Abstract
This study examined the effect of different apple cultivars upon the UV inactivation of Escherichia coli O157:H7 strains within unfiltered apple cider. Apple cider was prepared from eight different apple cultivars, inoculated with approximately 106 to 107 CFU of three strains of E. coli O157:H7 per ml (933, ATCC 43889, and ATCC 43895), and exposed to 14 mJ of UV irradiation per cm2. Bacterial populations for treated and untreated samples were then enumerated by using nonselective media. E. coli O157:H7 ATCC 43889 showed the most sensitivity to this disinfection process with an average 6.63-log reduction compared to an average log reduction of 5.93 for both strains 933 and ATCC 43895. The highest log reduction seen, 7.19, occurred for strain ATCC 43889 in Rome cider. The same cider produced the lowest log reductions: 5.33 and 5.25 for strains 933 and ATCC 43895, respectively. Among the apple cultivars, an average log reduction range of 5.78 (Red Delicious) to 6.74 (Empire) was observed, with two statistically significant (α ≤ 0.05) log reduction groups represented. Within the paired cultivar-strain analysis, five of eight ciders showed statistically significant (α ≤ 0.05) differences in at least two of the E. coli strains used. Comparison of log reductions among the E. coli strains to the cider parameters of °Brix, pH, and malic acid content failed to show any statistically significant relationship (R2 ≥ 0.95). However, the results of this study indicate that regardless of the apple cultivar used, a minimum 5-log reduction is achieved for all of the strains of E. coli O157:H7 tested.
Escherichia coli O157:H7 was first described as a human pathogen in 1982 (28); infections with E. coli O157:H7 have become an important cause of hemorrhagic colitis and hemolytic-uremic syndrome (18, 25). Many food-associated outbreaks caused by enterohemorrhagic E. coli have occurred as a result of consuming foods improperly handled or processed. Specific foods such as undercooked hamburger meat (14), yogurt (24), mayonnaise (8, 29), and roast beef (30) have been implicated in disease outbreaks. Recently, fruit and vegetable products, including apple cider, have been identified as vehicles for infection (3, 19). Apple cider was implicated in a disease outbreak caused by E. coli O157:H7 in the early 1980s in Canada (34), and the frequency of outbreaks has increased over the last decade. These particular outbreaks have raised concerns about the acid tolerance of E. coli O157:H7 and consequently the safety of high-acid foods. Studies have indicated that E. coli O157:H7 is acid tolerant, particularly at low temperatures (21, 23).
Fresh apple cider is defined as a fresh, unfermented, short-shelf-life juice extracted from apples that have not been clarified or heat treated. Refrigeration and chemical preservatives are the main techniques used to prolong the shelf life of fresh apple cider. The composition of apple cider has been determined for different varieties of apples (4, 12, 13, 17, 20, 22), finding differences between some apple cultivars. The physicochemical variation among apple cultivars is also affected by season and storage conditions. Some components (11, 27) and common additives (9) of apple cider have been shown to exhibit antimicrobial effects against E. coli O157:H7. However, apple cider made from stored apples shows less inhibition of E. coli O157:H7 than that made with freshly harvested apples (6, 7, 9, 27).
Researchers have studied the importance of different factors on the effect of growth inhibition of E. coli O157:H7. Dingman (7) has shown that the growth of E. coli O157:H7 was inhibited in the bruised tissue of freshly picked McIntosh apples, but not in the tissues of other cultivars. This observation suggests that a combination of intrinsic factors in apple cider may influence the growth and survival of E. coli O157:H7 and other E. coli strains. Acidity is one of the most important intrinsic factors in apple cider. The pH and types of organic acids influence the growth and survival of E. coli O157:H7 (5, 9). Research from Splittstoesser et al. (33) suggested that pH and malic acid content, but not °Brix, can influence the heat inactivation of E. coli within apple juice. However, research from Zhao et al. (36) and Dingman (7) did not indicate any correlation between acidity, °Brix of apple juice, and inhibition of E. coli. Instead, they postulated that other factors might be more important. Reinders et al. (27) associated the inhibition of E. coli with specific phenolic compounds in apple juice.
