SUMMARY
The frequency of human social and emotional disorders varies significantly between males and females. We have recently reported that oxytocin receptor interneurons (OxtrINs) modulate female sociosexual behavior. Here we show that in male mice OxtrINs regulate anxiety-related behaviors. We demonstrate that corticotropin releasing hormone binding protein (CRHBP), an antagonist of the stress hormone CRH, is specifically expressed in OxtrINs. Production of CRHBP blocks the CRH-induced potentiation of postsynaptic layer 2/3 pyramidal cell activity of male but not female mice, thus producing an anxiolytic effect. Our data identify OxtrINs as critical for modulation of social and emotional behaviors in both females and males, and reveal a molecular mechanism that acts on local mPFC circuits to coordinate responses to OXT and CRH. They suggest that additional studies of the impact of the OXT/OXTR and CRHBP/CRH pathways in males and females will be important in development of gender specific therapies.
Keywords: cerebral cortex, sexually dimorphic, Crhbp, male anxiety, OxtrINs, Oxytocin
INTRODUCTION
Gender specific differences in the occurrence of social and emotional disorders are well documented. Autism spectrum disorder (ASD), which is characterized by impaired social communication and restrictive, repetitive behaviors, is severalfold more prevalent in males than females (Rubenstein et al, 2015). In contrast, anxiety-related disorders such as post-traumatic stress disorder, generalized anxiety disorder, panic disorder, and social anxiety disorder occur approximately twice as frequently in females than males (Altemus et al, 2014; Maeng and Milad, 2015). Although recent studies in rodents (Yang et al, 2013; Lee et al, 2014; Kunwar et al, 2015; Scott et al, 2015) and humans (Wigton et al et al, 2015; Insel, 2016) have provided strong evidence that differences in hormonal balance and brain circuitry between males and females can contribute to complex behaviors, the specific mechanisms that are responsible for gender biases in the occurrence and treatment of social and emotional disorders are not understood.
Oxytocin (OXT) is a peptide hormone that has been shown to contribute to many aspects of social behavior (Insel, 2010; Neumann and Slattery, 2015; Guastella and Hickie, 2016). It is released in response to a variety of social cues and acts through oxytocin receptors (OXTR) that are widely dispersed in the brain and periphery. Studies of behaviors altered in response to systemic or local administration of OXT and analysis of behavioral phenotypes evident in knockout mice lacking Oxt or Oxtr have demonstrated definitively that this hormone system plays essential roles in maternal care, social cognition and affiliative behaviors. Stressful and anxiogenic stimuli result in release of OXT, which acts as a powerful modulator of anxiety and stress related behaviors. These studies have led to a great deal of interest in OXT as a potential treatment for human social disorders, resulting in a large number of clinical trials to assess its therapeutic efficacy. Although the results of these trials have been encouraging in the context of ASD and anxiety disorders, our understanding of the brain circuits engaged by endogenous or exogenously supplied OXT and their role in specific behaviors remains incomplete.
We have recently identified a specific class of interneurons (OxtrINs) in the mouse medial prefrontal cortex (mPFC) that express Oxtr and are activated in response to OXT (Nakajima et al, 2014). Genetic and pharmacological studies of these neurons revealed that they modulate female social interactions with male mice during the sexually responsive, estrus phase of their cycle. Although the number of OxtrINs in male and female mPFC is equivalent, social behavior of male mice was not altered by silencing of OxtrINs or blockade of OXT signaling in the mPFC. While it is possible that these neurons are dedicated to modulation of social behaviors in female mice, OXT administration can impact a wide range of behaviors in both males and females. Furthermore, human imaging studies have indicated that OXT elicits activity in cortical and subcortical sites in both sexes, although gender specific differences in these responses have been noted (Bethlehem et al, 2013; MacDonald, 2013). It seems probable, therefore, that OxtrINs in the mPFC of male mice also modulate behavior in response to changing levels of OXT, but that gender specific differences in physiology or circuitry result in sexually dimorphic behavioral outcomes.
Here we report that optogenetic activation of OxtrINs in male mice has a strong anxiolytic effect and no impact on social interaction, whereas activation of these neurons in female mice results in increased sociality and no change in anxiety-related behaviors. Postsynaptic responses to OxtrINs activation in cortical pyramidal cells are primarily inhibitory. Layer 2/3 neurons respond more robustly in male mice, whereas postsynaptic responses in layer 5 are enhanced in females. TRAP translational profiling (Heiman et al, 2008) revealed that expression of the corticotropin releasing hormone binding protein (Crhbp) gene (Van Den Eede et al, 2005), an inhibitor of the stress hormone CRH (Laryea et al, 2012), is specifically enriched in OxtrINs. Upon CRH application, induced activity of male but not female layer 2/3 pyramidal cells in mPFC slice recordings was potentiated. This response to CRH was blocked by optogenetic stimulation of OxtrINs, and enhanced by bath application of a CRHBP antagonist. Infusion of OXT into the mPFC resulted in a significant decrease of anxiety-related behavior in male mice. This anxiolytic effect was blocked by co-infusion of a CRHBP antagonist. Furthermore, shRNA mediated knockdown of Crhbp expression in OxtrINs of the mPFC resulted in increased anxiety related behaviors only in male mice. Finally, CRH expression in female mice is strongly elevated. Taken together, these data identify a molecular mechanism regulating male specific anxiety-related behaviors. They demonstrate that OXT acts through OxtrINs and CRHBP to moderate the anxiogenic effects of the stress hormone CRH in the mPFC. When taken together with our previous study of the role of OxtrINs in female sociosexual behavior, these results provide an important illustration of the concept that higher brain circuits regulating complex behaviors may be identical in males and females, yet retain the ability to generate gender specific behaviors based on differential sensitivity to sexually dimorphic hormones. They suggest that coordination of the local actions of OXT and CRH in the cerebral cortex by OxtrINs and CRHBP may be involved in the modulation of a wide variety of gender specific cognitive and behavioral functions.
RESULTS
OxtrINs in the mPFC of Male Mice Modulate Anxiety-Related Behaviors
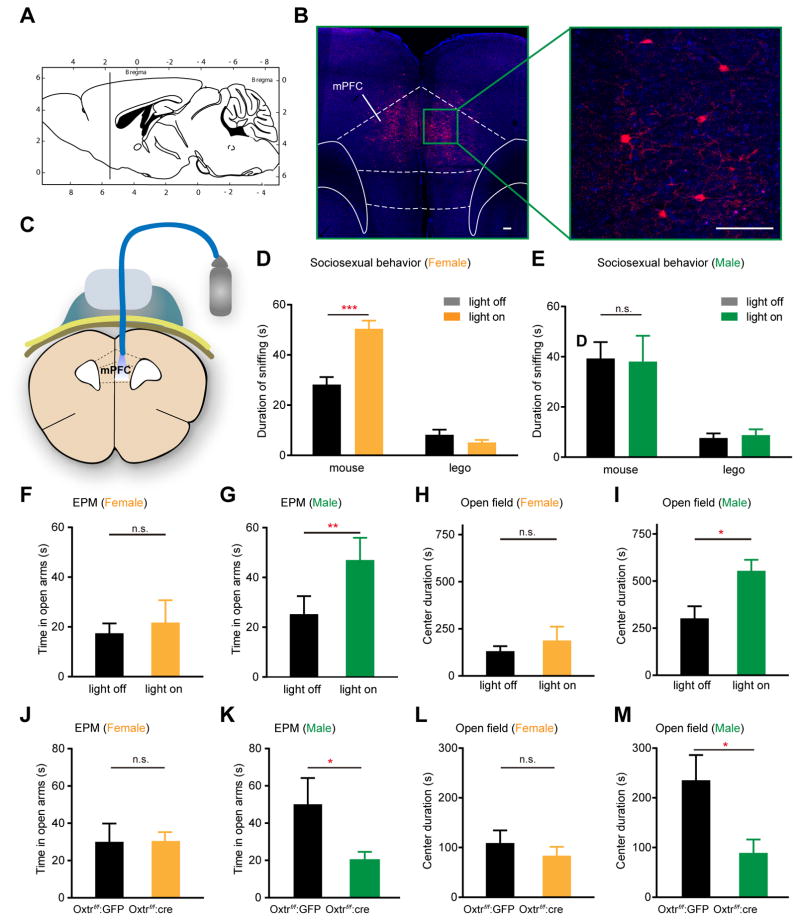
To investigate the function of OxtrINs in male mice, we performed two types of genetic manipulations. We first used Oxtr-Cre mice (Nakajima et al, 2014) for optogenetic activation of OxtrINs (Figure 1A–I). For this purpose, the mPFC of male and female Oxtr-Cre mice were stereotactically injected with a Cre-dependent AAV virus expressing channelrhodopsin (ChR2-H134R), and fiber-optic cannulas were implanted in the prelimbic cortex (Figure 1A–C; Figure S1A–D). Behavioral responses during blue light (BL) on and off periods were scored in the three-chamber social interaction test, the open field test (OF), and the elevated plus maze (EPM). Optogenetic activation of OxtrINs in the mPFC of female mice promoted social preference toward male mice (Figure 1D), consistent with the suppression of sociosexual preference observed by silencing these same neurons (Nakajima et al, 2014). However, male mice showed no significant changes in social interaction upon light activation of OxtrINs (Figure 1E). Given that the oxytocin system can also affect behavioral responses to stress (Neumann and Slattery, 2015), we next examined whether OxtrINs can contribute to the regulation of anxiety-related behaviors in males. Avoidance of open arms of the elevated plus maze and decreased exploration time in the center of an open field are established behavioral tests that are used to assess anxiety-like behavior in rodents. In female mice, activation of OxtrINs by blue light had no impact on the behavior of the animals in either of these assays (Figure 1F, H). However, optogenetic activation of OxtrINs in the mPFC of male mice resulted in more exploration of the open arms of the EPM (Figure 1G) and increased time in the center area of the OF arena (Figure 1I) compared to the light-off phase. As expected, the strength of the anxiety phenotype in male mice correlated directly with the number of OxtrINs expressing ChR2 (Figure S1A–C). These data demonstrate that activation of OxtrINs in the mPFC results in the induction of distinct gender specific behaviors. They confirm our previous studies demonstrating a role for OxtrINs in female sociosexual behavior (Nakajima et al, 2014), and establish a role for these same neurons in the modulation of anxiety-related behaviors in male mice.
