and K. H. Park. 1998. Molecular and enzymatic characterization of novel maltogenic amylase that hydrolyzes and transglycosylates acarbose. Eur. J. Biochem. 253:251-262. [DOI] [PubMed] [Google Scholar]
- 6.Dalgaard, J. Z., and R. A. Garrett. 1993. Archaeal hyperthermophile genes, p. 535-563. In M. Kates, D. J. Kusher, and A. T. Matheson (ed.), The biochemistry of Archaea (Archaebacteria). Elsevier, Amsterdam, The Netherlands.
- 7.Dong, G., C. Vieille, A. Savchenko, and J. G. Zeikus. 1997. Cloning, sequencing, and expression of the gene encoding extracellular α-amylase from Pyrococcus furiosus and biochemical characterization of the recombinant enzyme. Appl. Environ. Microbiol. 63:3569-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong, G., C. Vieille, and J. G. Zeikus. 1997. Cloning, sequencing, and expression of the gene encoding amylopullulanase from Pyrococcus furiosus and biochemical characterization of the recombinant enzyme. Appl. Environ. Microbiol. 63:3577-3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox, J. D., and J. F. Robyt. 1991. Miniaturization of three carbohydrate analyses using a microsample plate reader. Anal. Biochem. 195:93-96. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto, Y., T. Yamamoto, S. Fujiwara, M. Takagi, and T. Imanaka. 2001. Extracellular synthesis, specific recognition, and intracellular degradation of cyclomaltodextrins by the hyperthermophilic archaeon Thermococcus sp. strain B1001. J. Bacteriol. 183:5050-5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, J. S., S. S. Cha, H. J. Kim, T. J. Kim, N. C. Ha, S. T. Oh, H. S. Cho, M. J. Cho, M. J. Kim, H. S. Lee, J. W. Kim, K. Y. Choi, K. H. Park, and B. H. Oh. 1999. Crystal structure of a maltogenic amylase provides insights into a catalytic versatility. J. Biol. Chem. 274:26279-26286. [DOI] [PubMed] [Google Scholar]
- 12.Kim, T. J., M. J. Kim, B. C. Kim, J. C. Kim, T. K. Cheong, J. W. Kim, and K. H. Park. 1999. Modes of action of acarbose hydrolysis and transglycosylation catalyzed by a thermostable maltogenic amylase, the gene for which was cloned from a Thermus strain. Appl. Environ. Microbiol. 65:1644-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim, T. J., V. D. Nguyen, H. S. Lee, M. J. Kim, H. Y. Cho, Y. W. Kim, T. W. Moon, C. S. Park, J. W. Kim, B. H. Oh, S. B. Lee, B. Svensson, and K. H. Park. 2001. Modulation of the multisubstrate specificity of Thermus maltogenic amylase by truncation of the N-terminal domain and by a salt-induced shift of the monomer/dimer equilibrium. Biochemistry 40:14182-14190. [DOI] [PubMed] [Google Scholar]
- 14.Kim, T. J., C. S. Park, H. Y. Cho, S. S. Cha, J. S. Kim, S. B. Lee, T. W. Moon, J. W. Kim, B. H. Oh, and K. H. Park. 2000. Role of the glutamate 332 residue in the transglycosylation activity of Thermus maltogenic amylase. Biochemistry 39:6773-6780. [DOI] [PubMed] [Google Scholar]
- 15.Kuriki, T., and T. Imanaka. 1989. Nucleotide sequence of the neopullulanase gene from Bacillus stearothermophilus. J. Gen. Microbiol. 135:1521-1528. [DOI] [PubMed] [Google Scholar]
- 16.Lee, H. S., M. S. Kim, H. S. Cho, J. I. Kim, T. J. Kim, J. H. Choi, C. Park, H. S. Lee, B. H. Oh, and K. H. Park. 2002. Cyclomaltodextrinase, neopullulanase, and maltogenic amylase are nearly indistinguishable from each other. J. Biol. Chem. 277:21891-21897. [DOI] [PubMed] [Google Scholar]
- 17.Martin, S. L., B. Vrhovski, and A. S. Weiss. 1995. Total synthesis and expression in Escherichia coli of a gene encoding human tropoelastin of special interest. Gene 154:159-166. [DOI] [PubMed] [Google Scholar]
- 18.Miller, G. L. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31:426-428. [Google Scholar]
- 19.Miwa, I., J. Okudo, K. Maeda, and G. Okuda. 1972. Mutarotase effect on colorimetric determination of blood glucose with D-glucose oxidase. Clin. Chim. Acta 37:538-540. [DOI] [PubMed] [Google Scholar]
- 20.Nelson, K. E., R. A. Clayton, S. R. Gill, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, W. C. Nelson, K. A. Ketchum, L. McDonald, T. R. Utterback, J. A. Malek, K. D. Linher, M. M. Garrett, A. M. Stewart, M. D. Cotton, M. S. Pratt, C. A. Phillips, D. Richardson, J. Heidelberg, G. G. Sutton, R. D. Fleischmann, J. A. Eisen, O. White, S. L. Salzberg, H. O. Smith, J. C. Venter, and C. M. Fraser. 1999. Evidence for lateral gene transfer between Archaea and Bacteria from genome sequence of Thermotoga maritima. Nature 399:323-329. [DOI] [PubMed] [Google Scholar]
- 21.Park, K. H., T. J. Kim, T. K. Cheong, J. W. Kim, B. H. Oh, and B. Svensson. 2000. Structure, specificity and function of cyclomaltodextrinase, a multispecific enzyme of the α-amylase family. Biochim. Biophys. Acta 1478:165-185. [DOI] [PubMed] [Google Scholar]
- 22.Rauen, H. M. 1964. Biochemisches Taschenbuch, vol. 2, p. 90-104. Springer Verlag, Berlin, Germany.
- 23.Robyt, J. F., and R. Mukerjea. 1994. Separation and quantitative determination of nanogram quantities of maltodextrins and isomaltodextrins by thin-layer chromatography. Carbohydr. Res. 251:187-202. [Google Scholar]
- 24.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Schiraldi, C., and M. De Rosa. 2002. The production of biocatalysts and biomolecules from extremophiles. Trends Biotechnol. 20:515-521. [DOI] [PubMed] [Google Scholar]
- 26.Suvd, D., Z. Fujimoto, K. Takase, M. Matsumura, and H. Mizuno. 2001. Crystal structure of Bacillus stearothermophilus α-amylase: possible factors determining the thermostability. J. Biochem. 129:461-468. [DOI] [PubMed] [Google Scholar]
- 27.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tito, B. J. J., J. M. Ward, J. Hodgson, C. J. Gershater, H. Edwards, L. A. Wysocki, F. A. Watson, G. Sathe, and J. F. Kane. 1995. Effects of a minor isoleucyl tRNA on heterologous protein translation in Escherichia coli. J. Bacteriol. 177:7086-7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vihinen, M., P. Ollikka, J. Niskanen, P. Meyer, I. Suominen, M. Karp, L. Holm, J. Knowles, and P. Mäntsälä. 1990. Site-directed mutagenesis of a thermostable α-amylase from Bacillus stearothermophilus: putative role of three conserved residues. J. Biochem. 107:267-272. [DOI] [PubMed] [Google Scholar]