Abstract
We investigated the regulation of the central aerobic and hypoxic metabolism of the biocontrol and non-Saccharomyces wine yeast Pichia anomala. In aerobic batch culture, P. anomala grows in the respiratory mode with a high biomass yield (0.59 g [dry weight] of cells g of glucose−1) and marginal ethanol, glycerol, acetate, and ethyl acetate production. Oxygen limitation, but not glucose pulse, induced fermentation with substantial ethanol production and 10-fold-increased ethyl acetate production. Despite low or absent ethanol formation, the activities of pyruvate decarboxylase and alcohol dehydrogenase were high during aerobic growth on glucose or succinate. No activation of these enzyme activities was observed after a glucose pulse. However, after the shift to oxygen limitation, both enzymes were activated threefold. Metabolic flux analysis revealed that the tricarboxylic acid pathway operates as a cycle during aerobic batch culture and as a two-branched pathway under oxygen limitation. Glucose catabolism through the pentose phosphate pathway was lower during oxygen limitation than under aerobic growth. Overall, our results demonstrate that P. anomala exhibits a Pasteur effect and not a Crabtree effect, i.e., oxygen availability, but not glucose concentration, is the main stimulus for the regulation of the central carbon metabolism.
The ascomycetous yeast Pichia (Hansenula) anomala is an efficient biocontrol agent against mold infections in airtight stored grain (11, 37), in apples (21), and on grapevines (28). P. anomala has also been reported to be a non-Saccharomyces wine yeast (31, 44) and can be a spoilage yeast of food (23, 24) and fermented drinks (25). Growth and survival in these environments require rapid adaptation of central carbon and energy metabolism to aerobic or oxygen-limited conditions, i.e., regulation of respiration and fermentation. Moreover, products of the glucose metabolism of P. anomala, in particular the volatile compound ethyl acetate, produced in significant amounts by this yeast (8, 38, 49), are important for wine aroma (41).
Respiration and fermentation in the model yeast Saccharomyces cerevisiae are regulated by sugar availability (9, 19). A glucose pulse rapidly induces alcoholic fermentation and the activities of alcohol dehydrogenase (ADH) and pyruvate decarboxylase (PDC). This induction is correlated with the accumulation of certain glycolytic metabolites (3) and an enhanced specific growth rate (40, 52). The sugar regulation in S. cerevisiae is an exception to most other known yeasts that respond to oxygen availability rather than to glucose concentration (51, 54). In the Crabtree-negative yeasts Kluyveromyces lactis and Pichia stipitis, the induction of PDC and ADH activity does not depend on the glycolytic flux but on the O2 tension (22, 36). Contrary to the general conviction that Crabtree-negative yeasts lack glucose induction of the alcoholic fermentation, this has been reported for K. lactis (22). P. anomala is a Crabtree-negative yeast but is able to grow anaerobically (18).
Individual enzyme activities or production rates can provide important metabolic insight. However, integrating such data on isolated components into knowledge about the whole metabolic network is rather complicated. Metabolic flux analysis based on 13C labeling experiments provides a tool to study central carbon metabolism as a network (46). The general principle is that uptake and secretion rates are interpreted within a stoichiometric model of metabolism, together with nuclear magnetic resonance or mass spectrometry (MS) data on the distribution of 13C label from a tracer experiment. The calculated fluxes are thus a best fit to physiological and 13C data. Several yeasts were successfully investigated by this method, including S. cerevisiae (20, 26), Saccharomyces kluyveri (32), and P. stipitis (13). Important findings derived from flux analysis include the discovery of quantitative differences between yeasts with respect to the activity of the pentose phosphate (PP) pathway, the importance of anaplerosis for the contribution of oxaloacetate to the tricarboxylic acid (TCA) cycle, the activity of the TCA cycle, and the presence of a mitochondrial malic enzyme.
No data are available on the regulation of the respiratory and fermentative enzymes in P. anomala, nor on the metabolic fluxes or product formation under controlled aerobic and oxygen-limited conditions. This information is necessary to understand and predict the growth and metabolic activity of P. anomala in different habitats like grain, silage, and various food environments. In addition, regulation of the central energy metabolism needs to be understood for determining and improving the role of P. anomala in biocontrol or wine making. Using flux analysis, quantitative physiology, and in vitro enzyme activities, we attempt to provide a baseline study for central carbon metabolism and its global regulation in the yeast P. anomala.
MATERIALS AND METHODS
Yeast strain and growth media.
The haploid strain NRRL-Y-366-8 (+; CBS 1984), derived from the diploid type strain NRRL-Y-366 (CBS 5759) of P. anomala (BioloMICS Database; http://www.cbs.knaw.nl/databases/index.htm), was chosen for the growth experiments in this study. Defined media were used for all cultivations (10, 34) and either glucose (20 g liter−1), succinate (10 g liter−1), or ethanol (2 g liter−1) was used as the sole carbon source. The inocula were precultivated for 20 to 30 h at 25°C, 150 rpm, in YNB medium (Difco Laboratories, Detroit, Mich.) with the same carbon source as that for the subsequent batch cultivation and added to the fermentor vessel to an optical density at 600 nm (OD600) of 0.1 to 0.15. All growth experiments were repeated at least three times.
Batch cultivation under controlled conditions.
All experiments were performed in a Belach fermentor with a 1-liter working volume at 25°C. The pH was automatically kept constant at 5.0 ± 0.1 by titration with 2 M KOH. The dissolved oxygen tension was measured with an autoclavable O2 sensor (D100 series OxyProbe; Bradley James Corporation).
