Abstract
Background:
Microwave therapy is a minimal invasive procedure and has been employed in clinical practice for the treatment of various types of cancers. However, its therapeutic application in non-small-cell lung cancer and the underlying mechanism remains to be investigated. This study aimed to investigate its effect on Lewis lung carcinoma (LLC) tumor in vivo.
Methods:
Fifty LLC tumor-bearing C57BL/6 mice were adopted to assess the effect of microwave radiation on the growth and apoptosis of LLC tumor in vivo. These mice were randomly assigned to 10 groups with 5 mice in each group. Five groups were treated by single pulse microwave at different doses for different time, and the other five groups were radiated by multiple-pulse treatment of a single dose. Apoptosis of cancer cells was determined by terminal deoxynucleotidyl transferase dUTP nick-end labeling assay. Western blotting was applied to detect the expression of proteins.
Results:
Single pulse of microwave radiation for 5 min had little effect on the mice. Only 15-min microwave radiation at 30 mW/cm2 significantly increased the mice body temperature (2.20 ± 0.82)°C as compared with the other groups (0.78 ± 0.29 °C, 1.24 ± 0.52 °C, 0.78 ± 0.42 °C, respectively), but it did not affect the apoptosis of LLC tumor cells significantly. Continous microwave radiation exposure, single dose microwave radiation once per day for up to seven days, inhibited cell division and induced apoptosis of LLC tumor cells in a dose- and duration-dependent manner. It upregulated the protein levels of p53, Caspase 3, Bax and downregulated Bcl-2 protein.
Conclusions:
Multiple exposures of LLC-bearing mice to microwave radiation effectively induced tumor cell apoptosis at least partly by upregulating proapoptotic proteins and downregulating antiapoptotic proteins. Continuous radiation at low microwave intensity for a short time per day is promising in treating non-small-cell lung cancer.
Keywords: Apoptosis, Lewis Lung Carcinoma Cells, Microwave Radiation, Non-small-cell Lung Cancer
Introduction
Lung cancer is the most common cancer and the first leading cause of cancer-related death with 1.82 million new cases and 1.56 million deaths per year worldwide.[1,2] It is clinically divided into non-small-cell lung cancer (NSCLC) and small-cell lung cancer based on the origin of cancer cells.[3,4,5] NSCLC accounts for 85% of lung cancer and most cases are diagnosed at the late stages. The current common treatment strategies for lung cancer include palliative care, surgery, chemotherapy, and radiation therapy.[6,7,8,9] However, the facts that lots of primary NSCLCs have local invasion and distant metastasis to other tissues such as brain, bones, and liver and many lung tumors also develop drug resistance limit the effects of the current therapies.[10,11,12] The prognosis of lung cancer is poor with a 5-year survival rate below 15% after diagnosis.[4,13,14] It is urgent to develop novel and effective strategies for the management of lung cancer.
Microwave radiation has been successfully applied in clinical practice to treat various types of cancer. Clinical data indicated that microwave therapy prior to surgery is associated with a better prognosis.[15,16] The effect of microwave irradiation might be a combination of thermal effects, arising from the heating rate and nonthermal effects. Microwave ablation, a form of thermal ablation through interventional radiology, uses electromagnetic waves in the microwave energy spectrum (300 MHz to 300 GHz) to treat cancer by typical thermal effects of microwave irradiation.[17,18,19,20]
The oscillation of polar molecules produces frictional heating, ultimately resulting in tissue necrosis within solid tumors.[21,22] It is a minimally invasive procedure that delivers energy into the tumor through percutaneous needles.[17,23] Microwave ablation has been successfully used for the treatment of many cancers,[24,25] including primary lung malignancies.[26,27,28]
Clinical trials have indicated that microwave ablation alone or in combination with other treatments are efficient for treating early-stage primary lung cancer.[29] However, the therapeutic efficacy of microwave radiation for late-stage lung cancer, remains largely unknown. Meanwhile, little was known about the role of nonthermal effect, also known as the “specific microwave effect,” in lung tumors treated by microwave radiation in vivo.
In our previous study, we observed that microwave radiation inhibited the growth of lung cancer A549 cells and induced cell apoptosis in vitro.[30] In the present study, we adopted the Lewis lung carcinoma (LLC) tumor-bearing C57BL/6J mice to assess the efficacy of microwave radiation in treating lung cancer in vivo by a noncontact mode and to explore the thermal and nonthermal role of microwave radiation in this process so as to provide information to guide the clinical application of microwave therapy for the treatment of late-stage as well as metastatic lung cancers.
Methods
Cell lines, mice, and antibodies
The LLC cells were purchased from American Type Culture Collection (USA). Six-week-old male C57BL/6J mice were purchased from the local Vital River Laboratory Animal Technology. Immunohistochemistry reagents were purchased from Zhongshan Jinqiao Biotechnology (Beijing, China). Transferase dUTP nick-end labeling (TUNEL) staining kit was bought from Roche Applied Science (USA). The anti-vascular endothelial growth factor antibody was obtained from Boster Biological Technology (Wuhan, China). The antibodies for p53, Bcl-2, Bax, and cleaved-Caspase-3 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The source of microwave was obtained from the high-energy microwave dummy source with pulse width at 0.5 µs, frequency at 1.6 kHz, and peak power density at 65 kW. All animal experiments were carried out in a humane manner by trained personnel, following the Animal Care Instructions, and the experimental procedures were approved by Ethical Committee on Animal Care and Use of the Local University.
