Abstract
Uncultivated bacteria that densely colonize the midgut glands (hepatopancreas) of the terrestrial isopod Porcellio scaber (Crustacea: Isopoda) were identified by cloning and sequencing of their 16S rRNA genes. Phylogenetic analysis revealed that these symbionts represent a novel lineage of the Mollicutes and are only distantly related (<82% sequence identity) to members of the Mycoplasmatales and Entomoplasmatales. Fluorescence in situ hybridization with a specific oligonucleotide probe confirmed that the amplified 16S rRNA gene sequences indeed originated from a homogeneous population of symbionts intimately associated with the epithelial surface of the hepatopancreas. The same probe also detected morphotypically identical symbionts in other crinochete isopods. Scanning and transmission electron microscopy revealed uniform spherical bacterial cells without a cell wall, sometimes interacting with the microvilli of the brush border by means of stalk-like cytoplasmic appendages, which also appeared to be involved in cell division through budding. Based on the isolated phylogenetic position and unique cytological properties, the provisional name “Candidatus Hepatoplasma crinochetorum” is proposed for this new taxon of Mollicutes colonizing the hepatopancreas of P. scaber.
The common woodlouse, Porcellio scaber (Crustacea: Isopoda), is a cosmopolitan species that feeds mainly on decaying plant material, such as leaf litter, wood, and grass (29), which are rich in recalcitrant structural polymers, such as cellulose, hemicelluloses, and lignins (3). Together with other terrestrial isopods (Oniscidea), P. scaber is thought to play an important role in decomposition processes and nutrient recycling (29).
The hepatopancreas (digestive glands) of P. scaber, which consists of two pairs of tubular midgut ceca, functions in the secretion of digestive enzymes and the absorption of nutrients. The midgut ceca are densely colonized by microorganisms (8, 25, 27, 28, 31). It has been speculated that hepatopancreatic bacteria, like the gut symbionts of termites and other soil arthropods that feed on fiber-rich diets (3, 4), might be involved in the digestion of leaf litter, e.g., by producing cellulases or phenol oxidases (28, 30-32).
Like many previous attempts to isolate bacteria associated with invertebrates, all efforts to culture the symbionts in vitro have failed. This has so far prevented both identification of the symbionts and analysis of the nature of the symbiotic association. In a previous report, it was shown that the hepatopancreas of P. scaber is colonized by a novel lineage of rod-shaped, stalk-forming symbionts that fall into the alpha subclass of the Proteobacteria but are only distantly related to the Rickettsiales (<81% sequence similarity) (25). In the course of a broader survey of the distribution of such Rickettsia-like symbionts among different populations of P. scaber, we observed that in many specimens the hepatopancreas contained spherical symbiotic bacteria instead of the rod-shaped forms.
In the present study, we investigated the phylogenetic affiliation, diversity, and location of the spherical symbionts by cloning and sequencing 16S rRNA genes and performing denaturing gradient gel electrophoresis (DGGE) and hybridization with specific fluorescence-labeled oligonucleotide probes, and we determined the morphology and ultrastructure of these organisms by using scanning and transmission electron microscopy. Our results provide novel insights into the origin and diversity of the hepatopancreatic symbionts of isopods.
MATERIALS AND METHODS
Specimens.
P. scaber was collected from decaying wood in the botanical garden of Christian-Albrechts-Universität (Kiel, Germany) in August 2002. Only healthy adult isopods of both sexes were used for the experiments.
16S rRNA gene cloning and sequencing.
The midgut glands of five individuals were pooled, and total DNA was extracted and purified by using a bead-beating protocol and polyvinylpolypyrrolidone spin columns (7). 16S rRNA genes were amplified with a Bacteria-specific primer pair (S-D-Bact-0007-a-S-21 and S-D-Bact-1492-a-A-22) (26) and were cloned by using the pGEM-T vector cloning system, and they were transferred into competent Escherichia coli JM 109 cells by using the instructions of the supplier (Promega). Inserts of the expected size (approximately 1,500 bp) were amplified by PCR with the M13 forward and M13 reverse primers, and clones were grouped on the basis of the restriction patterns obtained after separate digestions with MspI, AluI, and RsaI endonucleases (MBI). For restriction analysis, 18 μl of PCR products, 2 μl of 10× Y+/Tango buffer (MBI), and 0.2 μl (1 U) of an enzyme were added to a sterile tube and incubated at 37°C for 15 h. Plasmids were extracted and purified with an Easy nucleic acid plasmid miniprep kit 1 (Peqlab), and the inserts were sequenced on both strands by using primers 27F (26), 533F (26), 907R (14), and 1492R (26) by GATC (http://www.gatc.de). Sequences were checked and assembled by using the DNAStar software (http://www.dnastar.com).
