Abstract
The spice oil components eugenol and cinnamaldehyde possess activity against both gram-positive and gram-negative bacteria, but the mechanisms of action remain obscure. In broth media at 20°C, 5 mM eugenol or 30 mM cinnamaldehyde was bactericidal (>1-log reduction in the number of CFU per milliliter in 1 h) to Listeria monocytogenes. At a concentration of 6 mM eugenol was bactericidal to Lactobacillus sakei, but treatment with 0.5 M cinnamaldehyde had no significant effect. To investigate the role of interference with energy generation in the mechanism of action, the cellular and extracellular ATP levels of cells in HEPES buffer at 20°C were measured. Treatment of nonenergized L. monocytogenes with 5 mM eugenol, 40 mM cinnamaldehyde, or 10 μM carbonyl cyanide m-chlorophenylhydrazone (CCCP) for 5 min prevented an increase in the cellular ATP concentration upon addition of glucose. Treatment of energized L. monocytogenes with 40 mM cinnamaldehyde or 10 μM CCCP caused a rapid decline in cellular ATP levels, but 5 mM eugenol had no effect on cellular ATP. Treatment of L. sakei with 10 mM eugenol prevented ATP generation by nonenergized cells and had no effect on the cellular ATP of energized cells. CCCP at a concentration of 100 μM had no significant effect on the cellular ATP of L. sakei. No significant changes in extracellular ATP were observed. Due to their rapidity, effects on energy generation clearly play a major role in the activity of eugenol and cinnamaldehyde at bactericidal concentrations. The possible mechanisms of inhibition of energy generation are inhibition of glucose uptake or utilization of glucose and effects on membrane permeability.
Spices have traditionally been used to preserve foods, as well as to enhance flavor and odor. The earliest description of antimicrobial effects of a spice was made by Antony van Leeuwenhoek. In a letter dated 9 October 1676, van Leeuwenhoek described the decline in the number and activity of “animalcules” in a sample of well water following the addition of pepper (6). The current interest in the use of compounds derived from spices as antimicrobial agents was sparked in the 1980s by changes in consumer attitudes toward the use of preservative agents such as nitrates and NaCl in foods (24). However, progress in the application of spice-derived compounds as antimicrobial agents in food products has been slow. The major problems include accurate identification of active components and the apparent requirement for concentrations that alter the sensory qualities of the food (18, 21).
The antimicrobial activity of spice oils has been attributed to a number of substituted aromatic molecules, such as eugenol, cinnamaldehyde, and carvacrol (Fig. 1) (10, 11, 17). Both eugenol and cinnamaldehyde are of interest for development as food antimicrobial agents due to their demonstrated activity against both gram-positive and gram-negative bacteria, including organisms that are of concern for safety. Eugenol has been reported to inhibit the growth of Escherichia coli O157:H7 and Listeria monocytogenes (2). Cinnamaldehyde has been reported to inhibit the growth of Clostridium botulinum (3), Staphylococcus aureus (4), E. coli O157:H7, and Salmonella enterica serovar Typhimurium (7).
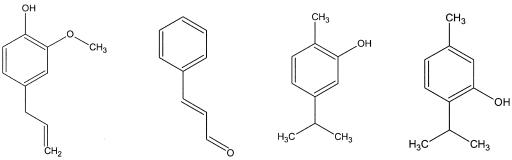
FIG. 1.
Structures of the substituted aromatic compounds (from left to right) eugenol, trans-cinnamaldehdye, carvacrol, and thymol.
To resolve the problem of high concentrations, it has been proposed that spice-derived compounds should be utilized in a system of antimicrobial agents in a form of hurdle technology (1, 2, 18, 21). The development of multicomponent antimicrobial systems for food products requires a greater understanding of the mechanisms of action of specific agents so that attention can be focused on potentially effective combinations.
Although it is common for reviewers of spice oils to ascribe the antimicrobial effects to interactions with the cell membrane, the precise mechanisms of bactericidal or inhibitory action remain unclear (5, 21). This problem is due to some extent to the use of methodologies that fail to adequately distinguish between inhibitory and lethal effects (18). The available experimental evidence for eugenol and cinnamaldehyde is contradictory, and there is evidence supporting both membrane interaction and inhibition of specific cellular processes or enzymes (7, 13, 28, 29).