Establishment of Lewis lung carcinoma tumor-bearing mouse model
The hair on the back of experimental mice was shaved, and 1 × 107 LLC cells in 0.1 ml phosphate-buffered saline (PBS) were injected subcutaneously into each mouse. Ten days after injection, mice with a tumor diameter of 5–10 mm were chosen for subsequent experiments.
Microwave treatment of Lewis lung carcinoma tumor-bearing mice
The tumor-bearing mice were randomly assigned to 10 different groups with 5 mice for each group. For the single-pulse microwave treatment, the groups included untreated control group (S1), mice treated with microwave at 10 mW/cm2 × 5 min (S2), mice treated with microwave at 10 mW/cm2 × 15 min (S3), mice treated with microwave at 30 mW/cm2 × 5 min (S4), and mice treated with microwave at 30 mW/cm2 × 15 min (S5). For multiple-pulse treatment of single dose, LLC tumor-bearing mice in each group were treated by microwave at the same dose as the single pulse above once per day for up to 7 days. The untreated tumor-bearing mice were defined as control group (C1), and the other four groups of tumor-bearing mice were defined as C2 (10 mW/cm2 × 5 min each time for continuous 7 days), C3 (10 mW/cm2 × 15 min each time for continuous 7 days), C4 (30 mW/cm2 × 5 min each time for continuous 7 days), and C5 (30 mW/cm2 × 15 min each time for continuous 7 days).
All mice were housed under specific pathogen-free conditions with a 12 h light/dark cycle and allowed food and water ad libitum. The mice were anesthetized with an overdose of chloral hydrate i.p. followed by exsanguination after the last treatment.
Histopathology and in situ apoptosis assay
The subcutaneous LLC tumor tissue in each mouse was dissected, fixed in 10% formaldehyde for 24 h, embedded in paraffin wax, and cut into 4 mm thick sections which were stained with hematoxylin and eosin (HE) to evaluate general morphology and other specific staining.
The paraffin-embedded sections were stained by terminal deoxynucleotidyl TUNEL using the in situ cell death detection kit (Roche) according to the manufacturer's instructions to detect the apoptotic cells. Apoptotic cells were manifested by brownish staining in the nuclei and visualized by light microscopy. Five microscopic fields (×400) of each section were randomly examined, and the cells were counted by a single-blinded observer in a coded randomized order. The apoptosis rate was presented as the percentage of TUNEL-positive cells to the total LLC cells in each microscopic field.
Immunohistochemistry
The sections were dewaxed in xylene and rehydrated through different concentrations of alcohol. To better reserve and recover the antigens, tumor sections were heated in a citrate buffer in a microwave oven for 30 min at 93°C, followed by three washes in PBS and incubation with 3% bovine serum albumin at 37°C for 30 min. The tumor sections were then immunostained with the primary antibodies including p53, Bax, and Bcl-2 at 4° overnight. On the following day, the sections were washed by PBS briefly three times and incubated with an appropriate second antibody at 37°C for 1 h. The sections were developed by diaminobenzidine solution according to the manufacturer's instructions. The expression level of specific proteins detected in tumor cells was assessed by determination of the integrated optical density of the positively stained cells. The assessment was performed by two independent experienced pathologists.
Statistical analysis
All statistical analyses were performed using the SPSS software version 16.0 statistical software package (SPSS Inc., Chicago, IL, USA). Data were presented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) followed by Scheffe post hoc test was performed to evaluate statistical significance between groups. P < 0.05 was considered statistically significant.
Results
Effect of single-microwave pulse on the body temperature of Lewis lung carcinoma tumor-bearing mice
The tumors were measured 10 days after the LLC cells were implanted subcutaneously into the back of mice. Mice bearing a tumor with 5–10 mm of diameter were chosen for the subsequent study, and there was no significant difference in the tumor volume between different groups.
To assess the thermal effect of microwave treatment on the LLC-bearing mice, a single pulse of microwave radiation was adopted, and the Veterinary Thermal Imaging Camera (Olympus, Japan) was applied to generate thermal images and detect core body temperature of the mice [Figure 1a]. The results demonstrated that 5 min microwave radiation of different doses did not significantly improve the body temperature of mice (0.78 ± 0.29°C increased in S2 and 0.78 ± 0.42°C in S4 group) as compared with that before the microwave treatment respectively, whereas 15 min microwave radiation of either dose increased the body temperature by over 1°C (1.24 ± 0.52°C increased in S3 and 2.20 ± 0.82°C in S5) [Figure 1b]. Single-microwave pulse at 30 mW/cm2 for 15 min significantly increased the mice body temperature as compared with the other treatments. These results indicated that the thermal effect of single-microwave pulse on the body temperature of LLC-bearing mice was associated with the power density and irradiation duration. Microwave radiation in Group S5 was more apt to affect the LLC tumor by thermal effect than that in the others, while nonthermal effects of microwave radiation in the other three groups might play a major role in affecting LLC tumor in vivo.
Figure 1.