The contamination of unpasteurized apple cider with E. coli O157:H7 varies according to the diversity of contamination sources during harvesting, handling, processing, and storage. Due to the increase in outbreaks linked to juice, the Food and Drug Administration (FDA) (10) initiated new regulations for all juice processing, requiring that juice manufacturers either obtain a minimum 5-log reduction of the pertinent pathogen in the finished juice or provide a warning label on the bottle. This regulation led to the investigation of new alternative technologies in order to safely process fresh juices.
As described above, the chemical composition of apple cider could provide some antimicrobial advantages/disadvantages in natural juice products. Existing thermal processing regimens rely on the apples' inherent chemical properties to establish time-temperature combinations as a means to achieve a safe finished product. However, cider manufacturers believe that off-flavors are generated in the finished product due to excessive heat exposure (33). Thus, nonthermal processing techniques present an alternative method for juice processors to produce a minimally processed food with few and minor quality and organoleptic changes. UV irradiation is a nonthermal process that has shown its ability to inactivate pathogens in apple cider (15, 16, 32, 35). Recent studies have demonstrated that doses of ≥6,500 μW s/cm2 were sufficient to achieve a 5- to 6-log reduction factor of E. coli in apple cider (26). This result demonstrates that UV technology can meet or exceed the established specifications set by the FDA.
As UV irradiation use has grown, further studies to expand the applicability and functionality of the technology have been undertaken. One question raised within this expansion is whether or not the effect of UV irradiation suffers from juice compositional variability, often associated with cultivar differences, as seen in thermal processing (33). This study reports the relationship between apple cider produced from different apple cultivars commonly used in apple cider and the inactivation of three different E. coli O157:H7 strains by UV irradiation.
MATERIALS AND METHODS
Preparation of apple cider.
Eight varieties of apples (Cortland, Rome, Golden Delicious, Red Delicious, McIntosh, Empire, Jonagold, and Northern Spy) were harvested from Cornell University Orchards at the New York State Agricultural Experiment Station in Geneva, N.Y. Apples were harvested between mid-August and early October according to the appropriate specific varietal ripeness parameters that include °Brix and flesh texture. Apple cider was prepared from the eight apple varieties in the Food Science pilot plant within 1 month of harvest. Washed apples were milled and pressed in a continuous cloth system using a rack and frame press in a batch operation. Extracted juice from each apple variety was analyzed for soluble solids (Atago refractometer; Japan), pH (Orion pH meter, Boston, Mass.), and titratable acidity as per method 22.059 described by the Association of Official Analytical Chemists (1). The apple cider was bottled in 2-liter plastic bottles and stored for up to 6 months at −23°C until used. The cider was thawed at room temperature prior to inoculation.
Bacterial strains and maintenance.
Three strains of E. coli O157:H7 were used: ATCC 43889, ATCC 43895 (obtained from Chuck W. Kaspar, University of Wisconsin—Madison), and 933 (meat isolate, obtained from M. P. Doyle, University of Georgia, Griffin). (Note that strain ATCC 43895 is listed as a 933 strain by the American Type Culture Collection, but is different from the other 933 strain in this study.) These cultures were stored and kept on tryptic soy agar at 5°C or up to 1 month following each glycerol stock startup. For the microbial inactivation experiments, strains were grown for 18 h at 37°C in tryptic soy broth (Difco, Sparks, Md.) on a rotary shaker at 250 rpm, at which point the cells were in stationary growth phase.
UV irradiation equipment.
