Abstract
Background:
Wheezing is common in early childhood and remains an important health concern. The aim of this study was to assess the lung function of wheezing infants and to investigate the relationship between lung function and respiratory outcome.
Methods:
Infants <2 years of age with acute lower respiratory tract infection (ALRTI) who had undergone lung function tests were included in the study. They were assigned to wheeze or no wheeze group based on physical examination. Infants without any respiratory diseases were enrolled as controls. Lung function was measured during the acute phase and 3 months after ALRTI. One-year follow-up for infants with ALRTI was achieved.
Results:
A total of 252 infants with ALRTI who had acceptable data regarding tidal breathing were included in the final analysis. Compared with the control and the no wheeze groups, infants in the wheeze group had significantly decreased time to peak tidal expiratory flow as a percentage of total expiratory time (TPTEF/TE) (20.1 ± 6.4% vs. 34.4 ± 6.2% and 26.4 ± 8.3%, respectively, P < 0.0001) and significantly increased peak tidal expiratory flow (PTEF) (90.7 ± 26.3 ml/s vs. 79.3 ± 18.4 ml/s and 86.1 ± 28.0 ml/s, respectively, P < 0.01), sReff and Reff. The infants in the wheeze group still had lower TPTEF/TE and volume to peak tidal expiratory flow as a percentage of total expiratory volume (VPTEF/VE) than the no wheeze infants 3 months after the ALRTI. Moreover, there was a significant inverse relationship between TPTEF/TE, VPTEF/VE, and the recurrence of wheezing and pneumonia.
Conclusions:
Impaired lung function was present in wheezing infants with ALRTI and the deficits persisted. In addition, the lower level of TPTEF/TE and VPTEF/VE was a risk factor for poor respiratory outcome.
Keywords: Acute Lower Respiratory Tract Infection, Infant, Lung Function, Respiratory Outcome; Wheeze
Introduction
Worldwide, many studies have reported a high prevalence of early life wheezing. Approximately, one in three children has at least one episode of wheezing before their third birthday,[1] and the prevalence of wheezing has been increasing significantly in recent years.[2] Moreover, most cases of persistent wheezing and asthma begin during early childhood.[3] Preschool children with wheezing consume a disproportionately high amount of health-care resources compared to adults with asthma.[4]
It is well documented that bronchiolitis in infancy increases the risk of asthma in childhood and even in adulthood.[5,6] Acute lower respiratory tract infection (ALRTI) due to respiratory syncytial virus[7] and rhinovirus[8] in early life has been implicated as contributing to asthma. In recent years, significant evidence from birth cohort studies has been indicating that reduced neonatal lung function and increased airway responsiveness are associated with respiratory symptoms and asthma later in life.[9,10,11,12,13] However, due to the difficulty in performing a routine lung function test, relatively little is known about the lung function in infants with wheezing after respiratory infection. In addition, the natural history of wheezing after respiratory infection has not been studied extensively, and the relationship between wheezing, pulmonary outcomes, and atopy is poorly understood.
Wheezing infants form a heterogeneous group with different phenotypes and with different outcomes;[6,14,15] different phenotypes predict different outcomes and may require different management strategies. However, the phenotypes’ classification is based on longitudinal symptomatology, and good predictors that allow prospective prediction are not available for wheezing infants. Therefore, it is important to identify predictors that are simple and more practical for clinical practice.
The aim of the present study was to investigate the lung function of wheezing infants with ALRTI and its changes after recovery and to further explore the role of lung function in predicting the respiratory outcome.
Methods
Patients seen at the outpatient Department of Respiration at the Children's Hospital of Fudan University from February 1, 2010, to January 31, 2013, were enrolled in the study. Inclusion criteria were as follows: (1) infants with ALRTI, (2) age <2 years (excluding neonates), (3) no respiratory tract infection in the preceding 2 months, and (4) informed consent for lung function tests obtained from their parents. ALRTI was defined according to the World Health Organization definition, and infants were eligible if they had clinical (fever, cough, tachypnea, or abnormal auscultatory findings) or radiologic evidence of ALRTI.[16] Exclusion criteria were as follows: (1) gestational age <36 weeks, (2) major congenital abnormalities, and (3) infants with previously wheezing, bronchopulmonary dysplasia, bronchiolitis obliterans, tuberculosis, immune defect, and with lung and airway malformations. The patients included were assigned to the wheeze or no wheeze group based on physical examination by an experienced respiratory pediatrician.