The aim of the experiments described in this paper was to investigate the role played by inhibition of ATP generation at bactericidal concentrations of eugenol and cinnamaldehyde. A bactericidal concentration is defined as a concentration that prevents reproduction of treated cells when they are transferred to a noninhibitory medium. More than one mechanism may be involved in the activity of eugenol and cinnamaldehyde. However, the relevance of alternate mechanisms can be discounted if rapid inhibition of energy generation occurs. This is because cells that are unable to generate energy are unable to reproduce or alter their metabolism to adapt to antimicrobial challenge.
Experiments were conducted with the gram-positive organisms Lactobacillus sakei and Listeria monocytogenes. These organisms were selected due to their relevance to a wide range of food products; L. monocytogenes is an important food-borne pathogen, and lactic acid bacteria such as L. sakei are important in spoilage and fermentation of many foods. Additionally, although these bacteria are not greatly dissimilar physiologically, their energy metabolism reactions are significantly different. L. monocytogenes possesses an electron transport chain and generates energy by respiration, whereas L. sakei generates energy solely by fermentative metabolism (12, 22).
MATERIALS AND METHODS
Materials.
All-purpose Tween (APT) broth, M17 broth, brain heart infusion (BHI) broth, dextrose (glucose), and Proteose Peptone no. 3 were supplied by Difco, Becton-Dickinson, Sparks, Md. Agar granulated and Trypticase soy broth (TSB) were supplied by BBL, Becton-Dickinson, Sparks, Md. Yeast extract (YE), eugenol (2-methoxy-4-[2-propenyl]phenol), carbonyl cyanide m-chlorophenylhydrazone (CCCP), DEAE-dextran chloride (average molecular weight, 500,000) (DEAE-dextran), an ATP assay mixture (FL-AAM), an ATP standard (FL-AAS), ATP assay mixture dilution buffer (FL-AAB), and a P5656 protein assay kit were supplied by Sigma Chemical, St. Louis, Mo. trans-Cinnamaldehyde was supplied by Aldrich Chemical Co., Milwaukee, Wis. HEPES was supplied by Fisher Biotech, Fairlawn, N.J. An Isotemp 1016S refrigerated circulator and a Micro-12 microcentrifuge were obtained from Fisher Scientific, Fairlawn, N.J. An LB 9509 Berthold Junior luminometer was obtained from Berthold Technologies GmbH & Co. KG, Bad Wildbad, Germany. HEPES, EDTA (disodium salt), and trichloroacetic acid (TCA) were supplied by Fisher Chemicals, Fisher Scientific. ISO-Grid membranes were supplied by Neogen Co., Lansing, Mich. An Ultrafree-MC 0.22-μm-pore-size filter unit with a Durapore membrane was supplied by Millipore Co., Bedford, Mass. The spiral plater used was an Autoplate 4000, which was supplied by Spiral Biotech, Norwood, Mass.
Bacteria and culture conditions.
The L. monocytogenes strain used was a somatic serotype 1 meat plant isolate (Canadian Research Institute for Food Safety culture collection number C717) (M. W. Griffiths, University of Guelph, Guelph, Ontario, Canada, personal communication). The L. sakei strain used was isolated from spoiled cured meats (8). Stock cultures were frozen at −75°C in glycerol. For experimental use L. monocytogenes was streak plated on BHI agar, and L. sakei was streak plated on APT agar.
Preparation of antimicrobial agents.
Suspensions of the antimicrobial agents to be tested were prepared immediately prior to use by addition of eugenol or cinnamaldehyde to sterile TSB containing 5 g of YE per liter (TSB+YE) (pH 7.0) or 25 mM HEPES buffer (pH 7.0) and vortex mixing.
Determination of bactericidal concentrations of antimicrobial agents.
A single colony of the bacterium to be tested was used to inoculate 10 ml of TSB+YE (pH 7.0) at 20°C and grown to the log phase (optical density at 650 nm [OD650], 0.1 to 0.3). The concentration of the culture was then adjusted by dilution with TSB+YE (pH 7.0) to obtain an OD650 of 0.1 ± 0.04, and then the culture was diluted 1/1,000 into fresh TSB+YE (pH 7.0). A 0.5-ml aliquot of the bacterial cell suspension was then added to a 1.5-ml microcentrifuge tube containing 1 ml of the antimicrobial agent to be tested suspended in TSB+YE (pH 7.0). Controls composed of cells in TSB+YE (pH 7.0) that were not treated with an antimicrobial agent were examined simultaneously. The microcentrifuge tubes were incubated for 60 min in a water bath at 20°C. At the start of the experiment and every 15 min for 60 min the tubes were mixed by vortexing, and duplicate samples spread plated with a spiral plater on BHI (L. monocytogenes) or APT (L. sakei) agar plates. The plates were incubated at 25°C for 48 h, and the organisms were enumerated.
