Abstract
Glucobrassicin in Brassica vegetables gives rise to indole-3-carbinol, a compound with potent anti-cancer effects in preclinical models. We previously showed that the urinary metabolite 3,3′-diindolylmethane (DIM) could discriminate between volunteers fed high and low doses of Brassica vegetables. However, the quantitative relationship between glucobrassicin exposure and urinary DIM level is unclear. We conducted a clinical trial to examine the hypotheses that a range of glucobrassicin exposure from Brassica vegetables is reflected in urinary DIM, and that this effect plateaus. Forty-five subjects consumed vegetables, a mixture of Brussels sprouts and/or cabbage, at 1 of 7 discrete dose levels of glucobrassicin ranging from 25 to 500 μmol, once daily for two consecutive days. All urine was collected for 24 hours after each vegetable-eating session. Urinary DIM was measured using our published liquid chromatography-electrospray ionization-tandem mass spectrometry-selected reaction monitoring (LC/ESI-MS/MS-SRM) method. Urinary DIM excretion increased predictably with increasing glucobrassicin dose and plateaued between 200 and 300 μmol of glucobrassicin. The association between glucobrassicin dose and urinary DIM was strong and positive (R2=0.68). The majority of DIM was excreted in the first 12 hours after vegetable consumption. We conclude that urinary DIM is a reliable biomarker of glucobrassicin exposure and I3C uptake, and that feeding glucobrassicin beyond 200 μmol did not consistently lead to more urinary DIM, suggesting a plateau in potential chemopreventive benefit.
Keywords: 3,3′-diindolylmethane; indole-3-carbinol; glucobrassicin; chemoprevention; biomarker
INTRODUCTION
The anti-cancer effect of cruciferous vegetables is widely thought to be mediated by their glucosinolates,(1) and epidemiologic evidence points to an association between high cruciferous vegetable consumption and decreased cancer risk,(2) but the association is inconsistent, due in large part to a lack of objective measures of phytochemical exposure and uptake. Upon plant cell damage (i.e., chewing), inert glucosinolates are converted to indoles and isothiocyanates by myrosinase. Glucobrassicin, a predominant glucosinolate in many common Brassica vegetables,(3, 4) is hydrolyzed to indole-3-carbinol (I3C, Figure 1). In the acidic environment of the stomach, I3C readily undergoes acid condensation, primarily to 3,3′-diindolylmethane (DIM, Figure 1).(5–10) Both I3C and DIM possess strong effects in vitro and in-vivo against cancers of the lung, colon, prostate, and breast.(11–14) Mice gavaged with NNK and then treated with I3C or DIM developed significantly fewer lung tumors compared to controls.(13, 15, 16) In a placebo-controlled clinical trial, I3C taken for 12 weeks resulted in regression of cervical intraepithelial neoplasia in approximately half of the 17 women randomized to either 200 mg or 400 mg daily compared to placebo.(17) Other studies demonstrated both I3C and DIM modulate estrogen metabolism in animals and humans to favor the formation of the anti-proliferative estrogen 2-hydroxyestrone (2-OHE) over the proliferation-inducing 16a-hydroxyestrone (16-OHE);(18–22) a higher 2-OHE:16-OHE ratio is associated with a decreased risk of estrogen-sensitive tumors such as breast cancer.(23) Because of the rapid condensation of I3C to DIM and other oligomers, development of an in vivo biomarker to detect I3C in humans is not realistic.(6, 7, 24) Alternatively, urine is a non-invasive and practical biospecimen ideal for use in large studies. We previously developed a novel method to quantify DIM in urine, and showed that consuming vegetables with divergent glucobrassicin concentrations was consistently reflected in urinary DIM levels.(25) To further define the utility of urinary DIM as a non-invasive biomarker of glucobrassicin exposure and I3C uptake from vegetables in humans, we conducted a clinical trial to determine the ability of urinary DIM to discriminate a wide range of glucobrassicin doses. We also sought to define the glucobrassicin dose at which this relationship plateaus, as observed with I3C and DIM administered as supplements.(24, 26)
Figure 1.
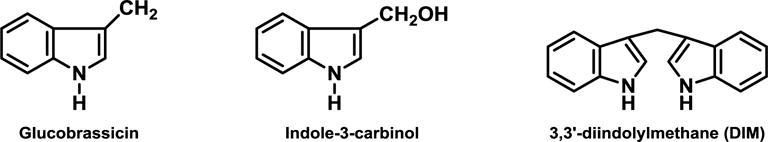
Chemical structures of glucobrassicin, indole-3-carbinol, and 3,3′-diindolylmethane.