Infants <2 years of age who presented to the Department of Child Health Care without any respiratory or cardiac disease were enrolled as controls. Infants who have upper or lower respiratory tract infection in the preceding 2 months of lung function tests were excluded from the study.
Informed consent was obtained from the parents before enrolling of the infants, and ethical approval was granted by the Research Ethics Committee of Children's Hospital of Fudan University (REC Number 276/97, 307/97, 089/99, 125/98, and 06/Q1702/104).
Baseline characteristics and follow-up data collection
Data on general information and clinical characteristics were collected by trained staff using a standard case report form, which included demographic characteristics, pre- and perinatal factors, parental demographics, social background, disease history, clinical presentation, primary disease diagnosis, accessory examinations, and treatment.
One-year follow-up for infants with ALRTI was performed by questionnaires. In each questionnaire, parents were asked about the recurrence of coughing and wheezing, readmissions, and occurrence of allergic diseases such as atopic eczema and rhinitis. Atopic eczema was defined as the current diagnosis made by a pediatric dermatologist. Allergic rhinitis was defined as a physician diagnosis of allergic rhinitis for which the child had taken medicine for that condition. The recurrence of wheezing is confirmed by pediatricians through physical examination. In addition, the anthropometrics and environmental factors including feeding patterns, second-hand smoking, siblings, and pets were also determined.
Pulmonary function tests
All the infants were sedated with orally administered chloral hydrate (30–50 mg/kg) prior to testing. During pulmonary function testing, the infants were lying supine with the head midline and the neck slightly extended to minimize airway or glottis obstruction. Pulmonary function tests were performed using the Jaeger MasterScreen BabyBody device (Erich Jaeger GmbH, v4.65, Würzburg, Germany) with a size 1 or size 2 mask in adherence with the American Thoracic Society/European Respiratory Society recommendations.[17,18,19] Measurement of tidal breathing, plethysmographic parameters including specific effective airway resistance (sReff), and functional residual capacity (FRC) were obtained according to international guidelines.[20,21] The main parameters of tidal breathing include tidal volume (VT), respiratory rate, time-to-peak tidal expiratory flow as a percentage of total expiratory time (TPTEF/TE), volume-to-peak tidal expiratory flow as a percentage of total expiratory volume peak tidal expiratory flow (VPTEF/VE), peak tidal expiratory flow (PTEF), and expiratory flow at 25% tidal volume (TEF25). Data of infants who failed to meet quality control standards of plethysmography testing were excluded from the analysis.
Lung function measurements of infants with ALRTI were conducted 1 day after diagnosis during the acute phase and 3 months after the first lung function test. Lung function tests were performed 1 day after enrollment in the control infants.
Statistical analysis
Continuous variables were expressed as mean (standard deviation) or median (inter quartile range). Categorical variables were presented as n (%). Student's t-test or Mann-Whitney U-test was used to compare differences between two groups, while one-way analysis of variance (ANOVA) or Kruskal-Wallis H-test was used to compare differences between groups for continuous variables. Univariate analyses on categorical data were performed using Chi-square test or Fisher's exact test when appropriate. Comparison between the first and follow-up pulmonary function parameters of the children was performed using the Wilcoxon rank-sum test. Pearson's correlation test was used for obtaining correlations between two variables. SPSS software version 17.0 (IBM Corporation, NY, USA) was used for statistical analysis. A value of P < 0.05 was considered statistically significant.
Results
Patient characteristics
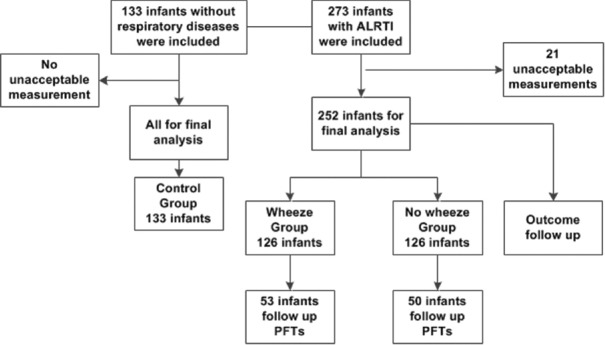
The study profile is shown in Figure 1. During the study period, 1859 infants with ALRTI were seen in the outpatient Department of Respiration of the Children's Hospital of Fudan University. In total, 273 (14.7%) infants with ALRTI were included in this study. In addition, 133 children without respiratory disease were included as the control group.
Figure 1.
Flow chart of the patient selection.