Figure 1. Sex-specific behavioral responses to OxtrINs function in the mPFC.
(A) Scheme indicating the mPFC area where AAV2.5-EF1-α-DIO-ChR2-mCherry was injected in Oxtr-Cre mice (referred to as Oxtr-Cre::ChR2)
(B) Coronal section Oxtr-Cre::ChR2 mice showing expression of ChR2-mCherry in OtxrINs in mPFC. Scale bars 100 μm.
(C) Diagram showing the optic fiber in the prelimbic cortex (PrL) for photostimulation of Oxtr interneurons in the mPFC in Oxtr-Cre::ChR2 mice.
(D) Photostimulation (light on, 10 Hz) in Oxtr-Cre::ChR2 female mice in the three-chamber social interaction test significantly increased social preference for male mice compared to time epochs without photostimulation (light off). n=9 mice per group. Two-way ANOVA, Bonferroni post test. *** p < 0.001.
(E) Photostimulation (light on, 10 Hz) in Oxtr-Cre::ChR2 male mice did not increase social preference for female mice compared to time epochs without photostimulation. n=8 mice per group. Two-way ANOVA, Bonferroni post-test. n.s. p>0.05
(F–I) Optogenetic stimulation of the mPFC in Oxtr-Cre::ChR2 increased open-arm time in the EPM (G) and center time on the OF (I) during light-on in males, but not in females (F, H). F to G, n=16 mice per group; Wilcoxon signed rank test p=0.78 in F and p=0.0017 in G; H to I, n=7 to 9, Mann Whitney test. p>0.9 in H and p=0.0164 in I.
(J–K) Conditional Oxtr female mice (Oxtrf/f) injected with AAV-Cre (Oxtrf/f:Cre), showed no changes in open-arm time (J) and center time in the OF (L) relative to Oxtrf/f:GFP controls. Male Oxtrf/f:Cre mice spent significantly less time in the open arms (K) and in the center of the OF (M) than controls. For J, n=10 to 20 mice per group; For K to M, n=7 to 9 mice per group. Mann Whitney test. p=0.64 in J and p=0.04 in K; p=0.82 in L and p=0.03 in M.
Data are represented as mean ± SEM.
See also Figure S1.
To determine whether the anxiolytic effect of OxtrINs in male mice requires OXT/OXTR signaling as we have shown previously for sociosexual behavior in females (Nakajima et al, 2014), we conducted a second set of experiments using Cre expressing AAV viruses injected into mice carrying a conditional allele of the Oxtr gene (Oxtrflox/flox) (Lee et al, 2008). We bilaterally injected the mPFC of adult male and female Oxtrflox/flox mice with AAV expressing Cre to delete the receptor (Oxtrf/f:Cre). As a control, we injected control Oxtrflox/flox mice with an AAV expressing GFP (Oxtrf/f:GFP) that cannot result in deletion of Oxtr. As shown in Figure 1 (J, L), deletion of the Oxtr in female mice has no significant effect on performance in either the OF or EPM assays. However, in male mice deletion of Oxtr resulted in a strong anxiogenic effect. Thus, male Oxtrf/f:Cre mice spent significantly less time in open arms of the EPM and in the center of the open field arena than did the control male Oxtrf/f:GFP mice (Figure 1K,M). There were no significant differences in anxiety levels between control males and females (Figure 1F–M), and between females during estrus and diestrus (Figure S1E). There were also no major differences in total locomotor behavior as a consequence of optogenetic activation or Oxtr deletion, although a slight increase in female locomotion in the open field test was observed with Oxtr deletion (Figure S1F–I). We conclude that OXT action on OxtrINs in the mPFC regulates distinct, gender specific behaviors.
Postsynaptic Responses to OxtrINs Activation are Primarily Inhibitory
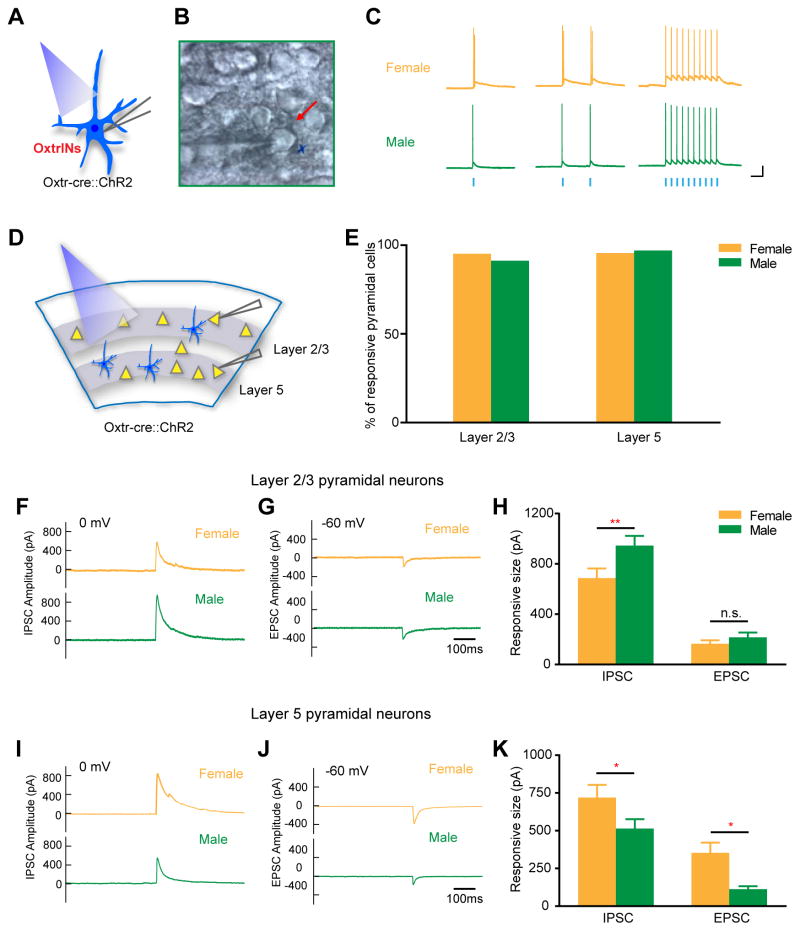
OxtrINs are regular spiking, GABAergic interneurons that increase their rate of firing in response to OXT (Nakajima et al, 2014). To investigate the mechanism by which OxtrINs activation regulates local circuit activity, we performed whole cell recordings of layer 2/3 and layer 5 pyramidal neurons in acute brain slices of the mPFC during photostimulation of ChR2 expressing OxtrINs (Figure 2). Brief light stimulation (470nm) efficiently evoked action potentials in ChR2-mCherry expressing OxtrINs in slice preparations (Figure 2A–C). We observed no differences in the response of male and female OxtrINs to BL at different frequencies (Figure 2C). To measure postsynaptic responses to OxtrIN activation, light-evoked responses of layer 2/3 and layer 5 pyramidal neurons (Figure 2D) were recorded at 0mV and −60mV holding potentials, allowing independent detection of both GABA and AMPA receptor mediated currents (Figure S2A, B). OxtrINs synapse extensively with layer 2/3 and layer 5 pyramidal neurons in both genders. Thus, in males 91.3% of layer 2/3 and 97% of layer 5 neurons responded to OxtrINs activation, whereas 95.2% of layer 2/3 and 95.6% of layer 5 pyramidal neurons responded in females (Figure 2E).
Figure 2. Postsynaptic Responses to OxtrINs Activation are Primarily Inhibitory.
(A–B) Diagram (A) and photomicrograph (B) of mPFC brain slice showing the recordings in OxtrINs expressing ChR2.
(C) Female and Male OxtrINs-ChR2 respond similarly to single and consecutive blue light pulses (blue squares). Scale bar: 100ms, 20mV.
(D) mPFC scheme showing the recordings in layer 2/3 and layer 5 pyramidal cells (yellow triangles) upon blue light stimulation of OxtrINs (blue) expressing ChR2.
(E) High percentage of pyramidal cells in layer 2/3 and layer 5 responded to OxtrINs optogenetic stimulation in female and male mice. n=21 to 29 cells.
(F–H) Representative traces and quantitation of IPSCs and EPSCs amplitudes in layer 2/3 cells in male and female mPFC slices. n=16 cells recorded in female and n=23 cells recorded in male. Two-way ANOVA, Bonferroni’s multiple comparison test. ** p<0.01.
(I–K) Representative traces and quantitation of IPSCs and EPSCs amplitudes in layer 5 pyramidal neurons in male and female. n=21 cells recorded in female and n=29 cells recorded in male. Two-way ANOVA, Bonferroni’s multiple comparison test. * p<0.05.
Data are presented as mean ± SEM.
See also Figure S2.
Analysis of the amplitudes of postsynaptic responses elicited by optogenetic activation of OxtrINs revealed that GABAergic responses to OxtrINs activation differed between males and females (Figure 2F–K). Inhibitory postsynaptic currents (IPSCs) elicited in response of BL activation were significantly larger in male layer 2/3 pyramidal neurons (Figure 2F, H), whereas IPSCs in layer 5 postsynaptic neurons were larger in females (Figure 2I, K). These differences in IPSCs between male and female were observed at different BL intensities (Figure S2C, D). Excitatory postsynaptic current (EPSC) amplitudes were also larger in females in layer 5 (Figure 2J, K). No differences in EPSC amplitudes in layer 2/3 (Figure 2G, H) were observed between male and female neurons. Thus, the electrophysiological evidence presented here establishes that there is broad connectivity between OxtrINs and pyramidal cells in the mPFC of both sexes, and that the strength of inhibition in male and female neurons in the mPFC is both laminar and gender specific.
