Abstract
Among the Rhizobiaceae, Bradyrhizobium japonicum strain USDA110 appears to be extremely salt sensitive, and the presence of glycine betaine cannot restore its growth in medium with an increased osmolarity (E. Boncompagni, M. Østerås, M. C. Poggi, and D. Le Rudulier, Appl. Environ. Microbiol. 65:2072-2077, 1999). In order to improve the salt tolerance of B. japonicum, cells were transformed with the betS gene of Sinorhizobium meliloti. This gene encodes a major glycine betaine/proline betaine transporter from the betaine choline carnitine transporter family and is required for early osmotic adjustment. Whereas betaine transport was absent in the USDA110 strain, such transformation induced glycine betaine and proline betaine uptake in an osmotically dependent manner. Salt-treated transformed cells accumulated large amounts of glycine betaine, which was not catabolized. However, the accumulation was reversed through rapid efflux during osmotic downshock. An increased tolerance of transformant cells to a moderate NaCl concentration (80 mM) was also observed in the presence of glycine betaine or proline betaine, whereas the growth of the wild-type strain was totally abolished at 80 mM NaCl. Surprisingly, the deleterious effect due to a higher salt concentration (100 mM) could not be overcome by glycine betaine, despite a significant accumulation of this compound. Cell viability was not significantly affected in the presence of 100 mM NaCl, whereas 75% cell death occurred at 150 mM NaCl. The absence of a potential gene encoding Na+/H+ antiporters in B. japonicum could explain its very high Na+ sensitivity.
Within the soil, rhizobia frequently encounter various stresses that affect their growth, the initial steps of symbiosis, and the efficiency of nitrogen fixation (36). Among several environmental conditions which are limiting factors, water stress and salinity are probably the most problematic. Increasing salt concentrations have a detrimental effect on rhizobial populations as a result of direct toxicity or through osmotic stress. Rhizobia show marked variations in salt tolerance, with fast-growing strains usually being more tolerant than slow-growing strains (12). A number of rhizobia from woody legumes show substantial salt tolerance: for example, strains isolated from Acacia, Prosopis, and Leucaena species are tolerant to 500 to 850 mM NaCl (37, 38). While less tolerant, various strains of Sinorhizobium meliloti can still grow at salt concentrations of more than 300 mM (2). In contrast, the growth of a number of strains of Bradyrhizobium japonicum is inhibited by NaCl concentrations lower than 100 mM (12).
Rhizobia exposed to increased salinity can maintain osmotic equilibrium across the membrane by exclusion of salts and via accumulation of compatible solutes, mainly organic osmolytes. Typically, such solutes have no net charge and are nontoxic and highly soluble molecules which do not inhibit normal metabolic reactions but stabilize proteins and membranes (6, 8, 17). Many of the best-characterized osmoregulatory mechanisms are designed to adjust compatible solute levels by modulating their biosynthesis, catabolism, uptake, and efflux. Whereas the composition of the set of endogenous compatible solutes accumulated by rhizobia varies at the species level, betaines are essential compatible solutes for most Rhizobiaceae (3). In S. meliloti, the best-characterized rhizobium, glycine betaine can be directly taken up or synthesized from choline or choline-O-sulfate. Two transport systems for betaines have been fully characterized: the Hut system, an ATP-binding cassette histidine transporter also involved in low-affinity glycine betaine transport (4), and the BetS system, a betaine choline carnitine transporter (BCCT) required for early osmotic adjustment (5). Choline transport activities have also been demonstrated (28), and the glycine betaine biosynthetic pathway from choline or choline-O-sulfate to glycine betaine has been well characterized at the molecular level (20, 29). Interestingly enough, we must emphasize that both compounds, glycine betaine and choline, are used as osmoprotectants by a large collection of rhizobia, whereas B. japonicum, the most salt-sensitive species, does not possess high-affinity uptake systems for the transport of these substances (3). However, B. japonicum is the most agriculturally important species of rhizobia, because it has the ability to form nodules on the soybean plant (Glycine max), which represents a major crop. Recently, the nucleotide sequence of the entire genome of B. japonicum USDA110 has been determined, and analysis of the characteristic features of the predicted genes has revealed that 9% of the 8,317 potential protein-encoding genes show similarity to genes involved in transport (15). Despite this rather high number, no significant similarity to any registered BCCT could be found.
In this report, we address the hypothesis that high-affinity betaine transport through the BCCT system (BetS) from S. meliloti provides an alternative mechanism for B. japonicum to acquire betaines for osmoprotection. The results of these studies demonstrated that betS expression allows B. japonicum to obtain and accumulate glycine betaine and proline betaine via an osmotically stimulated transport. In addition, functional expression of BetS leads to partial restoration of growth impaired by moderate salt stress.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Escherichia coli S17-1 cells (33) were grown at 37°C in Luria-Bertani medium (32). B. japonicum strain USDA110 and derivatives were routinely grown aerobically at 30°C in yeast extract-mannitol (YEM) medium (9). For physiological experiments, 7-day-old YEM cultures were harvested, washed in fresh medium, and used to inoculate (optical density at 600 nm [OD600] of 0.1 U) the defined minimal LSB medium. This medium was carbon- and nitrogen-free S medium (34) supplemented with vitamins, trace elements (1), and xylose and glutamate (2 g of each/liter). When appropriate, the following antibiotics were added to B. japonicum cultures at the given final concentrations: kanamycin (Km), 50 μg/ml, and spectinomycin (Spc), 200 μg/ml. When needed, filter-sterilized glycine betaine and proline betaine solutions were added at a final concentration of 1 mM. The osmotic strength of the media was increased by addition of an appropriate volume of stock solutions of 5 M NaCl or 20% mannitol (wt/vol). The osmolalities of the different media were determined by freezing point depression with a microosmometer (model H. Roebling; Bioblock Scientific, Illkirch, France). The minimal LSB medium had an osmotic pressure of 110 milliosmol (mOsm). When 80, 100, 150, or 300 mM NaCl was added to this medium, the osmotic pressure was 260, 300, 385, or 660 mOsm, respectively. Bacterial growth was monitored spectrophotometrically by measuring the OD600. Cell viability was measured by using cultures grown in LSB medium for 3 days and subjected to various NaCl concentrations for 24 h. Samples were diluted and plated on YEM medium. Colonies were counted after a 6-day incubation at 30°C.