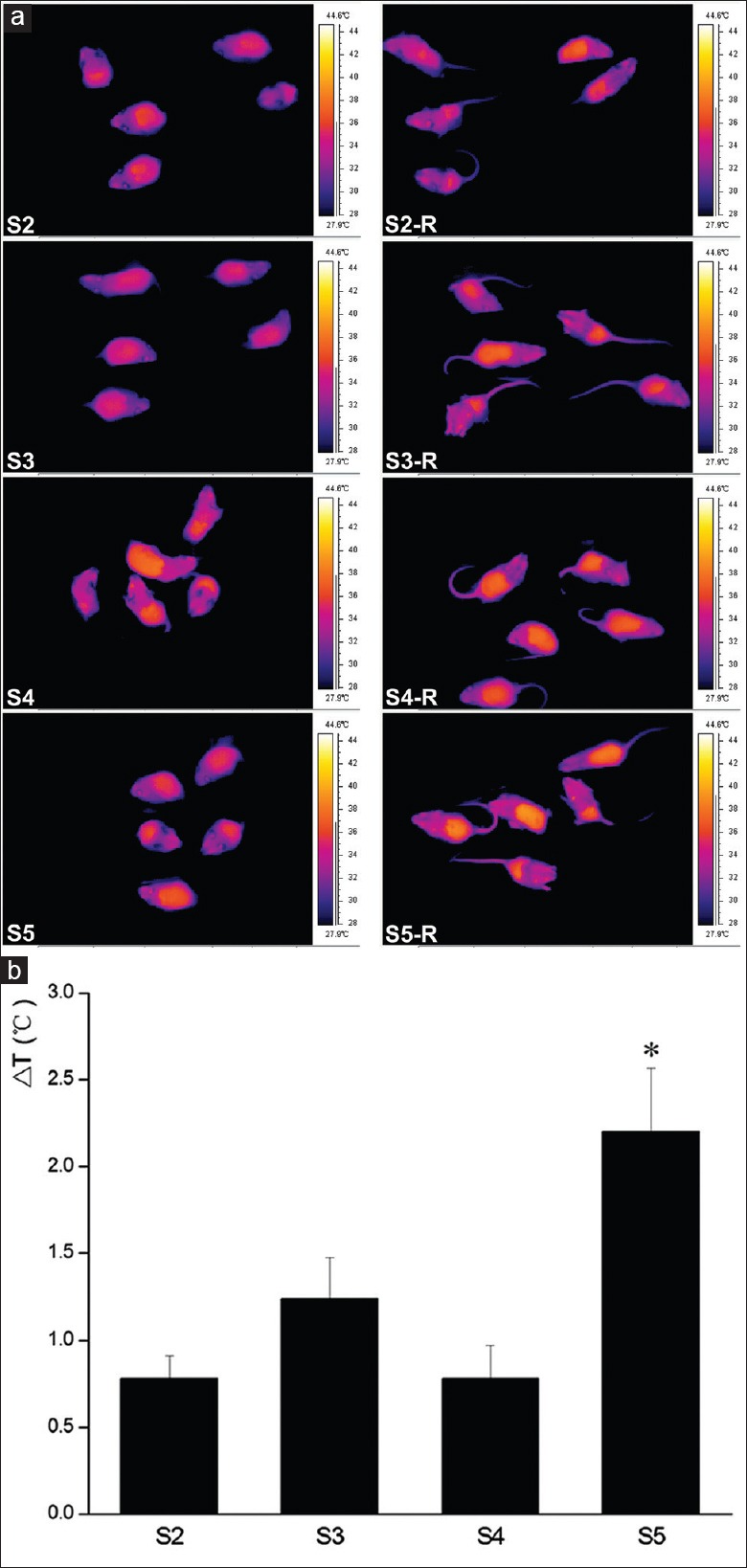
Effect of single-microwave pulse on the body temperature of Lewis lung carcinoma tumor-bearing mice. (a) Thermal images of mice captured by the Veterinary Thermal Imaging Camera; (b) changes of mice body temperature affected by microwave radiation. S2: 10 mW/cm2 × 5 min, S3: 10 mW/cm2 × 15 min, S4: 30 mW/cm2 × 5 min, and S5: 30 mW/cm2 × 15 min; *P < 0.01 versus S2 group, P < 0.05 versus S3 group, and P < 0.01 versus S4 group.
Effect of single-microwave pulse on the Lewis lung carcinoma tumor cells in vivo
HE staining was applied to observe the histopathological changes and mitotic cells. The results demonstrated that the LLC tumor cells in each group distributed diffusely with irregular size and morphology and abnormally large nuclei. Single-microwave radiation neither resulted in apparent nuclear condensation or necrotic foci nor did it affect the number of mitotic cells under light microscope. Moreover, no significant effect of single-microwave radiation on cell apoptosis or on the expression of proapoptotic proteins including cleaved-Caspase-3, p53, Bcl-2, and Bax was observed as compared with the untreated control, whatever the dose or radiation time was applied (data not shown). These results indicated that a single pulse of microwave at both power densities did not affect the growth or apoptosis of LLC tumors in vivo even when the radiation lasted for 15 min.
Effect of multiple pulses of microwave radiation on the growth of Lewis lung carcinoma tumor
To determine the cumulative effect of microwave on the growth and apoptosis of LLC tumors in vivo, tumor-bearing mice were microwave radiated by the same dose as the above single pulse once per day for up to 7 days. There was no significance in the tumor volume between the groups before the radiation. The tumor volumes in all mice were measured by vernier caliper on days 1, 3, 5, and 7. The results showed that the volumes of the LLC tumors in all groups increased with time prolonging. The microwave-radiated tumors on day 7, especially in the 30 mW/cm2 × 15 min group (C5), were evidently smaller than those in the untreated control group (C1) [Figure 2]. However, multiple microwave radiations did not significantly decrease the tumor volume as compared with that in the untreated control group. The weight of the tumor in each mouse was also weighed after the mice were sacrificed. The data demonstrated that 15 min radiation at both doses of microwave for continuous 7 days decreased the weight of the tumors as compared with the untreated control, but there was no statistical significance (data not shown).
Figure 2.