Translating Ribosome Affinity Purification (TRAP) Reveals Cell-Specific Enrichment of Corticotropin Releasing Hormone Binding Protein (CRHBP) in OxtrINs
Although the differences in postsynaptic inhibitory responses we observed between male and female neurons may explain the gender-specific anxiolytic effect of OXT, the response differences between genders suggested that additional factors might be involved. To gain insight into signaling pathways that are active in OxtrINs and to elucidate candidate mechanisms that might play a role in the anxiolytic effects of OxtrINs activation, we employed Translational Ribosome Affinity Purification (TRAP) to identify proteins actively synthesized in these cells in the mPFC. This was accomplished using Oxtr-Cre mice (Nakajima et al, 2014) crossed to EGFP-L10a reporter mice (Liu et al, 2014) (Figure S3A, B), followed by TRAP profiling as described previously (Heiman et al, 2008). Six replicate samples were collected from the mPFC of Oxtr-Cre/EGFP-L10a mice and the resulting RNA sequenced from each sample. Normalized values from each of the 6 replicates, and their averages, were obtained for both the INPUT and IP samples (Table S1). These data provided us with a more comprehensive and quantitative list of highly translated mRNAs than that available from our previous microarray analyses, and allowed us to confirm the reported (Nakajima et al, 2014) high expression and enrichment of somatostatin (Sst), glutamic acid decarboxylase 1 (Gad1), and Oxtr in OxtrINs. As shown in the scatter plot in Figure 3A, these markers are expressed at quite different levels, and each of them is significantly enriched in the OxtrINs IP data relative to the sample taken from the intact mPFC prior to immunoprecipitation (INPUT). To evaluate expressed genes as possible candidates for further analysis, we first removed genes whose mRNAs were present at low levels (Base Mean < 25.00), and then sorted for mRNAs that are highly enriched in the OxtrINs IP data (log2 Fold Change IP v INPUT). This resulted in the identification of a large number of known interneuron markers, and a variety of transcription factors, receptors and signaling proteins that were not known previously to be enriched in OxtrINs (Table S1).
Figure 3. TRAP profiling reveals cell-specific enrichment of corticotropin releasing hormone binding protein (CRHBP) in OxtrINs.
(A) Scatterplot of average TRAP IP samples (Y axis) versus INPUT samples (X axis) from the mPFC of Oxtr-EFGP-L10a mice. Sst, Gad1, Crhbp and Oxtr are highly enriched in the OxtrINs IP population (red). Crhr1 (green) is not enriched in the IP.
(B) The top ten most highly enriched translated mRNAs in OxtrINs. Base mean is the average expression in all samples (IP and INPUT). Log2 Fold Change is the enrichment in IP compared to INPUT.
(C) Analyses of cell-specific translated mRNAs displayed using the Integrated Genomics Viewer (IGV 2.3). Positive control genes expressed in each cell type are indicated on the top row. Chrbp (last column, red arrow) is specifically expressed at very high levels in OxtrINs, and at lower levels in a broad class of non-fast spiking interneurons that includes OxtrINs.
An intriguing candidate for further analysis, the corticotropin releasing hormone binding protein (Crhbp), was among the most highly enriched mRNAs in OxtrINs (log2 Fold Change = 4.64; Figure 3B). CRHBP is a small, secreted glycoprotein that binds to corticotropin releasing hormone (CRH) with equal or greater affinity than do the CRH receptors. The primary functional role of CRHBP is to bind and limit the bioavailability of CRH, thus diminishing the activity of CRH receptors (Westphal and Seasholtz, 2006). CRH and CRH receptors have been shown to be critical regulators of stress and anxiety (Van Den Eede et al, 2005), suggesting that production of CRHBP by OxtrINs may be important in the regulation of the male specific anxiety-related behaviors (Figure 1). To confirm that Crhbp expression is specific, two additional datasets were interrogated. First, comparative expression of Crhbp in 20 cell types was evaluated using our internal database of cell specific TRAPseq data. As shown in Figure 3C, Crhbp expression is very robust in OxtrINs relative to the other CNS cell types presented. The only other dataset in which Crhbp expression is evident is that collected from a broad population of Dlx1 expressing, non-fast spiking cortical interneurons that includes OxtrINs (Nakajima et al, 2014). Second, in situ hybridization data from the Allen Brain Institute (http://www.brain-map.org/.brain) was surveyed to determine whether the patterns of Crhbp and Crhr1 are consistent with our data (Figure S3C, D). Although specific cell type localization is not possible to determine from the ISH data, the scattered pattern of Crhbp positive cells in the mPFC is consistent with expression in interneurons. Given these data, we employed fluorescence in situ hybridization (FISH) to confirm that OxtrINs express CRHBP (Figure S3E). The limited sensitivity this approach did not allow accurate determination of the co-expression of Oxtr and Crhbp, although we reproducibly detected CRHBP in >50% of OxtrINs. The significance of this result is enhanced both by the finding that the CRH receptor (Crhr1), which was present in the input cortical samples but not enriched in the IP (Figure 3A), is expressed specifically in layer 2/3 pyramidal cells (Figure S3D) and by our recent demonstration that layer 2/3 pyramidal cells in the mPFC can mediate stress-induced depressive behaviors (Shrestha et al, 2015).
The Response of Layer 2/3 Pyramidal Neurons to CRH is Enhanced in Male Mice and Blocked by Optogenetic Stimulation of OxtrINs
The CRH/CRHR1 system is considered fundamental for the regulation of stress and anxiety in both experimental animals and humans (Altemus et al, 2014; Bangasser and Kawasumi, 2015). Genetic studies have established that loss of Crhr1 in glutamatergic cells in the cerebral cortex is anxiolytic (Refojo et al, 2011), and that CRH dysfunction can contribute to human anxiety related disorders (Weber et al, 2015; Smoller, 2016). Although no data suggesting an interaction of the OXT/OXTR and CRH/CRHR1 systems in the cerebral cortex has been reported previously, the specific and high expression of Crhbp in OxtrINs suggested to us that the anxiogenic effects of CRH on layer 2/3 pyramidal cells might be modulated by release of CRHBP from OxtrINs, and that differential sensitivity of this system may account for the male specific anxiolytic effects of OxtrINs activation. As a first test of this hypothesis, electrophysiological responses of layer2/3 pyramidal cells from female or male mPFC were assayed for their sensitivity to CRH, and for the influence of OxtrINs optogenetic activation on these responses (Figure 4). In each condition, the number of action potentials elicited (induced spikes) over an interval of 500ms was measured as the current was varied in steps of 50pA from −50pA to 400pA (Figure 4). Bath application of CRH (1μM) to female slice recordings resulted in a very small increase in activity at high current pulses (Figure 4B, C) that was insensitive to CRHR1 antagonists (Figure 4B, C). In contrast, in male slice recordings, CRH increased the number of induced spikes at all currents tested (Figure 4D, E). This response was suppressed by co-application of CRHR1 antagonist (Figure 4D, E). These results demonstrate that male layer 2/3 pyramidal cells are more sensitive to CRH than are those of female mice, and that this response is due to activation of the Crhr1 receptor. As predicted from the restricted expression of Crhr1 in layer 2/3 neurons, application of CRH to layer 5 pyramidal neurons did not alter induced spiking in recordings from either females or males (Figure S4A, B).
Figure 4. CRH enhances the response of layer 2/3 pyramidal neurons in males and this response is suppressed by optogenetic stimulation of OxtrINs.
(A) Scheme of the recordings in layer 2/3 neurons (in yellow) were recorded upon superfusion with CRH and upon blue light stimulation of OxtrINs (blue)
(B–C) Representative recordings and quantitation of induced spikes in female mice showing that addition of CRH (orange) and addition of CRH and CRHR1 antagonist (dark orange) do not increase action potential firing with respect to control conditions (grey) at increasing injected currents. n=8 to 15 cells per group. Two-way ANOVA, Bonferroni’s multiple comparison test. n.s. p>0.05.
(D–E) Representative recordings and quantitation of induced firing in male mice showing that CRH (green) significantly increases the number of induced spikes and that addition of a CRHR1 antagonist to CRH superfused slices (dark green) suppresses the response to CRH. n=14 to 25 cells per group. Two-way ANOVA, Bonferroni’s multiple comparison test. **** p<0.0001, n.s. p>0.05.
(F–G) Representative recordings and quantitation of induce spikes at different injected currents showing that blue light (blue) activation of OxtrINs suppresses the increase of firing mediated by CRH (green). n=10 to 25 cells per group. Two-way ANOVA, Bonferroni’s multiple comparison test. **** p<0.0001. n.s. p>0.05.
Data are presented as mean ± SEM.
See also Figure S4.
As shown in Figure 1, optogenetic stimulation of OxtrINs in the mPFC of male mice is anxiolytic. To determine whether activation of male layer 2/3 pyramidal cells by CRH could be modulated by OxtrINs, we again injected AAV-ChR2-H134R into the mPFC of Oxtr-Cre mice. Brain slices were prepared three weeks after viral injection to test whether CRH activation of layer 2/3 pyramidal cells could be measured in the presence or absence of OxtrINs stimulation by light. Optogenetic activation of OxtrINs in the slice preparation strongly diminished the activity of CRH on layer 2/3 male pyramidal cells at all current pulses (Figure 4F, G). BL alone did not significantly reduce induced spiking in male and female layer 2/3 neurons (Figure S4C, D). These data directly demonstrate that OxtrINs play an important role in the male mPFC circuit by inhibiting the activation of layer 2/3 neurons in response to the stress hormone CRH.
OxtrINs Production of CRHBP Regulates Anxiety in Male but not Female Mice
CRHBP binds to CRH and inhibits its ability to activate its receptors (Van Den Eede et al, 2005). Given the high and specific expression of Chrbp in OxtrINs (Figure 3 and S3), the robust potentiation of induced firing in pyramidal neurons of male mice by bath application of CRH, and the ability of optogenetic stimulation of OxtrINs to block the effects of CRH (Figure 4), we hypothesized that CRHBP released by OxtrINs binds to CRH and inhibits the stress inducing effects of CRH that are elicited by its binding to CRHR1 receptors in layer 2/3 pyramidal cells. To test this hypothesis, we performed electrophysiological recordings with an antagonist to CRHBP to determine whether ambient levels of CRHBP in the slice preparations influence the activity of layer 2/3 pyramidal cells. Application of a CRHBP antagonist augmented the number of induced spikes at increasing current clamp amplitudes (Figure 5A,B blue), indicating that CRHBP is present in the slice preparations and that it contributes to the observed activity of layer 2/3 pyramidal cells. Moreover, addition of exogenous CRH (Figure 5A, B green) further potentiated the effect of the CRHBP antagonist on induced activity. These data demonstrate that the balance of CRH and CRHBP is important for the regulation of local circuit activity in the mPFC, and that a major role for CRHBP is to limit the actions of CRH at CRHR1 receptors in layer 2/3 pyramidal cells.