Plasmids and cell transformation.
The plasmid pVKBS1, containing the betS gene of S. meliloti, was constructed by inserting the purified 3.4-kb SalI DNA fragment from the plasmid pBT58 (5) at the unique SalI restriction site of the vector pVK100 (16). Both plasmids, pVKBS1 and pVK100, were initially transferred into E. coli S17-1 and then into B. japonicum strain USDA110 by biparental mating. Such mating was done with E. coli S17-1 derivatives containing either pVKBS1 or pVK100 and grown in Luria-Bertani medium and with B. japonicum cells collected at stationary phase and grown in YEM medium. B. japonicum (10 ml) and E. coli (2.5 ml) cultures were mixed, collected by centrifugation, resuspended in 250 μl of YEM medium, and spread on solid YEM medium. Cells were grown at 30°C for 5 days, an aliquot was collected and resuspended in YEM medium, and USDA110 exconjuguants were selected on solid YEM medium as Kmr and Spcr cells.
Betaine transport assays and kinetics of accumulation.
Radioactive [methyl-14C]glycine betaine was prepared from [methyl-14C]choline (2.04 GBq/mmol; Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, England) as previously described (22). [U-14C]proline betaine (4.6 GBq/mmol) was obtained from the Commissariat à l'Energie Atomique (Gif-sur-Yvette, France). For transport assays, B. japonicum cells grown in LSB medium were harvested at late exponential phase (5 to 6 days), washed twice in the medium used for the cultures, and diluted to a final OD600 of 0.8. When needed, NaCl was added at the specified concentration 10 min before transport assays. All assays were carried out at 30°C for 10 min with 1 ml of cell suspension and radioactive substrate (100,000 dpm) at a final concentration of 40 μM. During such period, uptake was found to be linear with time. Uptake was terminated by rapid filtration through GF/F glass microfiber filters (Whatman, Maidstone, England), which were rinsed with 5 ml of the corresponding medium, as previously described (5). Under these rinsing conditions, no leakage of intracellular labeled substrate was observed. The radioactivity remaining on the filters was determined with a liquid scintillation spectrometer (model LS6000SC; Beckman Instruments, Villepinte, France).
For kinetics studies of intracellular betaine accumulation, B. japonicum cells were grown in LSB medium, supplemented or not supplemented with salt, and collected at late exponential phase. They were harvested as described above, and 10 ml of culture was maintained either in the presence of 40 μM [methyl-14C]glycine betaine for 2 h or in the presence of 400 μM [methyl-14C]glycine betaine for 6 or 24 h. Measurements of intracellular radioactivity were obtained as mentioned above for uptake assays. All data were calculated as mean values from at least two independent cultures, and each assay was run in duplicate. Protein concentration was measured according to the method described by Bradford (7) using bovine serum albumin as a standard. The total cell protein value was estimated to be 205 μg/ml per OD600 U at low osmolarity.
Glycine betaine efflux and fate.
Cells were grown in low-osmolarity LSB medium until late exponential phase and then incubated for 2 h in the presence of 40 μM [methyl-14C]glycine betaine added to LSB medium supplemented with 80 mM NaCl. At the end of this period, the cells were collected and washed once with glycine betaine-free LSB medium containing 80 mM NaCl, and the amount of radioactivity accumulated by the cells was measured. Aliquots of the same culture were then subjected to an osmotic downshock or upshock by suspending the pellets in LSB medium appropriately supplemented, i.e., with no salt or with 80 or 100 mM NaCl. Samples were withdrawn after 30 or 60 min of incubation at 30°C with shaking at 150 rpm. The bacteria were filtered as described above for transport assays, and both the radioactivity remaining on the filter and that excreted into the medium were measured, allowing determination of glycine betaine efflux.
In order to monitor the fate of cytosolic glycine betaine, cells were incubated for 2 h in LSB medium with [methyl-14C]glycine betaine (40 μM) and then maintained for 24 h in Warburg vials containing in the central cupule a small piece of filter paper moistened with 200 μl of 5 M KOH used as a CO2 trap. The cells were collected by filtration as described above for transport assays and quickly washed twice with LSB medium, and the labeled compounds were extracted with 80% (vol/vol) ethanol under vigorous shacking. The extract was centrifuged (12,000 × g for 20 min at 4°C). The supernatant (ethanol-soluble fraction [ESF]) was evaporated to dryness at 40°C, and the residue was dissolved in distilled water. The pellets (ethanol-insoluble fraction [EIF]) representing macromolecular components and cell envelopes were collected. The radioactivity of each fraction, ESF, EIF, and CO2 trapped by the KOH, was quantified. Aliquots from the ESF were submitted to high-voltage paper electrophoresis (2) in order to detect and quantify [methyl-14C]glycine betaine and its potential metabolites.