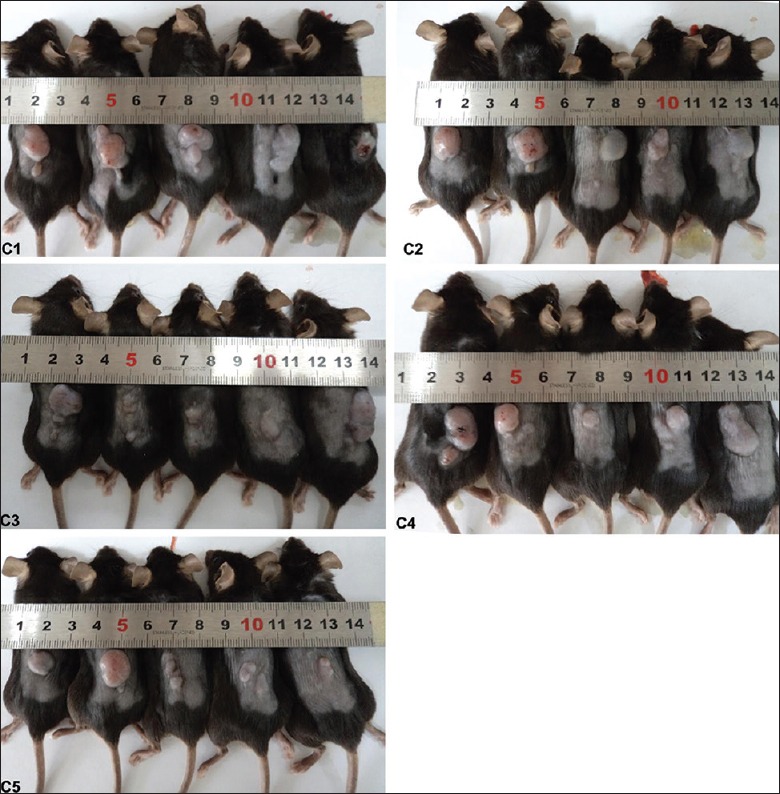
Effect of multiple pulses of microwave radiation on the volume of Lewis lung carcinoma tumors. C1: the untreated control group in the multiple-pulse treatment of single dose, C2: 10 mW/cm2 × 5 min each day, C3: 10 mW/cm2 × 15 min each day, C4: 30 mW/cm2 × 5 min each day, C5: 30 mW/cm2 × 15 min each day. All groups were treated for continuous 7 days.
Continuous microwave radiation-induced histopathological damages of Lewis lung carcinoma tumors
After continuous 7 days of repeated radiation by different microwave intensities and duration, there were lots of dispersal and multiple necrotic foci at the periphery of the tumor tissues, while little necrosis could be observed in the untreated LLC tumor. The percentage of mitotic cells in C2 group (6.00 ± 2.03) was significantly lower than that in the control C1 group (9.32 ± 1.78) (P = 0.47). As the microwave exposure dose increased and the time was prolonged (C5), the percentage of mitotic cells significantly decreased as compared with the untreated control (P = 0.004) [Figure 3].
Figure 3.
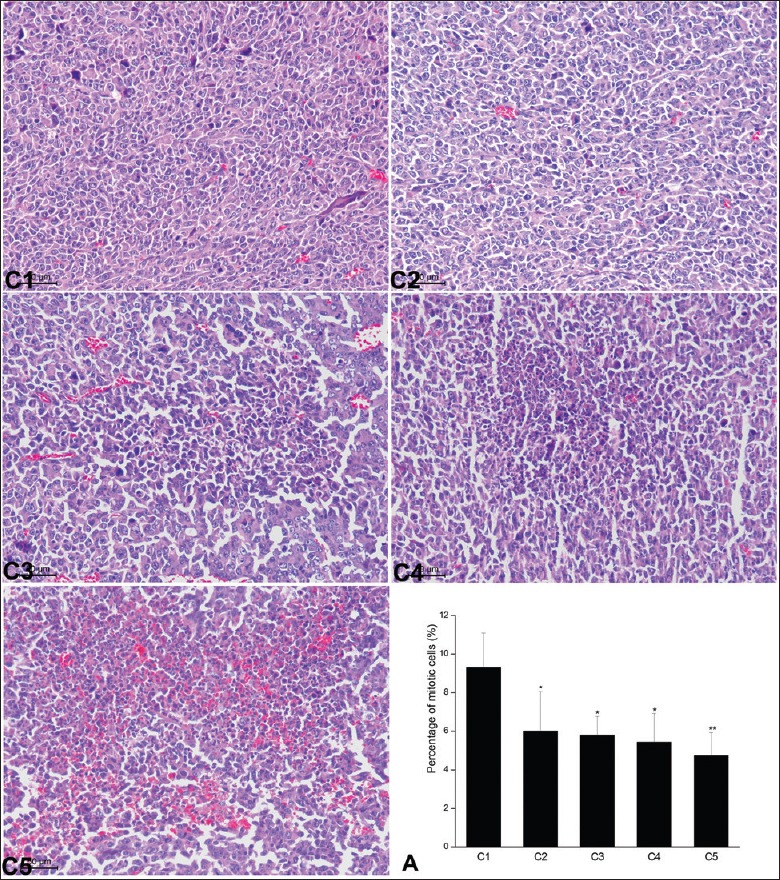
Effect of multiple pulses of microwave radiation on the histopathology of Lewis lung carcinoma tumors. C1–C5: Representative HE staining of Lewis lung carcinoma tumor sections (scale bars = 50 μm). A: the percentage of mitotic cells were counted. Continuous microwave radiation significantly decreased the percentage of mitotic cells as compared with the untreated control group. Data were presented as mean ± standard deviation (n = 5). *P < 0.05, **P < 0.01 versus the untreated control.