Of the 273 infants with ALRTI, 252 (92.3%) infants who had acceptable data on tidal breathing were included in the final analysis, while 21 (7.7%) infants were excluded due to unacceptable measurements. Lung function data in the control group were all technically acceptable. Of the 252 infants for final analysis, 126 infants were classified as the wheeze group and the other 126 infants as the no wheeze group. All the infants with ALRTI completed 1-year follow-up. Of these infants, 103 (53 in wheeze group and 50 in no wheeze group) completed the follow-up lung function tests 3 months later, which were technically satisfactory.
The baseline characteristics of the study population are summarized in Table 1. There was no difference in birth weight, sex, feeding practices, and household tobacco smoke exposure. All the infants with ALRTI had an acute course <5 days. There was no difference in the proportion of pneumonia infants between the wheeze and the no wheeze group (22/126 vs. 20/126). Moreover, no difference was found in the monthly distribution between the two groups [Figure 2].
Table 1.
Characteristics of infants participating in the study
| Characteristics | Wheeze (n = 126) | No wheeze (n = 126) | Control (n = 133) | F/χ2 | P |
|---|---|---|---|---|---|
| Birth weight (g)* | 3343 ± 550 | 3441 ± 472 | 3370 ± 390 | 1.662 | NS |
| Male, n (%)† | 94 (74.6) | 85 (67.5) | 94 (70.7) | 1.564 | NS |
| Breast feeding, n (%)† | 47 (37.3) | 53 (42.1) | 65 (48.9) | 3.585 | NS |
| Formula feeding, n (%)† | 27 (21.4) | 33 (26.2) | 21 (15.8) | 4.231 | NS |
| Mixed feeding, n (%)† | 52 (41.3) | 40 (31.7) | 47 (35.3) | 2.529 | NS |
| Family allergy, n (%)† | 65 (51.6)‡ | 56 (44.4) | 46 (34.6) | 7.701 | 0.021 |
| Household smoker, n (%)† | 66 (52.4) | 70 (55.6) | 59 (44.4) | 3.469 | NS |
| Household cigarettes* | 10.8 ± 2.8 | 10.9 ± 3.2 | 9.8 ± 3.0 | 2.362 | NS |
| Family members ≥5, n (%)† | 54 (42.8)‡ | 49 (39.1)§ | 33 (24.8) | 7.482 | 0.024 |
| Family income ≥2500 $/months, n (%)† | 16 (12.7) | 11 (8.7) | 9 (6.8) | 2.771 | NS |
| Cardiopulmonary history, n (%)† | 33 (26.2)‡ | 34 (27.0)§ | 18 (13.5) | 8.645 | 0.013 |
*Mean ± SD, demonstrated by one–way ANOVA; †n (%), evaluated by Chi–square test; P, differences among all the three groups; ‡Significant differences between wheeze and the control groups; §Significant differences between no wheeze and the control groups. SD: Standard deviation; NS: Not significant.
Figure 2.
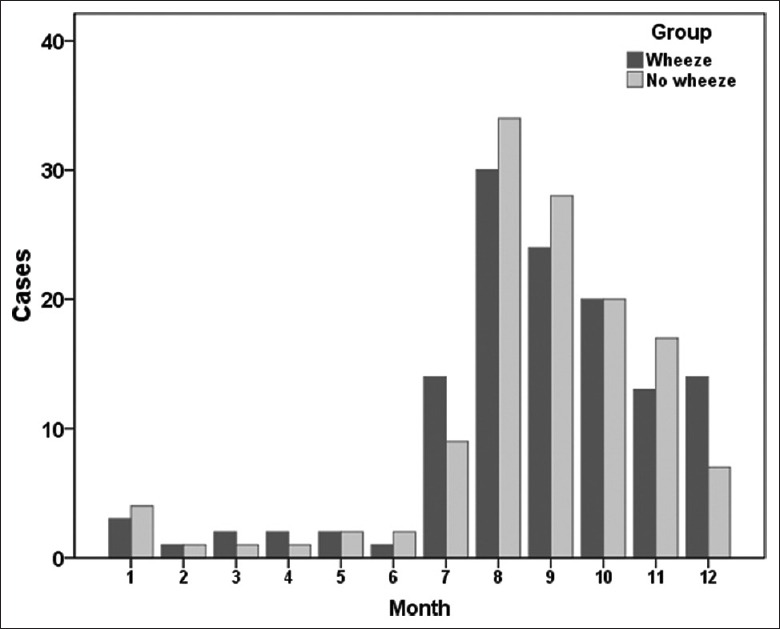
Monthly distribution of the infants with acute lower respiratory tract infection in both wheeze and no wheeze groups.