In aerobic cultivations the oxygen saturation was kept at 50% ± 5% by regulating the stirring velocity between 100 and 900 rpm at an aeration rate of 1 liter of air min−1. In the oxygen shift experiments, the culture was flushed with nitrogen (Air Liquid, Malmö, Sweden) until the dissolved oxygen tension reached 0%, which was achieved within 1 min. To avoid anaerobic conditions, the culture was aerated with 0.3 liters of air min−1 at a stirring speed of 90 rpm. The dissolved oxygen tension was always 0% after the shift, indicating that the oxygen demand of the yeast cells was higher than the oxygen transfer rate from the gas phase to the medium, i.e., the cultures were oxygen limited (12).
Experimental setup.
In the oxygen shift experiments, cells were grown aerobically until the culture reached an OD600 of 2 to 3. One sample was taken from the aerobic culture (t0) before the oxygen tension was shifted from 50 to 0%. Then samples were taken at 0.5, 2, 4, 6, 8, and 12 h after the oxygen shift. In the glucose pulse experiment, cells were grown aerobically with succinate as the sole carbon source until the culture reached an OD600 of 2 to 3. Samples were taken before glucose addition (t0) and then 0.5, 2, 4, 6, and 8 h after glucose addition (20 g liter−1).
Growth measurements.
Growth was estimated by measuring the OD600. The OD600 was correlated with the dry weight of cultures growing in aerobic and oxygen-limited conditions with controlled aeration. Dry weight was determined gravimetrically from triplicate 10-ml samples of culture supernatant as described earlier (36). One OD unit corresponded to 0.42 ± 0.03 mg of cells (dry weight) (cdw) ml−1.
Enzyme measurements.
Enzyme assays were done on fresh cell extracts at 30°C with a Beckman DU 600 spectrophotometer at 340 nm (Beckman Instruments, Inc., Fullerton, Calif.). Samples were taken 0 to 8 h after glucose pulse and 0 to 12 h after shift to oxygen limitation (see Results). Samples of 50 to 100 ml of the supernatant were withdrawn, and protein was extracted according to the method of Ciriacy and Breitenbach (7). Protease inhibitors (Complete; Roche Diagnostics, Mannheim, Germany) were added to the crude extract buffer (1 tablet, 50 ml−1). The protein concentrations were determined by comparison with standard solutions of bovine serum albumin by use of the Bio-Rad Laboratories protein assay based on the work of Bradford (5).
The activities of ADH (EC 1.1.1.1) (1), PDC (EC 4.1.1.1) (50), and the NAD+-dependent aldehyde dehydrogenase (ALD; EC 1.2.1.5) (4) were analyzed. One activity unit corresponds to the amount of enzyme catalyzing the conversion of 1 μmol of substrate min−1, and specific activities are expressed as units milligram of protein−1.
Determination of sugar and metabolite concentrations.
Glucose, succinate, and secreted metabolite concentrations were determined by high-pressure liquid chromatography analysis. Samples were prepared as previously described (17) and analyzed using an HC-75 column (305 by 7.8 mm; Hamilton, Reno, Nev.) and an RI detector (Agilent 1100 Series; Agilent Technologies, Waldbronn, Germany; 40°C). The column was eluted at 60°C with 5 mM sulfuric acid at a flow rate of 0.6 ml min−1. The metabolites were identified and quantified by comparison of peak areas with standard curves obtained by analyzing authentic reference compounds.
Determination of ethyl acetate.
To allow for analysis of the volatile ester ethyl acetate, the gas outflow of the fermentor was led through two serial tubes containing 4 ml of decane each (99%; Sigma). Oda et al. (33) showed that the organic solvent decane could be used as a hydrophobic trap for volatile esters. Ethyl acetate was quantified with a Hewlett-Packard 6890 gas chromatography column, equipped with a flame ionization detector and a capillary column (HP19091S-833; 250 μm by 30 m). The oven temperature was programmed to 60°C for 3 min followed by a temperature rise of 20°C min−1 to 250°C, where it was held for 2 min. Samples (1 μl) were injected in splitless mode with hydrogen as the carrier gas at 40 ml min−1. The compound was identified and quantified by comparison of retention time and peak areas to external standards of known concentrations of ethyl acetate (Sigma).
13C labeling experiments.
The labeling experiments were carried out in batch cultures assuming pseudo-steady-state conditions during the exponential growth phase (14, 47). 13C labeling of proteinogenic amino acids was achieved by growth on 5 g of glucose liter−1 (aerobic growth) or 10 g liter−1 (oxygen-limited growth), supplied as a mixture of 80% (wt/wt) unlabeled and 20% (wt/wt) uniformly labeled [U-13C]glucose (13C, >99%; Martek Biosciences Corp., Columbia, Md.). The inocula were pregrown under aerobic or oxygen-limited conditions, respectively, and added to the fermentor vessel to an OD600 of 0.01 (less than 1% of final OD) to minimize the influence of unlabeled glucose from the inocula. Aliquots were harvested (three to four per cultivation) by centrifugation when the culture reached an OD600 of 0.8 to 2 and washed with distilled water. Cellular protein was hydrolyzed by incubating resuspended cells in 6 M HCl for 24 h at 105°C. Subsequently, the samples were dried at 85°C. The free amino acids were derivatized at 85°C for 1 h with 25 μl of dimethyl formamide and 25 μl of N-(tert-butyldimethylsilyl)-N-methyl-trifluoroacetamide (15, 56). Gas chromatography-MS analysis was carried out as reported recently (14).