The UV processing unit, CiderSure 3500 (FPE, Inc., Macedon, N.Y.), was used for all trials. The UV apparatus comprises a stainless steel outer housing and an inner quartz tube. The cider is passed between the outer steel housing and inner quartz tube during UV treatment. Eight germicidal low-pressure mercury lamps are placed concentrically within the interior of the quartz-stainless steel cylinder to provide UV light exposure to the passing fluid. Two UV light sensors are located at the top and bottom of the cylinder. UV sensor readings were collected every 50 ms, and the values were directly relayed to the panel control. A calculated algorithm for the control panel which achieves a minimum 5-log reduction factor of E. coli O157:H7 in unfiltered apple cider with the UV apparatus was then used. The passage of the cider through the apparatus results in a temperature increase of ≤0.1°F throughout the unit. The CiderSure unit automatically accommodates for differences in color and solids content of different ciders through the UV sensor data acquisition. UV exposure readings automatically change the pump flow rate to ensure a specified dose is applied.
UV irradiance measurements.
The measurement of incident UV intensity on the liquid was made at a 254-nm wavelength, using two UVX-25 sensors (UVP, Inc., Calif.) calibrated by the Optical Technology Division of the National Institute of Standards & Technology (http://physics.nist.gov/Divisions/Div844/div844.html). The average intensity within the sample was calculated by an integration of Beer's law over the sample depth (0.762 mm). The UV dose was calculated with the equation UV dose (μW s/cm2) = irradiance × exposure time. The irradiance was determined by multiplying the sensor readings by the radial factor, reflection factor, and the absorption factor, which for this apple cider was 0.3546. A sensor factor value of 5.88 was used as provided by the manufacturer (26).
Inactivation treatments.
Twenty milliliters of each stationary culture was inoculated into 2 liters of cider to achieve final levels of 106 to 107 organisms per ml. Initial samples were collected aseptically and immediately analyzed by serial dilution in 0.1% (wt/vol) peptone water (Difco). The inoculated cider was pumped through the UV processing unit and irradiated as described above (26) with 14 mJ of UV irradiation/cm2 for 1.2 to 1.9 s. After UV treatment, cider was collected mid-stream in sterile 50-ml tubes and immediately plated in duplicate with plate count agar (PCA) (Acumedia Manufacturer, Inc., Baltimore, Md.), and then the plates were incubated at 37°C for 24 h. Plates were enumerated for total microbial count, which our previous results (not shown) have suggested provides a conservative estimate of E. coli inactivation. Prior to and following each treatment session, the CiderSure 3500 unit tubing was cleaned and sanitized with 200 ppm of hypochlorite solution and then rinsed with water. Each inactivation experiment was performed in triplicate for each apple cultivar and each E. coli O157:H7 strain. All CFU were counted before and after UV treatment and counts were averaged and expressed as log numbers. The log reduction for each treatment was then calculated for each E. coli O157:H7 strain and apple cultivar.
Statistical analysis.
The log reductions among the different E. coli O157:H7 strains, apple cultivars, and cultivar-strain combinations were statistically analyzed by analysis of variance. Comparisons using Tukey's Studentized Range (honestly significant difference) test at significance levels of α = 0.05 were performed to determine the statistical significance of differences between sample means, using SAS software (31). A summary of the relevant test statistics is given in Table 1. Comparisons between cultivar average log reduction and cider °Brix, pH, and malic acid content were done graphically with Microsoft Excel software using linear regression modeling.
TABLE 1.
