Abstract
Background:
Recent studies show that microRNA-145 (miRNA-145) might be an attractive tumor biomarker of considerable prognostic value, but little is known about their relationship with acute myocardial infarction (AMI). This study investigated the correlation between the level of miR-145 and AMI.
Methods:
One-hundred patients were divided into three groups: no coronary artery disease (CAD) group, non-ST segment elevation myocardial infarction group, and ST segment elevation myocardial infarction group. The plasma levels of miR-145 were quantified using real-time quantitative polymerase chain reaction. Logarithmic transformation of miRNA-145 levels (Ln_miRNA-145) was used for statistical analysis due to the skewed data distribution.
Results:
Plasma levels of miR-145 were significantly lower in patients with AMI compared to patients in the non-CAD group (−6.38 ± 0.11 vs. −4.47 ± 0.17, P < 0.0001). Compared to those without heart failure, the levels of miR-145 were significantly lower in patients with heart failure (−6.91 ± 0.20 vs. −5.35 ± 0.13, P < 0.0001). We also found that the lower plasma levels of miRNA-145 significantly correlated with increased serum levels of B-type natriuretic peptide (Spearman ρ= −0.60, P < 0.0001), troponin T (Spearman ρ= −0.62, P < 0.0001), and decreased ejection fraction (Spearman ρ= 0.65, P < 0.0001). In a multivariable linear regression analysis, AMI and heart failure were independently associated with lower Ln_miRNA-145 (estimate −0.99, standard error [SE] 0.28; P = 0.001 and estimate −0.62, SE 0.21; P = 0.004).
Conclusions:
Our results suggest that decreased plasma levels of miR-145 are associated with AMI. Circulating miR-145 may be useful in prognosticating cardiac function and the risk of developing heart failure.
Keywords: Acute Myocardial Infarction, Heart Failure, MicroRNA, MicroRNA-145
Introduction
Acute myocardial infarction (AMI) is one of the leading causes of death in the world.[1,2] Over the past two decades, much progress has been made in the diagnosis, treatment, and prognosis of AMI. Cardiac troponins are the most common biomarkers used for the diagnosis of AMI in clinical practice.[3,4] However, it is important to continue the search for novel biomarkers that offer higher sensitivity and specificity for the early diagnosis of AMI and to provide more information that accurately reflects the severity of disease. AMI is associated with the death of cardiac myocytes as well as other cell types such as smooth muscle and endothelial cells and results in the activation of various inflammatory cells and platelets. Therefore, myocardial infarction not only results in the release of cardiac proteins such as the biomarker troponin but also induces the release of cardiac myocyte microRNAs (miRNAs).[5,6] MiRNAs released from cardiac and noncardiac cells could serve as potential target biomarkers that provide meaningful information for the diagnosis of AMI.
MiRNAs are small noncoding RNA molecules that negatively regulate gene expression by triggering either translation repression or RNA degradation.[1,2,7,8] MiRNAs have been shown to be associated with various physiological and pathological conditions, including inflammation and cardiovascular disease.[9,10] Previous studies demonstrated that miRNAs can be detected in the peripheral circulation and are present in a remarkably stable form. The miRNAs in plasma and serum have been considered as promising novel biomarkers for the diagnosis and prognosis of cardiovascular diseases, particularly coronary artery disease (CAD).[11,12] MiRNA-145 is a mediator in the regulation of the proliferation and differentiation of vascular smooth muscle cells (VSMCs), in which it is highly expressed, and is the most abundant miRNA in healthy arterial walls.[13,14]
VSMCs play an important role in the stability of atherosclerotic plaques and mechanisms of plaque rupture. In addition, miRNA-145 regulates VSMC function and extracellular matrix synthesis and thus, in turn, influences plaque stability. Although the expression of different circulating miRNAs among CAD patients has been reported,[15] the functions of miRNA-145 in cardiovascular disease remain unknown. In the current study, we investigated the relationship between miRNA-145 levels and the diagnosis of AMI and evaluate the potential utility of plasma miRNA-145 as a clinical biomarker to determine the diagnosis and prognosis of AMI.