Multiple pulses of microwave radiation promoted Lewis lung carcinoma tumor cell apoptosis
Multiple pulses of microwave radiation induced LLC tumor cell apoptosis as shown by TUNEL staining. Microwave radiation at 10 mW/cm2 for 15 min resulted in a cell apoptosis rate of 13.20 ± 4.29 in the LLC tumors (C3), which was significantly higher than that in the control C1 group (P = 0.029). When the dose of microwave reached 30 mW/cm2, the apoptosis rate of LLC tumor cells increased significantly even when the tumor was radiated for only 5 min each time (C4) as compared with the untreated control [P = 0.044, Figure 4]. These results clearly demonstrated that multiple pulses of microwave radiation could efficiently induce cell apoptosis of LLC tumors.
Figure 4.
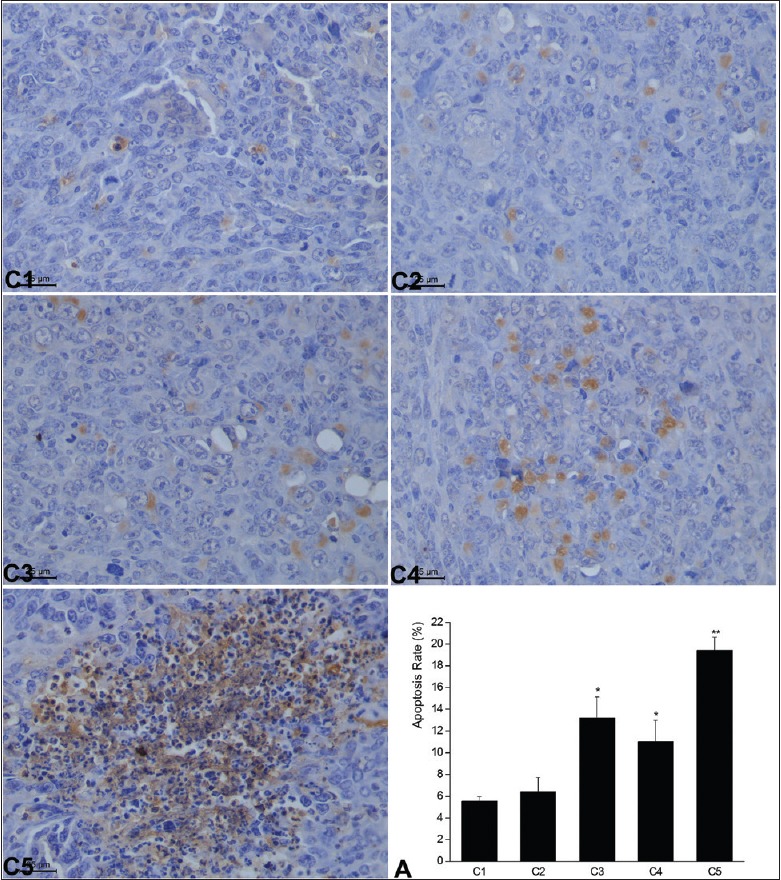
Multiple pulses of microwave radiation-induced cell apoptosis of Lewis lung carcinoma tumors assessed by transferase dUTP nick-end labeling staining. The percentage of apoptotic cells in each group on day 7 (mean ± standard deviation). *P < 0.05, **P < 0.01 versus the untreated control group (C1). C2: 10 mW/cm2 × 5 min each day, C3: 10 mW/cm2 × 15 min each day, C4: 30 mW/cm2 × 5 min each day, C5: 30 mW/cm2 × 15 min each day. All the groups were treated for 7 continuous days; A: The bar graph showed the cell apoptosis rate in the lung of each group. Data were presented as mean±SD of the apoptosis rate from all the mice in each group as indicated.