Phylogenetic analysis.
Sequences were aligned and phylogenetically analyzed by using the ARB software package (version 2.5b; O. Strunk and W. Ludwig, Technische Universität München; http://www.arb-home.de). 16S rRNA gene sequences were compared to sequences in public databases by using BLAST (1); closely related sequences were retrieved and integrated into the existing database of aligned sequences of Mollicutes with the Fast Aligner function of ARB. The automatic alignments were verified and manually corrected, taking into account the constraints of the secondary structure (17). For tree reconstruction, highly variable regions of the 16S rRNA gene sequences were excluded by using only those positions of the alignment that were identical in at least 50% of all sequences, which resulted in a filter that yielded 1,368 informative nucleotide positions for construction of the phylogenetic tree. Only sequences with more than 1,400 nucleotides were used for the alignment. For phylogenetic analysis we utilized the maximum-likelihood, maximum-parsimony, and neighbor-joining algorithms as implemented in ARB (17).
DGGE.
16S rRNA gene fragments (∼450 bp) were amplified by using a primer pair (S-D-Bact-0515-a-S-19 and S-D-Bact-0907-a-A-15 with a GC clamp) and were separated by DGGE (10) by using a linear denaturing gradient (40 to 65% denaturant gradient of urea and formamide [a 100% gradient is defined as 7.0 M urea plus 40% formamide, vol/vol]), a constant voltage of 65 V for 16 h, and a temperature of 60°C.
DAPI staining and whole-cell hybridization.
The midgut glands of individual isopods were thoroughly homogenized in 500 μl of phosphate-buffered saline (PBS), and formaldehyde was added to a final concentration of 4% (wt/vol). After fixation for 14 h at 4°C, the samples were centrifuged at 10,000 × g for 5 min. The pellets were washed three times in PBS, resuspended in a solution containing 250 μl of PBS plus 250 μl of ethanol (100%), and stored at −21°C for 1 to 2 weeks until analysis.
Samples were filtered onto polycarbonate filters (pore size, 0.2 μm) and dried at 46°C for 30 min. Samples were stained with DAPI (4′,6-diamidino-2-phenylindole) and hybridized with fluorescently labeled oligonucleotides as described by Wagner et al. (23); negative controls with an EUB338 antisense probe (5′-ACTCCTACGGGAGGCAGC-3′) (24) were used to exclude nonspecific probe binding. All probes were synthesized and 5′ labeled with the fluorescent cyanine dye Cy3 or with 5(6)-carboxyfluorescein-N-hydroxysuccinimide ester by Thermo Hybaid (http://www.interactiva.de). Samples were covered with Citifluor (Citifluor Ltd., London, United Kingdom) and examined at a magnification of ×1,000 with a Zeiss Axiophot epifluorescence microscope by using filter sets for DAPI, Cy3, and fluorescein. Images were recorded with a cooled charge-couple device camera (Magnafire; INTAS, Göttingen, Germany).
For in situ hybridization, thin (6-μm) sections of paraffin-embedded midgut glands were prepared as previously described (6). Samples were hybridized with the fluorescein-labeled Bacteria-specific probe EUB338 (5′-GCTGCCTCCCGTAGGAGT-3′) (2) and the Cy3-labeled group-specific probe PsSym352 (see below) as described above; control experiments were included to exclude nonspecific binding.
Probe design.
The group-specific oligonucleotide probe PsSym352 (Cy3-5′-GTGAAAAATTCCCTACTGCTG-3′; E. coli positions 352 to 372) was designed by using the probe design function of the ARB software. This probe matched exactly the target region of the 16S rRNA molecule of all clones obtained in this study, the Spiroplasma group, and the Mycoplasma pneumoniae group, but it had two or more mismatches with the sequences of all other bacteria in the Ribosomal Database Project database, including the Mycoplasma hominis group (5).