Summary of Tukey's Studentized Range (honestly significant difference) test statistics
| Statistical category | Comparison
|
||
|---|---|---|---|
| Strain | Cultivar | Strain-cultivar | |
| α | 0.05 | 0.05 | 0.05 |
| Error degrees of freedom | 46 | 46 | 46 |
| Error mean square | 0.177941 | 0.177941 | 0.177941 |
| Critical value | 3.42499 | 4.48929 | 2.0129 |
| Minimum significant difference | 0.2949 | 0.6312 | 0.6933 |
RESULTS
°Brix, pH, and malic acid in apple cultivars. Eight apple cultivars were used in this study. Table 2 shows the physical property measurements in cider obtained from each cultivar. The pH and °Brix values among the different apple ciders did not show notable differences. °Brix values fell between 11.2 for Empire cider to 13.8 for Golden Delicious cider, with a mean of 12.48. pH values fell between 3.24 for McIntosh cider to 4.01 for Jonagold cider, with a mean of 3.60. Some variation in malic acid content was observed among the apple cultivars. The average malic acid content was 0.445%, with a high of 0.630% for cider from Empire apples and a low of 0.210% for cider from Jonagold apples.
TABLE 2.
°Brix, pH, and percent malic acid for apple ciders of different cultivars before inoculation with E. coli O157:H7 strains
| Variety | Soluble solids °Brix | pH | Titratable acidity (g of malic acid/100 ml) |
|---|---|---|---|
| Rome | 11.8 | 3.76 | 0.380 |
| Jonagold | 13.4 | 4.01 | 0.210 |
| Golden Delicious | 13.8 | 3.51 | 0.475 |
| Empire | 11.2 | 3.36 | 0.630 |
| Cortland | 12.6 | 3.57 | 0.483 |
| McIntosh | 11.6 | 3.24 | 0.549 |
| Red Delicious | 13.0 | 3.91 | 0.295 |
| Northern Spy | 12.4 | 3.41 | 0.536 |
Inactivation of E. coli O157:H7 in cider produced from an individual apple cultivar.
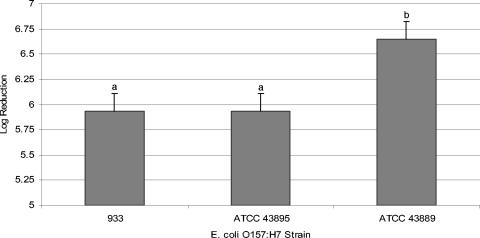
The effect of different E. coli O157:H7 strains in cider averaged over apple cultivars on the log reduction is shown in Fig. 1. In general, it can be observed that E. coli O157:H7 ATCC 43889 was the most sensitive bacterium, showing the highest average log reduction of 6.65 in the irradiated apple cider. E. coli O157:H7 strains 933 and ATCC 43895 each presented an average log reduction of 5.93 and were significantly different (α ≤ 0.05) from the log reduction of E. coli O157:H7 ATCC 43889.
FIG. 1.
Average apple cider UV inactivations for different E. coli O157:H7 strains. Error bars represent the error mean square. (The same letter indicates no significant difference at the 95% confidence level, using a least-significant-differences test.)
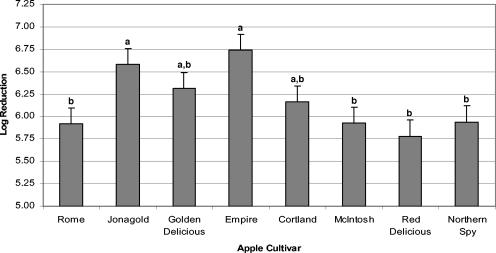
The effect of the apple cultivar averaged over the log reductions of the three strains of E. coli is shown in Fig. 2. Some statistically significant variation (α ≤ 0.05) was seen among the cultivars, as represented by the breakdown of the cultivars into two distinct significance classes. Jonagold and Empire ciders were in the higher-log-reduction group and were statistically significantly different from the lower-log-reduction group, which included Rome, McIntosh, Red Delicious, and Northern Spy apples. Golden Delicious and Cortland ciders spanned both groups and thus were not statistically different from any of the other ciders.
FIG. 2.
Average E. coli O157:H7 strain UV inactivations within different apple cultivar ciders. Error bars represent the error mean square. (The same letter indicates no significant difference at the 95% confidence level, using a least-significant-differences test.)