To confirm that the bactericidal effects of an antimicrobial agent were not due to inhibition of growth by residual antimicrobial agent, experiments at a bactericidal concentration were repeated with duplicate 100-μl samples recovered on ISO-Grid membranes. The samples were washed twice with 20-ml portions of 0.1% peptone before incubation of the filter on BHI or APT agar.
When ATP was measured, we found that it was necessary to use a concentration of cells that was 30 times greater than the concentration used in the experiments used to determine the bactericidal concentration. The lethality of the concentration used for cells suspended in 25 mM HEPES buffer was confirmed by spread plating.
Measurement of extracellular and intracellular ATP levels.
The bacterium to be tested was grown to the log phase in TSB+YE (pH 7.0) as described above. The culture was then harvested by centrifugation at 10,000 × g and washed twice with 25 mM HEPES buffer (pH 7.0) before it was resuspended in HEPES buffer to an OD650 of 0.1 ± 0.04. Then 0.3 ml of the resuspended cells was added to 9.7 ml of HEPES buffer, and the cell suspension was stored for 2 h at 20°C to deplete cellular ATP from the energized level. Depletion of cellular ATP under these conditions was confirmed during preliminary experiments.
The effect of an antimicrobial agent on ATP levels was determined for cells that were first energized with glucose or treated with the antimicrobial agent before addition of glucose.
In energized cell experiments 0.5 ml of cells in buffer was added to a 1.5-ml microcentrifuge tube containing 1.0 ml of buffer with 0.375% glucose. The microcentrifuge tubes were incubated for 5 min at 20°C to energize the cells, and a 200-μl sample was removed from each tube. Then 200 μl of the antimicrobial agent at 7.5× (final concentration) in 25 mM HEPES buffer with 0.25% glucose was immediately added to the tube, and incubation was continued for 15 min; 200-μl samples were taken every 5 min.
In experiments in which cells were first exposed to an antimicrobial agent, 0.5 ml of cells in buffer was added to a 1.5-ml microcentrifuge tube containing 1.0 ml of the antimicrobial agent in buffer at 1.5× (final concentration). The microcentrifuge tube was incubated for 5 min at 20°C, and a 200-μl sample was taken. Then 200 μl of the antimicrobial agent at the final concentration in buffer with 1.88% glucose was added to the tube, and incubation was continued for 15 min; samples were taken every 5 min.
At each time point, 200-μl samples were placed in Ultrafree-MC centrifuge filtration units (pore size, 0.22 μm). The cells in the samples were separated from the surrounding buffer by centrifugation for 30 s at 8,000 rpm in the Micro-12 microcentrifuge. A 100-μl aliquot of 2.5% TCA with 2 mM EDTA was added to the filter unit. After 10 min of incubation, the TCA solution was mixed with a pipettor, and a 20-μl aliquot was removed and diluted in 380 μl of FL-AAB and used as the cellular ATP sample. The filtrate was used as the external ATP sample. Samples were stored at −75°C until they were analyzed.
ATP analysis.
The ATP contents of cellular ATP and external ATP samples were assayed by a continuous light output luciferase reaction (16) in which the light output was amplified with DEAE-dextran (9). Each 75-μl sample was assayed by using 50 μl of FL-AAM luceriferase assay mixture with 25 μl of 1% DEAE-dextran in 5 mM HEPES (pH 7.8). The light output was expressed in relative light units (RLU) as determined with the Junior LB 9509 luminometer. Then 5 μl of an ATP standard was added and used as an internal standard. The ATP content of the sample was calculated from the ratio of the RLU of the sample to the RLU of the standard.
Measurement of protein content.
The protein contents of cell suspensions were determined by the modified Lowry method by using a protein assay kit according to the manufacturer's instructions.
Statistical analysis.
To determine whether there were significant differences between cells recovered by direct plating and ISO-Grid recovery, as well as between ATP measurements following different treatments, Student's t tests were used. The value for α for each analysis is indicated below.