Figure 5. Conditional Knockdown of Crhbp in OxtrINs increases anxiety in males but not females.
(A–B) Representative recordings in layer 2/3 pyramidal male neurons and quantitation of induced spikes show that application of CRHBP antagonist (1μM, blue) significantly increases induced firing with respect to control conditions (grey). Additional superfusion with CRH (1μM, green) further increases the number of induced spikes. n=10 to 15 cells per group. Two-way ANOVA, Bonferroni’s multiple comparison test. ****p<0.0001, **p<0.01.
(C–E) Oxtr-Cre female mice injected with Chrbp shRNA lentivirus showed no changes in EPM open-arm time (C), EPM open-arm entries (D) and open field center area entries (E) relative to Oxtr-Cre female mice injected with shRNA scramble control lentivirus. n=5 to 16 mice per group; Mann Whitney test, p=0.27 in C, p=0.41 in D and p=0.29 in E.
(F–H) Oxtr-Cre male mice injected with Chrbp shRNA lentivirus showed significantly reduced EPM open-arm time (F), EPM open-arm entries (G) and Open field center area entries (H) relative to Oxtr-Cre male mice injected with scramble shRNA lentivirus. n=8 to 14 mice per group; Mann Whitney test, p=0.04 in F, p=0.0006 in G and p=0.01 in H.
(I, J) Oxtr-Cre female (I) and male (J) mice injected with Chrbp shRNA lentivirus show no significant differences in the three-chamber social interaction test relative to Oxtr-Cre mice injected with shRNA scramble control. n=5 to 9 mice per group; Two-way ANOVA, Bonferroni’s multiple comparison test. n.s. p>0.05
(K) mPFC cannulated male mice were tested in the EPM after infusion of saline (light green), OXT (medium green) and OXT together with a CRHBP antagonist (dark green). Time in the open arms was significantly increased in male mice infused with OXT and the anxiolytic effect of OXT was blocked by coinfusion with the CRHBP antagonist (CRH 6–33). n=7 to 13 mice for each condition, One-way ANOVA, Bonferroni’s multiple comparison test. * p<0.05.
Data are presented as mean ± SEM.
See also Figure S5.
The simplest interpretation of the data we have presented thus far is that production of CRHBP in response to OXT activation of OxtrINs inhibits the anxiogenic effects of CRH, thus resulting in decreased anxiety-related behaviors. To assess this possibility in vivo and determine whether competition between CRH and CRHBP accounts for the anxiety phenotype in males, we designed lentiviral constructs to express shRNAs for conditional knockdown of Crhbp in Cre positive OxtrINs (LV-Crhbp shRNA and LV-scramble shRNA, Figure S5) (Ventura et al, 2004). Cre-dependent expression of the shRNA was monitored by western blot analyses of CHRBP in transfected cells (Figure S5C, D) and by loss of EGFP upon excision of the loxP flanked CMV-EGFP cassette preceding the shRNA cassette (Figure S5B). Cre-dependent shRNA expression reduced expression of CRHBP by 67% (Figure S5C, D). Given the strong inhibition observed in the in vitro studies, we next tested the effect of the shRNA in vivo. Accordingly, Oxtr-Cre mice were injected bilaterally in the mPFC with LV-Crhbp shRNA and LV-scramble shRNA and tested in the EPM and OF. Anxiety-like behaviors were not affected by shRNA mediated knockdown of Chrbp in female mice (Figure 5C–E). However, males expressing the Crhbp shRNA spent less time in the open arms, and entered the open arms and the center of the OF less frequently than did mice expressing the control scrambled shRNA (Figure 5F–H). These results clearly establish that Crhbp expression is essential for regulation of anxiety-related behaviors in male but not female mice. Furthermore, in spite of the critical role of OxtrINs in the regulation of social behavior and the expression of Crhbp in these neurons, knockdown of Crhbp in the mPFC of female mice had no impact on female sociosexual behavior (Figure 5I) or total locomotor behavior in male or female mice (Figure S5E, F). It is apparent, therefore, that CRHBP produced by OxtrINs acts as a key molecular modulator for anxiety related behaviors in male, but not female mice.
While the CRHBP knockdown data clearly establish that CRHBP production by OxtrINs is an essential component of the pathway regulating male anxiety, it remained possible that CRHBP acts chronically to establish sensitivity to CRH rather than to dynamically regulate its function. To address this possibility, OXT was infused into the prefrontal cortex of male and female mice in the presence or absence of the CRHBP antagonist. As shown in Figure 5K, administration of OXT results in a strong, acute anxiolytic effect in male mice that is blocked by the CRHBP antagonist. No change in anxiety-related behavior was evident in female mice in response to OXT. These data prove that OXT can act in the PFC to dynamically regulate anxiety in male mice, and that CRHBP acts downstream of OXT to mediate this anxiolytic effect.
CRH Expression is Elevated in Female Mice
The male specific anxiolytic effects of OxtrINs activation and its dependence on CRHBP could be the result of gender specific differences in the expression or activity of any of the components of this system. To explore this issue, we first measured the expression of Oxtr, Crhbp, Crhr1, and Xist in the mPFC (Figure 6A, B). Since Oxtr and Crhbp are both expressed in OxtrINs, we quantitated their expression in TRAPseq data from replicate samples of male and female IPs (Figure 6A, Table S2). Crhr1 is not expressed in OxtrINs but in nearby cortical neurons, and thus was measured in the INPUT samples (Figure 6A, Table S3). Crhr2 was not expressed at significant levels in the mPFC. Xist (X-inactive specific transcript) was measured as an internal control for gender specific expression in the INPUT of TRAP samples (Figure 6A, Table S3) because it is a non-coding RNA. No significant difference in expression of Oxtr, Crhbp or Crhr1 between male and female animals was observed (Figure 6A). RT-PCR experiments were performed (Figure 6B) to confirm the TRAP results, revealing no significant changes in the expression of Oxtr, Crhbp, or Crhr1 in the mPFC of males and females. It is interesting to note that in addition to the expected changes in expression of genes located on the X and Y chromosomes, a number of autosomal genes whose expression differs between genders are evident in these data (Table S2). While the magnitudes of these expression changes are small and none appear to be directly relevant to the OXT or CRH pathways, future studies of these genes may yield additional insights into gender specific functions of the mPFC.
Figure 6. Quantitation of Oxtr, Crhbp, Crhr1, Crh and Xist in female and male mice.
(A) Normalized TRAP values of IP samples showing no change in Oxtr, Crhbp and Crhr1 expression in mPFC. Female specific Xist expression is included as a gender specific control. n=3 mice per group, Two-way ANOVA, Bonferroni’s multiple comparison test. n.s. p>0.05. **** p<0.0001.
(B) RT-PCR of Oxtr, Crhbp and Crhr1 expression in mPFC samples. Normalization is to female values. n=6 mice per group, Two-way ANOVA, Bonferroni’s multiple comparison test. n.s. p>0.05.
(C–D) RT-PCR analyses of PVH samples normalized to male expression levels show that CRH is significantly higher in female mice. n=6 to 7 mice per group; Mann Whitney test, p=0.0047.
Data are presented as mean ± SEM.
See also Table S2.
We next turned our attention to CRH. Two facts support the hypothesis that differences in the levels of CRH between males and females might account for the male specific results presented thus far. First, the electrophysiological data we have presented in Figures 4 and 5 strongly support a model in which the balance of CRH and CRHBP determines the impact of this pathway on layer 2/3 pyramidal cell activity. Second, Crh expression is elevated in the female rat CNS (Iwasaki-Sekino et al, 2009). Thus, Crh expression was measured in the paraventricular nucleus of the hypothalamus (PVH) by RT-PCR. As shown in Figure 6C, D, the level of Crh present in the PVH of female mice is strongly elevated. These data both confirm previous studies cited above, and suggest that the high level of CRH present in female mice may account for female insensitivity to OxtrINs expression of Crhbp.
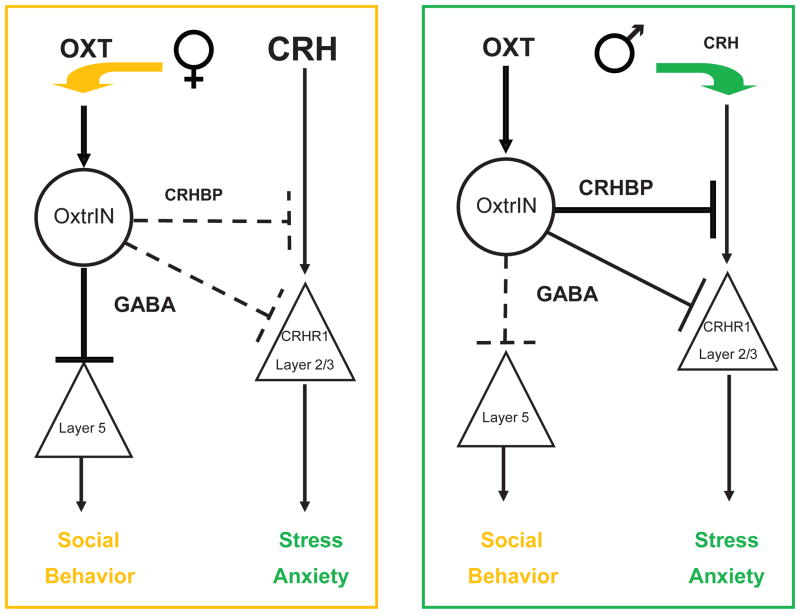
A Model to Illustrate the Sexually Dimorphic Role of OxtrINs in the mPFC
A model to illustrate the pathways involved in OxtrINs modulation of gender specific social and emotional behaviors is presented in Figure 7. The concept is simple: the prefrontal cortex is tuned to respond to the changing levels of OXT and CRH that occur in a variety of social and stressful situations. In both genders, OXT stimulation of OxtrINs results in release of the fast acting neurotransmitter GABA and the neuromodulator CRHBP. In female mice, the elevated levels of circulating CRH render the inhibitory action of CRHBP ineffective. Consequently, the dominant arm of the pathway in females (orange arrow) is GABAergic modulation of layer 5 pyramidal cells. These cells respond more robustly to GABA in the female mPFC, and we believe that it is this pathway that is responsible for the modulation of social behavior. In male mice, due to limiting amounts of CRH in the mPFC, the critical OxtrINs modulatory event (green panel) is the release of CRHBP, which intercepts CRH and inhibits its activation of layer 2/3 pyramidal cells expressing the CRHR1 receptor thus producing an anxiolytic effect. Although GABA may augment the actions of CRHBP in this arm of the circuit, CRHBP is absolutely required. The relative activities of these two pathways, and thus the behavioral output of the circuit, depends on three factors: the balance of CRHBP released from OxtrINs versus the circulating levels of CRH; the fine tuning of postsynaptic responses to GABA in layer 5 and layer 2/3; and the actions of the two sexually dimorphic hormones OXT and CRH.