However, the gestational age was higher in the control group than that of the wheeze group. The infants in the wheeze group more commonly had a family history of allergy than infants in the control group. Compared with the control group, infants with ALRTI (both wheeze and no wheeze groups) had more family members and a higher incidence of underlying cardiopulmonary condition. However, no difference was observed between the wheeze and no wheeze groups.
Lung function
There was no significant difference in age or body size between the three groups. During the acute phase, the sReff and effective airway resistance (Reff) were higher in the wheeze group than the no wheeze and control groups. The FRC in the wheeze group was higher than that in the control group. However, no difference was observed in the FRC made by plethysmography between the three groups. Infants with ALRTI had lower TPTEF/TE, VPTEF/VE, and TEF25 than infants in the control group. In addition, the PTEF level was higher in ALRTI infants compared with the control group. Importantly, the TPTEF/TE and VPTEF/VE levels of infants in the wheeze group were lower than that in the no wheeze group [Table 2].
Table 2.
Pulmonary function measurements for infants with ALRTI during the acute phase
| Items | Wheeze (n = 126) | No wheeze (n = 126) | Control (n = 133) | F/χ2 | P |
|---|---|---|---|---|---|
| Weight (kg)* | 8.95 ± 1.91 | 8.71 ± 1.99 | 9.03 ± 2.25 | 0.820 | NS |
| Length (cm)* | 72.5 ± 7.8 | 72.0 ± 7.3 | 73.6 ± 8.8 | 1.395 | NS |
| Age (months)* | 8.85 ± 4.97 | 8.58 ± 4.80 | 9.66 ± 5.37 | 1.599 | NS |
| VT/kg (ml/kg)* | 7.2 ± 1.4‡,|| | 7.6 ± 1.1 | 7.7 ± 1.1 | 4.889 | 0.008 |
| RR (breaths/min)* | 34.7 ± 9.7‡,|| | 32.3 ± 7.9 | 31.1 ± 6.4 | 4.610 | 0.011 |
| TPTEF/TE (%)* | 20.1 ± 6.4‡,|| | 26.4 ± 8.3§ | 34.4 ± 6.2 | 118.9 | <0.0001 |
| VPTEF/VE (%)* | 22.6 ± 5.3‡,|| | 27.7 ± 6.4§ | 34.3 ± 5.6 | 113.5 | <0.0001 |
| PTEF (ml/s)* | 90.7 ± 26.3‡ | 86.1 ± 28.0§ | 79.3 ± 18.4 | 6.969 | 0.001 |
| TEF25 (ml/s)* | 51.9 ± 14.5‡ | 54.7 ± 14.1§ | 61.3 ± 14.2 | 14.82 | <0.0001 |
| FRCP (ml)† | 170 (127–233)‡ | 165 (126–207) | 154 (108–207) | 6.123 | 0.022 |
| FRCP/kg (ml/kg)† | 18.9 (15.3–24.3) | 18.2 (14.7–21.3) | 17.9 (14.9–20.9) | 3.123 | NS |
| sReff (kPa·s)† | 0.67 (0.41–1.11)‡,|| | 0.51 (0.28–0.86) | 0.45 (0.29–0.72) | 19.11 | <0.0001 |
| Reff (kPa·s/L)† | 3.29 (1.94–5.22)‡,|| | 2.41 (1.42–4.79) | 2.25 (1.42–4.17) | 11.02 | 0.004 |
| Reff/kg† | 0.37 (0.21–0.60)‡ | 0.30 (0.16–0.59) | 0.26 (0.14–0.51) | 7.916 | 0.019 |
*Mean ± SD, demonstrated by one–way ANOVA; †Median (25th–75th percentiles), compared by Kruskal-Wallis H-test or Mann-Whitney U-test; P, differences among all the three groups; ‡Significant differences between wheeze and the control groups; §Significant differences between no wheeze and the control groups; ||Significant differences between wheeze and no wheeze groups. SD: Standard deviation; NS: Not significant; ALRTI: Acute lower respiratory tract infection; sReff: Specific effective airway resistance; Reff: Effective airway resistance; VT: Tidal volume; RR: Respiratory rate; TPTEF/TE: Time–to–peak tidal expiratory flow as a percentage of total expiratory time; VPTEF/VE: Volume–to–peak tidal expiratory flow as a percentage of total expiratory volume; FRCP: Functional residual capacity made by plethysmography.