Methods
Subjects
A total of 100 patients who underwent coronary angiography for the evaluation of chest pain were recruited among inpatients admitted to the Beijing Anzhen Hospital of Capital Medical University, China. CAD was defined as angiographic evidence of at least one segment of a main coronary with more than 50% luminal narrowing.[16] Two independent cardiologists who had no prior knowledge of the clinical history of the patients included in this study reviewed each angiogram. A diagnosis of ST segment elevation myocardial infarction (STEMI) was made using electrocardiographic criteria while non-STEMI (NSTEMI) was diagnosed on the basis of elevated serum troponin levels in addition to clinical symptoms consistent with cardiac ischemia. Non-AMI was characterized as the absence of elevated serum troponin levels and the absence of ST segment elevation on electrocardiograph. The criteria for diagnosing heart failure related to AMI was in keeping the American College of Cardiology/American Heart Association guidelines and reflected the clinical judgment of two experienced independent cardiologists.
Patients with a history of hepatitis, hepatic failure, end-stage renal failure, cardiomyopathy, congenital heart disease, bleeding disorders, previous thoracic irradiation therapy, or malignant disease were excluded from the study. This study was approved by the Beijing Anzhen Hospital Ethics Committee of Capital Medical University, and informed consent was obtained from each participant.
Sample collection and storage
Blood samples were collected in ethylenediaminetetraacetic acid (EDTA) containing tubes (BD, Franklin Lakes, NJ, USA). Plasma was isolated within 1 h using centrifugation at 1900 ×g for 10 min at 4°C to remove blood cells, and then at 16,000 ×g for 10 min at 4°C to remove additional cellular nucleic acid attached to cell debris. Plasma samples were transferred to RNase/DNase-free tubes and stored at −80°C.
RNA isolation and preparation
Total RNA in plasma was isolated using miRNeasy Plasma Kit (QIAGEN, Hilden, Germany) following the manufacturer's instructions. In brief, 200 μl of EDTA-containing plasma was mixed thoroughly with 1000 μl QIAzol in an RNase-free tube and incubated for 5 min at room temperature (15°C–25°C). To normalize for the plasma miRNA content, 3.5 μl (1.6 × 108 copies/μl) Caenorhabditis elegans miRNA-39 (cel-miR-39) was added to the samples, following the addition of QIAzol.[17] After thorough mixing, 200 μl of chloroform was added. Following vigorous vortex mix for 15 s, the tube containing the lysate was placed at room temperature for 2–3 min and then centrifuged for 15 min at 12,000 ×g at 4°C. The aqueous phase containing the RNA was then carefully removed.
Quantification of microRNA-145
Total RNA was polyadenylated and reverse transcribed using the miScript Reverse Transcription kit (QIAGEN) as per the manufacturer's instructions, using 100–200 ng of the total RNA per reaction. The obtained cDNA (2.5 μl of a 1/100 dilution) was then amplified with real-time polymerase chain reaction (PCR) using the miScript SYBR Green PCR kit (QIAGEN) in 25 μl reaction mixtures consisting of 12.5 μl Quantitect SYBR Green PCR master mix and 2.5 μl miRNA-145 primer with miScript universal primer. The reactions were incubated at 95°C for 15 min, followed by 45 cycles of 94°C for 15 s, 55°C for 30 s, and 70°C for 30 s. The relative level of expression of miR-145 was computed using the comparative computed tomography (CT) method. MiRNA expression was normalized to cel-miR-39. MiRNA levels are expressed as 2-CT (miRNA)/normalized cel-miR-39.[16]
Statistical analysis
Normality of distribution was assessed using the Kolmogorov–Smirnov test. Logarithmic transformation was performed to normalize plasma miR-145 values (Ln_miRNA-145).
Comparisons between two groups were performed using Fisher's exact test for categorical variables and Student's t-test or Mann–Whitney U-test as appropriate for continuous variables. For comparisons between more than two groups, one-way analysis of variance and Tukey–Kramer honestly significant difference tests were used. Pearson Chi-square test and Spearman rho test were used to compare qualitative and quantitative variables as appropriate. The correlation between plasma levels of miRNAs and other variables was assessed using Pearson's correlation coefficient for symmetrically distributed variables and Spearman's correlation coefficient for skewed variables. Univariate and multivariate linear regression analyses were performed to determine the association between clinical variables and Ln_miRNA-145. All tests were two-sided, and P < 0.05 was considered statistically significant. All statistical analyses were performed using JMP 10 software (SAS Institute, Inc., Cary, NC, USA).