Figure 7. A Model for mPFC Control of Social and Emotional Behaviors by OxtrINs.
Two pathways in the mPFC are coordinated by OxtrINs to generate prosocial or anxiolytic behaviors. In the OXT pathway (left side of both diagrams) activation of OxtrINs results in production of GABA and CRHBP. In females (orange diagram) this pathway predominates (orange arrow), because CRH levels are high (CRH large font) and release of CRHBP by OxtrINs has little effect. Consequently, in female mice pulses of OXT produce primarily a GABAergic inhibitory effect on layer 5 pyramidal neurons that regulate social behaviors. In contrast, in males (green diagram) the CRH pathway predominates (green arrow) because CRH levels are low (small font) and release of CRHBP is sufficient to bind to CRH, and suppress the activation of CRHR1 receptors on layer 2/3 pyramidal cells resulting in an anxiolytic effect.
DISCUSSION
Differences in social and emotional behaviors between males and females are common. We have recently identified OxtrINs as a class of mPFC interneurons that respond to OXT and modulate sociosexual behavior in female, but not male mice (Nakajima et al, 2014). Here we report that these same neurons in male, but not female mice regulate anxiety-related behaviors. This role for OxtrINs is also dependent on OXT. It is due to production of CRHBP by OxtrINs, which suppresses the anxiogenic effects of CRH on postsynaptic layer 2/3 pyramidal cells. Although the number of Oxtr interneurons and the expression of Oxtr, Crhbp, and Crhr1 in the mPFC do not differ between males and females, expression of Crh in the hypothalamus of female mice is strongly elevated. Thus, our results demonstrate that the sexually bivalent roles of mPFC OxtrINs in social and emotional behaviors arise from gender specific impacts on local circuitry that depend on distinct molecular pathways that confer sensitivity to sexually dimorphic hormones. They suggest that modulation of additional complex gender specific social, emotional and cognitive functions may rest upon the coordination of OXT and CRH actions by CRHBP expression in OxtrINs, or other cellular and molecular mechanisms that are fine-tuned to modulate local, higher order circuitry in response to remote sexually dimorphic cues.
Sexually Dimorphic Neural Circuits
Differences in male and female behaviors in mammals ultimately derive from the effects of gonadal hormones (estradiol, progesterone, testosterone) on the developing and adult nervous system (Pfaff and McEwen, 1883). While the consequences of these hormones on brain development and function must be many and varied, recent studies focusing on their impact on hypothalamic circuits have advanced substantially our knowledge of cell types controlling sexually dimorphic behaviors. For instance, ablation of progesterone receptor (PR) expressing neurons in the ventromedial hypothalamus (VMH) results in deficits in mating behavior in both sexes, yet reduces aggression in only males (Yang et al, 2013). PR expressing neurons differ in number, distribution and connectivity in the VMH of males and females, suggesting that the gender specific behavioral outcomes reflect the anatomical differences in PR expressing neurons between sexes (Yang et al, 2013). On the other hand, optogenetic activation of estrogen receptor (ESR1) expressing neurons in the VMH resulted in sexual approach behaviors in both males and females, and stimulated attack behavior in males only (Lee et al, 2014; Kunwar et al, 2015). In this case, ESR1 expressing cells are also sexually dimorphic, but attack behavior could be elicited simply by increasing photostimulation in male mice. It is clear from these studies that gender specific behaviors reflect sexually dimorphic features of VMH neuronal populations in this hypothalamic circuitry. This is also the case in the anteroventral periventricular (APVP) hypothalamic nucleus, where a sexually dimorphic population of tyrosine hydroxylase (TH) expressing neurons modulates parental care and oxytocin secretion specifically in females, while in males TH positive neurons in the APVP regulate inter-male aggression (Scott et al, 2015). These studies provide potent and precise examples of the importance of sexually dimorphic cells and circuits in the hypothalamus for regulation of gender specific sexual and aggressive behaviors in mammals. They suggest that identification and analysis of other sexually dimorphic modules will be a productive avenue for investigation of other sex specific behavioral biases (Yang and Shah, 2014).
Our studies of OxtrINs in the mPFC provide a different paradigm for the generation of gender specific complex behaviors. In this case, the critical cell type is not sexually dimorphic: OxtrINs number does not vary between males and females in the mPFC (Nakajima et al, 2014), and no gender specific differences in expression of the key signaling proteins that regulate female social behavior or male anxiety are evident (Figure 6). However, OxtrINs produce two mechanistically distinct modulatory substances, GABA and CRHBP, which act on local circuits in the mPFC to regulate behavior differentially. They do so because their function requires the sexually dimorphic hypothalamic hormones OXT and CRH, and because of the restricted expression of CRHR1 in superficial layers of the mPFC. Consequently, the high levels of OXT and CRH produced in females result predominantly in GABAergic control of postsynaptic cells by OxtrINs and modulation of social behavior. In males, the reduced production of CRH allows CRHBP released from OxtrINs to block activation of CRHR1 in layer 2/3 pyramidal cells, thus modulating anxiety-related behaviors. In this case, therefore, sexually dimorphic modules in the hypothalamus work remotely to regulate sexually bivalent behaviors through a uniform higher order circuit in the mPFC.
OXT, CRH, CRHBP, Stress and Anxiety
OXT is considered a “social” hormone because of the extensive literature documenting its ability to impact a variety of different social behaviors in many species, and because its production is dynamically regulated in response to changing social situations (Insel, 2010; Neumann and Slattery, 2015; Guastella and Hickie, 2016). OXT is produced in the hypothalamus, and acts through the OXTR in many brain regions. Progress on circuits responsible for its role in social behaviors has been substantial: in the nucleus accumbens, it acts together with serotonin (5-HT) to modulate social reward through regulation of synaptic properties of both Drd1 and Drd2 expressing medium spiny neurons (Dolen et al, 2013); in the auditory cortex, OXT increases the salience of infant distress calls to stimulate maternal behavior in experienced, but not virgin females (Marlin et al, 2013); and in the mPFC, OXT modulates social approach toward male mice during estrus (Nakajima et al, 2014). A second major role for OXT is the regulation of anxiety and stress (Neumann and Slattery, 2015). OXT is produced in response to stress, and its abilities to modulate stress and anxiety have been demonstrated both pharmacologically and genetically. Although it is quite clear that responses to OXT administration in humans can vary between genders (Donaldson and Young, 2008), little is known regarding higher order circuits that regulate the anxiolytic effects of OXT or its differential effects in men and women.
The main system regulating physiological responses to stress is the hypothalamic-pituitary-adrenal (HPA) axis (Smith and Vale, 2006). CRH plays a fundamental role in stress because its production in the PVN stimulates the HPA axis, resulting in myriad peripheral physiological responses. The receptors for CRH (CRHR1 and CRHR2) are expressed in distinct patterns in the periphery and the brain (Laryea et al, 2012). CRHR1, which has been strongly implicated in stress vulnerability (Labermaier et al, 2014), is highly expressed in layer 2/3 pyramidal cells of the cerebral cortex ((http://www.brain-map.org/.brain; Figure S3D). These cells are particularly vulnerable to stress, which typically induces alterations in their morphology and synaptic function (Shansky and Morrison, 2009; Moench and Wellman, 2014). Layer 2/3 pyramidal cells in the mPFC also express the Wolfram syndrome gene, and it has been demonstrated recently that they are required to modulate stress-induced depressive behaviors (Shrestha et al, 2015).
Although the central targets for OXT and CRH are largely distinct, stress and social behaviors are complex and intertwined. (Sandi and Haller, 2015). In general, stress results in decreased social motivation, reduced social interaction and increased antisocial behavior. While the influence of social interactions on stress responses is less studied, recent evidence strongly supports the concept that “social buffering” can protect against a variety of stress related behaviors (Beery and Kaufer, 2015). Administration of OXT in animal models promotes behavioral resilience to stress, and in humans it both stimulates social behavior and decreases anxiety. Despite the roles of both OXT and CRH in the regulation of stress and anxiety, and the comorbidity of social and emotional disorders in humans, it has remained unclear how these systems are modulated in higher brains areas to control behavior.
The data we have presented here provides important mechanistic insights into the central coordination of social and anxiety-related behaviors. First, the observation thatoptogenetic activation of sexually monomorphic OxtrINs in the mPFC results in bivalent, gender specific behaviors identifies these neurons as a critical node in social brain circuitry. Second, the discovery that this class of interneurons expresses CRHBP when activated, thus blocking the anxiogenic effects of CRH on layer 2/3 pyramidal cells in the cortex, provides a direct molecular mechanism through which OXT can both promote social behaviors and alleviate stress and anxiety. Third, the facts that OXT and CRH are circulating hormones whose actions are coordinated by OxtrINs in the mPFC, and that the receptors for OXT and CRH are expressed in many brain regions, suggest that CRHBP production in response to OXT may coordinate the actions of these hormones in other brain regions to modulate additional social and emotional behaviors. It will be of particular interest, for example, to determine whether OxtrINs production of CRHBP in the auditory cortex is involved in suppression of pup retrieval behavioral responses in virgin female or male mice (Marlin et al, 2015), and whether expression of CRHBP in the shell of the nucleus accumbens (http://mouse.brain-map.org) is involved in the modulation of social reward by OXT and serotonin (Dolen et al, 2013). Finally, the model we have presented allows for graded control of behavior. Thus, the balance between the prosocial and anxiolytic effects of OXT will depend ultimately on the vast variety of circumstances that are known to alter the levels of OXT and CRH in the brain.