MATERIALS AND METHODS
Study Design
Healthy, non-smoking, non-vegetarian adult subjects age 18–60 years were recruited. Kidney and liver function were required to be normal. Subjects taking H2-blockers, proton pump inhibitors, or calcium carbonate regularly, and those recently treated with antibiotics were excluded. Questionnaires were administered to collect demographic information, and histories of tobacco, medication, and alcohol use. At enrollment, subjects were randomly assigned to one of 7 doses of glucobrassicin – 25, 50, 100, 200, 300, 400 or 500 μmol. Four subjects were recruited at each dose level. To determine inter-individual variation in urinary DIM between glucobrassicin doses, an additional 6 subjects were recruited for three dose levels – 50, 200 and 500 μmol. Subjects refrained from cruciferous vegetable consumption for a minimum of 5 days prior to the study intervention. Salads comprising fixed proportions of Brussels sprouts and cabbage to attain the desired glucobrassicin dose (Table 1 in Supplemental Data) were freshly prepared with minimal chopping on the day of the study intervention. Subjects fasted 2 h, provided a spot urine collection, and then consumed the assigned dose of study vegetables once daily for 2 consecutive days at the study center as quickly as possible. This was followed by another 2 h fasting period. All urine was collected for 24 h following each vegetable-eating session, divided into the following periods: 0–2 h, 2–6 h, 6–12 h, and 12–24 h. This was done to inform whether briefer collection periods could be used in future studies. Urine volume was measured and aliquots stored at −20°C. Self-reported food diaries were kept throughout the study period and reviewed for obvious dietary intake of cruciferous vegetables. The protocol and consent form were approved by the Institutional Review Board at the University of Minnesota. All subjects provided informed consent.
Analysis of Glucobrassicin Concentration in the Vegetables
‘Blue Dynasty’ cabbage and ‘Jade Cross’ Brussels sprouts were grown specifically for this study (see Supplemental Materials). Approximately 200 g, bulked from different heads of cabbage (n=4) and Brussels sprouts (n=4), were taken for determination of glucobrassicin concentration at one time point shortly after harvest. Glucobrassicin concentration was analyzed using a published technique.(25)
Urine Sample Preparation
Urine samples were prepared using a previously published technique.(25) Analysts were blinded, but to minimize intra-individual variability, all samples from a given subject were prepared together in a single set.
LC-ESI-MS/MS-SRM
Analysis was performed as previously published,(25) with slight modifications to increase sample throughput. Briefly, a TSQ Vantage instrument was used in addition to the TSQ Quantum Discovery Max instrument used previously. The TSQ Vantage was coupled to an Eskigent NanoLC Ultra (Eskigent Technologies) liquid chromatographic system; MS parameters on the TSQ Vantage were comparable to those previously published.(25) The chromatographic method was shortened to decrease analysis time. Initial conditions were 40% 10mM NH4OAc and 60% methanol, percent methanol was then increased to 70% over 15 min and held for 1 min, then increased to 95% over 1 min and held for 2 min, the system was then returned to initial conditions over 1 min and allowed to re-equilibrate for 10 min before injection of the next sample. Flow rate was held constant at 10 μL/min throughout the separation. The column was equipped with a KrudKatcher 0.5μ pre-filter (Phenomenex), which was exchanged after approximately 50 sample injections or when significant peak-broadening was observed. Typical retention times for DIM and [2H2]DIM on the Vantage system were 14.7 and 14.8 min respectively, and 16.6 and 16.5 on the Discovery. The detection limit of the assay was 0.4 pmol/mL.
Quality Control
This laboratory method for quantifying urinary DIM has been previously validated.(25) To ensure continued quality control, each set included a minimum of two water blanks, spiked with [2H2]DIM to monitor recovery, as well as four positive controls consisting of urine samples known to contain DIM, pooled from subjects who had consumed Brussels sprouts. Spiked control samples were included in select sets to confirm reproducibility at the lower and upper ends of the observed DIM concentration range in study samples. Briefly, baseline urine samples from 9 subjects, which had been confirmed to contain no measurable DIM, were pooled and then spiked to yield final DIM concentrations of 35 pmol/mL or 0.90 pmol/mL, or slightly over 2 times the detection limit of the assay. Samples with an apparent recovery below 5% were re-assayed.