RESULTS
Response of L. monocytogenes and L. sakei to cinnamaldehyde and eugenol.
Experiments were conducted to determine the concentration of eugenol and cinnamaldehyde required for a bactericidal effect on log-phase cells of L. monocytogenes and L. sakei. A bactericidal effect was defined as a >1-log reduction in the number of CFU recovered compared to untreated controls within 1 h.
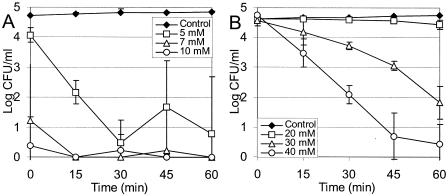
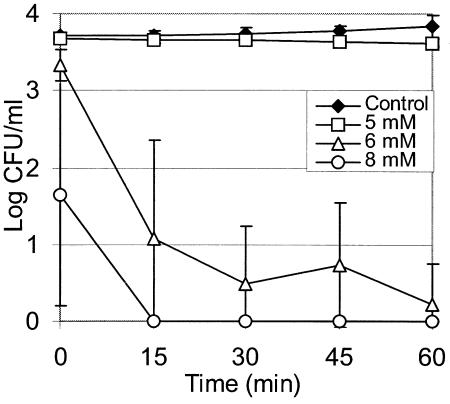
The minimum concentrations of antimicrobial agents required for a bactericidal effect on L. monocytogenes in TSB+YE (pH 7.0) were found to be 5 mM for eugenol (Fig. 2A) and 30 mM for cinnamaldehyde (Fig. 2B). A concentration of eugenol of 6 mM was required for a bactericidal effect on L. sakei in TSB+YE (pH 7.0) (Fig. 3), but treatment with up to 0.5 M cinnamaldehyde had no effect over a 1-h incubation (results not shown). When experiments were repeated with cells recovered on an ISO-Grid filter after washing with 0.1% peptone, there was no significant difference (α = 0.05, as determined by a Student t test) between the number of cells and the number of unwashed cells recovered following direct plating on agar (results not shown).
FIG. 2.
Effects of eugenol (A) and cinnamaldehyde (B) on L. monocytogenes in TSB+YE at 20°C and pH 7.0. The data are averages for three experiments, and the error bars indicate one standard deviation.
FIG. 3.
Effect of eugenol on L. sakei in TSB+YE at 20°C and pH 7.0. The data are averages for three experiments, and the error bars indicate one standard deviation.
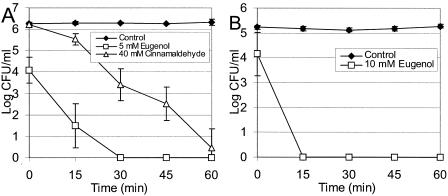
The lethality of the antimicrobial treatments for bacteria suspended in 25 mM HEPES (pH 7.0) with 0.25% glucose was verified (Fig. 4).
FIG. 4.
Effects of antimicrobial agents on L. monocytogenes (A) and L. sakei (B) in 25 mM HEPES (pH 7.0) at 20°C with 0.25% dextrose. The data are averages of results from two experiments, and the error bars indicate one standard deviation.
Inhibitory effects on ATP generation by L. monocytogenes.
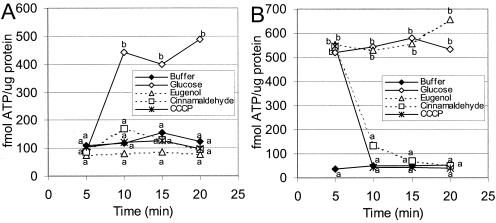
When 0.25% glucose was supplied to L. monocytogenes cells in 25 mM HEPES (pH 7.0) following 5 min of incubation at 20°C, the cellular ATP levels increased significantly compared to the levels in controls without glucose (Fig. 5A). Incubation of L. monocytogenes with 5 mM eugenol, 40 mM cinnamaldehyde, or 10 μM CCCP for 5 min prior to addition of glucose prevented a significant increase in the cellular ATP level (Fig. 5A).
FIG. 5.