The stringency of hybridization was optimized for probe PsSym352 by using hepatopancreatic symbionts as the target and Acidithiobacillus ferrooxidans as the nontarget reference organism with two mismatches. The formamide concentration in the hybridization buffer was varied between 0 and 35%, while the sodium chloride concentration in the posthybridization buffer was adjusted accordingly (15). The fluorescence signal conferred by probe PsSym352 to target cells was stable; the intensity of this signal was stable at 0 and 10% formamide and decreased slightly at 20% formamide, and the signal was almost not detectable at 30 and 35% formamide. With nontarget cells, there was no signal even under low-stringency conditions (no formamide). Therefore, we routinely used 10% formamide for single hybridizations and 20% formamide for double hybridizations with EUB338.
Electron microscopy.
For scanning electron microscopy, freshly dissected midgut glands were dehydrated in an ethanol series and then subjected to critical-point drying with CO2. The dried samples were mounted on aluminum stubs with double-sided adhesive tape. The samples were coated with gold with a sputter coater (SCD 050; Zeiss) and observed with a LEO 420 scanning electron microscope (LEO Electron Microscopy Ltd., Cambridge, England).
For transmission electron microscopy, freshly dissected midgut glands were immediately fixed in a drop of 3.5% glutaraldehyde in 0.05 M cacodylate buffer (pH 7.4) for 30 min. To ensure rapid penetration of fixative into the lumen, holes were punched into the hepatopancreas with a sterile needle. The glands were then fixed in the same buffer for 14 h at 4°C. After it was washed with 0.075 M cacodylate buffer (pH 7.4) for 30 min, the tissue was postfixed with 1% OsO4 in 0.05 M cacodylate buffer for 2 h at 4°C. After additional washing for 30 min, the hepatopancreas specimens were dehydrated in a graded ethanol series at room temperature and embedded in Agar 100 resin (Agar Scientific Ltd., Essex, England). Ultrathin (60-nm) sections were contrasted with 2.5% uranyl acetate and lead citrate (21) and analyzed by using a Philips CM 10 electron microscope.
Nucleotide sequence accession numbers.
The nucleotide sequence accession numbers for the 16S rRNA genes cloned in this study are AY500249 (clone 48) and AY500250 (clone 59).
RESULTS
Density and morphology.
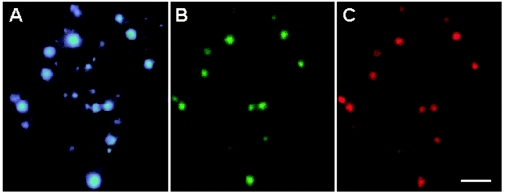
DAPI staining and whole-cell hybridization of homogenates with fluorescence-labeled oligonucleotide probe EUB338 revealed dense bacterial colonization in each of the hepatopancreatic ceca of P. scaber, at levels ranging from 2.2 × 106 to 1.4 × 108 cells per animal (n = 15) with a cell density of about 2 × 106 to 1 × 108 cells per mg (dry mass). The cells were spherical and fell into two size classes with diameters of 0.2 to 0.3 μm (small spheres) and 0.5 to 0.8 μm (large spheres) (Fig. 1). Most specimens (12 of 15 specimens) harbored both small and large spheres, whereas a few had either large spheres or small spheres.
FIG. 1.
Symbiotic bacteria in a midgut gland homogenate of P. scaber. The three epifluorescence photomicrographs show the same microscopic field of a preparation stained with DAPI (A) and double-hybridized with oligonucleotide probes specific for Bacteria (EUB338) (B) and for the clones obtained in this study (PsSym352) (C). The hybridization signals of small spherical symbiotic bacteria faded rapidly during the exposure. Scale bar = 2.5 μm.
Cloning and sequencing.
To identify the hepatopancreatic symbiotic bacteria, DNA was extracted from isolated midgut glands, and bacterial 16S rRNA genes were amplified with universal bacterial primers and cloned in E. coli. The screened positive clones that were digested with the MspI, AluI, and RsaI endonucleases resulted in two different restriction fragment length polymorphism groups, comprising 12 and 6 clones, which differed only slightly in their restriction patterns with each of the three enzymes. Sequencing of two representative clones from each restriction fragment length polymorphism group yielded almost complete 16S rRNA genes (1,517 bp for clone 48 and 1,515 bp for clone 59) with virtually identical sequences (99.8% similarity), which indicated the possibility that two closely related bacterial lineages were present in the hepatopancreas of P. scaber.