Statistical Analysis
The primary objective of this study was to characterize the relationship between glucobrassicin dose and urinary DIM, specifically to identify the maximum dose where the urinary DIM ‘response’ levels off (plateau). The two 24 h DIM measurements from each subject were averaged. Any DIM detected in the baseline spot urine sample prior to vegetable intake was subtracted from this value. The model used to determine the dose-response curve of glucobrassicin fed (dose) and urinary DIM (response) is based on the “median effect principle” of pharmacology(27) and is expressed as a four parameter logistic equation:
The four parameters estimated by this model are β1 and β2, the intercept and slope of the linear portion of the sigmoidal curve, and β3 and β4, the minimum and maximum levels, respectively, of urinary DIM where the logistic curve flattens out at lower and higher doses. These parameters were estimated using the non-linear (NLIN) procedure in SAS version 9.3 (SAS Institute Inc., USA). The dose that produces a 50% response (ED50) is calculated by the estimates of the intercept and slope: ED50= exp[ (− 1)(intercept/slope)]. The minimum, maximum DIM and EC50 are reported with their 95% confidence intervals. The minimum and maximum estimates were entered into the above equation to calculate (C/1-C), which results in a simple linear expression in the logarithmic scale and, therefore the R-squared between glucobrassicin dose and urinary DIM was calculated.
All means are reported with their SE and/or 95% confidence intervals unless otherwise noted.
RESULTS
Subject Characteristics and Study Compliance
Nineteen males and 26 females ranging in age from 19–56 years (mean 31.5 ± 1.5 years) completed the study. The accrual goal was 46; one subject at the 50 μmol dose dropped out due to issues unrelated to the study and was not replaced. Thirty-five (78%) were Caucasian, 5 were Asian (11%), 2 were African-American (4%) and 3 (7%) reported more than one race. At the 500 μmol dose level, two subjects could not finish due to the taste of the raw Brussels sprouts and were reassigned to the 50 μmol dose after a minimum 7 day washout period. Additionally, subject 40 at the 500 μmol dose finished only 162.1 g (66.8%) of the Brussels sprouts on day 1 and 210.5 g (86.7%) on day 2. Subject 43 at the 500 μmol dose finished 90.5 g (37.3%) on day 1 and 188.1 g (77.5%) on day 2. Subject 36 at the 500 μmol dose finished 128.1 g (52.3%) on day 1 and 184.8 g (76.1%) on day 2. At the 200 μmol dose level, one subject finished only 52% of the vegetables on day 1 and 75% of the vegetables on day 2 due to taste intolerance. Since these salads were a mixture of cabbage and Brussels sprouts, it was not possible to determine the exact amount of each consumed. Of the 352 total urine collection periods (8 collection periods per subject × 44 subjects), only 4 partial urine voids were missed. Subject 39 missed one void on day 2 during the 2–6 h collection period. Subject 44 missed collecting 2 voids on day 2 during the 6–12 h urine collection. Urine collection compliance was based on self-report. All subjects who consumed any study vegetables were included in the data analysis.
Glucobrassicin concentration
The cabbage (n=4 samples) contained 33.5 ± 4.0 μmol per 100 grams food weight. The Brussels sprouts (n=4 samples) contained 206.0 ± 12.9 μmol per 100 grams food weight. Both are consistent with prior results.(25)
Quality Control
Inter-day precision was 8.7% and intra-day precision was 3.9% (n=68 across 12 sets). Measured DIM concentration (N=14 samples, split between 5 sets) in the 35 pmol/mL spiked samples was 33.3 ± 2.5 (SD) pmol DIM/mL (CV 7.5%) and in the 0.9 pmol/mL spiked samples was 0.87 ± 0.08 (SD) pmol DIM/mL (CV 9.2%).
Dose-response between glucobrassicin exposure and urinary DIM excretion
Baseline urinary DIM levels, 24 h DIM concentrations after consumption of the study vegtables, mean 24 h DIM concentration, and percent DIM excreted in the first 12 hours of the 24 h urine collection period are shown in Table 1. Very few subjects had DIM in the urine at baseline. On average, 95.1 ± 1.2% (95% CI 92.7, 97.5) on day 1 and 96.1 ± 0.8% (95% CI 94.5, 97.7) on day 2 of the total 24 h DIM was excreted in the first 12 hours after vegetable consumption. In contrast, on average, only 76.9% (95% CI 70.4, 83.4) % on day 1 and 80.4 (95% CI 75.7, 85.1) on day 2 of total 24 h DIM was excreted in the first 6 hours. DIM excretion by collection period is shown in Table 2 (supplemental materials). As shown in Figure 3, urinary DIM excretion plateaued between glucobrassicin doses of 200 and 300 μmol. The R2 between measured DIM and predicted DIM across all subjects was 0.68, indicating a strong association between dose and urinary DIM. Excluding the three subjects at the 500 μmol dose who could not finish the Brussels sprouts due to taste intolerance resulted in a very similar predictive value for the dose (R2=0.67). We then analyzed the intraclass correlation (ICC), or how similar the two 24 h urinary DIM levels from an individual compare to the similarity in other individuals. The ICC at the three expanded dose level cohorts is shown in Table 2. At 50 μmol, variability in 24 h urinary DIM levels appears to stem from both within an individual and between indivduals. At the 200 and 500 μmol dose levels, most of the variability is coming from between individuals rather than within an individual.