Cellular ATP concentrations expressed as femtomoles per microgram of protein from L. monocytogenes in 25 mM HEPES buffer (pH 7.0) at 20°C. (A) Cells were exposed to antimicrobial agents at zero time, and all treatments except the buffer treatment were energized with 0.25% glucose at 5 min. (B) All treatments except the buffer treatment were energized with 0.25% glucose at zero time, and antimicrobial agents were added at 5 min. The treatments included buffer, glucose, eugenol (5 mM), cinnamaldehyde (40 mM), and CCCP (10 μM). The data are averages for four experiments, and values that are significantly different as determined by a Student t test (α = 0.05) are indicated by different letters.
When L. monocytogenes cells were energized by 5 min of incubation with 0.25% glucose before addition of the antimicrobial agent, we observed that 40 mM cinnamaldehyde and 10 μM CCCP resulted in a significant reduction in the level of cellular ATP. However, 5 mM eugenol was found to have no significant effect on the cellular ATP levels when energized cells were challenged (Fig. 5B).
No significant difference in extracellular ATP levels was observed between controls and treatments for L. monocytogenes that was treated first with either glucose or an antimicrobial agent (results not shown) (α = 0.05, as determined by a Student t test).
Inhibitory effects on ATP generation by L. sakei.
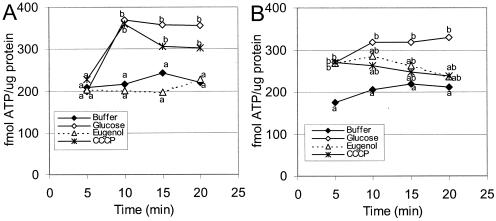
L. sakei cells treated with 10 mM eugenol had significantly smaller amounts of cellular ATP following addition of 0.25% glucose compared to cells in buffer (Fig. 6A). The cellular ATP levels of L. sakei cells treated with 100 μM CCCP were not significantly different than the levels in controls that were treated with glucose alone (Fig. 6A).
FIG. 6.
Cellular ATP concentrations expressed as femtomoles per microgram of protein from L. sakei in 25 mM HEPES buffer (pH 7.0) at 20°C. (A) Cells were exposed to antimicrobial agents at zero time, and all treatments except the buffer treatment were energized with 0.25% glucose at 5 min. (B) All treatments except the buffer treatment were energized with 0.25% glucose at zero time, and antimicrobial agents were added at 5 min. The treatments included buffer, glucose, eugenol (10 mM), and CCCP (100 μM). The data are averages for four experiments, and values that are significantly different as determined by a Student t test (α = 0.10) are indicated by different letters.
Treatment of energized L. sakei with 10 mM eugenol or 100 μM CCCP resulted in cellular ATP values that were not significantly different from the values for either energized or unenergized controls (Fig. 6B).
Again, there was no significant difference in extracellular ATP levels between controls and treatments when L. sakei was treated first with either glucose or an antimicrobial agent (results not shown) (α = 0.10, as determined by a Student t test).
DISCUSSION
Responses of L. monocytogenes and L. sakei to cinnamaldehyde and eugenol.
The experiments described here demonstrate that above a minimum threshold concentration eugenol has a dose-dependent bactericidal effect on growing log-phase cells of both L. monocytogenes and L. sakei within 15 min after exposure. Cinnamaldehyde was a less effective bactericidal agent than eugenol, requiring a concentration that was six times greater for a bactericidal effect on L. monocytogenes. Additionally, no effect on L. sakei was observed with up to 0.5 M cinnamaldehyde. The failure of cinnamaldehyde to have any appreciable effect on L. sakei suggests three possibilities: (i) L. sakei possesses a cell envelope that is less permeable to cinnamaldehyde than the L. monocytogenes cell envelope; (ii) L. sakei possesses a resistance mechanism which allows it to export or inactivate cinnamaldehyde; and (iii) L. sakei does not possess the target for cinnamaldehyde activity found in L. monocytogenes. If the third possibility is true, then it seems that the mechanism of cinnamaldehyde action is different from that of eugenol, which affects the two organisms at similar concentrations.
Effects of eugenol on ATP generation by L. monocytogenes and L. sakei.
When supplied to L. monocytogenes cells prior to glucose, eugenol was observed to prevent the increase in cellular ATP levels that occurred in untreated controls. However, eugenol was not observed to cause ATP depletion from cells previously energized with glucose, whereas the protonophore CCCP prevented ATP generation from glucose by nonenergized cells and caused rapid ATP depletion in energized L. monocytogenes cells.