Lung function tests were repeated 3 months after the ALRTI. No difference was found in the sReff, Reff, and Reff/kg between the two groups. Notably, the infants in the wheeze group still had remarkably lower TPTEF/TE and VPTEF/VE levels than infants in the no wheeze group 3 months later [Table 3]. Moreover, the level of TEF25 was lower in wheezing infants than that of nonwheezing infants.
Table 3.
Comparison of pulmonary function between the wheeze and no wheeze infants 3 months after ALRTI
| Items | Wheeze (n = 53) | No wheeze (n = 50) | t/Z | P |
|---|---|---|---|---|
| VT/kg (ml/kg) | 7.1 ± 1.2 | 7.6 ± 1.1 | -2.681 | 0.007 |
| RR (breaths/min) | 35.5 ± 6.2 | 29.3 ± 6.3 | 0.781 | 0.434 |
| TPTEF/TE (%) | 20.6 ± 6.8 | 27.2 ± 8.5 | -4.069 | <0.001 |
| VPTEF/VE (%) | 23.2 ± 5.1 | 29.0 ± 8.4 | -4.369 | <0.001 |
| PTEF (ml/s) | 96.9 ± 29.9 | 97.0 ± 23.0 | -0.018 | 0.986 |
| TEF25 (ml/s) | 57.6 ± 11.9 | 65.5 ± 17.3 | -2.698 | 0.008 |
| FRCP (ml) | 188 (138–251) | 171 (116–218) | -1.172 | 0.241 |
| FRCP/kg (ml/kg) | 17.8 (12.8–22.6) | 17.3 (14.0–22.6) | -0.330 | 0.741 |
| sReff (kPa·s) | 0.47 (0.30–0.77) | 0.46 (0.30–1.05) | -0.250 | 0.803 |
| Reff (kPa·s/L) | 2.03 (1.22–3.21) | 2.46 (1.48–4.14) | -0.762 | 0.446 |
| Reff/kg | 0.20 (0.11–0.38) | 0.27 (0.14–0.39) | -0.867 | 0.386 |
Data were presented as mean ± SD or Median (25th–75th percentiles). ALRTI: Acute lower respiratory tract infection; TEF25: Expiratory flow at 25% tidal volume; PTEF: Peak tidal expiratory flow; VPTEF/VE: Volume-to-peak tidal expiratory flow as a percentage of total expiratory volume; TPTEF/TE: Time-to-peak tidal expiratory flow as a percentage of total expiratory time; VT: Tidal volume; RR: Respiratory rate; FRCP: Functional residual capacity made by plethysmography; sReff: Specific effective airway resistance; Reff: Effective airway resistance.
One-year respiratory outcomes
As expected, infants in the wheeze group had higher recurrence of wheezing (49.2% vs. 25.4%, P < 0.0001) and more wheezing exacerbations (1.73 ± 0.77/years vs. 1.38 ± 0.70/years, P = 0.031) than the infants in the no wheeze group. Interestingly, we found that infants in the wheeze group showed a higher prevalence of eczema (36.5% vs. 15.8%, P < 0.0001) than nonwheezing infants. However, no significant difference was found in allergic rhinitis between the two groups.
No difference was observed in either recurrence or readmission of respiratory infections such as bronchitis and pneumonia between the wheeze and no wheeze groups.
Association between lung function and respiratory outcomes
We found a significant inverse relationship between TPTEF/TE, VPTEF/VE, and the recurrence of wheezing. Moreover, significant inverse relationships were also observed between the levels of TPTEF/TE, VPTEF/VE, and recurrence of pneumonia [Table 4]. We also found an inverse correlation between the TPTEF/TE level and the allergy and bronchitis recurrence [Table 4]. However, there was no relationship between PTEF, TEF25, and the recurrence of wheezing and lower respiratory tract infection. We also did not observe a relationship between the FRC and respiratory outcomes.
Table 4.