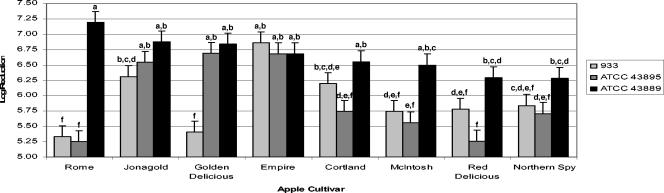
Within the paired cultivar-strain analysis (Fig. 3), three of the five ciders showed statistically significant (α ≤ 0.05) differences for at least two of the E. coli strains used. Rome and McIntosh ciders showed significant differences (α ≤ 0.05) between ATCC 43889 log reduction and both 933 and ATCC 43895. Red Delicious and Cortland ciders showed a significant difference (α ≤ 0.05) between ATCC 43895 and ATCC 43889 log reductions. Golden Delicious cider showed significant differences (α ≤ 0.05) between 933 and both ATCC 43895 and ATCC 43889. Empire, Jonagold, and Northern Spy ciders showed no significant differences between the strains' log reductions.
FIG. 3.
Analysis of UV inactivation within different apple cider cultivar-E. coli O157:H7 pairings. Bars represent the error mean square. (The same letter indicates no significant difference at the 95% confidence level, using a least-significant-differences test.)
Significant differences (P ≤ 0.05) were found with strains of E. coli O157:H7 as well as with the apple cultivars used. Linear regression modeling comparing the average log reduction seen within each cultivar to the corresponding cider °Brix, pH, and malic acid content failed to show any significant correlation (R2 ⩾ 0.95) and in fact showed almost zero correlation.
DISCUSSION
Unpasteurized apple cider is a documented vehicle of foodborne illness (2, 34). Apple ciders typically have a pH range of 3.3 to 4.1 (22) and previously were not considered a potential vector for foodborne pathogens. However, due to the enhanced acid resistance of E. coli O157:H7, its contamination of acidic fruit juices has led to foodborne outbreaks. Apple cider is made from different apple cultivars, so physical and chemical properties of apple ciders are different. These properties are affected by season, variety, and storage conditions of apples. This investigation examined the effects of apple variety on different E. coli O157:H7 strains in terms of inactivation in UV-irradiated apple cider. The apple varieties used in this study were typical apple cultivars commonly used in making apple cider in the Northeastern United States. Most ciders in production are the result of blending among a number of cultivars. Preliminary studies were performed on blended ciders, and the single-variety cider inactivation work presented here represents the extreme of each blend with regards to pH, °Brix, and phenolic content, thus allowing the most accurate analysis of varietal influence upon E. coli UV inactivation.
The apple ciders had pHs ranging from 3.24 to 4.01 and °Brix values ranging from 11.2 to 13.8, both of which are typical values for apple ciders. The effects of these physical properties in apple ciders are not clear, and in this study they failed to correlate with the variation seen in the inactivation of the E. coli O157:H7 strains during UV irradiation of the apple ciders. Similar findings reported by Dingman (6, 7) suggest that pH and °Brix values of the same apple cultivars were not important factors that influence E. coli growth in apple cider. Recently, studies have demonstrated that pH does not significantly affect the inactivation of the E. coli in apple cider with UV irradiation at different doses (26). This study appears to support those findings. The titratable acidity, expressed as malic acid, for cider among apple cultivars has been reported by Mattick and Moyer (22), with an average value of 0.42%. Empire apple cider had the highest acidity (0.630%) among the eight apple varieties studied and did show the highest average log reduction, 6.74, seen among the apple cultivars. However, this relationship was not significantly correlated, and in fact the Jonagold cultivar, which had the second highest average log reduction, 6.58, had the lowest malic acid content (0.21%). Splittstoesser et al. (33) showed that acidity in apple juice could be an important factor that increased cell sensitivities of E. coli O157:H7 to thermal-processing. Other research showed that acidity influences the growth of E. coli in apple juice (36). The effects of organic acids from apple juice on the inactivation and growth of E. coli O157:H7 have been studied. In the case of this study, pH alone does not seem to be responsible for the variation seen.