RESULTS
Heterologous expression of S. meliloti betS in B. japonicum USDA110.
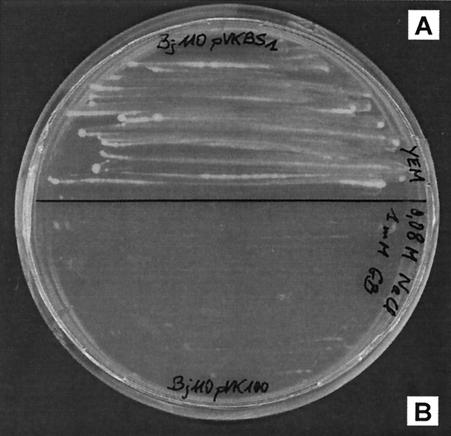
The whole nucleotide sequence of betS, including the promoter, was cloned into the broad-host-range vector pVK100, and the resulting plasmid pVKBS1 was transferred into B. japonicum USDA110. Cells were also transformed with the empty vector pVK100 as controls. The resultant transformed cells were grown on solid YEM medium supplemented with NaCl. Since B. japonicum displays a very low tolerance of osmotic stress, growth of strain USDA110(pVK100) was totally inhibited in medium containing NaCl at a concentration as low as 80 mM, and addition of glycine betaine had no beneficial effect. In contrast, in the same experimental conditions (80 mM NaCl and 1 mM glycine betaine), cells containing pVKBS1 (betS) were able to grow (Fig. 1), and the growth could not be distinguished from that of the parental strain USDA110 in no-salt medium. These results suggest that the betS gene of S. meliloti is expressed in B. japonicum, and as a consequence BetS activity probably allows sufficient glycine betaine accumulation to sustain growth under an osmotic pressure of 260 mOsm. To our knowledge, this is the first report of heterologous transformation of this slow-growing Rhizobiaceae.
FIG. 1.
Glycine betaine confers osmoprotection to B. japonicum USDA110 cells expressing the S. meliloti betS gene. The transformed cells, strain USDA110(pVKBS1) (A), and the control strain, USDA110(pVK100) (B), were spread on YEM medium containing 80 mM NaCl and 1 mM glycine betaine.
Betaine transport activities in B. japonicum cells expressing BetS.
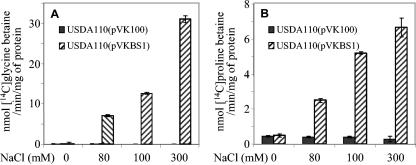
BetS-mediated betaine transport in S. meliloti is the consequence of direct activation of the transporter by high osmolarity, most likely through posttranslational activation (5). We wanted to determine whether BetS activity and activation by the osmolarity also occur in transformed B. japonicum cells. Thus, betaine uptake was quantified in strains USDA110(pVKBS1) and USDA110(pVK100) grown in LSB medium and subjected to a sudden osmotic upshift by the addition of NaCl at final concentrations ranging from 0 to 300 mM. The uptake of glycine betaine or proline betaine used at a final concentration of 40 μM was measured on exponentially growing cells 10 min after the upshock. In strain USDA110(pVK100), whatever the osmolarity of the medium, [methyl-14C]glycine betaine transport was almost null: less than 0.2 nmol/min/mg of protein (Fig. 2). In USDA110(pVKBS1) cells expressing betS maintained at low osmolarity, transport activity was also extremely low. In contrast, the addition of NaCl strongly stimulated [methyl-14C]glycine betaine uptake. At a final concentration of 80 mM NaCl, uptake activity reached 7 nmol/min/mg of protein, and this activity was stimulated 4.2-fold at 300 mM NaCl. At this NaCl concentration, glycine betaine transport activity was twofold higher than that previously measured in S. meliloti at the same osmolarity (5). However, it should be noted that the betS gene expressed in B. japonicum was carried on a low-copy-number plasmid, while the S. meliloti strain was bearing a unique genomic copy of betS. Thus, the difference between BetS activities obtained in the two bacteria might be due to variations in the amount of BetS proteins rather than in activity. When [14C]proline betaine was used as a substrate, the transport rate was very low (0.5 nmol/min/mg of protein) in both the control cells, USDA110(pVK100), grown at all salinities, and the transformed cells, USDA110(pVKBS1), grown at low osmolarity. Upon addition of 80 or 300 mM NaCl, uptake was stimulated 5- and 13-fold, respectively, in the USDA110(pVKBS1) strain but remained very low in the control strain. Despite the strong stimulation, BetS-mediated proline betaine transport at 300 mM was fourfold less than glycine betaine uptake, a feature already noticed for S. meliloti. Altogether, these results demonstrated that BetS is functional in B. japonicum and regulated by the osmolarity, as previously observed for S. meliloti.
FIG. 2.
Effect of medium osmolarity on BetS-mediated betaine uptake in B. japonicum cells. Strain USDA110(pVKBS1), expressing the S. meliloti betS gene, and the control strain, USDA110(pVK100), were grown in low-osmolarity LSB medium and subjected to a sudden upshock by addition of NaCl at the indicated final concentrations. Transport assays were performed 10 min after NaCl addition. Uptake measurements were done for 10 min at a final concentration of 40 μM [methyl-14C]glycine betaine (A) or [14C]proline betaine (B). During this period, uptake was linear with time. Values shown are means of results from duplicates of at least two independent cultures; standard deviations are also shown.