Relationship between lung function and respiratory outcome
| Index | Wheezing recurrence | Pneumonia recurrence | Allergy disease | Bronchitis recurrence | ||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| sReff | 0.014 | 0.914 | 0.148 | 0.251 | 0.024 | 0.857 | 0.039 | 0.765 |
| Reff | 0.085 | 0.513 | 0.245 | 0.055 | −0.014 | 0.914 | 0.078 | 0.550 |
| Reff/kg | 0.050 | 0.700 | 0.281 | 0.027 | −0.077 | 0.554 | 0.098 | 0.450 |
| TPTEF/TE | −0.442 | 0.002 | −0.342 | 0.032 | −0.237 | 0.036 | −0.239 | 0.035 |
| VPTEF/VE | −0.368 | 0.018 | −0.230 | 0.042 | −0.168 | 0.139 | −0.184 | 0.107 |
| PTEF | 0.137 | 0.230 | −0.051 | 0.654 | 0.343 | 0.002 | 0.082 | 0.474 |
| TEF25 | 0.152 | 0.183 | −0.058 | 0.610 | 0.354 | 0.001 | 0.073 | 0.527 |
TEF25: Expiratory flow at 25% tidal volume; PTEF: Peak tidal expiratory flow; VPTEF/VE: Volume–to–peak tidal expiratory flow as a percentage of total expiratory volume; TPTEF/TE: Time-to-peak tidal expiratory flow as a percentage of total expiratory time; sReff: Specific effective airway resistance; Reff: Effective airway resistance.
Discussion
In this study, we found that wheezing infants had lower lung function than infants who were not wheezing, and the function was still decreased 3 months after the acute infection. Moreover, the TPTEF/TE and VPTEF/VE were negatively associated with later respiratory outcome.
We observed that wheezing infants with lower respiratory tract infection have reduced lung function compared with control children and nonwheezing infants. Wheezing infants had increased sReff, Reff, and FRC than nonwheezing infants. Moreover, they had obvious deficits such as VT/kg, TPTEF/TE, and TEF25. The damage of plethysmographic indices was recovered 3 months later. However, the deficit in TPTEF/TE and VPTEF/VE still existed after recovery. These results suggested underlying air trapping and peripheral airway obstruction in the wheezing infants.[22] They also indicated that children with wheezing lower respiratory illness might have premorbid abnormal lung function.[14] Several recent studies have demonstrated that the lung function deficit may have already been present at a much younger age.[11,12,13] Thus, the reduced lung function level in wheezing infants may reflect an underlying mechanism that preceded lung function deficit due to some characteristics of the infants and might predispose them to wheezing after infections.
The infants with wheezing were at a risk of subsequent persistent wheezing as reported previously.[7] It has been suggested that a predisposition to allergies is a risk factor for wheezing. Eczema has been documented associated with the wheezing and asthma,[23] and eczema during the 1st year of life was associated with persistent wheezing.[1] Consistent with the previous studies, we found that wheezing infants had a higher incidence of eczema. No correlation was found between wheezing and allergic rhinitis in this study. Grad and Morgan have reported that early-onset rhinitis was associated with late-onset wheezing.[24] Our patients were infants <2 years old, the phenotype of wheezing was different from that. Moreover, as allergic rhinitis is rare in young children, the association between wheezing and rhinitis should be evaluated in further long-term follow-up.
We found that the level of TPTEF/TE and VPTEF/VE was associated with the occurrence of wheezing and respiratory disease during the following year. Previously, studies have also shown that the level of TPTEF/TE was associated with subsequent wheezing.[1,25] One possible explanation could be that the decreased level of TPTEF/TE might reflect reduced airway size, and the early wheezing is most likely to be associated with reduced airway caliber. Moreover, the Reff is associated with the occurrence of pneumonia. Van Putte-Katier et al. showed that a higher resistance was associated with an increased risk of coughing and wheezing.[11] There are two possible mechanisms that may contribute to this relationship. First, the abnormal TPTEF/TE and Reff reflect the reduced airway caliber, which may be contributed to the predisposition to airway spasm and respiratory tract infection. Second, many pneumonia cases in infants are due to viral infections, and the two conditions may be due to a common infective cause.[26,27] We observed an association between PTEF, TEF25, and the occurrence of allergy diseases unexpectedly. The exact mechanism under this remains unclear. However, monitoring the lung function of symptomatic children early during a respiratory tract infection may enable physicians to identify children at risk for persistent disease and may be an important factor in modifying outcomes later in life.
There are some limitations in our study. As the study was performed in the outpatient department, the data on clinical treatment were not analyzed due to the difficulty in collecting complete patient record. However, since all the infants were treated by the same trained pediatric respiration team under the same guideline, it was a big issue that only about 50% of the infants complete the lung function 3 months later. The major reason for this is that many infants were living in the country of other provinces and towns and passed the follow-up of lung function at recovery phase. However, the severity of disease was comparable and there was no difference in the baseline characters between the two groups.