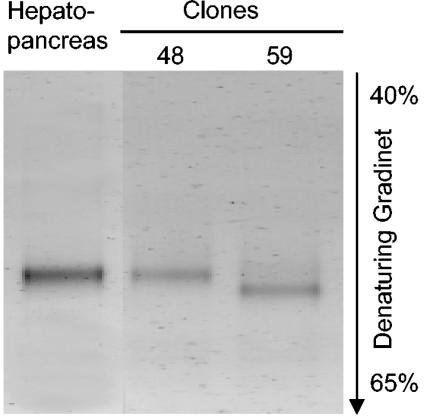
However, DGGE of 16S rRNA gene fragments amplified by PCR from hepatopancreatic DNA extracts with Bacteria-specific primers resulted in electropherograms that contained only a single band, which had the same position in the gel as the band of representative clone 48 (Fig. 2). The PCR product of the closely related clone 59, representing the second, less frequent ribotype in the clone library, had a slightly different melting point; a corresponding band was not present in the hepatopancreatic sample.
FIG. 2.
DGGE profiles (negative images) of PCR amplicons obtained from the DNA extracted from the hepatopancreas of P. scaber and from two representative clones from the clone library.
Phylogenetic analysis.
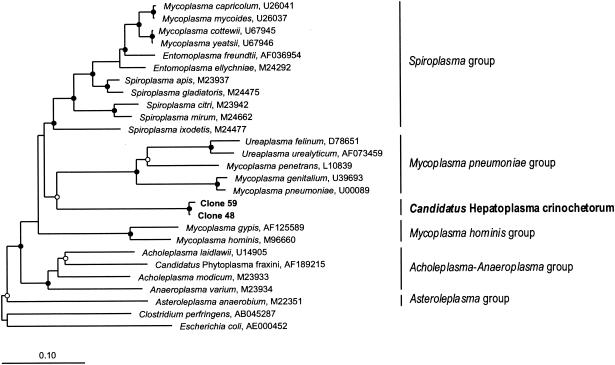
BLAST searches resulted in only low levels of similarity of the hepatopancreatic clones to other bacteria in public databases, but the results indicated that there is an affiliation with the bacterial class Mollicutes. A detailed phylogenetic analysis based on an extensive data set comprising all major taxa of Mollicutes revealed that the clones are a novel lineage, representing a sister group of the Spiroplasma group and the M. pneumoniae group (<82% sequence identity), which contain parasites and pathogens of other animals (Fig. 3). The early branching point at the base of the Mycoplasmatales was confirmed by all phylogenetic analyses, in which neighbor-joining, maximum-parsimony, and maximum-likelihood algorithms were used, and was also supported by bootstrap analysis (Fig. 3).
FIG. 3.
Phylogenetic tree (16S rRNA gene sequences; maximum-likelihood method) showing the evolutionary relationship of the hepatopancreatic symbionts of P. scaber (boldface type) to selected species belonging to the main phylogenetic clades of the Mollicutes (indicated by vertical lines). The tree was rooted by using E. coli and Clostridium perfringens as outgroups. Nodes with bootstrap values (DNAPARS; 1,000 replicates) of >99% (•) and >50% (○) are marked. All marked nodes were observed in all other phylogenetic analyses in which maximum-parsimony and neighbor-joining algorithms were used (data not shown). Bar = 10 substitutions per 100 nucleotides.
In situ identification and localization.
To exclude the possibility that the apparent homogeneity of the symbiont population was due to PCR bias and to confirm that the clones truly originated from the symbiotic bacteria in the hepatopancreas of P. scaber and not from contamination, midgut gland homogenates were subjected to whole-cell hybridization with fluorescence-labeled oligonucleotide probes. In homogenates from 15 different individuals, all DAPI-stained cells (Fig. 1A) hybridized with a Bacteria-specific probe (Fig. 1B) and with the group-specific probe PsSym352 (Fig. 1C). The weak hybridization signal of the small spheres bleached rapidly and is difficult to detect on the image in Fig. 1. Spherical bacterial cells hybridizing with the group-specific probe PsSym352 were also detected in hepatopancreatic homogenates of other crinochete isopods (Alloniscus perconvexus, Philoscia muscorum, Oniscus asellus, Trachelipus rathkii, and Armadillidium vulgare). Detailed results will be published in the context of a comparative study of the symbionts in the hepatopancreases of different isopods.