Glycine betaine accumulation.
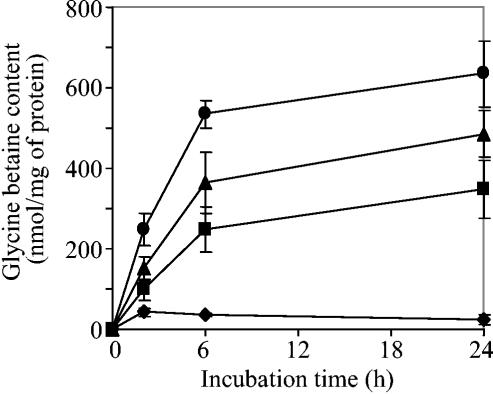
It is well established that to offset the deleterious effect of high osmolarity on cell division, bacteria can accumulate high intracellular concentrations of glycine betaine and that the amount accumulated depends on the stress intensity. Glycine betaine uptake measurements (Fig. 2) have shown high transport activity in strain USDA110(pVKBS1) maintained at salt concentrations higher than 80 mM. However, such assays did not provide evidence for intracellular glycine betaine accumulation. Thus, to establish whether BetS-mediated glycine betaine uptake allowed a durable increase of the intracellular pool of this compound, a kinetic analysis of glycine betaine accumulation was performed on B. japonicum USDA110(pVKBS1) cells grown under low osmotic pressure and transferred into medium of increased salinity (no NaCl or 80, 100, or 150 mM NaCl). [methyl-14C]glycine betaine accumulation was measured after 2, 6, or 24 h of incubation in the presence of the substrate (Fig. 3). In cells maintained in no-salt medium, the intracellular level of glycine betaine was always very low, whatever the incubation time. Upon addition of 80 mM NaCl, 2.3- and 5.8-fold increases were observed after 2 and 6 h, respectively. Then, the increase in glycine betaine content continued to rise but at a much lower rate. Addition of salt at a higher concentration (100 or 150 mM NaCl) was followed by a much greater glycine betaine accumulation. Whatever the intensity of the NaCl upshock, the strain USDA110(pVK100), used as a control, was unable to accumulate a significant amount of glycine betaine. Even after 24 h, the glycine betaine content was less than 17 nmol/mg of protein (data not shown). Hence, the endogenous pool of glycine betaine taken up by cells expressing betS appeared to be clearly dependent on the salt concentration. Nevertheless, the rapid initial rate of accumulation was followed by a much slower increase, and it is likely that the intracellular content of glycine betaine reached a maximum level between 6 and 24 h. Assuming that the cellular volume of B. japonicum cells is approximately equivalent to that of S. meliloti, i.e., 2.6 μl/mg of protein in the presence of 150 mM NaCl (27), the intracellular glycine betaine concentration was estimated to be 240 ± 30 mM after 24 h of accumulation.
FIG. 3.
Effect of increased NaCl concentration in the medium on glycine betaine accumulation in B. japonicum strain USDA110 cells expressing the S. meliloti betS gene. Cells were grown in low-osmolarity LSB medium (⧫), and NaCl (▪, 80 mM; ▴, 100 mM; •, 150 mM) was supplemented 10 min before the addition of [methyl-14C]glycine betaine. In order to avoid deficiency in the radiolabeled substrate, [methyl-14C]glycine betaine was used at 40 μM for 2 h of incubation or at 400 μM for an incubation period of 6 or 24 h. Values shown are means of results from duplicates of at least two independent cultures, and standard deviations are also shown.
Fate and efflux of glycine betaine in B. japonicum expressing betS.
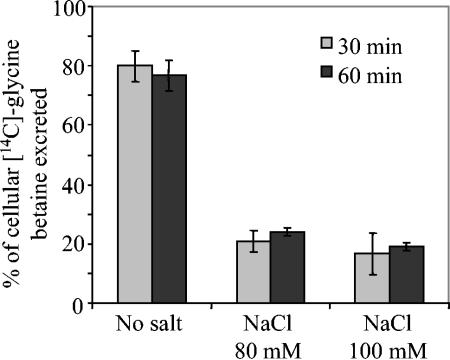
The steady-state level of glycine betaine accumulation which was observed could represent an equilibrium between transport, degradation, and efflux of glycine betaine. Therefore, the capacity of the USDA110(pVKBS1) strain to eliminate intracellular glycine betaine by catabolism or by activation of efflux channels was investigated. First, in order to follow the fate of cytosolic glycine betaine, B. japonicum cells were maintained for 2 h in the presence of 80 mM NaCl and 40 μM [methyl-14C]glycine betaine. The cells were then transferred for 24 h to LSB medium without salt or with 80 or 100 mM NaCl. All of the cell-incorporated radioactive material was recovered in the ESF, and no radioactivity could be detected in the EIF or in the CO2 fraction (see Materials and Methods). It should be pointed out that the amount of radioactivity recovered in cells transferred at low osmolarity was very low compared to the 14C found in salt-treated cells. Electrophoretic analysis of the ESF revealed only a single 14C-labeled compound which moved to the same position as pure glycine betaine. These results demonstrated that intracellular glycine betaine is free in the cytoplasm, and unlike many rhizobia (3), B. japonicum strain USDA110 cannot catabolize this solute. Second, to test the capability of the USDA110(pVKBS1) cells to remove glycine betaine from the cytosol by excretion, an osmotic downshock experiment was conducted. Cells were preloaded with [methyl-14C]glycine betaine for 2 h at elevated osmolarity (80 mM NaCl), and the amount of glycine betaine excreted after transfer to free-substrate medium was monitored (Fig. 4). After incubation for 30 min under a low-osmolarity condition, most of the [methyl-14C]glycine betaine previously taken up was recovered in the medium. By contrast, excretion by cells maintained under isosmotic condition (80 mM NaCl) was limited to about 20% of the radioactivity previously taken up. Very limited efflux of glycine betaine was also observed from cells transferred at 100 mM NaCl. Hence, the glycine betaine efflux from strain USDA110(pVKBS1) was clearly dependent on the medium's osmolarity, allowing a modulation of the endogenous pool with response to the external osmolarity. These results also indicated that B. japonicum USDA110 possesses osmoregulated channels used for glycine betaine efflux during osmotic downshift. Indeed, such a mechanism could be important for the survival of B. japonicum cells expressing betS, since glycine betaine accumulation, which induced elevated intracellular osmotic pressure, might be detrimental to cells exposed to a sudden reduction in the external osmolarity.