Results
Basic clinical characteristics of the study participants
Table 1 shows the baseline clinical characteristics of the study population. The mean age was 58.0 ± 12.1 years and males made up 65% of the total number of the study participants. The prevalence of traditional CAD risk factors, such as hypertension, diabetes mellitus, dyslipidemia, and smoking, was 66%, 27%, and 75%, respectively. Mean Ln_miRNA-145 in the whole population was −5.7 ± 1.3.
Table 1.
Baseline characteristics in patients who underwent coronary angiography (n = 100)
| Characteristics | Data |
|---|---|
| Age (years) | 58.0 ± 12.0 |
| Gender, n (%) | |
| Male | 65 (65) |
| BMI (kg/m2) | 26.0 ± 3.5 |
| Hypertension, n (%) | 66 (66) |
| Diabetes, n (%) | 27 (27) |
| Dyslipidemia, n (%) | 75 (75) |
| Smoking, n (%) | 40 (40) |
| Blood glucose (mmol/L) | 6.0 ± 2.4 |
| Triglycerides (mmol/L) | 1.8 ± 1.2 |
| Total cholesterol (mmol/L) | 4.3 ± 1.0 |
| Low–density lipoprotein (mmol/L) | 2.7 ± 0.9 |
| High–density lipoprotein (mmol/L) | 1.00 ± 0.24 |
| hsCRP (mg/L) | 7.4 ± 3.7 |
| Serum creatinine (μmol/L) | 74.7 ± 15.0 |
| EF (%) | 57 ± 11 |
| LVEDD (mm) | 49.2 ± 6.0 |
| BNP (pg/ml) | 239.2 ± 23.4 |
| Troponin T (ng/ml) | 26.0 ± 2.4 |
| Ln_miRNA–145 | −5.7 ± 1.3 |
Continuous data are expressed as mean ± SD; categorical data are expressed as frequencies. hsCRP: High–sensitivity C–reactive protein; Ln_miRNA–145: Logarithmic_miRNA–145; LVEDD: Left ventricular end diastolic diameter; BMI: Body mass index; BNP: Brain natriuretic peptide; SD: Standard deviation; EF: Ejection fraction.
Correlation between plasma microRNA-145 levels and traditional risk factors for coronary artery disease
Table 2 demonstrates the univariate linear regression analysis for the association between clinical variables and Ln_miRNA-145. Diabetes mellitus was significantly associated with lower Ln_miRNA-145 (P = 0.03). Smoking had a borderline significant association with lower Ln_miRNA-145 (P = 0.09).
Table 2.
Univariate linear regression analysis for plasma levels of Ln_miRNA–145
| Variable | Estimate | SE | P |
|---|---|---|---|
| Age | 0.01 | 0.01 | 0.38 |
| Female | 0.41 | 0.28 | 0.15 |
| BMI | 0.06 | 0.04 | 0.15 |
| Hypertension | −0.33 | 0.26 | 0.23 |
| Diabetes | −0.67 | 0.30 | 0.03 |
| Dyslipidemia | −0.05 | 0.29 | 0.11 |
| Smoking | −0.46 | 0.27 | 0.09 |
Data are expressed as parameter estimates with SEs. All continuous variables were standardized to a mean of 0 and a SD of 1; all dichotomous variables were coded as 0 = presence and 1 = absence. SEs: Standard errors; SD: Standard deviation; BMI: Body mass index; Ln_miRNA–145: Logarithmic_miRNA–145.
Correlation between plasma levels of microRNA-145 and vessel disease number
To further evaluate the relationship between plasma miRNA-145 levels and AMI, we assessed the association between the severity of CAD with plasma miRNA-145 levels, by performing a subanalysis among the AMI population (n = 69). Using coronary angiography, 15 (22%) AMI patients had single-vessel disease, 29 (42%) had double-vessel disease, and 25 (36%) had triple-vessel disease. The levels of miRNA-145 in patients with three-vessel disease were significantly lower than those with one- or two-vessel disease (Ln_miRNA-145 −7.09 ± 1.01 vs. −5.68 ± 1.03; P < 0.0001 and −7.09 ± 1.01 vs. −6.14 ± 0.40; P < 0.0001, respectively); however, levels between patients with one- and two-vessel disease were not significantly different (P = 0.09) [Figure 1]. In univariate and multivariate linear regression analyses, the number of diseased vessels was significantly associated with lower Ln_miRNA-145 (Estimate, −0.70, standard error [SE], 0.09; P < 0.0001) [Table 3].