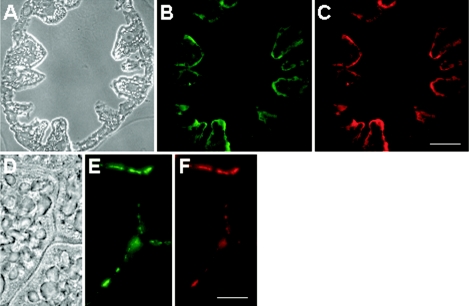
In situ hybridization of thin sections of midgut glands revealed that the symbiotic bacteria were not spread over the lumen of the hepatopancreatic ceca but were mostly associated with the wall, which is formed by a monolayer of endothelial cells (Fig. 4A). Tangential sections substantiated that the bacteria were not located within the cytoplasm but colonized the surfaces of the epithelium (Fig. 4B). The same results were obtained with thin sections from specimens harboring only small spheres (data not shown), which supports the conclusion that the small spheres and the large spheres represent a homogeneous population rather than two very closely related lineages.
FIG. 4.
Thin sections of the midgut glands of P. scaber, hybridized with fluorescently labeled oligonucleotide probes. The photomicrographs show the same microscopic fields of a transverse section (A to C) and a tangential section (D to F), viewed with phase-contrast microscopy (A and D) and with epifluorescence microscopy by using filter sets for probe EUB338 (B and E), which is specific for Bacteria, and for probe PsSym352 (C and F), a group-specific probe. (C) Scale bar = 100 μm. (F) Scale bar = 10 μm.
Morphology and ultrastructure.
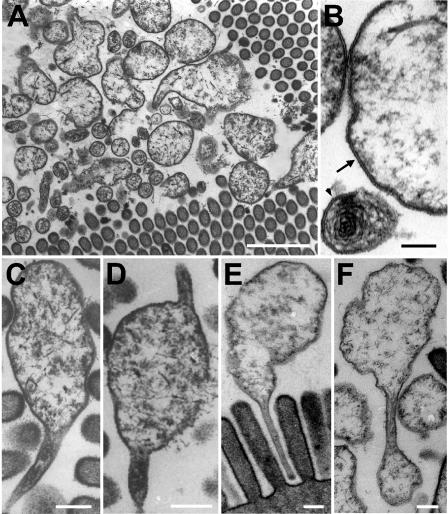
Both transmission electron microscopy and scanning electron microscopy revealed an intimate association of the symbionts with the epithelial surfaces of the hepatopancreas. Every cecum investigated contained a dense population of bacteria located close to the endothelial cell surface, often occupying the grooves between the protruding epithelial cells (Fig. 5A and Fig. 6A). The bacterial cells were spherical, and large reticulate cells and small cells with dense cytoplasm could be distinguished (Fig. 5A). No cell wall was visible, and the cells were surrounded by a single unit membrane (Fig. 5B). Flagella were not observed, but the large cells frequently had stalk-like appendages at one cell pole (Fig. 5C and E and Fig. 6B) or both cell poles (Fig. 5D). The stalks were cytoplasmic protrusions surrounded by a cell membrane (Fig. 5C), had a diameter of 50 to 100 nm, and varied in length (0.3 to 1.1 μm). In some cases, the stalks appeared to be involved in budding (Fig. 5F), with the small cells possibly representing the result of unequal cell division. Often, stalks were in contact with the microvillous brush border and inserted into the space between the microvilli (Fig. 5E).
FIG. 5.
Transmission electron micrographs of bacterial symbionts in the midgut glands of P. scaber, showing large and small spherical cells in close association with the microvilli (A); both large and small cells lacking a cell wall and bound by a single unit membrane, indicated by an arrow (large cell) and an arrowhead (small cell) (B); the frequently observed stalk on one cell pole (C), on both cell poles (D), and inserted into the gaps between the microvilli of the brush border (E); and cell division by budding from the stalks (F). (A) Scale bar = 1 μm; (B) Scale bar = 0.1 μm; (C to F) Scale bars = 0.2 μm.
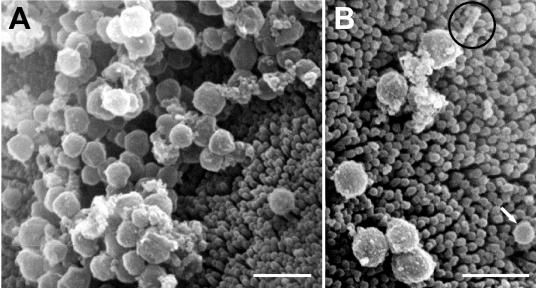
FIG. 6.