Table 1.
Urinary DIM at baseline and after consuming escalating glucobrassicin doses from cabbage and Brussels sprouts
| Subject # | glucobrassicin dose (μmol) | Baseline DIM (pmol/mL) | 24 h DIM (pmol/mL)
|
Mean 24 h DIM ± SE (pmol/mL) | DIM excreted in 12 hours (%)
|
||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 1 | Day 2 | ||||
|
| |||||||
| 1 | 25 | <LOD | 7.91 | 1.84 | 4.88 ± 3.04 | 100 | 100 |
| 2 | ND | 7.27 | 4.70 | 5.99 ± 1.29 | 91.8 | 100 | |
| 3 | <LOD | 28.1 | 49.1 | 38.6 ± 10.5 | 92.2 | 100 | |
| 4 | 1.24 | 8.83 | 3.83 | 6.33 ± 2.50 | 93.2 | 100 | |
|
| |||||||
| 5 | 50 | ND | 3.19 | 12.7 | 7.95 ± 4.76 | 100 | 100 |
| 6 | <LOD | 2.00 | 2.71 | 2.36 ± 0.36 | 60.0 | 81.5 | |
| 7* | <LOD | 11.9¶ | 10.6 | 11.3 ± 0.7 | 95.0 | 95.3 | |
| 8 | ND | 14.7 | 15.6 | 15.2 ± 0.5 | 100 | 100 | |
| 9* | <LOD | 54.6 | 15.1 | 34.9 ± 19.8 | 98.9 | 100 | |
| 10 | 0.82 | 13.8 | 22.1∞ | 18.0 ± 4.2 | 81.2 | 84.2 | |
| 11 | 0.82 | 5.99 | 43.3 | 24.6 ± 18.7 | 88.3 | 98.6 | |
| 12 | ND | 5.69 | 2.54 | 4.12 ± 1.58 | 100 | 100 | |
| 13 | <LOD | 49.2 | 47.2 | 48.2 ± 1.0 | 97.6 | 96.8 | |
|
| |||||||
| 14 | 100 | <LOD | 175 | 171 | 173 ± 2 | 99.5 | 98.5 |
| 15 | ND | 122 | 127 | 125 ± 3 | 98.8 | 95.7 | |
| 16 | 2.45 | 35.4 | 66.2 | 50.8 ± 15.4 | 98.3 | 96.4 | |
| 17 | <LOD | 106 | 47.2 | 76.6 ± 29.4 | 96.9 | 100 | |
|
| |||||||
| 18 | 200 | 2.71 | 340 | 58.4 | 199 ± 141 | 99.2 | 91.3 |
| 19 | <LOD | 300 | 129 | 215 ± 86 | 96.5 | 91.1 | |
| 20 | ND | 1150 | 1060 | 1105 ± 45 | 98.7 | 95.6 | |
| 21 | <LOD | 49.1 | 26.5 | 37.8 ± 11.3 | 97.8 | 96.6 | |
| 22 | <LOD | 126 | 193 | 160 ± 34 | 96.2 | 99.4 | |
| 23 | <LOD | 107 | 39.0 | 73.0 ± 34.0 | 98.2 | 84.7 | |
| 24 | 1.30 | 347 | 226 | 287 ± 61 | 94.8 | 100 | |
| 25 | <LOD | 509 | 614 | 562 ± 53 | 97.8 | 100 | |
| 26 | ND | 502 | 335 | 419 ± 84 | 93.1 | 100 | |
| 27 | <LOD | 16.7 | 24.7 | 20.7 ± 4.0 | 95.8 | 93.1 | |
|
| |||||||
| 28 | 300 | <LOD | 517 | 554 | 536 ± 19 | 99.7 | 99.6 |
| 29 | <LOD | 91.2 | 134 | 113 ± 21 | 97.5 | 97.9 | |
| 30 | <LOD | 64.9§ | 106 | 85.5 ± 20.6 | 96.1 | 95.5 | |