Multiple pulses of microwave treatment increased the protein levels of p53 and apoptotic-associated proteins
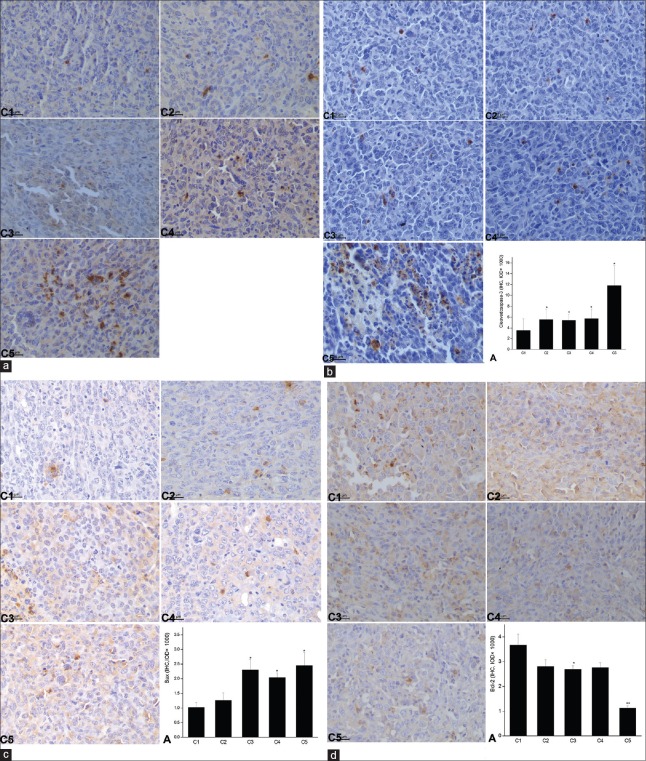
To further explore the molecular mechanism by which multiple pulses of microwave radiation induced LLC tumor cell apoptosis, we detected the expression of proapoptotic proteins including p53, cleaved-Caspase-3, and Bax by immunochemistry [Figure 5a–5c]. The results demonstrated that microwave radiation significantly increased the protein levels of p53, cleaved-Caspase-3, and Bax proteins as compared with the control group (P < 0.05). The strongest positive brown staining of these proteins was observed in the tumor tissues from C5 group which was radiated by a stronger microwave for a longer time as compared with other groups radiated by microwave. Meanwhile, we detected the expression of antiapoptotic regulator Bcl-2 protein in the LLC tumor tissues from different groups and found that microwave radiation at 30 mW/cm2 (C5 group) for 15 min significantly decreased Bcl-2 expression as compared with the control group (P = 0.002). While there was no significance between the 5 min groups and the control group, no matter the microwave dose was 10 mW/cm2 (C2 group) or 30 mW/cm2 (C4 group) [Figure 5d]. The results above demonstrated that 30 mW/cm2 microwave radiation had a stronger influence on the gene expression in LLC tumor cells than the 10 mW/cm2 did for the same radiation time. The expressions of p53, Caspase-3, and Bax were positively correlated with the intensity of microwave radiation whereas the expression of Bcl-2 was negatively correlated with the intensity of microwave treatment. These results revealed that multiple pulses of microwave radiation could induce LLC tumor cell apoptosis by upregulating the expressions of proapoptotic proteins.
Figure 5.
Immunohistochemistry analysis of the protein expressions (p53, cleaved-Caspase-3, Bax, and Bcl-2) affected by multiple pulses of microwave treatment. (a) p53, (b) cleaved-Caspase-3, (c) Bax, and (d) Bcl-2. Data were presented as mean ± standard deviation (n = 5). *P < 0.05, **P < 0.01 versus the untreated control. Positive immunoreactivity was characterized by brown staining. C1: the untreated control, C2: 10 mW/cm2 × 5 min each day, C3: 10 mW/cm2 × 15 min each day, C4: 30 mW/cm2 × 5 min each day, C5: 30 mW/cm2 × 15 min each day. All the groups were microwave-treated for continuous 7 days; A: The bar graph showed the IOD values for the positive-staining in the mice lungs of each group as indicated.
Discussion
Chemotherapy and radiotherapy are two major treatment strategies for NSCLC, especially for patients with late-stage cancers.[31,32,33] However, both treatment methods are associated with a high toxicity. Microwave is a nonionizing radiation and shows less toxicity than chemotherapy and radiotherapy in cancer patients. It has both thermal and nonthermal effects. The thermal effects of microwave on local tumors have been widely recognized, whereas the nonthermal effects of noncontact microwave radiation on lung tumor still remain to be elucidated. In the present study, the result that single-microwave radiation (10 mW/cm2 or 30 mW/cm2) for 5 min had little effect on the body temperature revealed that 5 min microwave radiation played a role in this study mainly in a nonthermal effect manner. Single-microwave radiation for 15 min elevated mice body temperature by over 1°C, and the body temperature in 30 mW/cm2 group was significantly higher than that in 10 mW/cm2 group. These results revealed that the thermal effect of microwave was related to the irradiation intensity and duration, and 15 min microwave exposure affected the LLC-tumor-bearing mice by both nonthermal and thermal effects even at a lower intensity of 10 mW/cm2.
Our previous study in vitro demonstrated that low-intensity or short-term microwave radiation induced tumor cell apoptosis 3–6 h after a single radiation, while increasing radiation intensity or time led to immediate tumor cell death, as is called coagulation necrosis.[30] In this study, we did not observe apparent LLC tumor cell apoptosis 6 h or 24 h after the LLC-tumor-bearing mice received a single 30 mW/cm2 microwave radiation for 15 min. This revealed that the thermal effect caused by the irradiation dose adopted in this study was not strong enough to directly induce LLC cell apoptosis or coagulation necrosis. However, continuous microwave radiation once a day for up to 7 days efficiently induced LLC tumor cell apoptosis even at the low intensity of 10 mW/cm2 for 5 min each time. These results revealed that nonthermal effect played a critical role in the induction of tumor cell apoptosis in a cumulative manner in the LLC-bearing mice exposed to noncontact microwave. Although the study of nonthermal effect on microwave-induced apoptosis or inhibition of cancer growth is in its infancy, further studies on the underlying mechanism are potentially important to facilitate the application of microwave radiation in clinical practice.
p53 is a major tumor suppressor and has been described as “the guardian of the genome” because of its pivotal role in maintaining genome stability.[34] Normally, p53 is expressed at a very low level, and high expression of p53 can promote cell apoptosis.[35] In the present study, microwave radiation significantly upregulated the expression of p53 in a dose- and duration-dependent manner which revealed that p53 was implicated in microwave radiation-induced LLC cell apoptosis.
It is well documented that a change in the ratio between proapoptotic and antiapoptotic proteins affected cell apoptosis.[36] In the present study, continuous microwave radiation upregulated the protein levels of proapoptotic proteins such as cleaved-Caspase-3 and Bax and downregulated the expression of antiapoptotic protein Bcl-2. This revealed that these apoptosis-associated proteins were implicated in microwave radiation-induced LLC cell apoptosis in vivo. However, it still remains to be elucidated whether the upregulation of proapoptotic and downregulation of antiapoptotic proteins were attributed to the upregulation of p53 induced by microwave radiation in tumor cells.