FIG. 4.
Modulation of glycine betaine efflux by osmolarity in B. japonicum strain USDA110 cells expressing the S. meliloti betS gene. Cells were preloaded for 2 h with 40 μM [methyl-14C]glycine betaine at 80 mM NaCl and then transferred in glycine betaine-free medium for 30 or 60 min at the indicated NaCl concentrations. Results are expressed as a percentage of the initial intracellular amount of glycine betaine taken up. Values shown are means of results from duplicates of two independent cultures; standard deviations are shown.
betS expression conferred osmotic tolerance to B. japonicum.
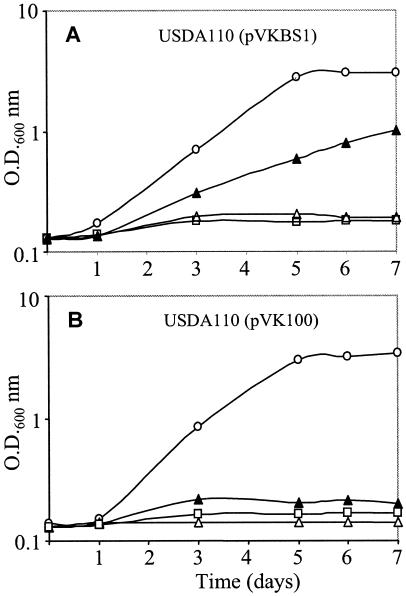
Since glycine betaine transport and accumulation in strain USDA110(pVKBS1) was effective at elevated salt concentrations, to assess the contribution of the BetS transporter to osmoprotection by betaines, we monitored bacterial growth. Thus, USDA110(pVK100) and USDA110(pVKBS1) cells were grown in LSB medium at different osmotic strengths in the presence (1 mM) or absence of glycine betaine or proline betaine. As already known, in the absence of osmoprotectant, the growth of both strains was strongly reduced by 50 mM NaCl (data not shown) and completely abolished in the presence of 80 mM NaCl or more (Fig. 5). Addition of glycine betaine to a medium of low osmolarity had no effect on the growth rate or growth yield of either strain. A striking difference was obtained with 80 mM NaCl: while the growth of strain USDA110(pVK100) was still suppressed (Fig. 5B), the growth inhibition of the betS-transformed strain was alleviated, and after 7 days, the final yield was 50% of the yield observed in the absence of salt (Fig. 5A). However, attempts to grow the transformant cells in medium containing 100 mM NaCl and glycine betaine were unsuccessful. When proline betaine (1 mM) was used instead of glycine betaine, similar growth data were obtained (data not shown). To determine whether or not the growth inhibition was due primarily to the presence of Na+ or Cl− ions rather than to the increased osmotic strength, a nonelectrolyte, mannitol, was added to the growth medium. At a concentration osmotically equivalent to 100 mM NaCl (160 mM mannitol), the growth of both strains was not affected. Thus, the inhibition observed at 100 mM NaCl was not simply a consequence of elevated external osmotic pressure but essentially resulted from the toxic effect of ions. However, increasing the mannitol concentration to the equivalent of 300 mM NaCl was harmful, without any restoration of growth by the addition of glycine betaine (data not shown). From all of these growth experiments, we can conclude (i) that the expression of the betS gene in B. japonicum cells enhanced the osmotolerance of strain USDA110 but only at moderately elevated osmolarity, (ii) that the intrinsic osmotic tolerance of the transformed cells still remained rather low compared to that of other rhizobia, and (iii) that the ionic strength was particularly toxic.
FIG. 5.
Growth of B. japonicum strains USDA110(pVKBS1) (A) and USDA110(pVK100) (B). Cells were grown in LSB medium without salt (○), with 80 mM NaCl (▵), with 80 mM NaCl and 1 mM glycine betaine (▴), or with 100 mM NaCl and glycine betaine (□). Values shown are means of results from three independent cultures, with variations of less than 5%.
Cell viability of the transformed strain was also measured. Whereas growth was totally abolished in the presence of 100 mM NaCl, the viability of the cells was not significantly affected. However, increasing the NaCl concentration to 150 mM was strongly deleterious, with 75% cell death, and no improvement could be seen in the presence of glycine betaine (data not shown). Thus, increasing the Na+ concentration also had a strong adverse impact on cell survival.