Metabolic flux ratio analysis.
The gas chromatography-MS data include the mass isotopomer distributions of the following amino acids: alanine, aspartate, glutamate, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tyrosine, and valine. For each amino acid fragment α, one mass isotopomer distribution vector (MDV) was assigned,
 |
(1) |
with m0 being the fractional abundance of the lowest mass and mi>0 being the abundances of molecules with higher masses. The MDVα were corrected for naturally occurring isotopes as described previously (14), to obtain the exclusive mass isotopomer distributions of the carbon skeletons, which were used to calculate the amino acid (MDVAA) and metabolite (MDVM) mass distribution vectors. Metabolic flux ratios were calculated from the MDVM as described previously (2).
13C-constrained flux balancing.
Intracellular fluxes were calculated with a flux model that comprised all major pathways of yeast central carbon metabolism (see Fig. 4). The resulting stoichiometric model of 27 linear equations is underdetermined, thus having an infinite solution space. Hence, additional constraints were required to uniquely solve the mass balance equations and to estimate the in vivo fluxes. For this purpose, a set of linearly independent equations were obtained from METAFoR analysis to render the system solvable (see also Table 2).
FIG. 4.
Metabolite flux distributions in P. anomala. The numbers represent the percentages of carbon passing through each reaction mixture. The upper numbers in each set represent flux distribution for an aerobic culture, whereas the lower numbers are those of an oxygen-limited culture.
TABLE 2.
Metabolic flux ratios in P. anomala when grown aerobically and under oxygen limitation
| Metabolic flux ratio | Aerobic growth | Oxygen-limited growth |
|---|---|---|
| PEP through PP pathway (upper bound) | 0.21 ± 0.05 | 0.06 ± 0.05 |
| PEP from oxaloacetatecyt | 0 ± 0.01 | 0 ± 0.01 |
| Oxaloacetatemit from anaplerosis | 0.37 ± 0.02 | 0.86 ± 0.02 |
| Pyruvatemit from oxaloacetatemit (upper bound) | 0.11 ± 0.02 | 0.6 ± 0.13 |
| Pyruvatecyt from oxaloacetatemit (lower bound) | 0.07 ± 0.01 | 0.08 ± 0.01 |
The set included the upper bound of PEP through the PP pathway:
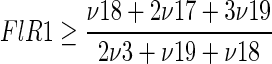 |
(2) |
the fraction of mitochondrial oxaloacetate derived through anaplerosis:
 |
(3) |
the fraction of PEP originating from cytosolic oxaloacetate:
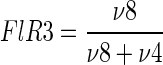 |
(4) |
and the upper and lower bounds for mitochondrial pyruvate derived through the malic enzyme (from mitochondrial malate):
 |
(5/6) |
The biomass composition and the resulting precursor requirements were used as described for S. cerevisiae (20). The minimization of errors from the extracellular fluxes, the metabolite balances, and the flux ratios was carried out as recently described (15).
RESULTS
Growth and fermentation profiles during batch cultivation of P. anomala.
To characterize the regulation of the respiratory and fermentative metabolism, the yeast strain was cultivated in controlled aerobic and oxygen-limited batch cultures. Under aerobic conditions, P. anomala grew exponentially with a growth rate of 0.22 h−1 and a biomass yield of 0.59 g of cdw g of glucose−1 (Table 1). Only low amounts of ethanol, acetate, glycerol, and ethyl acetate were detected (Table 1; Fig. 1), indicating a preferentially respiratory metabolism. When the oxygen tension was reduced from 50 to 0%, growth was arrested for approximately 0.5 h. Growth resumed within 2 h, with a much lower growth rate than that for aerobic growth (Fig. 2 and Table 1). Under our experimental conditions, we could not conclude whether the growth of the yeast cells was oxygen limited. Such limitation is characterized by a linear growth curve (12). Indeed, linear regression of the growth curve yielded an R2 value of 0.99. However, a fit to an exponential function also resulted in an R2 value of 0.99, with a growth rate of 0.056 h−1. The biomass yield was reduced sixfold to 0.11 g of cdw g of glucose−1. The production rates of ethanol, glycerol, and ethyl acetate increased considerably (Table 1; Fig. 1). The ethanol yield was close to the theoretical maximum, indicating a preferentially fermentative metabolism. The glucose consumption rate increased more than twofold in response to oxygen limitation (Table 1). The ethanol production rate of 6.2 ± 0.6 mmol g of cdw−1 h−1 during fermentative growth was slightly lower than that in K. lactis (10 mmol g of cdw−1 h−1) and P. stipitis (0.38 g g of cdw−1 h−1, equal to 8.3 mmol g of cdw−1 h−1) (22, 48) and significantly lower than the 24 mmol g of cdw−1 h−1 that has been described for anaerobically cultivated S. cerevisiae (42).
TABLE 1.