These results appear to contradict the ion transport model for activity proposed by Ultee et al. (27) for carvacrol, which, like eugenol, is a substituted phenolic. Carvacrol is bactericidal to Bacillus cereus at a concentration of 1.5 to 2 mM in HEPES buffer at 8°C (25). Carvacrol (2 mM) was observed to cause a rapid decline in the cellular ATP pools and a slight increase in the extracellular ATP level of glucose-energized B. cereus (26). Addition of 0.15 mM carvacrol was shown to dissipate the membrane potential, as measured with the fluorescent dye 3,3-dipropylthiacarbocyanine, and 1 mM was sufficient to dissipate the membrane gradients of pH and potassium (26). When the effect of carvacrol on the growth rate of B. cereus was compared with the effects of structurally related molecules (thymol, menthol, carvacrol methylester, and cymene), it was found that lower concentrations of molecules possessing a hydroxyl group were required for growth inhibition (27). Based on this observation and the observed effects on membrane ion gradients without apparent ATP leakage, Ultee et al. (27) proposed an ion transporter model of carvacrol action. In this model the molecule inserts into the membrane, disrupting its structure, but the hydroxyl group allows carvacrol to act “as a transmembrane carrier of monovalent cations by exchanging its hydroxyl proton for another ion such as a potassium ion.” However, it is difficult to distinguish between membrane disruption and ion transport, and 6.7 mM carvacrol has been reported to increase ethidium bromide staining of Pseudomonas aeruginosa and S. aureus, a clear indicator of membrane disruption (14).
If eugenol acted as an ion transporter, it would be expected to cause ATP depletion from energized cells, as was observed with CCCP, a known ion transporter. CCCP and other protonophores can be predicted to cause depletion of ATP pools since to maintain its normal intracellular pH, pH 8, L. monocytogenes uses electron transport and ATPase to export H+ (23). Since ATP is not depleted following addition of eugenol to energized L. monocytogenes, either the proton gradient of the membrane is not dissipated or the activity of the F1F0 ATPase is inhibited. ATPase inhibition is a very real possibility as Rico-Munoz et al. (20) reported that the phenolic compounds tertiary butylhydroquinine and propyl gallate can reduce the activity of S. aureus ATPase in isolated membranes. However, in the same study a significant increase in ATPase activity was noted in samples treated with butylated hydroxyanisole.
The absence of any observed increase in the extracellular ATP level for energized cells does not support disruption of the cell membrane as an explanation for lethality. The results observed are consistent with inhibition of glucose import or utilization by L. monocytogenes. If this is the case, inhibition of glucose utilization rather than inhibition of uptake seems more probable. L. monocytogenes is known to possess two glucose import systems, a high-affinity phosphoenolpyruvate-dependent phosphotransferase system (PTS) (Km = 0.11 mM) and a low-affinity proton motive force-dependent system (Km = 2.9 mM) (19). It seems unlikely that eugenol inactivates both transport mechanisms by interaction with the enzymes involved. If inhibition of glucose utilization occurs, then it most probably involves inhibition of an enzyme involved in glycolysis, as inhibition of the tricarboxylic acid cycle or respiration would allow the cell to continue generating ATP by fermentation.
Membrane effects cannot be discounted, as Walsh et al. (28) have reported potassium leakage from E. coli (3.03 mM) and S. aureus (6.06 mM) treated with eugenol. If eugenol acts as an ion transporter or causes membrane leakage, it is conceivable that glucose uptake by the PTS could continue, and ATP pools could be maintained by fermentative metabolism. Cells in this position would still be severely injured and could be expected to expend most of the energy generated on futile attempts to reestablish membrane gradients.
Similar conclusions can be drawn from the effects of eugenol treatment on cellular ATP levels of L. sakei. Like L. monocytogenes, L. sakei imports glucose by the PTS and a poorly characterized non-PTS system (15). Furthermore, since in L. sakei generation of ATP is purely fermentative, if inhibition of glucose utilization occurs, it must involve a key step in glycolysis. This conclusion is supported by the failure of 100 μM CCCP to alter ATP generation by L. sakei, which is presumably able to continue ATP generation by substrate level phosphorylation.
Although Wendakoon and Sakaguchi (29) found that eugenol at a concentration of >6 mM is inhibitory for the histidine decarboxylase of Enterobacter aerogenes, the role of inhibition of biosynthetic enzymes can be discounted, as the rapidity of effects on energy metabolism at a bactericidal concentration make any such activity irrelevant.