DISCUSSION
In this study, we reported the expression of the S. meliloti betS gene in B. japonicum USDA110 and addressed the question of possible accumulation and role of glycine betaine in transformed cells submitted to salt stress. B. japonicum is one of the most salt-sensitive rhizobia and possesses neither high-affinity uptake systems for betaines or choline nor the choline-glycine betaine biosynthetic pathway (3). These deficiencies were the rationale to install the betaine transport activity. Analysis of the B. japonicum transformed cells revealed strong glycine betaine and proline betaine uptake activities in an osmotically stimulated manner. It is also noteworthy that the stimulated BetS-mediated glycine betaine transport in B. japonicum leads to a large accumulation of this compatible solute, up to 240 mM, which is not used as sole carbon and nitrogen sources, in contrast to many other members of the family Rhizobiaceae. Genetic engineering has also proved fruitful in the freshwater cyanobacterium Synechococcus sp. strain PCC7942 that does not produce glycine betaine. The codA gene for choline oxidase from Arthrobacter globiformis was introduced in the cyanobacterium, and the resultant cells which accumulated 80 mM glycine betaine when choline was supplied exogenously exhibited enhanced tolerance to salt stress (11). Similarly, Synechococcus cells transformed with the bet operon from E. coli, which encodes choline transport and glycine betaine biosynthesis from choline, accumulated the betaine, grew better than control cells under salt stress, and exhibited enhanced stability of photosystems I and II (18). However, metabolic engineering of glycine betaine synthesis might induce disturbances in endogenous pathways of primary metabolism. Overexpressed choline oxidase might compete for choline with the enzymes involved in the synthesis of phosphatidylcholine, an essential component of membrane phospholipids, particularly in the Rhizobiaceae (10). Indeed, it has been suggested that the availability of choline limits the production of glycine betaine in transgenic plants (14, 19). In such plants, as in transgenic cyanobacteria, levels of accumulated glycine betaine increased significantly only when exogenous choline was available. In our study, in order to avoid this difficulty, we have chosen to introduce a betaine transporter in B. japonicum instead of a choline uptake system associated with the biosynthetic choline-glycine betaine pathway. One advantage of such an approach is that the cells were transformed by a single gene, whereas the expression of the glycine betaine biosynthesis pathway would have needed at least two genes, one for choline uptake and one for choline conversion. Furthermore, the betS gene was a particularly suitable candidate, since its expression is constitutive, and the BetS-mediated betaine uptake is the consequence of immediate activation of the transporter by high osmolarity (5). Moreover, BetS has a narrow specificity for betaines and presents a high affinity (Km in the μΜ range) for betaines. Such features should allow the bacterial cell to acquire betaines from the environment even when these osmoprotectants are present at very low concentrations. Although the availability of betaines in the soil has been poorly studied, these compounds are mainly plant-derived molecules which are likely released by plant roots and seeds or through decaying plant material (24). Indeed, glycine betaine is produced by several families of higher plants, particularly Chenopodiaceae, Amaranthaceae, and Gramineae (31), and proline betaine is found mainly in some species of Plumbaginaceae, Compositae, and Leguminosae (13). In addition, alfalfa seeds release a substantial amount of proline betaine during germination (up to 8 to 16 nmol per seed), and this compound is a major component of seed exudate (23). Thus, even if the availability of betaines is likely to vary considerably in the upper layers of soil, the growth of the recombinant strain of B. japonicum will be favored by effective mechanisms for the acquisition of the osmoprotectants from the environment.
One particularly relevant point of our results is that B. japonicum cells expressing BetS have the capacity to transport and accumulate glycine betaine and proline betaine. These new functions helped the bacterium to grow at increased osmolarity, up to 80 mM NaCl, whereas growth of the wild-type strain was almost totally abolished at 50 mM NaCl. However, the defect in growth improvement at 100 mM NaCl, despite a large accumulation of glycine betaine, was surprising. Since our results demonstrated that B. japonicum cannot catabolize glycine betaine, it was important to know whether the lack of osmoprotection by glycine betaine might have resulted from a too-large intracellular betaine pool associated with the loss of the ability to regulate the cytoplasmic turgor pressure. Usually, mechanosensitive channels play an essential role in such regulation by controlling ion contents (Na+ and K+) and organic compatible compounds through immediate excretion (30). The data presented here describe the existence of a rapid glycine betaine release during osmotic downshock (Fig. 4). Such results are consistent with the recent identification of mechanosensitive channel MscL in B. japonicum (26). Thus, we can reasonably assume that the intracellular turgor regulation during osmotic downshock was not affected by the introduction of the betS gene.