Scanning electron micrographs of bacterial symbionts in the midgut glands of P. scaber, showing uniform spherical cells in close association with the microvilli of the brush border (A) and a large sphere with a stalk (circle) and a small sphere (arrow) (B). Scale bars = 1 μm.
DISCUSSION
Phylogeny and evolution of Mycoplasma-like symbionts.
At this time, the Mollicutes are considered to consist of five separate phylogenetic clades: the Asteroleplasma group, the Acholeplasma-Anaeroplasma group, the M. hominis group, the M. pneumoniae group, and the Spiroplasma group (18). All phylogenetic analyses supported the conclusion that the hepatopancreatic symbionts of P. scaber represent a sixth lineage in the Mollicutes.
Although Mollicutes are widespread commensals or pathogens of humans, mammals, reptiles, fish, plants, and other arthropods (20), little is known about their occurrence in crustaceans. Apart from a report of a pathogenic mollicute-like bacterium occurring intracellularly in the hepatopancreatic epithelium of shrimp (Penaeus vannamei) (13), this is the first study demonstrating the colonization of the hepatopancreatic lumen of a crustacean host by extracellular Mycoplasma-like symbionts.
Symbiotic association.
Many Mollicutes cause only mild and chronic infections, rarely harming their hosts markedly or killing them (20), which is considered indicative of an evolutionary advancement towards mutualistic symbiosis. Hence, it is conceivable that the Mycoplasma-like symbionts of P. scaber are either benign pathogens, commensals, or mutualistic symbionts. The benefit for the host is difficult to predict, but the presence of the symbionts might diminish the risk of infection of the hepatopancreas by more harmful parasitic or pathogenic microorganisms. Thus, harboring hepatopancreatic symbionts might have enabled isopods to colonize new habitats and extend their geographic distribution. Previous work, based on antibiotic treatment and analysis of the activity of cellulases and phenol oxidases, led to the hypothesis that symbiotic bacteria in midgut glands might facilitate the digestion of terrestrial food sources (leaf litter) (28, 31). Our results make this less likely since there is to date no evidence of degradation of cellulose in Mollicutes.
Stalk function.
A flask- or club-like cell shape with a protruding tip or bleb structure has been observed in pathogenic mycoplasmas (12, 19), and there is evidence for the presence of cytoskeletal elements in M. pneumoniae (9). While the tip-like structures of Mycoplasma penetrans and Mycoplasma genitalium are involved in the invasion of host cells (11, 16), the morphologically different stalks of the Mycoplasma-like symbionts of P. scaber appear to serve merely in adhesion to the host endothelium. An analogous function has been proposed for the stalks of the candidate species “Hepatincola porcellionum,” a Rickettsia-like symbiont colonizing the hepatopancreas in other populations of P. scaber (25). The occurrence of stalk-like structures that insert between the microvilli of the epithelial brush border is rather unusual among the bacterial symbionts of invertebrates and may be a case of convergent evolution, driven by the same functional necessities. Whether the latter lies, e.g., in prevention of complete washout of the symbionts into the digestive tract together with the hepatopancreatic secretions or in the facilitation of nutrient exchange with the host epithelium remains open to speculation and further study.
Small and large spheres.
The results of the sequencing analysis documented the presence of two closely related 16S rRNA genes in the DNA extracted from the hepatopancreas of P. scaber. Coincidentally, two types of bacterial cells, small and large spheres, were observed to colonize the hepatopancreatic lumen. These two cell types both hybridized with the sequence-specific probe PsSym352 and possessed the same ultrastructural features. The probe did not allow us to differentiate between the two clone groups, and the fact that the DGGE analysis showed that there was only a single band may be explained by PCR bias. However, at this time it is not possible to decide whether there are two separate, albeit closely related, lineages of symbionts or only a single, morphologically variable strain with two slightly divergent copies of the 16S rRNA gene.
As the 16S rRNA genes of the symbionts in the hepatopancreas of P. scaber exhibit <82% sequence identity with the 16S rRNA genes of their closest relatives in the Spiroplasma group and M. pneumoniae group of the Mollicutes, these organisms cannot be assigned to any recognized taxon in this class. Following the recommendation of Stackebrandt et al. (22), who encouraged the use of the Candidatus concept for well-characterized but as-yet-uncultured organisms (22), we propose provisional classification of the symbionts as “Candidatus Hepatoplasma crinochetorum.”