The mPFC, OxtrINs, Gender and Therapy
OXT is now being investigated intensively as a treatment for social/emotional disorders, including autism spectrum disorders (Guastella and Hickie, 2015), social anxiety disorders (Neumann and Slattery, 2015), and schizophrenia (Insel, 2010; MacDonald and Feifel, 2011). In the Cntnap2 mouse model of ASD, both exogenous and evoked OXT can restore normal social behaviors (Penagarikano et el, 2015). Given the prominent role of OXT in female reproductive biology and behavior, gender is recognized as a critically important factor in studies of its therapeutic effects and development of improved treatments (Insel, 2015). The sexually dimorphic role we have demonstrated for OxtrINs in the mPFC, the mechanistic relationship between OXT and CRH that we have discovered, and the arrangement of OxtrINs and Crhr1 pyramidal cells suggest that both gender and environment will be critically important for understanding the therapeutic efficacy of OXT. Thus, the mechanism we have described in the mPFC is sensitive to the levels of both hormones, which vary independently as a consequence of sex, social situation and stress. Since it is capable of generating a graded response to the levels of OXT and CRH, the behavioral outcome of this system will be complex and dependent on all of the factors influencing OXT and CRH levels in vivo. Consequently, we expect that OXT therapy will be strongly dependent on the effective dose reaching the mPFC, the degree of target engagement, and the relative contributions of the mPFC and other sites to the overall clinical outcome. We speculate that in some settings it may be beneficial to reduce the impacts of gender and environment by artificially titrating these pathways through combined therapy employing OXTR agonists and CRHR1 antagonists.
METHODS AND RESOURCES
CONTACT FOR REAGENT AND RESOURCE SHARING
Nathaniel Heintz, The Rockefeller University, heintz@rockefeller.edu
MTAs required.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
All procedures involving mice were approved by The Rockefeller University Institutional Animal Care and Use Committee (IACUC) and were in accordance with the National Institutes of Health guidelines.
All mouse lines were bred to C57BL/6J (Jackson Stock #000664) for more than 7 generations. Male and female transgenic mice were bred in our facility and were 2–3 months of age, 22–26 g weight, at the beginning of each experiment. Oxtr-Cre ON82 transgenic mice previously generated (Nakajima et al. 2014) were crossed with knock-in Rosa-flox-EGFP-L10a line (Liu et al 2014) to generate the Oxtr-EGFP-L10a (TRAP) line. The conditional knockout mouse line of the oxytocin receptor (Oxtr-CKO) was donated by Dr. W. Scott Young III (Lee et al. 2008). Mice were housed in a specific-pathogen free facility, in groups of four to five per cage under a 12-h light-dark cycle with food and water ad libitum. Littermates of the same sex were randomly assigned to different experimental groups.
293TN producer cell line
293TN cells were grown in DMEM 10%FCS for 2 passages.
METHOD DETAILS
Behavioral Testing
Sociosexual Behavior
Sociosexual behavior was measured using the three-chamber social interaction chamber as previously described (Nakajima 2014). The apparatus is comprised of a rectangular, three chamber box. Each dividing wall has an open middle section, which allows free access to each chamber. Two identical wire cups are placed in each side chamber. Animal behaviors were scored three weeks after surgery and virus injection. Prior to behavioral testing, the test mouse was moved to a holder cage and the fiber optic cannula was connected to the fiberoptic patchcord. The sociosexual behavior test comprised two parts: a habituation session and 2 trial sessions. During the habituation session, two empty wire cups were placed in the left and right chambers. The test mouse was placed in the middle chamber and allowed to explore all three chambers of the apparatus for 10 min. The doors to the side chambers were closed, the test mouse was placed in the middle chamber, and a stranger mouse of the opposite sex and a novel lego were placed inside the left or right wire cups randomly. Doors between chambers were opened to allow the test mouse to explore the three chambers freely. For mice expressing Cre dependent ChR2-mCherry in the mPFC, the test mouse was allowed to explore and interact with the stranger animal or lego for 2 trial sessions and each trial lasted for 10min. Blue light was generated by 473nm laser at 1mW and driven at 10Hz to activate OxtrINs cell bodies. During each social interaction session, the light was off during the first 3 min and on during the following 3 min. Two trial sessions were performed and the total duration that the mice spent in sniffing the stranger mouse or lego during the 6 min light-off and 6 min light-on phases were analyzed. Sociosexual behavior tests in Oxtr-cKO and Crhbp shRNAinjected mice did not require optogenetic stimulation and comprised a habituation period and one trial session during which the test mouse was allowed to explore the chamber for 10 min and sniffing behaviors were scored during this period. Mouse behavior was videotracked (Noldus system) and sniffing time was manually scored with Nodules software. The experimenter was blind to the type of virus injected besides for AAV-DIO-ChR2 injected mice. No mice were excluded from the Sociosexual Behavior.
Elevated Plus Maze (EPM)
The apparatus consisted of two open arms, two closed arms and a center zone. Light intensity on the center zone and two open arms was always measured by light meters at the beginning of each experimental day. For EPM tests combined with optogenetics (Oxtr-ChR2 mice), the light intensity in the center zone of EPM was adjusted to 130 Lux. Prior to behavioral testing, the test mouse was moved to a holder cage and the fiber optic cannula was connected to the fiber optic patchcord. The test mouse was placed in the center zone of the EPM facing an open arm. The EPM trials consisted of 5 min light off and 5 min light on phases. For EPM tests in Oxtr-cKO and Crhbp shRNA experimental groups, which did not require optogenetics, light intensity in the center zone was maintained at 60 lux. Mice were allowed to move freely in the maze for 5 min. The distance moved, the time spent in each arm and the number of entries into each arm were automatically analyzed by Noldus software. The experimenter was blind to the type of virus injected besides for AAV-DIO-ChR2 injected mice. Animals falling off from open arms were excluded from analyses.
Open Field Test (OF)
Animal behavior in the open field was automatically measured by fusion software. The test mice were introduced into a corner of the open arena (50*50*30cm) and allowed to explore for 30 min. Parameters including total distanced travelled and time spent in the center of the open field were measured by the Fusion tracking software. For OPT tests in Crhbp shRNA experimental groups, the number of entries into the center zone during the first 5 min were automatically analyzed by the Fusion tracking software. The experimenter was blind to the type of virus injected besides for AAV-DIO-ChR2 injected mice. Mice injected with AAV-DIO-ChR2 virus were randomly divided into light on and light off groups. No mice were excluded from the Open field test.
Brain Slice Preparation
Adult mice (2–3 months) were anesthetized with Ketamine/Xylazine and perfused with 20 ml chilled dissection buffer (25.0 mM NaHCO3, 1.25 mM NaH2PO4, 2.5 mM KCl, 0.5 mM CaCl2, 7.0 mM MgCl2, 25.0 mM glucose, 110.0 mM choline chloride, 11.6 mM ascorbic acid and 3.1 mM pyruvic acid, gassed with 95% O2 and 5% CO2). Coronal mPFC slices of 300μm thickness were sectioned in chilled dissection buffer with a VT1000s vibratome (Leica). Slices were incubated in oxygenated artificial cerebrospinal fluid (ACSF; 118 mM NaCl, 2.5 mM KCl, 26 mM NaHCO3, 1 mM NaH2PO4, 10 mM glucose, 1.3 mM MgCl2 and 2.5 mM CaCl2, gassed with 95% O2 and 5% CO2), let recover for 90 min at 32°C and transferred to room temperature until electrophysiological recordings.
Electrophysiological Recordings
Slices were continuously perfused with ACSF in the recording chamber and Patch-clamp experiments were performed at 32°C using a temperature control system. Signals were amplified using EPC10-2 amplifier (HEKA Elektronik, Lambrecht, Germany). Slices were visualized under a Zeiss microscope station equipped with camera. The pipette resistance was in the range of 4–6 MΩ. For voltage clamp recordings, the intracellular solution contained (in mM): 115 CsMeSO3, 20 CsCl, 10 HEPES, 2.5 MgCl2, 4 Na2-ATP, 0.4 Na-GTP, 10 Na-phosphocreatine, and 0.6 EGTA. For current-clamp recordings, the composition of the intracellular solution was (in mM): 130 K-Gluconate, 5 KCl, 10 HEPES, 2.5 MgCl2. 4 Na2ATP, 0.4 Na3GTP, 10 Na-phosphocretine, 0.6 EGTA. Current clamp recordings were filtered at 2.5kHz and sampled at 5kHz. Voltage-clamp recordings were filtered at 2.5kHz and sampled at 10kHz using Pathmaster program (HEKA Elektronik). Data were analyzed by Fitmaster (HEKA Elektronik). To measure light-evoked postsynaptic currents, 5 ms 470nm light pulses were delivered to the mPFC via an optic fiber attached to a laser. Layer 2/3 pyramidal cells were clamped at 0mV and −60mV. Ten sweeps were taken at each potential. To confirm the excitatory or inhibitory currents evoked by light, picrotoxin (100μM), D-APV (100μM) and CNQX (20μM) were added during the recordings. Peak amplitude was calculated by subtracting the baseline. Synaptic latency was determined as the duration from current onset time to peak time. To examine the effect of CRH (1μM, BACHEM) and the CRHBP antagonist (CRF 6–33, 1μM, BACHEM) on excitability of layer 2/3 pyramidal neurons, we injected sequential currents from −50pA to 400pA in a 50pA step during 500ms. Current was injected every 60 s in current clamp. To measure the effect of light on excitability, continuous blue light was delivered at the same time of current injection. Littermates were randomly used to prepare brain slices treated with different drugs. Two to four independent data sets were collected.
Stereotactic surgeries
Adult male and female mice (2–3 month old) were anesthetized with a mix of ketamine (100mg/ml) and xylazine (1mg/ml) by intraperitoneal injection. Oxtr-Cre mice received unilateral virus infusion of AAV5-DIO-hChR2(H134R)-mcherry in the mPFC using the coordinates: (A-P: 1.84 to 1.50 mm; M-L: 0.28 mm; D-V: 2.2 from dura). We injected 0.25 μl of AAV virus per site. Fiber optic cannulae were implanted after virus injection using the coordinates: (A-P: 1.60 mm; M-L: 0.28 mm; D-V 1.9 mm). Oxtrf/f mice received AAV8-cre expressing virus bilaterally in the mPFC for conditional deletion of oxtr. Lentivirus expressing Crhbp shRNA (1μl virus per site) were bilaterally injected into Oxtr-cre mice to silence Crhbp in OxtrINs. The coordinates were the same as above. Behavioral experiments were performed 3 weeks after the surgery.