In this study, although multiple pulses of single dose microwave radiation once per day for up to seven days did not significantly decreased the tumor volume or weight as compared with that in the untreated control group, low dose at 10 mW/cm2 for 5 minutes each time upregulated the expression of cleaved-caspase-3, which played a key role in the apoptosis of LLC tumor cells. The results in the present study provided important clues that low dose microwave radiation had the potential to sensitize cancer cells in vivo. Further studies on the role of microwave radiation in the sensibilization of cancer cells to conventional chemotherapy and/or radiotherapy might be helpful to develop new therapeutic strategies for lung cancer.
In summary, continuous exposure of LLC-bearing mice to microwave radiation induced lung cancer cell apoptosis at least partly by upregulating the proapoptotic proteins p53, cleaved-Caspase-3, and Bax and downregulating the antiapoptotic protein Bcl-2. Our findings suggested that multiple-pulse microwave radiation at low intensity for a short time per day is promising in treating NSCLC.
Financial support and sponsorship
This work was supported by grants from Science and Technology Commission of Shanghai Municipality (No.134119a0302) and Shanghai Municipal Commission of Health and Family Planning (No. 2009114).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Pillai RN, Owonikoko TK. Small cell lung cancer: Therapies and targets. Semin Oncol. 2014;41:133–42. doi: 10.1053/j.seminoncol.2013.12.015. doi: 10.1053/j.seminoncol.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sundar R, Soong R, Cho BC, Brahmer JR, Soo RA. Immunotherapy in the treatment of non-small cell lung cancer. Lung Cancer. 2014;85:101–9. doi: 10.1016/j.lungcan.2014.05.005. doi: 10.1016/j.lungcan.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Travis WD, Rekhtman N. Pathological diagnosis and classification of lung cancer in small biopsies and cytology: Strategic management of tissue for molecular testing. Semin Respir Crit Care Med. 2011;32:22–31. doi: 10.1055/s-0031-1272866. doi: 10.1055/s-0031-1272866. [DOI] [PubMed] [Google Scholar]
- 6.Chan BA, Coward JI. Chemotherapy advances in small-cell lung cancer. J Thorac Dis. 2010;5(Suppl 5):S565–78. doi: 10.3978/j.issn.2072-1439.2013.07.43. doi: 10.3978/j.issn.2072-1439.2013.07.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gadgeel SM, Ramalingam SS, Kalemkerian GP. Treatment of lung cancer. Radiol Clin North Am. 2012;50:961–74. doi: 10.1016/j.rcl.2012.06.003. doi: 10.1016/j.rcl.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Inoue M, Sawabata N, Okumura M. Surgical intervention for small-cell lung cancer: What is the surgical role? Gen Thorac Cardiovasc Surg. 2012;60:401–5. doi: 10.1007/s11748-012-0072-9. doi: 10.1007/s11748-012-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McElnay P, Lim E. Adjuvant or neoadjuvant chemotherapy for NSCLC. J Thorac Dis. 2010;6(Suppl 2):S224–7. doi: 10.3978/j.issn.2072-1439.2014.04.26. doi: 10.1016/s0169-5002(05)81568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arslan C, Dizdar O, Altundag K. Systemic treatment in breast-cancer patients with brain metastasis. Expert Opin Pharmacother. 2010;11:1089–100. doi: 10.1517/14656561003702412. doi: 10.1517/14656561003702412. [DOI] [PubMed] [Google Scholar]
- 11.Chen YT, Feng B, Chen LB. Update of research on drug resistance in small cell lung cancer chemotherapy. Asian Pac J Cancer Prev. 2012;13:3577–81. doi: 10.7314/apjcp.2012.13.8.3577. doi: 10.7314/apjcp.2012.13.8.3577. [DOI] [PubMed] [Google Scholar]
- 12.Kallianos A, Rapti A, Zarogoulidis P, Tsakiridis K, Mpakas A, Katsikogiannis N, et al. Therapeutic procedure in small cell lung cancer. J Thorac Dis. 2013;5(Suppl 4):S420–4. doi: 10.3978/j.issn.2072-1439.2013.09.16. doi: 10.1016/0169-5002(89)90486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sudhindra A, Ochoa R, Santos ES. Biomarkers, prediction, and prognosis in non-small-cell lung cancer: A platform for personalized treatment. Clin Lung Cancer. 2011;12:360–8. doi: 10.1016/j.cllc.2011.02.003. doi: 10.1016/j.cllc.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Suh JH. Current readings: Pathology, prognosis, and lung cancer. Semin Thorac Cardiovasc Surg. 2013;25:14–21. doi: 10.1053/j.semtcvs.2013.01.003. doi: 10.1053/j.semtcvs.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Perez CA, Kopecky W, Baglan R, Rao DV, Johnson R. Local microwave hyperthermia in cancer therapy. Preliminary report. Henry Ford Hosp Med J. 1981;29:16–23. doi: 10.1016/0360-3016(81)90555-1. [PubMed] [Google Scholar]