Alternatively, Reinders et al. (27) evaluated the inhibitory effect of caffeic acid on E. coli O157:H7 in a model apple juice and concluded that phenolic acids such as caffeic acid may play an important role in the survival and growth of E. coli in apple juice. This variable was not analyzed here, but such analysis would certainly be warranted in future studies. The variations seen within the various treatments of this study suggest some inherent characteristic of the cider is affecting the survivability of E. coli within the ciders. Non-UV-exposed control samples within the study showed no significant difference between counts for the same strain (data not shown). This indicates that at least in the short time interval of exposure involved in the study, direct cider-to-bacterium interaction does not account for the variation seen. Rather, interactions between cider, bacteria, and UV appear more plausible. Phenolic compounds within cider, as well as other conjugated double-bond-containing compounds, which can interact with and absorb UV energy, certainly seem like a plausible explanation for the variations seen here. Future studies might aim to examine this possibility.
In conjunction with the cultivar-based variation in inactivation of E. coli O157:H7 strains in UV-irradiated apple cider, differences in UV sensitivities between different E. coli O157:H7 strains were also observed. E. coli O157:H7 ATCC 43889 showed the highest UV sensitivity for all apple cultivars tested. While overall strains 933 and ATCC 43895 (a 933 strain itself) showed the same average log reduction factor, the two varied significantly in their log reduction in more than one apple cultivar. The physical properties of apple cider, such as pH, °Brix, and malic acid content, could influence these results even if these physical properties were similar to those of the other apple cultivars. A similar finding was obtained by Dingman (7). Unfortunately, no bacterium-specific variables were investigated here, preventing any inference as to what effect, if any, bacterial cell differences may have on the various cultivar-associated UV responses witnessed here.
Based on these results, apple cider, regardless of the apple cultivars used, processed by 14 mJ of UV irradiation achieved a greater than 5-log reduction in all E. coli O157:H7 strains tested. This nonthermal processing meets the FDA (10) requirement for a 5-log reduction factor in apple cider, representing an alternative to traditional thermal processing.
Acknowledgments
This research was funded by USDA-CSREES no. 9804732 and Regional Project S-295 no. 0186502.
We thank Phil Hartman (FPE, Inc., Macedon, N.Y.) for his assistance in the operation of the UV equipment.
REFERENCES
- 1. Association of Official Analytical Chemists 1970. Official methods of analysis, 11th ed., p. 377. Association of Official Analytical Chemists. Washington, D.C.
- 2.Besser, R. E., S. M. Lett, J. T. Weber, M. P. Doyle, T. J. Barret, J. G. Wells, and P. M. Griffin. 1993. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. JAMA 269:2217-2220. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 1996. Outbreak of Escherichia coli O157:H7 infections associated with drinking unpasteurized commercial apple juice—British Columbia, California, Colorado, and Washington, October 1996. Morb. Mortal. Wkly. Rep. 45:975. [PubMed] [Google Scholar]
- 4.Cilliers, J. J. L., V. L. Solimar, and R. M. Lamuela-Raventos. 1990. Total polyphenols in apples and ciders: correlation with chlorogenic acid. J. Food Sci. 55:1458-1459. [Google Scholar]