| 31 | <LOD | 457 | 106 | 282 ± 176 | 99.6 | 97.2 | |
|
| |||||||
| 32 | 400 | ND | 738 | 957 | 848 ± 110 | 95.8 | 91.5 |
| 33 | <LOD | 625 | 482 | 544 ± 72 | 98.4 | 98.5 | |
| 34 | <LOD | 76.3 | 159 | 118 ± 41 | 95.0 | 97.5 | |
| 35 | <LOD | 151¶ | 406 | 279 ± 128 | 73.6 | 91.2 | |
|
| |||||||
| 36 | 500 | 0.74 | 157 | 134 | 146 ± 12 | 82.3 | 76.3 |
| 37 | <LOD | 302ˆ | 787 | 545 ± 243 | 99.1 | 99.3 | |
| 38 | <LOD | 352ˆ | 314ˆ | 333 ± 19 | 97.9 | 97.3 | |
| 39 | ND | 2260 | 2100 | 2180 ± 80 | 99.5 | 99.0 | |
| 40 | 5.72 | 959 | 2044 | 1500 ± 543 | 96.2 | 92.1 | |
| 41 | ND | 1265 | 109# | 687 ± 578 | 100 | 93.7 | |
| 42 | <LOD | 591ˆ | 911ˆ | 751 ± 160 | 99.4 | 99.4 | |
| 43 | <LOD | 109 | 74.9 | 92.0 ± 17.1 | 97.7 | 95.3 | |
| 44 | <LOD | 122ˆ | 348ˆ | 235 ± 113 | 100 | 100 | |
| 45 | <LOD | 180 | 108† | 144 ± 36 | 93.1 | 85.7 | |
Originally randomized to 500 μmol dose level but reassigned to 50 μmol dose level due to intolerance
Missed one void during the 12–24 hour collection period
Missed one void during 6–12 hour collection period
One void from 6–12 hour collection period inadvertently collected in the container allocated to the 12–24 hour collection period
Did not eat all of the assigned vegetables
Missed one void during 2–6 hour collection period
Missed 2 voids during the 6–12 hour collection period
LOD Level of detection
ND Not detectable
Table 2.
Intraclass correlation (ICC) in urinary DIM at 3 glucobrassicin dose levels.
ICC here is a measure of how similar urinary DIM levels from the two 24 h collections within an individual compare to the similarity between subjects at the same dose level.
| Glucobrassicin dose (μmol) | ICC |
|---|---|
|
| |
| 50 | 0.41 |
| 200 | 0.94 |
| 500 | 0.71 |
Figure 3.
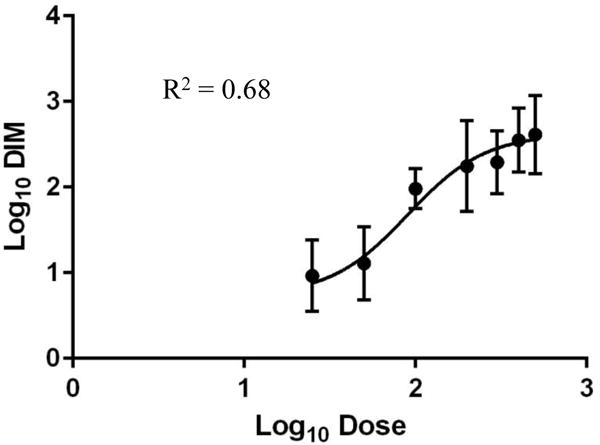
Dose curve between glucobrassicin dose (25–500 μmol) and urinary DIM. Bars represent standard deviation. Glucobrassicin dose ranged from 25 μmol to 500 μmol. Estimated parameters in the original scale (95% CI): Maximum DIM 421.5 pmol/mL (154.7, 1148.4), minimum DIM 5.4 pmol/mL (0.7, 44.3), EC50 90.2 μmol (29.1, 151.3).