- 16.Zhang W, Lei P, Dong X, Xu C. The new concepts on overcoming drug resistance in lung cancer. Drug Des Devel Ther. 2014;8:735–44. doi: 10.2147/DDDT.S60672. doi: 10.2147/DDDT.S60672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gharib H, Hegedüs L, Pacella CM, Baek JH, Papini E. Clinical review: Nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J Clin Endocrinol Metab. 2013;98:3949–57. doi: 10.1210/jc.2013-1806. doi: 10.1210/jc.2013-1806. [DOI] [PubMed] [Google Scholar]
- 18.Hernández JI, Cepeda MF, Valdés F, Guerrero GD. Microwave ablation: State-of-the-art review. Onco Targets Ther. 2015;8:1627–32. doi: 10.2147/OTT.S81734. doi: 10.2147/OTT.S81734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paliwal BR, Shrivastava PN. Microwave hyperthermia. Principles and quality assurance. Radiol Clin North Am. 1989;27:489–97. [PubMed] [Google Scholar]
- 20.Wallen CA, Michaelson SM. Proceedings: Microwave-induced hyperthermia as an adjuvant to cancer therapy. J Microw Power. 1976;11:175–6. [PubMed] [Google Scholar]
- 21.Brace CL. Microwave ablation technology: What every user should know. Curr Probl Diagn Radiol. 2009;38:61–7. doi: 10.1067/j.cpradiol.2007.08.011. doi: 10.1067/j.cpradiol.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang P, Wang Y. Microwave ablation of hepatocellular carcinoma. Oncology. 2007;72(Suppl 1):124–31. doi: 10.1159/000111718. doi: 10.1159/000112594. [DOI] [PubMed] [Google Scholar]
- 23.Kelekis AD, Thanos L, Mylona S, Ptohis N, Malagari K, Nikita A, et al. Percutaneous radiofrequency ablation of lung tumors with expandable needle electrodes: Current status. Eur Radiol. 2006;16:2471–82. doi: 10.1007/s00330-006-0270-x. doi: 10.1007/s00330-006-0270-x. [DOI] [PubMed] [Google Scholar]
- 24.Chu KF, Dupuy DE. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199–208. doi: 10.1038/nrc3672. doi: 10.1038/nrc3672. [DOI] [PubMed] [Google Scholar]
- 25.Ward RC, Healey TT, Dupuy DE. Microwave ablation devices for interventional oncology. Expert Rev Med Devices. 2013;10:225–38. doi: 10.1586/erd.12.77. doi: 10.1586/erd.12.77. [DOI] [PubMed] [Google Scholar]
- 26.Baisi A, De Simone M, Raveglia F, Cioffi U. Thermal ablation in the treatment of lung cancer: Present and future. Eur J Cardiothorac Surg. 2013;43:683–6. doi: 10.1093/ejcts/ezs558. doi: 10.1093/ejcts/ezs558. [DOI] [PubMed] [Google Scholar]
- 27.Ido K, Isoda N, Sugano K. Microwave coagulation therapy for liver cancer: Laparoscopic microwave coagulation. J Gastroenterol. 2001;36:145–52. doi: 10.1007/s005350170121. doi: 10.1007/s005350170121. [DOI] [PubMed] [Google Scholar]
- 28.Sherar MD, Trachtenberg J, Davidson SR, Gertner MR. Interstitial microwave thermal therapy and its application to the treatment of recurrent prostate cancer. Int J Hyperthermia. 2004;20:757–68. doi: 10.1080/02656730410001734146. doi: 10.1080/02656730410001734146. [DOI] [PubMed] [Google Scholar]
- 29.Wei Z, Ye X, Yang X, Zheng A, Huang G, Li W, et al. Microwave ablation in combination with chemotherapy for the treatment of advanced non-small cell lung cancer. Cardiovasc Intervent Radiol. 2015;38:1–8. doi: 10.1007/s00270-014-0895-0. doi: 10.1007/s00270-014-0895-0. [DOI] [PubMed] [Google Scholar]
- 30.Song XL, Wang CH, Hu HY, Yu C, Bai C. Microwave induces apoptosis in A549 human lung carcinoma cell line. Chin Med J. 2011;124:1193–8. doi: 10.3760/cma.j.issn.0366-6999.2011.08.013. [PubMed] [Google Scholar]
- 31.Kepka L, Sprawka A, Casas F, Abdel-Wahab S, Agarwal JP, Jeremic B. Combination of radiotherapy and chemotherapy in locally advanced NSCLC. Expert Rev Anticancer Ther. 2009;9:1389–403. doi: 10.1586/era.09.121. doi: 10.1586/era.09.121. [DOI] [PubMed] [Google Scholar]
- 32.Soffietti R, Costanza A, Laguzzi E, Nobile M, Rudà R. Radiotherapy and chemotherapy of brain metastases. J Neurooncol. 2005;75:31–42. doi: 10.1007/s11060-004-8096-3. doi: 10.1007/s11060-004-8096-3. [DOI] [PubMed] [Google Scholar]
- 33.Zaric B, Stojsic V, Tepavac A, Sarcev T, Zarogoulidis P, Darwiche K, et al. Adjuvant chemotherapy and radiotherapy in the treatment of non-small cell lung cancer (NSCLC) J Thorac Dis. 2013;5(Suppl 4):S371–7. doi: 10.3978/j.issn.2072-1439.2013.05.16. doi: 10.21037/jtd.2016.02.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller PA, Vousden KH. p53 mutations in cancer. Nat Cell Biol. 2013;15:2–8. doi: 10.1038/ncb2641. doi: 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- 35.Carvajal LA, Manfredi JJ. Another fork in the road – Life or death decisions by the tumour suppressor p53. EMBO Rep. 2013;14:414–21. doi: 10.1038/embor.2013.25. doi: 10.1038/embor.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]