Description of “Candidatus Hepatoplasma crinochetorum”
Hepatoplasma crinochetorum (he.pa.to.plas'ma. Gr. n. hepar liver, Gr. neut. n. plasma something formed or molded, N.L. neut. n. hepatoplasma something from the liver [hepatopancreas]; cri.no.che.to'rum. N.L. gen. pl. crinochetorum of isopods belonging to the taxon Crinocheta).
The short description is as follows: spherical cells (diameter, 0.5 to 0.8 μm); no cell wall and outer membrane; forms stalk-like cytoplasmic appendages at one or two cell poles that attach to host epithelia or are involved in budding. Basis of assignment: 16S ribosomal DNA sequences (accession numbers AY500249 and AY500250), hybridization with 16S rRNA-targeted oligonucleotide probe (5′-GTG AAA AAT TCC CTA CTG CTG-3′). Symbiont of P. scaber and other terrestrial isopods belonging to the taxon Crinocheta, located exclusively in the lumen of the hepatopancreas; so far uncultivated.
Acknowledgments
Y.W. and U.S. were supported by grants from the Deutsche Forschungsgemeinschaft to M.Z. and A.B., respectively.
We thank Antje Thomas for technical assistance, Hans G. Trüper for etymological advice, and Karen A. Brune for editing the manuscript.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann, R. I., B. J. Binder, R. J. Olson, S. W. Chisholm, R. Devereux, and D. A. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breznak, J. A., and A. Brune. 1994. Role of microorganisms in the digestion of lignocellulose by termites. Annu. Rev. Entomol. 39:453-487. [Google Scholar]
- 4.Brune, A. 2003. Symbionts aiding digestion, p. 1102-1107. In V. H. Resh and R. T. Cardé (ed.), Encyclopedia of insects. Academic Press, New York, N.Y.
- 5.Cole, J. R., B. Chai, T. L. Marsh, R. J. Farris, Q. Wang, S. A. Kulam, S. Chandra, D. M. McGarrell, T. M. Schmidt, G. M. Garrity, and J. M. Tiedje. 2003. The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res. 31:442-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubilier, N., O. Giere, D. L. Distel, and C. M. Cavanaugh. 1995. Characterization of chemoautotrophic bacterial symbionts in a gutless marine worm (Oligochaeta, Annelida) by phylogenetic 16S rRNA sequence analysis and in situ hybridization. Appl. Environ. Microbiol. 61:2346-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedrich, M. W., D. Schmitt-Wagner, T. Lueders, and A. Brune. 2001. Axial differences in community structure of crenarchaeota and euryarchaeota in the highly compartmentalized gut of the soil-feeding termite Cubitermes orthognathus. Appl. Environ. Microbiol. 67:4880-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hames, C. A. C., and S. P. Hopkin. 1989. The structure and function of the digestive system of terrestrial isopods. J. Zool. (London) 217:599-627. [Google Scholar]
- 9.Hegermann, J., R. Herrmann, and F. Mayer. 2002. Cytoskeletal elements in the bacterium Mycoplasma pneumoniae. Naturwissenschaften 89:453-458. [DOI] [PubMed] [Google Scholar]
- 10.Henckel, T., M. Friedrich, and R. Conrad. 1999. Molecular analyses of the methane-oxidizing microbial community in rice field soil by targeting the genes of the 16S rRNA, particulate methane monooxygenase, and methanol dehydrogenase. Appl. Environ. Microbiol. 65:1980-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen, J. S., J. Blom, and K. Lind. 1994. Intracellular location of Mycoplasma genitalium in cultured Vero cells as demonstrated by electron microscopy. Int. J. Exp. Pathol. 75:91-98. [PMC free article] [PubMed] [Google Scholar]
- 12.Kirchhoff, H., R. Rosengarten, W. Lotz, M. Fischer, and D. Lopatta. 1984. Flask-shaped mycoplasmas: properties and pathogenicity for man and animals. Isr. J. Med. Sci. 20:848-853. [PubMed] [Google Scholar]
- 13.Krol, R., W. Hawkins, and R. Overstreet. 1991. Rickettsial and mollicute infections in hepatopancreatic cells of cultured Pacific white shrimp (Penaeus vannamei). J. Invertebr. Pathol. 57:362-370. [DOI] [PubMed] [Google Scholar]
- 14.Lane, D. J., B. Pace, G. J. Olsen, D. A. Stahl, M. L. Sogin, and N. R. Pace. 1985. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc. Natl. Acad. Sci. USA 82:6955-6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lathe, R. 1985. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J. Mol. Biol. 183:1-12. [DOI] [PubMed] [Google Scholar]
- 16.Lo, S. C., M. M. Hayes, H. Kotani, P. F. Pierce, D. J. Wear, P. B. Newton, J. G. Tully, and J. W. Shih. 1993. Adhesion onto and invasion into mammalian cells by Mycoplasma penetrans: a newly isolated mycoplasma from patients with AIDS. Mod. Pathol. 6:276-280. [PubMed] [Google Scholar]
- 17.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Forster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. Konig, T. Liss, R. Lussmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K. H. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Razin, S. 8 September 2001, posting date. The genus Mycoplasma and related genera (class Mollicutes). In M. Dworkin (ed.), The prokaryotes: an evolving electronic resource for the microbiological community, 3rd ed. [Online.] Springer-Verlag, New York, N.Y. http://link.springer-ny.com/link/service/books/10125/.