Specific growth rate, production and consumption rates, and yieldsa in P. anomala when grown aerobically, exposed to a shift in oxygen tension or to a glucose pulseb
| Parameter | Aerobic growth (glucose) | After shift to 0% oxygen (glucose) | After glucose pulse (succinate and glucose) |
|---|---|---|---|
| Specific growth rate (h−1) | 0.22 ± 0.02 | 0.056 ± 0.009 | 0.21 ± 0.04 |
| Generation time (h) | 3.2 ± 0.3 | 12.4 ± 1.7 | 3.3 ± 0.5 |
| Specific consumption or production rate (mmol g of biomass−1 h−1) | |||
| Glucose | 2.1 ± 0.4 | 4.6 ± 0.5 | 2.65 ± 0.6 |
| Ethanol | 0.14c | 6.2 ± 0.6 | 0 ± 0 |
| Glycerol | 0.12c | 0.61 ± 0.06 | NDd |
| Acetate | 0.22c | 0 | ND |
| Yield (g g of glucose−1) | |||
| Biomass | 0.59 ± 0.13 | 0.11 ± 0.04 | ND |
| Ethanol | 0.03 ± 0.004 | 0.48 ± 0.09 | ND |
| Glycerol | 0.05 ± 0.02 | 0.08 ± 0.02 | ND |
| Acetate | 0.06 ± 0.009 | 0 ± 0 | ND |
| Carbon balancee (%) | 80 | 100 |
The yield coefficients were calculated on data from samples taken during exponential phase (aerobic culture) and at 4 to 6 h after oxygen limitation (when maximum yield of ethanol was obtained).
Values are averages ± standard deviations based on the results from three independent cultivations. Carbon sources are given in parentheses.
Rate estimation from intermediate production of acetate, ethanol, and glycerol during aerobic growth.
ND, not determined.
CO2 production was not measured, but theoretical yields were calculated from ethanol production.
FIG. 1.
Semiquantitative analysis (see text) of ethyl acetate production in aerobic (t0) and oxygen-limited batch cultures. Values are means ± standard deviations (n = 3).
FIG. 2.
Growth of P. anomala in batch cultures grown under aerobic conditions (▴) and after a shift to oxygen limitation (▪), on glucose as the sole carbon source.
Ethyl acetate production increased 10-fold within 1 h after oxygen limitation (Fig. 1). The production rate then decreased but was still higher than that during aerobic conditions (4 to 8 h after the shift; Fig. 1). The contact time between the gas outflow (containing ethyl acetate) and decane in the tubes was only a few seconds (aeration rate, 0.3 or 1 liter min−1 in 2 × 4 ml of decane). Oda et al. (33) as well as Rojas et al. (45) measured ester concentration in decane that had been in contact with the yeast cultures for 24 to 48 h. We therefore used two serial tubes to trap as much ethyl acetate as possible without creating a dilution effect from large decane volumes. During aerobic growth, all ethyl acetate was found in the first tube, indicating a low and continuous production. In the first sample after the oxygen shift (0 to 1 h), the concentration was almost equally high in both tubes, indicating that the contact time was too short to trap all ethyl acetate. In the subsequent samples (4 to 8 h after oxygen shift) the concentration in the second tube was 10 to 50% of that in the first. This shows that the decane trap method is only semiquantitative at high production rates and that the increase in ethyl acetate production rate is probably even higher than that shown in Fig. 1.
To investigate if alcoholic fermentation in P. anomala can also be induced by glucose, a glucose pulse was applied to cultures grown on succinate. With succinate as a carbon source, P. anomala grew linearly. After the pulse, the cultures resumed exponential growth within 6 h (Table 1). The growth rate (0.21 h−1) and the glucose consumption rate (2.65 mmol g of biomass−1 h−1) correspond to the values obtained from the aerobic batch cultures grown on glucose (Table 1). The glucose pulse did not induce ethanol formation within the time of analysis (8 h), but small amounts of glycerol, arabitol, and acetate were detected in the growth medium (data not shown). Arabitol was detected in low amounts under all growth conditions during late exponential and stationary phase, independent of glucose concentration or oxygen tension (data not shown).
There seems to be no strict glucose repression of the consumption of ethanol, acetate, or succinate, since these compounds were coconsumed with glucose when present in the culture (data not shown). The results show that P. anomala is a respiratory yeast that lacks a Crabtree effect, i.e., having no induction of alcoholic fermentation in response to a high glucose concentration.
Enzyme activities during batch cultivation of P. anomala.
To investigate whether the oxygen-dependent changes in the respiratory and fermentative metabolism are connected to enzyme regulation, we determined activities of the fermentative key enzymes, PDC and ADH, as well as ALD, an enzyme in the pyruvate bypass, under aerobic (t0) and oxygen-limited conditions (0.5 to 12 h after the shift) in glucose-grown cultures. The three enzymes had significant activities in the aerobic cultures (Fig. 3) with ADH having the highest activity (2 U mg of protein−1) and ALD having the lowest (100-fold lower than that of ADH). When the culture conditions were shifted from aerobic to oxygen limited, PDC and ADH were activated threefold and reached maximum activity after 6 h (Fig. 3a and b). ALD was only slightly activated and reached its highest activity 12 h after the oxygen shift (Fig. 3c). The high enzyme activities of PDC and ADH in aerobic batch culture might indicate a glucose-dependent induction of these fermentative enzymes. To further investigate the role of glucose in the induction of the fermentative pathways, the same enzyme activities were measured in cultures before (t0) and after the addition of a glucose pulse (0.5 to 8 h after the pulse). We observed considerable activities for PDC, ADH, and ALD in cultures growing on succinate (Fig. 3). Both ADH and ALD activities were higher than in cells grown aerobically on glucose. After glucose addition, the ADH and ALD activities were down regulated (Fig. 3b and c). The activity of ADH reached the level of the aerobic glucose culture after 8 h (Fig. 3b). The activity of PDC was lower during growth on succinate than during growth on glucose and was not affected by the addition of glucose within the 8 h of analysis (Fig. 3a).