Effects of cinnamaldehyde on ATP generation by L. monocytogenes.
As observed with eugenol, the possibility that the mechanism of cinnamaldehyde activity involves inhibition of cell wall synthesis (2.36 mM, B. cereus) (13) or inhibition of biosynthetic enzymes (>7.5 mM, histidine decarboxylase) (29) is unlikely because of the rapidity of ATP inhibition or depletion.
Treatment with 40 mM cinnamaldehyde was observed to have an effect identical to the effect of 10 μM CCCP in preventing an increase in the cellular ATP level following addition of glucose to cells preexposed to inhibitor and also in causing rapid depletion of the cellular ATP of energized cells. The hypothesis that cinnamaldehyde functions as a ion transporter like CCCP can be dismissed as cinnamaldehyde does not have any chemical group, such as a hydroxyl, which would allow it to function in such a manner. The results seen were consistent with a mechanism for cinnamaldehyde action in which interaction with the cell membrane causes disruption sufficient to disperse the proton motive force by leakage of small ions without leakage of larger cell components, such as ATP. The observed results are also consistent with either inhibition of glucose import or inhibition of glycolysis.
Conclusions.
The results of our experiments clearly indicate that there is rapid inhibition of the energy metabolism of L. monocytogenes and L. sakei when the cells are exposed to bactericidal concentrations of eugenol and cinnamaldehyde. Further experiments are necessary to determine whether the mode of inhibition involves glucose utilization or membrane interactions. Additionally, the possibility of ATPase inhibition by eugenol should be investigated.
Acknowledgments
This project was supported by a research grant from the Natural Sciences and Engineering Research Council of Canada.
We thank Emefa Monu, Kit Bee Tan, and Jocelyn Lam for their technical assistance.
REFERENCES
- 1.Adams, M., and E. Smid. 2003. Nisin in multifactorial food preservation, p. 11-33. In S. Roller (ed.), Natural antimicrobials for the minimal processing of foods. Woodhead Publishing Ltd., Cambridge, United Kingdom
- 2.Blaszyk, M., and R. A. Holley. 1998. Interaction of monolaurin, eugenol and sodium citrate on growth of common meat spoilage and pathogenic organisms. Int. J. Food Microbiol. 39:175-183. [DOI] [PubMed] [Google Scholar]
- 3.Bowles, B. L., and A. J. Miller. 1993. Antibotulinal properties of selected aromatic and aliphatic aldehydes. J. Food Prot. 56:788-794. [DOI] [PubMed] [Google Scholar]
- 4.Bowles, B. L., S. K. Sackitey, and A. C. Williams. 1995. Inhibitory effects of flavor compounds on Staphylococcus aureus WRRC B124. J. Food Saf. 15:337-347. [Google Scholar]
- 5.Brul, S., and P. Coote. 1999. Preservative agents in foods: mode of action and microbial resistance mechanisms. Int. J. Food Microbiol. 50:1-17. [DOI] [PubMed] [Google Scholar]
- 6.Dobell, C. 1960. Antony van Leeuwenhoek and his “little animals,” p. 141-142. Dover Publications, Inc. New York, N.Y.
- 7.Helander, I. M., H. L. Alakomi, K. Latva-Kala, T. Mattila-Sandholm, L. Pol, E. J. Smid, L. G. M. Gorris, and A. von Wright. 1998. Characterization of the action of selected essential oil components on Gram-negative bacteria. J. Agric. Food Chem. 46:3590-3595. [Google Scholar]
- 8.Holley, R. A., G. Doyon, J. Fortin, N. Rodrigue, and M. Charbonneau. 1996. Post-process packaging induced fermentation of delicatessen meats. Food Res. Int. 29:35-48. [Google Scholar]
- 9.Ishida, A., T. Yoshikawa, T. Nakazawa, and T. Kamidate. 2002. Enhanced firefly bioluminescence assay of ATP in the presence of ATP extractants by using diethylaminoethy-dextran. Anal. Biochem. 305:236-241. [DOI] [PubMed] [Google Scholar]
- 10.Jay, J. J., and G. M. Rivers. 1984. Antimicrobial activity of some food flavoring compounds. J. Food Saf. 6:129-139. [Google Scholar]
- 11.Juven, B. J., J. Kanner, F. Schved, and H. Weisslowicz. 1994. Factors that interact with the antibiotic action of thyme essential oil and its active constituents. J. Appl. Bacteriol. 76:626-631. [DOI] [PubMed] [Google Scholar]
- 12.Kandler, O., and N. Weiss. 1986. Genus Lactobacillus, p. 1209-1234. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology vol. 2. Williams and Wilkins, Baltimore, Md.