Figure 1.
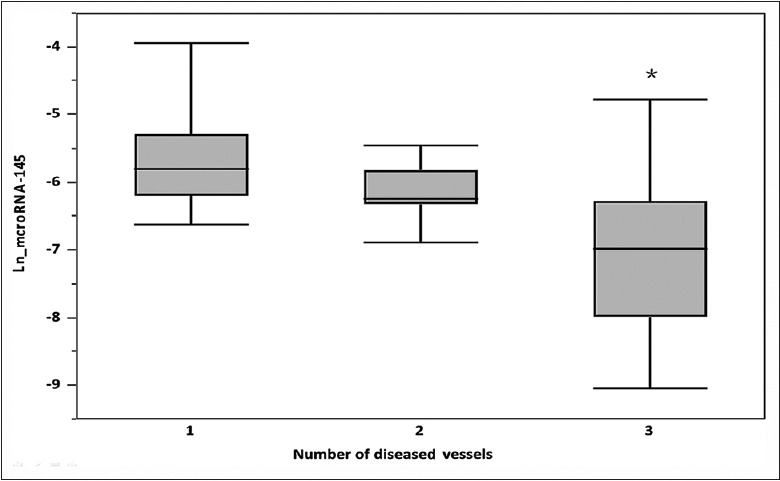
Association between plasma miRNA-145 levels and number of diseased vessels. Bars represent average plasma miRNA-145 levels among patients with single-, double-, and triple-vessel CAD. T-bars indicate standard deviation. *Statistically different versus other groups. MiRNA-145: MicroRNA-145; CAD: Coronary artery disease; Ln: Logarithmic.
Table 3.
Univariate linear regression analysis for plasma levels of Ln_miRNA–145
| Variables | Univariate | Adjusted for traditional risk factors | ||||
|---|---|---|---|---|---|---|
| Estimate | SE | P | Estimate | SE | P | |
| AMI | −1.91 | 0.20 | <0.001 | −0.99 | 0.28 | 0.001 |
| Troponin T | −0.04 | 0.00 | <0.001 | −0.01 | 0.01 | 0.002 |
| BNP | −0.00 | 0.00 | <0.001 | −0.00 | 0.00 | 0.003 |
| EF | 0.08 | 0.01 | <0.001 | 0.05 | 0.01 | <0.0001 |
| Number of diseased vessels | −0.86 | 0.07 | <0.0001 | −0.70 | 0.09 | <0.0001 |
| HF | −1.56 | 0.24 | <0.0001 | −0.62 | 0.21 | 0.004 |
Data are expressed as parameter estimates with SEs. All continuous variables were standardized to a mean of 0 and a SD of 1; all dichotomous variables were coded as 0 = presence and 1 = absence. Traditional risk factors include age, male gender, BMI, diabetes, hypertension, dyslipidemia, and smoking. AMI: Acute myocardial infarction; EF: Ejection fraction; HF: Heart failure; SEs: Standard errors; BNP: Brain natriuretic peptide; SD: Standard deviation; Ln_miRNA–145: Logarithmic_miRNA–145; BMI: Body mass index.
Circulating cardiac-associated microRNA-145 levels were decreased in acute myocardial infarction patients
Levels of plasma miRNA-145 were lower in patients with AMI, particularly those who had sustained a STEMI. Figure 2 demonstrates the relative differences in Ln_miRNA-145 levels between patients with non-CAD (n = 30), NSTEMI (n = 40), and STEMI (n = 30). Patients with AMI had significantly lower Ln_miRNA-145 levels compared to those with non-CAD, suggesting an altered expression of miRNA-145 in patients with AMI (all P < 0.0001). In multivariate linear regression analysis, AMI and heart failure was associated with significantly lower Ln_miRNA-145 levels after the adjustment for traditional CAD risk factors (Estimate, −0.99; SE, 0.28; P = 0.006 and Estimate, −0.62; SE, 0.21; P = 0.004, respectively) [Table 3].
Figure 2.