FIG. 3.
Specific enzymatic activities of PDC (a), ADH (b), and ALD (c) in crude extracts from cells grown on glucose under initially aerobic conditions (t0) after a shift to oxygen limitation (•) and from cells grown on succinate (t0) pulsed with glucose (▪). The curves show representative results from three independent cultivations.
13C metabolic flux analysis in aerobic and oxygen-limited batch cultures.
To monitor the global effects of oxygenation on metabolism, the metabolic flux distributions in P. anomala were determined by metabolic flux analysis. For this purpose, extracellular fluxes (Table 2) and the known precursor requirements for the determined biomass formation were balanced in a stoichiometric model. The analytically determined flux ratios (Table 2) were used as constraints in the calculation to obtain a unique flux solution (15).
The intracellular flux distribution changed significantly in response to oxygen availability (Table 2; Fig. 4). Under aerobic conditions, about 30% of the glucose-6-phosphate (glucose-6-P) was directly oxidized and entered the PP pathway (Fig. 4), whereas only 10% of the glucose-6-P was metabolized via the glucose-6-P dehydrogenase under oxygen limitation. The flux response to the two aeration regimens was even more drastic at the pyruvate branching point. Under aerobic conditions, the pyruvate flux into the mitochondria was 59%, but under oxygen limitation the flux was only 7% and pyruvate was mainly decarboxylated by PDC. Reducing oxygen availability caused a switch in operation of the TCA cycle from a cyclic activity to a two-branched pathway that sustained only synthesis of the biomass precursors oxaloacetate and α-ketoglutarate. Akin to S. cerevisiae and P. stipitis (13), no flux was detected via the gluconeogenic PEP-carboxykinase that catalyzes the reaction from oxaloacetate to PEP. Finally, low in vivo activities of the mitochondrial malic enzyme were detected in the aerobic culture (Fig. 4). This contribution to the mitochondrial pyruvate pool was also reported for S. cerevisiae (20).
DISCUSSION
A decrease in oxygen tension was the inducing stimulus for the activation of fermentation, and the doubling of the glucose uptake rate shows that P. anomala exhibits a clear Pasteur effect. The activities of fermentative key enzymes PDC and ADH were induced threefold by oxygen limitation but not by a glucose pulse. In addition, glucose availability did not induce ethanol formation. This regulation pattern is in sharp contrast to S. cerevisiae, where a glucose pulse activates PDC, the fermentative ADH (ADH1), and ethanol production (53, 55).
Three major differences were observed in the flux distributions of cultures grown under different aeration regimens. The flux through the PP pathway was lower during oxygen-limited growth; more reduced metabolites, mainly glycerol and ethanol, were produced; and the TCA cycle operated as a cycle under aerobic growth but as a two-branched pathway under oxygen limitation. Our analysis shows that P. anomala is very similar to S. cerevisiae in the general flux distribution under respiratory and fermentative metabolism (13, 20). However, the signals that induce respiratory and fermentative metabolism are completely different. We obtained respiratory growth from aerobic batch cultures with a high glucose concentration, and fermentation could be induced only by shifting the cultures to oxygen limitation. In contrast, S. cerevisiae shows fermentative behavior when cultivated in aerobic batch cultivation (19). This confirms that oxygen availability is the main inducer of the fermentative metabolism in P. anomala. Among the respiratory yeasts, P. anomala is similar to K. lactis (22) with only minor fermentation despite the considerable activities of PDC and ADH, but different from P. stipitis (36).
The ADH and ALD activities were higher in cells grown on succinate than in those grown aerobically on glucose. This was unexpected, as no ethanol was formed during these cultivations and no ethanol was present as a carbon source. Possibly, the ethanol assimilation pathway is controlled by glucose repression but constitutively expressed when no glucose is present. It is also possible that it fulfills other, as yet unknown functions in the metabolism. Moreover, we also found considerable PDC activity during growth on succinate. We can presently only speculate about the function of the PDC under these conditions. Flikweert et al. (16) demonstrated that in S. cerevisiae the PDC is indispensable for growth on glucose. Possibly, in P. anomala this enzyme is also required for growth on gluconeogenetic carbon sources.
The regulation of ethyl acetate production by P. anomala has not previously been studied under controlled oxygenation. This compound essentially contributes to the aroma of wine (41). Ethyl acetate was produced during all aeration conditions, but the production rate increased considerably after a shift to oxygen limitation. This result differs from those of Rojas et al. (45), who demonstrated a stronger ethyl acetate production from a P. anomala wine strain under higher aeration. However, these experiments were done with shake-flask cultures where oxygen limitation, despite rotation, is likely to occur (6). We recently demonstrated that oxygen limitation rapidly occurred in P. anomala shake-flask cultures even at low cell densities (17); thus, the high values from the wine yeast might be due to a beginning oxygen limitation. In yeasts, ethyl acetate is produced from ethanol and acetyl coenzyme A (CoA) by the action of an alcohol acetyltransferase (EC 2.3.1.84) or from ethanol and acetate by the reversed action of an esterase (EC 3.1.1.1) (27, 30, 39, 43, 58). The contribution of these two enzymes to the production of ethyl acetate differs among several species (27, 57). Proposed reasons for the ethyl acetate production are that the ester synthesis is (i) a detoxification mechanism to remove the toxic compounds acetate and ethanol from the cells or (ii) a recirculation pathway for free CoA (CoA-SH), which is liberated in the condensation reaction between acetyl-CoA and ethanol (29). The molar amount of ethyl acetate is only 1% of the ethanol produced, and thus the removal is not significant. However, the removal of acetate may be important under oxygen limitation, if the cell is more sensitive to intracellular organic acids under these conditions. However, the second hypothesis is more likely since the ester production is induced during oxygen limitation when the lipid synthesis and the resulting release of CoA-SH are reduced. Moreover, the yeast grows in many different environments and shows considerable antimicrobial activity (35). We recently found an antifungal activity of ethyl acetate during airtight storage of cereal grain (18a). Thus, the volatile compound might contribute to the competitiveness of P. anomala in closed environments, i.e., with limited gas exchange with the surroundings.