- 13.Kwon, J. A., C. B. Yu, and H. D. Park. 2003. Bactericidal effects and inhibition of cell separation of cinnamic aldehyde on Bacillus cereus. Lett. Appl. Microbiol. 37:61-65. [DOI] [PubMed] [Google Scholar]
- 14.Lambert, R. J. W., P. N. Skandamis, P. J. Coote, and G.-J. E. Nychas. 2001. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 91:453-462. [DOI] [PubMed] [Google Scholar]
- 15.Lauret, R., F. Morel-DeVille, F. Berthier, M. Champomier-Vergès, P. Postma, S. D. Ehrlich, and M. Zagorec. 1996. Carbohydrate utilization in Lactobacillus sakei. Appl. Environ. Microbiol. 62:1922-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lundin, A. 2000. Use of firefly luciferase in ATP-related assay of biomass, enzymes and metabolites. Methods Enzymol. 305:346-370. [DOI] [PubMed] [Google Scholar]
- 17.Moleyar, V., and P. Narasimham. 1992. Antibacterial activity of essential oil components. Int. J. Food Microbiol. 16:337-342. [DOI] [PubMed] [Google Scholar]
- 18.Nychas, G.-J. E., and P. N. Skandamis. 2003. Antimicrobials from herbs and spices, p. 176-200. In S. Roller (ed.), Natural antimicrobials for the minimal processing of foods. Woodhead Publishing Ltd., Cambridge, United Kingdom.
- 19.Parker, C., and R. W. Hutkins. 1997. Listeria monocytogenes Scott A transports glucose by high-affinity and low-affinity glucose transport systems. Appl. Environ. Microbiol. 63:543-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rico-Munoz, E., E. E. Bargiota, and P. M. Davidson. 1987. Effect of selected phenolic compounds on the membrane-bound adenosine triphosphatase of Staphylococcus aureus. Food Microbiol. 4:239-249. [Google Scholar]
- 21.Roller, S., and R. G. Board. 2003. Naturally occurring antimicrobial systems, p. 262-290. In N. J. Russell and G. W. Gould (ed.), Food preservatives, 2nd ed. Kluwer Academic, New York, N.Y.
- 22.Seeliger, H., and D. Jones. 1986. Genus Listeria, p. 1235-1245. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology vol. 2. Williams and Wilkins, Baltimore, Md.
- 23.Shabala, L., B. Budde, T. Ross, H. Siegumfeldt, M. Jakobsen, and T. McMeekin. 2002. Responses of Listeria monocytogenes to acid stress and glucose availability revealed by a novel combination of fluorescence microscopy and microelectrode ion-selective techniques Appl. Environ. Microbiol. 68:1794-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shelef, L. A. 1983. Antimicrobial effects of spices. J. Food Saf. 6:29-44. [Google Scholar]
- 25.Ultee, A., L. G. M. Gorris, and E. J. Smid. 1998. Bactericidal activity of carvacrol towards the food-borne pathogen Bacillus cereus. J. Appl. Microbiol. 85:211-218. [DOI] [PubMed] [Google Scholar]
- 26.Ultee, A., E. P. W. Kets, and E. J. Smid. 1999. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 65:4606-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ultee, A., M. H. J. Bennik, and R. Moezelaar. 2002. The phenolic hydroxyl group of carvacrol is essential for action against food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 68:1561-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh, S. E., J. Y. Maillard, A. D. Russell, C. E. Catrenich, D. L. Charbonneau, and R. G. Bartolo. 2003. Activity and mechanisms of action of selected biocidal agents on Gram-positive and -negative bacteria. J. Appl. Microbiol. 94:240-247. [DOI] [PubMed] [Google Scholar]
- 29.Wendakoon, C. N., and M. Sakaguchi. 1995. Inhibition of amino acid decarboxylase activity of Enterobacter aerogenes by active components in spices. J. Food Prot. 58:280-283. [DOI] [PubMed] [Google Scholar]