- 5.Conner, D. E., and J. S. Kotrola. 1994. Growth and survival of Escherichia coli O157:H7 under acid conditions. Appl. Environ. Microbiol. 61:382-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dingman, D. 1999. Prevalence of Escherichia coli in apple cider manufacturer in Connecticut. J. Food Prot. 62:567-573. [DOI] [PubMed] [Google Scholar]
- 7.Dingman, D. 2000. Growth of Escherichia coli O157:H7 in bruised apple (Malus domestica) tissue as influenced by cultivar, date of harvest, and source. Appl. Environ. Microbiol. 66:1077-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson, J. P., J. W. Stamer, M. Hayes, D. N. McKenna, and L. A. van Alstide. 1995. An assessment of Escherichia coli O157:H7 contamination risks in commercial mayonnaise from pasteurized eggs and environmental sources, and behavior in low-pH dressings. J. Food Prot. 58:1059-1064. [DOI] [PubMed] [Google Scholar]
- 9.Fisher, T. L., and D. A. Golden. 1998. Survival of Escherichia coli O157:H7 in apple cider as affected by dimethyl dicarbonate, sodium bisulfite, and sodium benzoate. J. Food Sci. 63:904-906. [Google Scholar]
- 10.Food and Drug Administration. 2001. Hazard analysis and critical control point (HAACP): procedures for the safe and sanitary processing and importing of juice. Final rule. Fed. Regist. 66:6137-6202. [Google Scholar]
- 11.Friedman, M., and H. S. Jurgens. 2000. Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 48:2101-2110. [DOI] [PubMed] [Google Scholar]
- 12.Fuleki, T., E. Pelabo, and R. B. Pelabay. 1995. Carboxylic acid composition of varietal juices produced from fresh and stored apples. J. Agric. Food Chem. 43:598-607. [Google Scholar]
- 13.Gökmen, V., A. Nevzat, and A. Jale. 2001. Effects of various clarification treatments on patulin, phenolic compound and organic acid compositions of apple juice. Eur. Food Res. Technol. 213:194-199. [Google Scholar]
- 14.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60-97. [DOI] [PubMed] [Google Scholar]
- 15.Hanes, D. E., R. W. Worobo, P. A. Orlandi, D. H. Burr, M. D. Miliotis, M. G. Robl, J. W. Bier, M. J. Arrowood, J. J. Churey, and G. J. Jackson. 2002. Inactivation of Cryptosporidium parvum oocysts in fresh apple cider by UV irradiation. Appl. Environ. Microbiol. 68:4168-4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrington, W. O., and C. H. Hills. 1968. Reduction of the microbial population of apple cider by ultraviolet irradiation. Food Technol. 22:117-120. [Google Scholar]
- 17.Hartmann, B. G., and F. Hilling. 1934. Acid constituents of food products. Special reference to citric, malic and tartaric acids. Assoc. Off. Agric. Chem. J. 17:522-531. [Google Scholar]
- 18.Hilborn, E. D., P. A. Mshar, T. R. Fiorentino, Z. F. Dembek, T. J. Barrett, R. T. Howard, and M. L. Cartter. 2000. An outbreak of Escherichia coli O157:H7 infections and haemolytic uremic syndrome associated with consumption of unpasteurized apple cider. Epidemiol. Infect. 124:31-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilborn, E. D., J. H. Mermin, P. A. Mshar, J. L. Hadler, A. Voetsch, C. Wojtkunski, M. Swartz, R. Msha, M. A. Lambert-Fair, A. J. Farrar, M. K. Glynn, and L. Slutsker. 1999. A multistate outbreak of Escherichia coli O157:H7 infections associated with consumption of mesclun lettuce. Arch. Intern. Med. 159:1758-1764. [DOI] [PubMed] [Google Scholar]
- 20.Lee, H. S., and R. E. Wrolstad. 1988. Apple juice composition: sugar, nonvolatile acid, and phenolic profiles. J. Assoc. Off. Anal. Chem. 71:789-794. [PubMed] [Google Scholar]