For infusion experiments, double guide cannulae (C235GS-5-0.5/SP, Plastic One) were implanted following bilateral craniotomy and attached to the skull using dental cement (S380, C&B-Metabond). Behavioral experiments were performed 3 weeks after the surgery. Twenty five minutes before the EPM behavioral test, either saline, or OXT (1μg/μl H-2510, BACHEM) or OXT and CRHBP antagonist (CRF 6–33; 50μg/μl, BACHEM) were infused in a volume of 1μl through the internal cannula inserted in the guide cannula.
TRAP (Translating Ribosome Affinity Purification) and RNA-SEQ
Three male and three female Oxtr-EGFP-L10a mice were used for independent TRAP replicates. The TRAP procedure was performed as described previously (Mellén et al. 2012). In brief, medial prefrontal cortices were dissected from individual mice. Tissue was immediately homogenized with a motor-driven Teflon glass homogenizer. Polyribosomes were immunoprecipitated by monoclonal anti-EGFP antibodies (custom made, a mix of 19C8 and 19F7)-coated protein L magnetic beads. RNAs from polyribosomes were extracted and further purified with an Rneasy Plus Micro Kit. RNA quantity and quality were determined with an Agilent 2100 Bioanalyzer. cDNA was synthesized from 5ng of mRNA from IP and input samples and further amplified by Oviation RNA-seq Kit. cDNA fragments of 200 bp were end-repaired and ligated with adapters for HiSeq 2000 (Ilumina Inc., San Diego, CA, USA) technology using TruSeq Nano DNA Sample kit (Illumina). Quality of libraries was assessed using HT DNA High Sensitivity Chip (Agilent) for 2100 Bioanalyzer. RNA-seq reads were aligned to the UCSC mm10 reference genome using STAR (version 2.3.0e_r291). Aligned reads were quantified by htseq-count module, part of the ‘HTSeq’ framework (version 0.6.0). Differentially expressed genes were identified by performing a negative binomial test using DESeq2 (R-package version 1.4.5) with default settings. Significant P-values were corrected to control the false discovery rate of multiple testing at 0.05 threshold.
FISH (Fluorescence in situ hybridization)
FISH was performed as described previously (Nakajima 2014). 12μm brain sections were collected on slides. Crhbp probe template was generated by PCR using gene-specific primers (F: CCCTCTCCTGCTCTTCAGTG, R: ATGGCTCCAGCTGACGATAC; from Crhbp cDNA plasmid). The PCR product was used to make Digoxigenin (DIG) labeled RNA probes using a T7-based in vitro transcription kit (Roche). Sections were hybridized with RNA probe for 16 hours at 55°C. Slides were washed and treated with the anti-DIG polyclonal antibodies and then incubated with biotin conjugated anti-sheep IgG antibodies (Chemicon) in PBS for 1 hr. After washing, samples were reacted with the HRP-conjugated streptavidin (PerkinElmer) and signals were visualized by incubating the samples with TSA-Cy3 (Perkin Elmer). After ISH, slides were incubated with chicken anti-EFGP (Aves) (1:500) for overnight and visualized with Alexa-fluor conjugated secondary antibodies (Invitrogen).
Lentivirus Packaging
Lentivirus was generated as described previously (Auer et al, 2010.) In brief, 293TN cells were co-transfected with the expression vector plasmid (pSico-Crhbp-shRNA), together with packaging and envelope plasmids, using PEI solution (Polysciences INC #24765-2). Two days after transfection, viral particles were harvested and the supernatant was concentrated by ultracentrifugation. The titer of concentrated lentivirus was measured by transducing 1 × 105 293TN cells per well in a 24-well cell culture plate with virus dilutions and quantification of EGFP-positive cells by fluorescence-activated cell sorting (FACS) analysis after 3 days. TU= (P*N/100*V)*1/DF. (p=%GFP+ cells; N=number of cell at the time of transfection; V=volume if dilution added to each well; DF=dilution factor). The titer of lenti-crhbp-shRNA virus is 4.6×108 transducing units/ml.
shRNA Design
The Cre regulated conditional RNA interference plasmid, pSico was purchased from addgene (#11578). The 19-nt sequence (GAGCCATTCGAGCTAGAAA) used for knockdown of Crhbp mRNA was generated by pSicoOligomaker and the knock down efficiency was validated by western blotting. The sense oligo and antisense oligo containing Crhbp shRNA generated by pSicoOligomaker were digested by SacII and NotI after annealing and cloned into the pSico vector (Ventura A et al., 2004).
Western Blot
To test the knockdown efficiency of the shRNA crhbp, 293TN cells were co-transfected with pSico-crhbp-shRNA and pCMV-crhbp-Myc (MR204660, ORIGENE) and a cre-expressing vector. Cell lysates were collected 48 hours after transfection. Protein extracts were loaded in 4–12% Bis-Tris gel, transferred to PDVF membrane for analysis and immunobloted with anti-CRHBP primary antibodies (1:500, sc-20630, Santa Cruz) and anti-b actin (1:2000).
Real Time PCR (RT-PCR)
Tissues were dissected from the mPFC and PVH of male and female wild type mice. Total RNA was homogenized and isolated by RNAeasy Mini Kit (QIAGEN). The first strand cDNA was synthesized by SuperScript IV reverse transcriptase (Invitrogen). Quantitative real-time PCR was performed using 480 SYBR Green I Master in a LightCycler 480II system (Roche). Quantification was analyzed by the delta Ct method with Gapdh as an endogenous control. Brain samples of same age male and female mice were collected in the same day and processing of the samples was performed in parallel.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical parameters including the exact value of n, precision measures (mean± SEM) and statistical significance are reported in the Figures and the Figure Legends. Data is judged to be statistically significant when p<0.05 by two tailed Mann Whitney test, One-way ANOVA or Two-way ANOVA. In figures, asterisks denote statistical significance (*, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001) as compared to controls. Pearson correlation coefficients were calculated with PRISM and statistical significance is based upon the assumption that values exhibit a Gaussian distribution. Statistical analysis was performed in Graph Pad PRISM 6.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-CRHBP Antibody | Santa Cruz | sc-20630 |
| Mouse monoclonal anti-beta-actin Antibody | ABGENT | AM1829B |
| Mouse monoclonal anti-EGFP antibody | Laboratory of Nathaniel Heintz | N/A |
| Chicken anti-EGFP antibody | AVES | GFP-1020 |
| Sheep anti-DIG polyclonal antibodies | Roche | 11333089001 |
| Biotinylated Rabbit anti-sheep IgG antibody | Vector lab | BA-6000 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Oxytocin acetate salt | BACHEM | H-2510.0025 |
| CRF (human, rat) acetate salt | BACHEM | H-2435.0001 |
| CRF (6-33) (human, rat) | BACHEM | H-3456.1000 |
| Picrotoxin | TOCRIS | 1128 |
| D-AP5 | TOCRIS | 0106 |
| CNQX disodium salt | TOCRIS | 1045 |
| Polyethylenimine HCI MAX | Polysciences, Inc. | 24765-2 |
| Critical Commercial Assays | ||
| TSA plus cyanine 3 system | PerkinElmer | NEL744001KT |
| RNAeasy Mini Kit | QIAGEN | 74106 |
| LightCycler 480 SYBR Green I Master | Roche | 04887352001 |
| DIG RNA Labeling Kit (SP6/T7) | Roche | 11175025910 |
| Dynabeads MyOne Streptavidin T1 | Invitrogen | 65601 |
| RNeasy Plus Micro Kit | QIAGEN | 74034 |
| Ovation RNA-seq System V2 | Nugen | 7102 |
| TruSeq Nano DNA LT kit | illumina | 15041757 |
| C&B Metabond | parkell | S380 |
| SuperScript IV First-Strand Synthesis System | Invitrogen | 18091050 |
| NuPAGE 4-12% Bis-Tris Protein Gels | Invitrogen | NP0321BOX |
| Deposited Data | ||
| No | ||
| Experimental Models: Cell Lines | ||
| 293TN producer cell line | System Biosciences | LV900A-1 |
| Experimental Models: Organisms/Strains | ||
| C57BL/6J | The Jackson Laboratory | 000664 |
| Oxtr-cre ON82 | Laboratory of Nathaniel Heintz (Nakajima N et al.,2014) | N/A |
| Rosa26-flox-EGFP-L10a | The Jackson Laboratory | 022367 |
| Oxtr-EGFP-L10a | Laboratory of Nathaniel Heintz | N/A |
| Oxtrflox/flox | The Jackson Laboratory | 008471 |
| Recombinant DNA | ||
| AAV5-EF1a-DIO-hChR2(H134R)-mCherry | University of Pennsylvania vector core | AAV-5-20297P |
| AAV8-hSyn-GFP-Cre | University of North Carolina vector Core | N/A |
| AAV8-CMV-GFP | University of North Carolina vector Core | N/A |
| Sequence-Based Reagents | ||
| Crhbp probe for ISH Forward: CCCTCTCCTGCTCTTCAGTG Reverse: ATGGCTCCAGCTGACGATAC |
This paper | N/A |
| pCMV-Crhbp cDNA (Myc-DDK-Tagged) | Origene | MR204660 |
| pSico | Ventura A et al.,2004 ( Addgene ) | 11578 |
| 19-nt sequence used for knockdown of Crhbp mRNA GAGCCATTCGAGCTAGAAA | This paper | Generated by pSicoOligomaker |
| Sense oligo containing Crhbp shRNA: TGAGCCATTCGAGCTAGAAATTCAAGAGATTTCTAG CTCGAATGGCTCTTTTTTC Antisense oligo containing Crhbp shRNA: TCGAGAAAAAAGAGCCATTCGAGCTAGAAATCTCTT GAATTTCTAGCTCGAATGGCTCA |
This paper | Generated by pSicoOligomaker |
| RT-PCR primers of Oxtr Forward: CTTCCTCAGATTCCACACCTG Reverse: TTCCAGAACATTCAGCTCCAG |
This paper | N/A |
| RT-PCR primers of CRH Forward: AAAGAAGAGAAAGGAGAAGAGGAAG Reverse: CCGCAGCCGCATGTTAG |
This paper | N/A |
| RT-PCR primers of CrhR1 Forward: CAGCCGCCTACAACTACTTCC Reverse: GGTGGAGTACGTGAGTACGATG |
This paper | N/A |
| RT-PCR primers of Crbhp Forward: TGATGCCCTTAGCAGACCTGT Reverse: CACACGGTTAATGTGTTTCCCA |
This paper | N/A |
| RT-PCR primers of GAPDH Forward: CATGGCCTTCCGTGTTCCTA Reverse: GCCTGCTTCACCACCTTCTT |
This paper | N/A |
| Software and Algorithms | ||
| MainScript | Laboratory of Nathaniel Heintz | https://gensat.rockefeller.edu/heintzp30/Bioinformatics_Flow_Chart.jsp |
| Other | ||
| Double guide canula | Plastic one | C235GS-5-0.5/SP |
| Fiber-optic cannula | Doric lenses | MFP_200/230-0.37 _1m_ZF1.25_FLT |
Supplementary Material
Acknowledgments
This work was supported by the HHMI (N.H), the NIH/NIDA: grant 1P30 DA035756-01 (I.I.T and N.H.), the Leon Black Family Foundation (N.H.) and the HFSP postdoctoral fellowship LT000271/2015-L (K.L.). We wish to thank Cuidong Wang (Rockefeller University, New York) for technical assistance. The authors declare no conflicts of interest.