- 19.Razin, S. 1985. Mycoplasma adherence, p. 161-202. In S. Razin and M. F. Barile (ed.), The mycoplasmas: mycoplasma pathogenicity. Academic Press, Orlando, Fla.
- 20.Razin, S., D. Yogev, and Y. Naot. 1998. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 62:1094-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds, E. S. 1963. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17:208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stackebrandt, E., W. Frederiksen, G. M. Garrity, P. A. Grimont, P. Kämpfer, M. C. Maiden, X. Nesme, R. Rossello-Mora, J. Swings, H. G. Trüper, L. Vauterin, A. C. Ward, and W. B. Whitman. 2002. Report of the Ad Hoc Committee for the Re-evaluation of the Species Definition in Bacteriology. Int. J. Syst. Evol. Microbiol. 52:1043-1047. [DOI] [PubMed] [Google Scholar]
- 23.Wagner, M., R. Amann, H. Lemmer, and K. H. Schleifer. 1993. Probing activated sludge with oligonucleotides specific for proteobacteria: inadequacy of culture-dependent methods for describing microbial community structure. Appl. Environ. Microbiol. 59:1520-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallner, G., R. Amann, and W. Beisker. 1993. Optimizing fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometry 14:136-143. [DOI] [PubMed] [Google Scholar]
- 25.Wang, Y., U. Stingl, F. Anton-Erxleben, M. Zimmer, and A. Brune. 2004. “Candidatus Hepatincola porcellionum” gen. nov., sp. nov., a new, stalk-forming lineage of Rickettsiales colonizing the midgut glands of a terrestrial isopod. Arch. Microbiol. 181:299-304. [DOI] [PubMed] [Google Scholar]
- 26.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood, S., and B. S. Griffith. 1988. Bacteria associated with the hepatopancreas of the woodlice Oniscus asellus and Porcellio scaber (Crustacea, Isopoda). Pedobiologia 31:89-94. [Google Scholar]
- 28.Zimmer, M. 1999. The fate and effects of ingested hydrolysable tannins in Porcellio scaber. J. Chem. Ecol. 25:611-628. [Google Scholar]
- 29.Zimmer, M. 2002. Nutrition in terrestrial isopods (Isopoda: Oniscidea): an evolutionary-ecological approach. Biol. Rev. 77:455-493. [DOI] [PubMed] [Google Scholar]
- 30.Zimmer, M., J. P. Danko, A. R. Danford, T. H. Carefoot, A. Ziegler, and R. F. Uglow. 2002. Cellulose digestion and phenol oxidation in coastal isopods (Custacea: Isopoda). Mar. Biol. 140:1207-1213. [Google Scholar]
- 31.Zimmer, M., and W. Topp. 1998. Microorganisms and cellulose digestion in the gut of Porcellio scaber (Isopoda: Oniscidea). J. Chem. Ecol. 24:1395-1408. [Google Scholar]
- 32.Zimmer, M., and W. Topp. 1998. Nutritional biology of terrestrial isopods (Isopoda: Oniscidea): copper revisited. Isr. J. Zool. 44:453-462. [Google Scholar]