The defect in growth improvement at 100 mM NaCl remains unclear. The existence of a cotransport glycine betaine/Na+ through BetS (5) prompts us to suggest that the entry of Na+ during glycine betaine accumulation might be deleterious for the cells. Control of membrane permeability to Na+ ions and the counteracting K+ ions is one of the most important aspects of the acclimation of microorganisms to increased salinity, and Na+/H+ antiporters are membrane proteins essential for the maintenance of the ionic balance in bacterial cells (21). In E. coli, mutants deficient in the two genes for Na+/H+ antiporters, NhaA and NhaB, are hypersensitive to sodium (25). A search for homologous NhaA and NhaB sequences in the B. japonicum genome sequence (http://www.kazusa.or.jp/rhizobase) could not detect any potential protein-encoding Na+/H+ antiporters. In contrast, a similar whole-genome analysis of both S. meliloti and Mesorhizobium loti revealed one sequence for each bacterium, Sma1913 and Mlr5309, with 39 and 51% identity to NhaA, respectively. In addition, in the M. loti genome, one sequence showed 20% identity to NhaB, whereas no homologue could be found in S. meliloti. Given the crucial role of Na+/H+ antiporters in salt tolerance, it is tempting to postulate that, in cells grown with 100 mM NaCl, the expulsion of Na+ could not compensate for the entry of Na+, which is amplified as a consequence of stimulated glycine betaine uptake through BetS. In the absence of osmotic downshock, the mechanosensitive channels remained closed, and the effectiveness of glycine betaine cannot reverse the toxicity of a high intracellular Na+ concentration. In this context, it is interesting that overexpression of a Na+/H+ antiporter gene from a halotolerant cyanobacterium (ApNhaP) drastically improved the salt tolerance of the freshwater cyanobacterium Synechococcus, making it capable of growth in seawater (35). It remains to be determined whether the expression of Na+/H+ antiporter in B. japonicum could control the Na+ status of the cells and confer radical improvement in salt tolerance.
Acknowledgments
This work was funded by the Centre National de la Recherche Scientifique. A.B. received a doctoral fellowship from the Ministère de la Recherche et de l'Enseignement supérieur.
We are grateful to E. Giraud for helpful suggestions about the biparental mating between B. japonicum and E. coli.
REFERENCES
- 1.Allen, G. C., D. T. Grimm, and G. H. Elkan. 1991. Oxygen uptake and hydrogen-stimulated nitrogenase activity from Azorhizobium caulinodans ORS571 grown in a succinate-limited chemostat. Appl. Environ. Microbiol. 57:3220-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernard, T., J. A. Pocard, B. Perroud, and D. Le Rudulier. 1986. Variations in the response of salt-stressed Rhizobium strains to betaines. Arch. Microbiol. 29:189-198. [Google Scholar]
- 3.Boncompagni, E., M. Østerås, M. C. Poggi, and D. Le Rudulier. 1999. Occurrence of choline and glycine betaine uptake and metabolism in the family Rhizobiaceae and their roles in osmoprotection. Appl. Environ. Microbiol. 65:2072-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boncompagni, E., L. Dupont, T. Mignot, M. Østerås, A. Lambert, M. C. Poggi, and D. Le Rudulier. 2000. Characterization of a Sinorhizobium meliloti ATP-binding cassette histidine transporter also involved in betaine and proline uptake. J. Bacteriol. 182:3717-3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boscari, A., K. Mandon, L. Dupont, M. C. Poggi, and D. Le Rudulier. 2002. BetS is a major glycine betaine/proline betaine transporter required for early osmotic adjustment in Sinorhizobium meliloti. J. Bacteriol. 184:2654-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourot, S., O. Sire, A. Trautwetter, T. Touzé, L. F. Wu, C. Blanco, and T. Bernard. 2000. Glycine betaine-assisted protein folding in a lysA mutant of Escherichia coli. J. Biol. Chem. 275:1050-1056. [DOI] [PubMed] [Google Scholar]
- 7.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 8.Csonka, L. N. 1989. Physiological and genetic responses of bacteria to osmotic stress. Microbiol. Rev. 53:121-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniel, R. M., and C. A. Appleby. 1972. Anaerobic-nitrate, symbiotic and aerobic growth of Rhizobium japonicum: effects on cytochrome P450, other haemoproteins, nitrate and nitrite reductases. Biochim. Biophys. Acta 275:347-354. [DOI] [PubMed] [Google Scholar]
- 10.de Rudder, K. E. E., I. M. López-Lara, and O. Geiger. 2000. Inactivation of the gene for phospholipid N-methyltransferase in Sinorhizobium meliloti: phosphatidylcholine is required for normal growth. Mol. Microbiol. 37:763-772. [DOI] [PubMed] [Google Scholar]
- 11.Deshnium, P., D. A. Los, H. Hayashi, L. Mustardy, and N. Murata. 1995. Transformation of Synechococcus with a gene for choline oxidase enhances tolerance to salt stress. Plant Mol. Biol. 29:897-907. [DOI] [PubMed] [Google Scholar]
- 12.El-Sheikh, E. A. E., and M. Wood. 1990. Salt effects on survival and multiplication of chick pea and soybean rhizobia. Soil Biol. Biochem. 22:343-347. [Google Scholar]