DISCUSSION
We showed, for the first time in humans, that urinary DIM accurately reflects exposure to a wide range of glucobrassicin concentrations in vegetables. Ours is also the first study to demonstrate that urinary DIM excretion plateaus after eating vegetables. Similar pharmacokinetic profiles were observed when I3C or DIM was administered to humans as supplements.(24, 26) Interestingly, the dose of glucobrassicin at which the plateau in urinary DIM occurs after vegetable consumption is strikingly lower than when I3C or DIM are taken as supplements, suggestive of a vast difference in bioavailability. DIM, the predominant acid condensation heterodimer of I3C, has poor bioavailability as a supplement due to its poor water solubility, although an absorption-enhanced form is available.(28) The factors that influence the relative bioavailability of I3C or DIM after food consumption and the major route of their excretion in humans remain largely uncharacterized, although gastric pH influences the relative abundance of oligomers derived from I3C, whereby more acidic conditions favor the formation of higher order oligomers.(5) For this reason, participants in our study were asked to fast and were not on medications that might affect gastric pH. However, our approach of using urinary DIM to quantify I3C uptake potentially bypasses this issue and can mitigate other sources of variation in glucobrassicin/I3C exposure (despite individuals eating identical amounts of glucobrassicin) that occur prior to gastric absorption, such as cultivar, growing conditions, and preparation method.(3, 29) Furthermore, DIM appears to be a good surrogate biomarker, as I3C itself is rapidly hydrolyzed and is therefore quickly undetectable in vivo, whereas DIM is quite stable.(10, 24) Importantly, our data support the notion that the cancer-preventive properties that might be derived from cruciferous vegetable consumption may not require a large quantity of vegetables nor high-dose supplements, which certainly has practical implications.(30) The optimal dose and duration of vegetable consumption remain to be worked out.
Our results indicate that DIM is excreted in the urine rapidly after vegetable consumption, consistent with pharmacokinetic studies done after single- and multiple-dose I3C(24) and single-dose DIM,(26) and consistent with our prior study.(25) This implies that daily consumption may be necessary to maximize phytochemical exposure. Additionally, our data suggest that a full 24 h urine collection may not be necessary to capture the magnitude of I3C exposure.
Consistent with prior studies,15,(31) we observed inter-individual variability in 24 h urinary DIM levels at each glucobrassicin dose level. However, the correlation between measured and predicted DIM remained very strong. At moderate to higher doses (200 and 500 μmol glucobrassicin), we did not see significant variability between the two 24 h urinary DIM measurements from an individual subject. In other words, the majority of the variability within a dose level occurs between subjects, not within a subject. Variability at the 50 μmol dose level was high, likely due to the very small amount of vegetables fed at this dose level. These data suggest that a single urine collection after consuming a known dose of glucobrassicin, in amounts that would reasonably be consumed in a meal, is fairly reliable. Additionally, the inter-individual variability we observed may reflect the relative benefit an individual derives from consuming glucobrassicin from vegetables, responsive not only to how much glucobrassicin was consumed, but also to variations in I3C uptake and DIM metabolism, many of which are not characterized. We will explore this hypothesis in future studies.
In conclusion, the amount of DIM excreted in the urine correlates with the amount of glucobrassicin consumed from vegetables, making it an easily accessible, non-invasive biomarker of glucobrassicin exposure and I3C uptake. Feeding glucobrassicin beyond 200 μmol, or ~100 g of raw Brussels sprouts, did not consistently lead to more urinary DIM. This represents a first step in defining a biologically relevant dose of Brassica vegetable consumption based on glucobrassicin. Our work sets the stage for objectively quantifying I3C uptake from the diet in epidemiological studies, overcoming major limitations of observational studies. Furthermore, we can now correlate I3C uptake in glucobrassicin-based vegetable feeding interventions with outcomes in chemoprevention studies.
Supplementary Material
Figure 2.
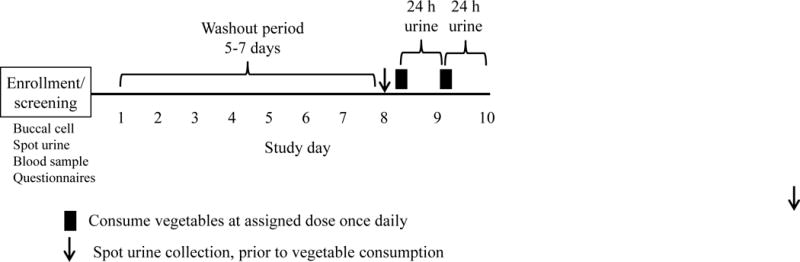
Clinical trial schema. The study intervention consisted of consuming the assigned dose of Brussels sprouts, cabbage, or a mixture for two consecutive days. Each vegetable-feeding session was followed by a 24 h urine collection.