- 21.Lin, J., M. P. Smith, K. C. Chapin, H. S. Baik, G. N. Bennett, and J. W. Foster. 1996. Mechanisms of acid resistance in enterohemorrhagic Escherichia coli. Appl. Environ. Microbiol. 62:3094-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattick, L. R., and J. C. Moyer. 1983. Composition of apple juice. J. Assoc. Off. Anal. Chem. 66:1251-1255. [PubMed] [Google Scholar]
- 23.Miller, L. G., and C. W. Kaspar. 1994. Escherichia coli O157:H7 acid-tolerance and survival in apple cider. J. Food Prot. 57:460-464. [DOI] [PubMed] [Google Scholar]
- 24.Morgan, D., C. P. Newman, D. N. Hutchinson, A. M. Walter, B. Rowe, and F. Majid. 1993. Verotoxin producing Escherichia coli O157:H7 infections associated with consumption of yogurt. Epidemiol. Infect. 111:181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Padhye, N. V., and M. P. Doyle. 1992. Escherichia coli O157:H7 epidemiology, pathogenesis, and methods for detection in food. J. Food Prot. 55:555-556. [DOI] [PubMed] [Google Scholar]
- 26.Quintero-Ramos, A., J. J. Churey, P. Hartman, J. Barnard, and R. W. Worobo. 2004. Modeling of Escherichia coli inactivation by UV irradiation at different pH values in apple cider. J. Food Prot. 67:1153-1156. [DOI] [PubMed] [Google Scholar]
- 27.Reinders, R. D., S. Biesterveld, and P. G. H. Bijker. 2001. Survival of Escherichia coli O157:H7 ATCC 43895 in a model apple juice medium with different concentrations of proline and caffeic acid. Appl. Environ. Microbiol. 67:2863-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riley, L. W., R. S. Remis, S. D. Helgerson, H. B. McGee, J. G. Wells, B. R. Davis, R. J. Hebert, E. S. Olcott, L. M. Johnson, N. T. Hargrett, P. A. Blake, and M. L. Cohen. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308:681-685. [DOI] [PubMed] [Google Scholar]
- 29.Riordan, T., R. J. Gross, S. M. Scotland, and S. M. Johnston. 1985. An outbreak of food-borne enterotoxigenic Escherichia coli diarrhea in England. J. Infect. 11:167-171. [DOI] [PubMed] [Google Scholar]
- 30.Rodrigue, D. C., E. E. Mast, K. D. Greene, J. P. Davis, M. A. Hutchinson, J. G. Well, T. J. Barret, and P. M. Griffin. 1995. A university outbreak of Escherichia coli O157:H7 infections associated with roast beef and an unusually benign clinical course. J. Infect. Dis. 172:1122-1125. [DOI] [PubMed] [Google Scholar]
- 31.SAS. 1987. Statistical Analysis System, version 5.0. SAS Institute, Inc., Cary, N.C.
- 32.Siobain, D., J. J. Churey, R. W. Worobo, and D. W. Schaffner. 2000. Analysis and modeling of the variability associated with UV inactivation of Escherichia coli in apple cider. J. Food Prot. 63:1587-1590. [DOI] [PubMed] [Google Scholar]
- 33.Splittstoesser, D. F., M. R. McLellan, and J. J. Churey. 1996. Heat resistance of Escherichia coli O157:H7 in apple juice. J. Food Prot. 59:226-229. [DOI] [PubMed] [Google Scholar]
- 34.Steele, B. T., N. Murphy, G. S. Arbus, and C. P. Rance. 1982. An outbreak of hemolytic uremic syndrome associated with the ingestion of fresh apple juice. J. Pediatr. 101:963-965. [DOI] [PubMed] [Google Scholar]
- 35.Wright, J. R., S. S. Summer, C. R. Hackney, M. D. Pierson, and B. W. Zoecklein. 2000. Efficacy of ultraviolet light for reducing E. coli O157:H7 in unpasteurized apple cider. J. Food Prot. 63:563-567. [DOI] [PubMed] [Google Scholar]
- 36.Zhao, T., M. P. Doyle, and R. E. Besser. 1993. Fate of enterohemorrhagic Escherichia coli O157:H7 in apple cider with and without preservatives. Appl. Environ. Microbiol. 59:2526-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]