- 13.Hanson, A. D., B. Rathinasabapathi, J. Rivoal, M. Burnet, D. O. Dilon, and D. A. Gage. 1994. Osmoprotective compounds of the Plumbaginaceae: a natural experiment in metabolic engineering of stress tolerance. Proc. Natl. Acad. Sci. USA 91:306-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang, J., R. Hirji, L. Adam, K. L. Rozwadowski, J. K. Hammerlindl, W. A. Keller, and G. Selvaraj. 2000. Genetic engineering of glycine betaine production towards enhancing stress tolerance in plants: metabolic limitations. Plant Physiol. 122:747-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaneko, T., Y. Nakamura, S. Sato, K. Minamisawa, T. Uchiumi, S. Sasamoto, A. Watanabe, K. Idesawa, M. Iriguchi, K. Kawashima, M. Kohara, M. Matsumoto, S. Shimpo, H. Tsuruoka, T. Wada, M. Yamada, and S. Tabata. 2002. Complete genomic sequence of nitrogen-fixing symbiotic bacterium Bradyrhizobium japonicum USDA110. DNA Res. 9:189-197. [DOI] [PubMed] [Google Scholar]
- 16.Knauf, V. C., and E. W. Nester. 1982. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid 8:45-54. [DOI] [PubMed] [Google Scholar]
- 17.Le Rudulier, D., A. R. Strøm, A. M. Dandekar, L. T. Smith, and R. C. Valentine. 1984. Molecular biology of osmoregulation. Science 224:1064-1068. [DOI] [PubMed] [Google Scholar]
- 18.Nomura, M., T. Hibino, T. Takabe, T. Sugiyama, A. Yokota, H. Miyake, and T. Takabe. 1998. Transgenically produced glycine betaine protects ribulose 1,5-bisphosphate carboxylase/oxygenase from inactivation in Synechococcus sp. PCC7942 under salt stress. Plant Cell Physiol. 39:425-432. [Google Scholar]
- 19.Nuccio, M. L., B. L. Russell, K. D. Nolte, B. Rathinasabapathi, D. A. Gage, and A. D. Hanson. 1998. The endogenous choline supply limits glycine betaine synthesis in transgenic tobacco expressing choline monooxygenase. Plant J. 16:487-498. [DOI] [PubMed] [Google Scholar]
- 20.Østerås, M., E. Boncompagni, N. Vincent, M. C. Poggi, and D. Le Rudulier. 1998. Presence of a gene encoding choline sulfatase in Sinorhizobium meliloti bet operon: choline-O-sulfate is metabolized into glycine betaine. Proc. Natl. Acad. Sci. USA 95:11394-11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Padan, E., and S. Schuldiner. 1994. Molecular physiology of the Na+/H+ antiporter in Escherichia coli. J. Exp. Biol. 196:443-456. [DOI] [PubMed] [Google Scholar]
- 22.Perroud, B., and D. Le Rudulier. 1985. Glycine betaine transport in Escherichia coli: osmotic regulation. J. Bacteriol. 161:393-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips, D. A., C. M. Joseph, and C. A. Maxwell. 1992. Trigonelline and stachydrine released from alfalfa seeds activate NodD2 protein in Rhizobium meliloti. Plant Physiol. 99:1526-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips, D. A., and W. R. Streit. 1997. Applying plant-microbe signalling concepts to alfalfa: roles for secondary metabolites, p. 319-342. In B. D. McKersie and D. C. W. Brown (ed.), Biotechnology and the improvement of forage legumes. CAB International, Wallingford, England.
- 25.Pinner, E., E. Padan, and S. Schuldiner. 1992. Cloning, sequencing and expression of nhaB gene, encoding a Na+/H+ antiporter in Escherichia coli. J. Biol. Chem. 267:11064-11068. [PubMed] [Google Scholar]
- 26.Pivetti, C. D., M. R. Yen, S. Miller, W. Busch, Y. H. Tseng, I. R. Booth, and M. H. Saier, Jr. 2003. Two families of mechanosensitive channel proteins. Microbiol. Mol. Biol. Rev. 67:66-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pocard, J.-A. 1987. Ph.D. thesis. Université de Rennes I, Rennes, France.
- 28.Pocard, J.-A., T. Bernard, L. T. Smith, and D. Le Rudulier. 1989. Characterization of three choline transport activities in Rhizobium meliloti: modulation by choline and osmotic stress. J. Bacteriol. 171:531-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pocard, J.-A., N. Vincent, E. Boncompagni, L. T. Smith, M. C. Poggi, and D. Le Rudulier. 1997. Molecular characterization of the bet genes encoding glycine betaine synthesis in Sinorhizobium meliloti 102F34. Microbiology 143:1369-1379. [DOI] [PubMed] [Google Scholar]
- 30.Poolman, B., and E. Glaasker. 1998. Regulation of compatible solute accumulation in bacteria. Mol. Microbiol. 29:397-407. [DOI] [PubMed] [Google Scholar]
- 31.Rhodes, D., and A. D. Hanson. 1993. Quaternary ammonium and tertiary sulphonium compounds in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 44:357-384. [Google Scholar]
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Simon, R., U. Priefer, and A. Pülher. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Biotechnology 1:784-791. [Google Scholar]
- 34.Smith, L. T., J. A. Pocard, T. Bernard, and D. Le Rudulier. 1988. Osmotic control of glycine betaine biosynthesis and degradation in Rhizobium meliloti. J. Bacteriol. 170:3142-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waditee, R., T. Hibino, T. Nakamura, A. Incharoensakdi, and T. Takabe. 2002. Overexpression of a Na+/H+ antiporter confers salt tolerance on a freshwater cyanobacterium, making it capable of growth in sea water. Proc. Natl. Acad. Sci. USA 99:4109-4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zahran, H. H. 1999. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. 63:968-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zahran, H. H., L. A. Rasanen, M. Karsisto, and K. Lindstrom. 1994. Alteration of lipopolysaccharide and protein profiles in SDS-PAGE of rhizobia by osmotic and heat stress. World J. Microbiol. Biotechnol. 10:100-105. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, X. P., M. Karsisto, R. Harper, and K. Lindstrom. 1991. Diversity of Rhizobium bacteria isolated from the root nodules of leguminous trees. Int. J. Syst. Bacteriol. 41:104-113. [Google Scholar]