Acknowledgments
N. Fujioka was supported in part by NHLBI training grant 5T32HL007062-34 (Gregory Vercellotti, PI). This work was supported in part by NIH P30 CA77598 (Douglas Yee, PI) utilizing the Masonic Cancer Center Analytical Biochemistry shared resource.
Footnotes
Conflict of Interest Disclosure: The authors have no conflicts of interest to disclose.
References
- 1.Herr I, Büchler MW. Dietary constituents of broccoli and other cruciferous vegetables: Implications for prevention and therapy of cancer. Cancer Treat Rev. 2010;36:377–83. doi: 10.1016/j.ctrv.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:733–48. [PubMed] [Google Scholar]
- 3.Ciska E, Martyniak-Przybyszewska B, Kozlowska H. Content of glucosinolates in cruciferous vegetables grown at the same site for two years under different climatic conditions. J Agric Food Chem. 2000;48:2862–7. doi: 10.1021/jf981373a. [DOI] [PubMed] [Google Scholar]
- 4.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci USA. 1997;94:10367–72. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grose KR, Bjeldanes LF. Oligomerization of indole-3-carbinol in aqueous acid. Chem Res Toxicol. 1992;5(2):188–93. doi: 10.1021/tx00026a007. [DOI] [PubMed] [Google Scholar]
- 6.Dashwood RH, Uyetake L, Fong AT, Hendricks JD, Bailey GS. In vivo disposition of the natural anti-carcinogen indole-3-carbinol after PO administration to rainbow trout. Food Chem Toxicol. 1989;27:385–92. doi: 10.1016/0278-6915(89)90144-0. [DOI] [PubMed] [Google Scholar]
- 7.Stresser DM, Williams DE, Griffin DA, Bailey GS. Mechanisms of tumor modulation by indole-3-carbinol. Disposition and excretion in male Fischer 344 rats. Drug Metab Dispos. 1995;23:965–75. [PubMed] [Google Scholar]
- 8.Anderton MJ, Jukes R, Lamb JH, Manson MM, Gescher A, Steward WP, et al. Liquid chromatographic assay for the simultaneous determination of indole-3-carbinol and its acid condensation products in plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;787:281–91. doi: 10.1016/s1570-0232(02)00923-6. [DOI] [PubMed] [Google Scholar]
- 9.Bjeldanes LF, Kim JY, Grose KR, Bartholomew JC, Bradfield CA. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc Natl Acad Sci USA. 1991;88:9543–7. doi: 10.1073/pnas.88.21.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderton MJ, Manson MM, Verschoyle RD, Gescher A, Lamb JH, Farmer PB, et al. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin Cancer Res. 2004;10:5233–41. doi: 10.1158/1078-0432.CCR-04-0163. [DOI] [PubMed] [Google Scholar]
- 11.International Agency for Research on Cancer. IARC handbooks of cancer prevention. Lyon, France: IARC Press; 2004. Cruciferous vegetables, isothiocyanates and indoles. [Google Scholar]
- 12.Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;4:1201–15. doi: 10.4161/cc.4.9.1993. [DOI] [PubMed] [Google Scholar]
- 13.Kassie F, Anderson LB, Scherber R, Yu N, Lahti D, Upadhyaya P, et al. Indole-3-carbinol inhibits 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone plus benzo(a)pyrene–induced lung tumorigenesis in A/J mice and modulates carcinogen-induced alterations in protein levels. Cancer Res. 2007;67:6502–11. doi: 10.1158/0008-5472.CAN-06-4438. [DOI] [PubMed] [Google Scholar]
- 14.Song JM, Qian X, Teferi F, Pan J, Wang Y, Kassie F. Dietary diindolylmethane suppresses inflammation-driven lung squamous cell carcinoma in mice. Cancer Prev Res. 2015;8:77–85. doi: 10.1158/1940-6207.CAPR-14-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kassie F, Matise I, Negia M, Upadhyaya P, Hecht SS. Dose-dependent inhibition of tobacco smoke carcinogen–induced lung tumorigenesis in A/J mice by indole-3-carbinol. Cancer Prev Res. 2008;1:568–76. doi: 10.1158/1940-6207.CAPR-08-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian X, Melkamu T, Upadhyaya P, Kassie F. Indole-3-carbinol inhibited tobacco smoke carcinogen-induced lung adenocarcinoma in A/J mice when administered during the post-initiation or progression phase of lung tumorigenesis. Cancer Lett. 2011;311:57–65. doi: 10.1016/j.canlet.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell MC, Crowley-Nowick P, Bradlow HL, Sepkovic DW, Schmidt-Grimminger D, Howell P, et al. Placebo-controlled trial of indole-3-carbinol in the treatment of CIN. Gynecol Oncol. 2000;78:123–9. doi: 10.1006/gyno.2000.5847. [DOI] [PubMed] [Google Scholar]