This metabolic and physiological study demonstrates that P. anomala is a respiratory yeast. Oxygen is the main regulator of the central energy metabolism; glucose does not repress respiration and does not even completely repress the utilization of alternative carbon sources. These findings also show that regulating oxygen availability may be the main factor in manipulating the activity of P. anomala in its numerous habitats, including biocontrol and wine making or as a spoilage yeast in beverages or yoghurt.
Acknowledgments
This work has been financially supported by The Foundation for Strategic Environmental Research (MISTRA). Lars M. Blank gratefully acknowledges financial support by the Deutsche Akademie der Naturforscher Leopoldina (BMBF-LPD/8-78).
REFERENCES
- 1.Bergmeyer, H. (ed.). 1974. Methods in enzymatic analysis, vol. 1. Academic Press, New York, N.Y.
- 2.Blank, L., and U. Sauer. 2004. TCA cycle activity in Saccharomyces cerevisiae is a function of the environmentally determined specific growth and glucose uptake rates. Microbiology 150:1085-1093. [DOI] [PubMed] [Google Scholar]
- 3.Boles, E., J. Heinisch, and F. K. Zimmermann. 1993. Different signals control the activation of glycolysis in the yeast Saccharomyces cerevisiae. Yeast 9:761-770. [DOI] [PubMed] [Google Scholar]
- 4.Bradbury, S., J. Clark, C. Steinman, and C. Jakoby. 1975. Aldehyde dehydrogenase from baker's yeast. Methods Enzymol. 41:354-360. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Büchs, J. 2001. Introduction to advantages and problems of shaken cultures. Biochem. Eng. J. 7:91-98. [DOI] [PubMed] [Google Scholar]
- 7.Ciriacy, M., and I. Breitenbach. 1979. Physiological effects of seven different blocks in glycolysis in Saccharomyces cerevisiae. J. Bacteriol. 139:152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies, R., E. Falkiner, J. Wilkinson, and J. Peel. 1951. Ester formation by yeast. 1. Ethyl acetate formation by Hansenula species. Biochem. J. 49:58-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Deken, R. 1966. The Crabtree effect: a regulatory system in yeast. J. Gen. Microbiol. 44:149-156. [DOI] [PubMed] [Google Scholar]
- 10.Dellweg, H., M. Rizzi, H. Methner, and D. Debus. 1984. Xylose fermentation by yeasts, comparison of Pachysolen tannophilus and Pichia stipitis. Biotechnol. Lett. 6:395-400. [Google Scholar]
- 11.Druvefors, U., N. Jonsson, M. E. Boysen, and J. Schnürer. 2002. Efficacy of the biocontrol yeast Pichia anomala during long-term storage of moist feed grain under different oxygen and carbon dioxide regimens. FEMS Yeast Res. 2:389-394. [DOI] [PubMed] [Google Scholar]
- 12.Elsworth, R., V. Williams, and R. Harris-Smith. 1957. The effect of oxygen supply on the rate of growth of Aerobacter cloacea. J. Appl. Chem. 7:269-274. [Google Scholar]
- 13.Fiaux, J., Z. P. Cakar, M. Sonderegger, K. Wüthrich, T. Szyperski, and U. Sauer. 2003. Metabolic-flux profiling of the yeasts Saccharomyces cerevisiae and Pichia stipitis. Eukaryot. Cell 2:170-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer, E., and U. Sauer. 2003. Metabolic flux profiling of Escherichia coli mutants in central carbon metabolism using GC-MS. Eur. J. Biochem. 270:880-891. [DOI] [PubMed] [Google Scholar]
- 15.Fischer, E., N. Zamboni, and U. Sauer. 2004. High-throughput metabolic flux analysis based on gas-chromatography-mass spectrometry derived 13C constraints. Anal. Biochem. 325:308-316. [DOI] [PubMed] [Google Scholar]
- 16.Flikweert, M. T., L. van der Zanden, W. Janssen, H. Y. Steensma, J. P. van Dijken, and J. T. Pronk. 1996. Pyruvate decarboxylase: an indispensable enzyme for growth of Saccharomyces cerevisiae on glucose. Yeast 12:247-257. [DOI] [PubMed] [Google Scholar]
- 17.Fredlund, E., A. Broberg, M. E. Boysen, L. Kenne, and J. Schnürer. 2004. Metabolite profiles of the biocontrol yeast Pichia anomala J121 grown under oxygen limitation. Appl. Microbiol. Biotechnol. 64:403-409. [DOI] [PubMed] [Google Scholar]
- 18.Fredlund, E., U. Druvefors, K.-J. Lingsten, M. E. Boysen, and J. Schnürer. 2002. Physiological characteristics of the biocontrol yeast Pichia anomala J121. FEMS Yeast Res. 2:395-402. [DOI] [PubMed] [Google Scholar]
- 18a.Fredlund, E., U. Ä. Druvefors, M. N. Olstorpe, V. Passoth, and J. Schnürer. 2004. Influence of ethyl acetate production and ploidy on the anti-mould activity of Pichia anomala. FEMS Microbiol. Lett. 238:133-137. [DOI] [PubMed] [Google Scholar]