Footnotes
DATA AND SOFTWARE AVAILABILITY
Data Resources: Supplemental files: table S1 and S2.
ADDITIONAL RESOURCES
All TRAP-seq data were subjected to the work flow described in https://gensat.rockefeller.edu/heintzp30/Bioinformatics_Flow_Chart.jsp
AUTHOR CONTRIBUTIONS
Conceptualization, I.I.T. and N.H; Investigation, K.L; Validation and Resources, M.N.; Writing - Original Draft, N.H; Writing Review & Editing, K.L., I.I.T. and N.H; Visualization K.L, I.I.T. and N.H; Supervision I.I.T. and N.H; Funding Acquisition N.H.
References
- Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol. 2014;35:320–330. doi: 10.1016/j.yfrne.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y, Watanabe T, Abe O, Kuwabara H, Yahata N, Takano Y, Iwashiro N, Natsubori T, Takao H, Kawakubo Y, et al. Oxytocin’s neurochemical effects in the medial prefrontal cortex underlie recovery of task-specific brain activity in autism: a randomized controlled trial. Mol Psychiatry. 2014;20:447–453. doi: 10.1038/mp.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer S, Stürzebecher AS, Jüttner R, Santos-Torres J, Hanack C, Frahm S, Liehl B, Ibañez-Tallon I. Silencing neurotransmission with membrane-tethered toxins. Nat Methods. 2010;7:229–236. doi: 10.1038/nmeth.1425. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Kawasumi Y. Cognitive disruptions in stress-related psychiatric disorders: A role for corticotropin releasing factor (CRF) Horm Behav. 2015;76:125–135. doi: 10.1016/j.yhbeh.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Kaufer D. Stress, social behavior, and resilience: insights from rodents. Neurobiol Stress. 2015;1:116–127. doi: 10.1016/j.ynstr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlehem RA, van Honk J, Auyeung B, Baron-Cohen S. Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology. 2013;38:962–974. doi: 10.1016/j.psyneuen.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Dolen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Hickie IB. Oxytocin Treatment, Circuitry, and Autism: A Critical Review of the Literature Placing Oxytocin Into the Autism Context. Biol Psychiatry. 2015;79:234–242. doi: 10.1016/j.biopsych.2015.06.028. [DOI] [PubMed] [Google Scholar]
- Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. Translating Oxytocin Neuroscience to the Clinic: A National Institute of Mental Health Perspective. Biol Psychiatry. 2016;79:153–154. doi: 10.1016/j.biopsych.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology. 2009;34:226–237. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Kunwar PS, Zelikowsky M, Remedios R, Cai H, Yilmaz M, Meister M, Anderson DJ. Ventromedial hypothalamic neurons control a defensive emotion state. eLife. 2015;4 doi: 10.7554/eLife.06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labermaier C, Kohl C, Hartmann J, Devigny C, Altmann A, Weber P, Arloth J, Quast C, Wagner KV, Scharf SH, et al. A polymorphism in the Crhr1 gene determines stress vulnerability in male mice. Endocrinology. 2014;155:2500–2510. doi: 10.1210/en.2013-1986. [DOI] [PubMed] [Google Scholar]
- Laryea G, Arnett MG, Muglia LJ. Behavioral Studies and Genetic Alterations in Corticotropin-Releasing Hormone (CRH) Neurocircuitry: Insights into Human Psychiatric Disorders. Behav Sci (Basel) 2012;2:135–171. doi: 10.3390/bs2020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Caldwell HK, Macbeth AH, Tolu AK, Scott Young W., 3rd A Conditional Knockout Mouse Line of the Oxytocin Receptor. Endocrinology. 2008;149(7):3256–3263. doi: 10.1210/en.2007-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Kim DW, Remedios R, Anthony TE, Chang A, Madisen L, Zeng H, Anderson DJ. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature. 2014;509:627–632. doi: 10.1038/nature13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Krautzberger AM, Sui SH, Hofmann OM, Chen Y, Baetscher M, Grgic I, Kumar S, Humphreys BD, Hide WA, McMahon AP. Cell-specific translational profiling in acute kidney injury. J Clin Invest. 2014;124:1242–1254. doi: 10.1172/JCI72126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald K, Feifel D. Oxytocin’s role in anxiety: a critical appraisal. Brain Res. 2014;1580:22–56. doi: 10.1016/j.brainres.2014.01.025. [DOI] [PubMed] [Google Scholar]
- Marlin BJ, Mitre M, D’Amour JA, Chao MV, Froemke RC. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature. 2015;520:499–504. doi: 10.1038/nature14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellén M, Ayata P, Dewell S, Kriaucionis S, Heintz N. MeCP2 binds to 5hmC enriched within active genes and accessible chromatin in the nervous system. Cell. 2012;151(7):1417–30. doi: 10.1016/j.cell.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima N, Görlich A, Heintz N. Oxytocin modulates female sociosexual behavior through a specific class of prefrontal cortical interneurons. Cell. 2014;159(2):295–305. doi: 10.1016/j.cell.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Slattery DA. Oxytocin in General Anxiety and Social Fear: A Translational Approach. Biol Psychiatry. 2015;79:213–221. doi: 10.1016/j.biopsych.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Owen SF, Tuncdemir SN, Bader PL, Tirko NN, Fishell G, Tsien RW. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature. 2013;500:458–462. doi: 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagarikano O, Lazaro MT, Lu XH, Gordon A, Dong H, Lam HA, Peles E, Maidment NT, Murphy NP, Yang XW, et al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med. 2015;7:271ra278. doi: 10.1126/scitranslmed.3010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, McEwen BS. Actions of estrogens and progestins on nerve cells. Science. 1983;219:808–814. doi: 10.1126/science.6297008. [DOI] [PubMed] [Google Scholar]
- Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM, Dedic N, Schumacher M, von Wolff G, Avrabos C, et al. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science. 2011;333:1903–1907. doi: 10.1126/science.1202107. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345:771–776. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein E, Wiggins LD, Lee LC. A Review of the Differences in Developmental, Psychiatric, and Medical Endophenotypes Between Males and Females with Autism Spectrum Disorder. J Dev Phys Disabil. 2015;27:119–139. doi: 10.1007/s10882-014-9397-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabihi S, Durosko NE, Dong SM, Leuner B. Oxytocin in the prelimbic medial prefrontal cortex reduces anxiety-like behavior in female and male rats. Psychoneuroendocrinology. 2014;45:31–42. doi: 10.1016/j.psyneuen.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci. 2015;16:290–304. doi: 10.1038/nrn3918. [DOI] [PubMed] [Google Scholar]
- Scott N, Prigge M, Yizhar O, Kimchi T. A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature. 2015;525:519–522. doi: 10.1038/nature15378. [DOI] [PubMed] [Google Scholar]
- Shrestha P, Mousa A, Heintz N. Layer 2/3 pyramidal cells in the medial prefrontal cortex moderate stress induced depressive behaviors. eLife. 2015;4:e08752. doi: 10.7554/eLife.08752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW. The Genetics of Stress-Related Disorders: PTSD, Depression, and Anxiety Disorders. Neuropsychopharmacology. 2016;41:297–319. doi: 10.1038/npp.2015.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Eede F, Van Broeckhoven C, Claes SJ. Corticotropin-releasing factor-binding protein, stress and major depression. Ageing Res Rev. 2005;4:213–239. doi: 10.1016/j.arr.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Ventura A, Meissner A, Dillon CP, McManus M, Sharp PA, Van Parijs L, Jaenisch R, Jacks T. Cre-lox-regulated conditional RNA interference from transgenes. Proc Natl Acad Sci U S A. 2004;101(28):10380–5. doi: 10.1073/pnas.0403954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Richter J, Straube B, Lueken U, Domschke K, Schartner C, Klauke B, Baumann C, Pane-Farre C, Jacob CP, et al. Allelic variation in CRHR1 predisposes to panic disorder: evidence for biased fear processing. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.125. [DOI] [PubMed] [Google Scholar]
- Westphal NJ, Seasholtz AF. CRH-BP: the regulation and function of a phylogenetically conserved binding protein. Front Biosci. 2006;11:1878–1891. doi: 10.2741/1931. [DOI] [PubMed] [Google Scholar]
- Wigton R, Radua J, Allen P, Averbeck B, Meyer-Lindenberg A, McGuire P, Shergill SS, Fusar-Poli P. Neurophysiological effects of acute oxytocin administration: systematic review and meta-analysis of placebo-controlled imaging studies. J Psychiatry Neurosci. 2015;40:E1–22. doi: 10.1503/jpn.130289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, Juntti SA, Unger EK, Wells JA, Shah NM. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell. 2013;153:896–909. doi: 10.1016/j.cell.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Shah NM. Representing sex in the brain, one module at a time. Neuron. 2014;82:261–278. doi: 10.1016/j.neuron.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar I, Weinstock M. Differential effect of prenatal stress on the expression of corticotrophin-releasing hormone and its receptors in the hypothalamus and amygdala in male and female rats. J Neuroendocrinol. 2011;23:320–328. doi: 10.1111/j.1365-2826.2011.02117.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.