In conclusion, wheezing infants with respiratory infections had obvious deficits in lung function and delayed improvement during the asymptomatic stage. Moreover, the levels of TPTEF/TE and VPTEF/VE were associated with recurrent wheezing and pneumonia. Measuring lung function in symptomatic children may enable targeting of children who are most likely to benefit from treatment intervention and monitoring. Future studies should focus on elucidating the mechanism of wheezing-associated loss of lung function, and the clinical use of lung function to estimate respiratory disease outcomes in wheezing infants.
Financial support and sponsorship
The work was supported by a grant from the Shanghai Committee of Science and Technology, China (No. 134119a4200).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
References
- 1.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 2.Kuehni CE, Davis A, Brooke AM, Silverman M. Are all wheezing disorders in very young (preschool) children increasing in prevalence? Lancet. 2001;357:1821–5. doi: 10.1016/S0140-6736(00)04958-8. doi: 10.1016/S0140-6736(00)04958-8. [DOI] [PubMed] [Google Scholar]
- 3.Lowe LA, Simpson A, Woodcock A, Morris J, Murray CS, Custovic A NAC Manchester Asthma and Allergy Study Group. Wheeze phenotypes and lung function in preschool children. Am J Respir Crit Care Med. 2005;171:231–7. doi: 10.1164/rccm.200406-695OC. doi: 10.1164/rccm.200406-695OC. [DOI] [PubMed] [Google Scholar]
- 4.Ducharme FM, Tse SM, Chauhan B. Diagnosis, management, and prognosis of preschool wheeze. Lancet. 2014;383:1593–604. doi: 10.1016/S0140-6736(14)60615-2. doi: 10.1016/S0140-6736(14)60615-2. [DOI] [PubMed] [Google Scholar]
- 5.Bessa OA, Leite ÁJ, SoléD, Mallol J. Prevalence and risk factors associated with wheezing in the first year of life. J Pediatr (Rio J) 2014;90:190–6. doi: 10.1016/j.jped.2013.08.007. doi: 10.1016/j.jped.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Piippo-Savolainen E, Korppi M. Wheezy babies – Wheezy adults?Review on long-term outcome until adulthood after early childhood wheezing. Acta Paediatr. 2008;97:5–11. doi: 10.1111/j.1651-2227.2007.00558.x. doi: 10.1111/j.1651-2227.2007.00558.x. [DOI] [PubMed] [Google Scholar]
- 7.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–5. doi: 10.1016/S0140-6736(98)10321-5. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 8.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–72. doi: 10.1164/rccm.200802-309OC. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner SW, Palmer LJ, Rye PJ, Gibson NA, Judge PK, Young S, et al. Infants with flow limitation at 4 weeks: Outcome at 6 and 11 years. Am J Respir Crit Care Med. 2002;165:1294–8. doi: 10.1164/rccm.200110-018OC. doi: 10.1164/rccm.200110-018OC. [DOI] [PubMed] [Google Scholar]
- 10.Palmer LJ, Rye PJ, Gibson NA, Burton PR, Landau LI, Lesouëf PN. Airway responsiveness in early infancy predicts asthma, lung function, and respiratory symptoms by school age. Am J Respir Crit Care Med. 2001;163:37–42. doi: 10.1164/ajrccm.163.1.2005013. doi: 10.1164/ajrccm.163.1.2005013. [DOI] [PubMed] [Google Scholar]
- 11.van Putte-Katier N, van der Gugten AC, Uiterwaal CS, de Jong BM, Numans ME, Kimpen JL, et al. Early life lung function and respiratory outcome in the first year of life. Eur Respir J. 2012;40:198–205. doi: 10.1183/09031936.00175910. doi: 10.1183/09031936.00175910. [DOI] [PubMed] [Google Scholar]
- 12.van der Gugten AC, Uiterwaal CS, van Putte-Katier N, Koopman M, Verheij TJ, van der Ent CK. Reduced neonatal lung function and wheezing illnesses during the first 5 years of life. Eur Respir J. 2013;42:107–15. doi: 10.1183/09031936.00214711. doi: 10.1183/09031936.00214711. [DOI] [PubMed] [Google Scholar]