- 19.Gancedo, J. M. 1998. Yeast carbon catabolite repression. Microbiol. Mol. Biol. Rev. 62:334-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gombert, A. K., M. M. dos Santos, B. Christensen, and J. Nielsen. 2001. Network identification and flux quantification in the central metabolism of Saccharomyces cerevisiae under different conditions of glucose repression. J. Bacteriol. 183:1441-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jijakli, M., and P. Lepoivre. 1998. Characterization of an exo-β-1,3-glucanase produced by Pichia anomala strain K, antagonist of Botrytis cinerea on apples. Phytopathology 88:335-343. [DOI] [PubMed] [Google Scholar]
- 22.Kiers, J., A. M. Zeeman, M. Luttik, C. Thiele, J. I. Castrillo, H. Y. Steensma, J. P. van Dijken, and J. T. Pronk. 1998. Regulation of alcoholic fermentation in batch and chemostat cultures of Kluyveromyces lactis CBS 2359. Yeast 14:459-469. [DOI] [PubMed] [Google Scholar]
- 23.Kosse, D., H. Seiler, R. Amann, W. Ludwig, and S. Scherer. 1997. Identification of yoghurt-spoiling yeasts with 18S rRNA-targeted oligonucleotide probes. Syst. Appl. Microbiol. 20:468-480. [Google Scholar]
- 24.Lanciotti, R., M. Sinigaglia, F. Gardini, and M. E. Guerzoni. 1998. Hansenula anomala as spoilage agent of cream-filled cakes. Microbiol. Res. 153:145-148. [DOI] [PubMed] [Google Scholar]
- 25.Loureiro, V., and M. Malfeito-Ferreira. 2003. Spoilage yeasts in the wine industry. Int. J. Food Microbiol. 86:23-50. [DOI] [PubMed] [Google Scholar]
- 26.Maaheimo, H., J. Fiaux, Z. P. Cakar, J. E. Bailey, U. Sauer, and T. Szyperski. 2001. Central carbon metabolism of Saccharomyces cerevisiae explored by biosynthetic fractional C-13 labeling of common amino acids. Eur. J. Biochem. 268:2464-2479. [DOI] [PubMed] [Google Scholar]
- 27.Malcorps, P., J. Cheval, S. Jamil, and J. P. Dufour. 1992. A new model for the regulation of ester synthesis by alcohol acetyltransferase in Saccharomyces cerevisiae during fermentation. ASBC J. 49:47-53. [Google Scholar]
- 28.Masih, E. I., I. Alie, and B. Paul. 2000. Can the grey mould disease of the grape-vine be controlled by yeast? FEMS Microbiol. Lett. 189:233-237. [DOI] [PubMed] [Google Scholar]
- 29.Mason, A. B., and J. P. Dufour. 2000. Alcohol acetyltransferases and the significance of ester synthesis in yeast. Yeast 16:1287-1298. [DOI] [PubMed] [Google Scholar]
- 30.Mauricio, J., J. Moreno, E. Valero, L. Zea, M. Medina, and J. Ortega. 1993. Ester formation and specific activities of in vitro alcohol acetyltransferase and esterase by Saccharomyces cerevisiae during grape must fermentation. J. Agric. Food Chem. 41:2086-2091. [Google Scholar]
- 31.Mingorance-Cazorla, L., J. M. Clemente-Jiménez, S. Martínez-Rodríguez, F. J. Las Heras-Vázquez, and F. Rodríguez-Vico. 2003. Contribution of different natural yeasts to the aroma of two alcoholic beverages. World J. Microbiol. Biotechnol. 19:297-304. [Google Scholar]
- 32.Moller, K., B. Christensen, J. Förster, J. Piskur, J. Nielsen, and L. Olsson. 2002. Aerobic glucose metabolism of Saccharomyces kluyveri: growth, metabolite production, and quantification of metabolic fluxes. Biotechnol. Bioeng. 77:186-193. [DOI] [PubMed] [Google Scholar]
- 33.Oda, S., Y. Inada, A. Kobayashi, A. Kato, N. Matsudomi, and H. Ohta. 1996. Coupling of metabolism and bioconversion: microbial esterification of citronellol with acetyl coenzyme A produced via metabolism of glucose in an interface bioreactor. Appl. Environ. Microbiol. 62:2216-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Passoth, V., and B. Hahn-Hägerdal. 2000. Production of a heterologous endo-1,4-β-xylanase in the yeast Pichia stipitis with an O2-regulated promoter. Enzyme Microb. Technol. 26:781-784. [DOI] [PubMed] [Google Scholar]
- 35.Passoth, V., and J. Schnürer. 2003. Non-conventional yeasts in antifungal application, p. 297-330. In H. de Winde (ed.), Functional genetics of industrial yeasts, vol. 2. Springer Verlag, Berlin, Germany. [Google Scholar]
- 36.Passoth, V., M. Zimmermann, and U. Klinner. 1996. Peculiarities of the regulation of fermentation and respiration in the Crabtree-negative, xylose-fermenting yeast Pichia stipitis. Appl. Biochem. Biotechnol. 58:201-212. [DOI] [PubMed] [Google Scholar]
- 37.Petersson, S., and J. Schnürer. 1998. Pichia anomala as a biocontrol agent of Penicillium roqueforti in high-moisture wheat, rye, barley, and oats stored under airtight conditions. Can. J. Microbiol. 44:471-476. [Google Scholar]