- 18.Wong GY, Bradlow L, Sepkovic D, Mehl S, Mailman J, Osborne MP. Dose-ranging study of indole-3-carbinol for breast cancer prevention. J Cell Biochem Suppl. 1997;28–29:111–6. doi: 10.1002/(sici)1097-4644(1997)28/29+<111::aid-jcb12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 19.Bradlow HL, Michnovicz JJ, Halper M, Miller DG, Wong GY, Osborne MP. Long-term responses of women to indole-3-carbinol or a high fiber diet. Cancer Epidemiol Biomarkers Prev. 1994;3:591–5. [PubMed] [Google Scholar]
- 20.Michnovicz JJ, Adlercreutz H, Bradlow HL. Changes in levels of urinary estrogen metabolites after oral indole-3-carbinol treatment in humans. J Natl Cancer Inst. 1997;89:718–23. doi: 10.1093/jnci/89.10.718. [DOI] [PubMed] [Google Scholar]
- 21.Michnovicz JJ, Bradlow HL. Altered estrogen metabolism and excretion in humans following consumption of indole-3-carbinol. Nutr Cancer. 1991;16:59–66. doi: 10.1080/01635589109514141. [DOI] [PubMed] [Google Scholar]
- 22.Dalessandri KM, Firestone GL, Fitch MD, Bradlow HL, Bjeldanes LF. Pilot study: effect of 3,3′-diindolylmethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr Cancer. 2004;50:161–7. doi: 10.1207/s15327914nc5002_5. [DOI] [PubMed] [Google Scholar]
- 23.Muti P, Bradlow HL, Micheli A, Krogh V, Freudenheim JL, Schünemann HJ, et al. Estrogen metabolism and risk of breast cancer: a prospective study of the 2:16α-hydroxyestrone ratio in premenopausal and postmenopausal women. Epidemiology. 2000;11:635–40. doi: 10.1097/00001648-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Reed GA, Arneson DW, Putnam WC, Smith HJ, Gray JC, Sullivan DK, et al. Single-dose and multiple-dose administration of indole-3-carbinol to women: pharmacokinetics based on 3,3′-diindolylmethane. Cancer Epidemiol Biomarkers Prev. 2006;15:2477–81. doi: 10.1158/1055-9965.EPI-06-0396. [DOI] [PubMed] [Google Scholar]
- 25.Fujioka N, Ainslie-Waldman CE, Upadhyaya P, Carmella SG, Fritz VA, Rohwer C, et al. Urinary 3,3′-diindolylmethane: a biomarker of glucobrassicin exposure and indole-3-carbinol uptake in humans. Cancer Epidemiol Biomarker Prev. 2014;23:282–7. doi: 10.1158/1055-9965.EPI-13-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed GA, Sunega JM, Sullivan DK, Gray JC, Mayo MS, Crowell JA, et al. Single-dose pharmacokinetics and tolerability of absorption-enhanced 3,3′-diindolylmethane in healthy subjects. Cancer Epidemiol Biomarkers Prev. 2008;17:2619–24. doi: 10.1158/1055-9965.EPI-08-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Reg. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 28.Anderton MJ, Manson MM, Verschoyle R, Gescher A, Steward WP, Williams ML, et al. Physiological modeling of formulated and crystalline 3,3′-diindolylmethane pharmacokinetics following oral administration in mice. Drug Metab Dispos. 2004;32:632–8. doi: 10.1124/dmd.32.6.632. [DOI] [PubMed] [Google Scholar]
- 29.Rosen CJ, Fritz VA, Gardner GM, Hecht SS, Carmella SG, Kenney PM. Cabbage yield and glucosinolate concentrations as affected by nitrogen and sulfur fertility. HortScience. 2005;40:1493–8. [Google Scholar]
- 30.Fahey JW, Talalay P, Kensler TW. Notes from the field: “green” chemoprevention as frugal medicine. Cancer Prev Res. 2012;5:179–88. doi: 10.1158/1940-6207.CAPR-11-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Getahun SM, Chung F-L. Conversion of glucosinolates to isothiocyanates in humans after ingestion of cooked watercress. Cancer Epidemiol Biomarkers Prev. 1999;8:447–51. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