- 13.Pike KC, Rose-Zerilli MJ, Osvald EC, Inskip HM, Godfrey KM, Crozier SR, et al. The relationship between infant lung function and the risk of wheeze in the preschool years. Pediatr Pulmonol. 2011;46:75–82. doi: 10.1002/ppul.21327. doi: 10.1002/ppul.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castro-Rodríguez JA, Holberg CJ, Morgan WJ, Wright AL, Halonen M, Taussig LM, et al. Relation of two different subtypes of croup before age three to wheezing, atopy, and pulmonary function during childhood: A prospective study. Pediatrics. 2001;107:512–8. doi: 10.1542/peds.107.3.512. doi: 10.1542/peds.107.3.512. [DOI] [PubMed] [Google Scholar]
- 15.Henderson J, Granell R, Heron J, Sherriff A, Simpson A, Woodcock A, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax. 2008;63:974–80. doi: 10.1136/thx.2007.093187. doi: 10.1136/thx.2007.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klig JE. Office pediatrics: Current perspectives on the outpatient evaluation and management of lower respiratory infections in children. Curr Opin Pediatr. 2006;18:71–6. doi: 10.1097/01.mpo.0000192520.48411.fa. doi: 10.1097/01.mpo.0000192520.48411.fa. [DOI] [PubMed] [Google Scholar]
- 17.Frey U, Stocks J, Sly P, Bates J. Specification for signal processing and data handling used for infant pulmonary function testing. ERS/ATS task force on standards for infant respiratory function testing. European Respiratory Society/American Thoracic Society. Eur Respir J. 2000;16:1016–22. doi: 10.1183/09031936.00.16510160. [DOI] [PubMed] [Google Scholar]
- 18.Frey U, Stocks J, Coates A, Sly P, Bates J. Specifications for equipment used for infant pulmonary function testing. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. European Respiratory Society/American Thoracic Society. Eur Respir J. 2000;16:731–40. doi: 10.1034/j.1399-3003.2000.16d28.x. [DOI] [PubMed] [Google Scholar]
- 19.Reinmann B, Stocks J, Frey U. Assessment of an infant whole-body plethysmograph using an infant lung function model. Eur Respir J. 2001;17:765–72. doi: 10.1183/09031936.01.17407650. [DOI] [PubMed] [Google Scholar]
- 20.Hülskamp G, Hoo AF, Ljungberg H, Lum S, Pillow JJ, Stocks J. Progressive decline in plethysmographic lung volumes in infants: Physiology or technology? Am J Respir Crit Care Med. 2003;168:1003–9. doi: 10.1164/rccm.200303-460OC. doi: 10.1164/rccm.200303-460OC. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen TT, Hoo AF, Lum S, Wade A, Thia LP, Stocks J. New reference equations to improve interpretation of infant lung function. Pediatr Pulmonol. 2013;48:370–80. doi: 10.1002/ppul.22656. doi: 10.1002/ppul.22656. [DOI] [PubMed] [Google Scholar]
- 22.Borrego LM, Stocks J, Leiria-Pinto P, Peralta I, Romeira AM, Neuparth N, et al. Lung function and clinical risk factors for asthma in infants and young children with recurrent wheeze. Thorax. 2009;64:203–9. doi: 10.1136/thx.2008.099903. doi: 10.1136/thx.2008.099903. [DOI] [PubMed] [Google Scholar]
- 23.Belgrave DC, Simpson A, Semic-Jusufagic A, Murray CS, Buchan I, Pickles A, et al. Joint modeling of parentally reported and physician-confirmed wheeze identifies children with persistent troublesome wheezing. J Allergy Clin Immunol. 2013;132:575–83. doi: 10.1016/j.jaci.2013.05.041. doi: 10.1016/j.jaci.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 24.Grad R, Morgan WJ. Long-term outcomes of early-onset wheeze and asthma. J Allergy Clin Immunol. 2012;130:299–307. doi: 10.1016/j.jaci.2012.05.022. doi: 10.1016/j.jaci.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zedan M, Nasef N, El-Bayoumy M, El-Assmy M, Attia G, Zedan M, et al. Does decline of lung function in wheezy infants justify the early start of controller medications? Indian J Pediatr. 2012;79:1176–80. doi: 10.1007/s12098-012-0694-z. doi: 10.1007/s12098-012-0694-z. [DOI] [PubMed] [Google Scholar]
- 26.Juvén T, Mertsola J, Waris M, Leinonen M, Meurman O, Roivainen M, et al. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J. 2000;19:293–8. doi: 10.1097/00006454-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 27.McIntosh K. Community-acquired pneumonia in children. N Engl J Med. 2002;346:429–37. doi: 10.1056/NEJMra011994. doi: 10.1056/NEJMra011994. [DOI] [PubMed] [Google Scholar]