- 38.Plata, C., C. Millan, J. C. Mauricio, and J. M. Ortega. 2003. Formation of ethyl acetate and isoamyl acetate by various species of wine yeasts. Food Microbiol. 20:217-224. [Google Scholar]
- 39.Plata, M. D., J. C. Mauricio, C. Millan, and J. M. Ortega. 1998. In vitro specific activity of alcohol acetyltransferase and esterase in two flor yeast strains during biological aging of sherry wines. J. Ferment. Bioeng. 85:369-374. [Google Scholar]
- 40.Postma, E., C. Verduyn, W. A. Scheffers, and J. P. van Dijken. 1989. Enzymic analysis of the Crabtree effect in glucose-limited chemostat cultures of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 55:468-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rapp, A., and H. Mandary. 1986. Wine aroma. Experientia 42:873-884. [Google Scholar]
- 42.Rodrigues, F., M. Côrte-Real, C. Leão, J. P. van Dijken, and J. T. Pronk. 2001. Oxygen requirements of the food spoilage yeast Zygosaccharomyces bailii in synthetic and complex media. Appl. Environ. Microbiol. 67:2123-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rojas, V., J. V. Gil, P. Manzanares, R. Gavara, F. Piñaga, and A. Flors. 2002. Measurement of alcohol acetyltransferase and ester hydrolase activities in yeast extracts. Enzyme Microb. Technol. 30:224-230. [Google Scholar]
- 44.Rojas, V., J. V. Gil, F. Piñaga, and P. Manzanares. 2003. Acetate ester formation in wine by mixed cultures in laboratory fermentations. Int. J. Food Microbiol. 86:181-188. [DOI] [PubMed] [Google Scholar]
- 45.Rojas, V., J. V. Gil, F. Piñaga, and P. Manzanares. 2001. Studies on acetate ester production by non-Saccharomyces wine yeasts. Int. J. Food Microbiol. 70:283-289. [DOI] [PubMed] [Google Scholar]
- 46.Sauer, U. 2004. High-throughput phenomics: experimental methods for mapping fluxomes. Curr. Opin. Biotechnol. 15:58-63. [DOI] [PubMed] [Google Scholar]
- 47.Sauer, U., D. Lasko, J. Fiaux, M. Hochuli, R. Glaser, T. Szyperski, K. Wüthrich, and J. Bailey. 1999. Metabolic flux ratio analysis of genetic and environmental modulations of Escherichia coli central carbon metabolism. J. Bacteriol. 181:6679-6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skoog, K., H. Jepsson, and B. Hahn-Hägerdal. 1992. The effect of oxygenation on glucose fermentation with Pichia stipitis. Appl. Biochem. Biotechnol. 34/35:369-375. [Google Scholar]
- 49.Tabachnick, J., and M. Joslyn. 1953. Formation of esters by yeast. I. The production of ethyl acetate by standing surface cultures of Hansenula anomala. J. Bacteriol. 65:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ullrich, J. 1970. Yeast pyruvate decarboxylase (2-oxoacid carboxy-lyase, EC 4.1.1.1.) assay of thiamine pyrophosphate. Methods Enzymol. 18:109-115. [Google Scholar]
- 51.van Dijken, J., and A. Scheffers. 1986. Redox balances in the metabolism of sugars by yeasts. FEMS Microbiol. Rev. 32:199-224. [Google Scholar]
- 52.van Hoek, P., J. P. van Dijken, and J. T. Pronk. 2000. Regulation of fermentative capacity and levels of glycolytic enzymes in chemostat cultures of Saccharomyces cerevisiae. Enzyme Microb. Technol. 26:724-736. [DOI] [PubMed] [Google Scholar]
- 53.Van Urk, H., W. S. L. Voll, W. A. Scheffers, and J. P. Van Dijken. 1990. Transient-state analysis of metabolic fluxes in Crabtree-positive and Crabtree-negative yeasts. Appl. Environ. Microbiol. 56:281-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Visser, W., W. A. Scheffers, W. H. Batenburg-Van der Vegte, and J. P. van Dijken. 1990. Oxygen requirements of yeasts. Appl. Environ. Microbiol. 56:3785-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wills, C., and J. Phelps. 1975. A technique for the isolation of yeast alcohol dehydrogenase mutants with altered substrate specificity. Arch. Biochem. Biophys. 167:627-637. [DOI] [PubMed] [Google Scholar]
- 56.Wittman, C., M. Hans, and E. Heinzle. 2002. In vivo analysis of intracellular amino acid labeling by GC/MS. Anal. Biochem. 307:379-382. [DOI] [PubMed] [Google Scholar]
- 57.Yoshioka, K., and N. Hashimoto. 1983. Cellular fatty acid and ester formation by brewer's yeast. Agric. Biol. Chem. 47:2287-2294. [Google Scholar]
- 58.Yoshioka, K., and N. Hashimoto. 1981. Ester formation by alcohol acetyltransferase from brewer's yeast. Agric. Biol. Chem. 45:2183-2190. [Google Scholar]