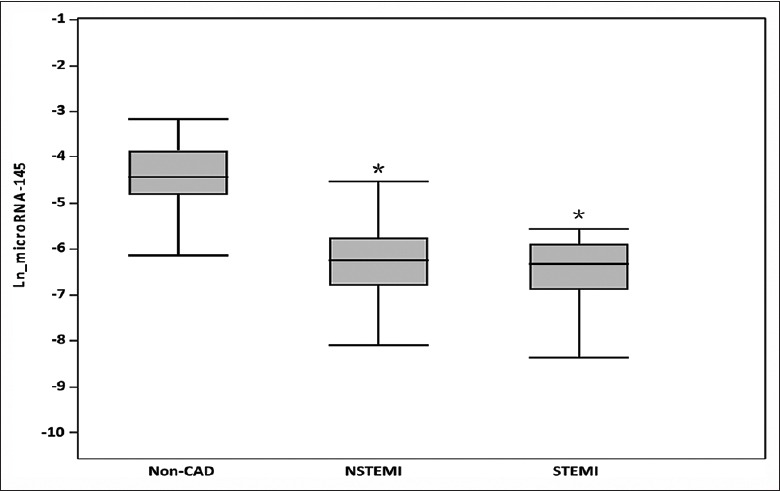
Plasma miRNA-145 levels and clinical presentation. Bars represent averages log miRNA-145 levels among different clinical presentations of acute myocardial infarction compared with non-CAD patients. T-bars indicate standard deviation. *Statistically different versus non-CAD. MiRNA-145: MicroRNA-145; CAD: Coronary artery disease; NSTEMI: Non-ST segment elevation myocardial infarction; STEMI: ST segment elevation myocardial infarction; Ln: Logarithmic.
Correlation between microRNA-145 and troponin levels
Cardiac troponins are considered as the gold standard among biomarkers used for the diagnosis of myocardial infarction. In this study, we found that levels of miRNA-145 correlated with infarct size as determined by troponin T release. These findings indicate that single miRNAs and especially miRNA signatures derived from the peripheral blood could serve as valuable biomarkers for AMI. We found that peak levels of miR-145 correlated with peak levels of troponin I (Spearman ρ = −0.62, P < 0.0001) [Figure 4], suggesting that they may be used as markers of acute cardiac myocyte cell death.
Figure 4.
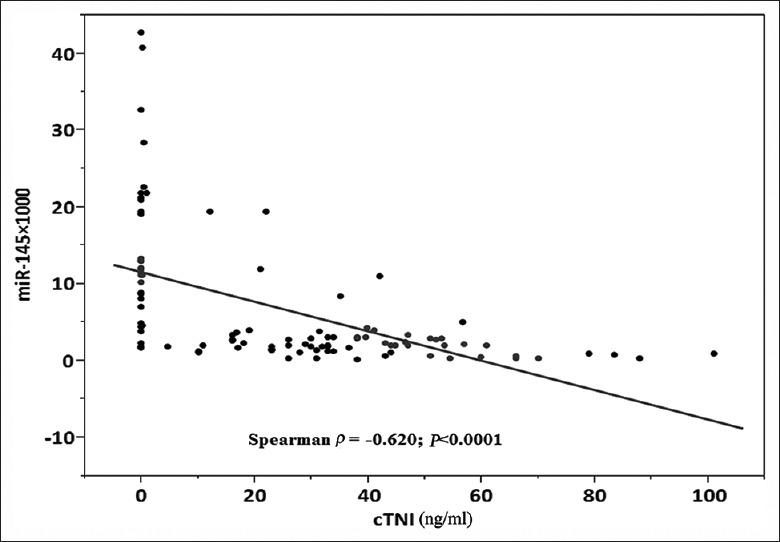
Correlation between cTNI levels and plasma miRNA-145. Scatter diagram demonstrating the correlation between miRNA-145 and cTNI levels (n = 100). MiRNA-145: MicroRNA-145; cTnI: Cardiac troponin I; miRNA-145: MicroRNA-145.
Correlation between levels of microRNA-145 and cardiac function
We show a correlation between the circulating miRNA levels and ejection fraction (EF) determined at echocardiography among patients following myocardial infarction. The peak troponin I levels correlated significantly with the EF (P < 0.0001, r2 = 0.52) [Figure 3]. We found negative correlations between the EF and peak miRNA-145 at day 1 post-AMI (Spearman ρ = 0.65, P < 0.0001) [Figure 5]. The peak amount of miRNA-145 in the blood correlated negatively with the EF. Patients with HF had significantly lower levels of Ln_miRNA-145 compared with patients without HF (P < 0.0001, Figure 6).
Figure 3.
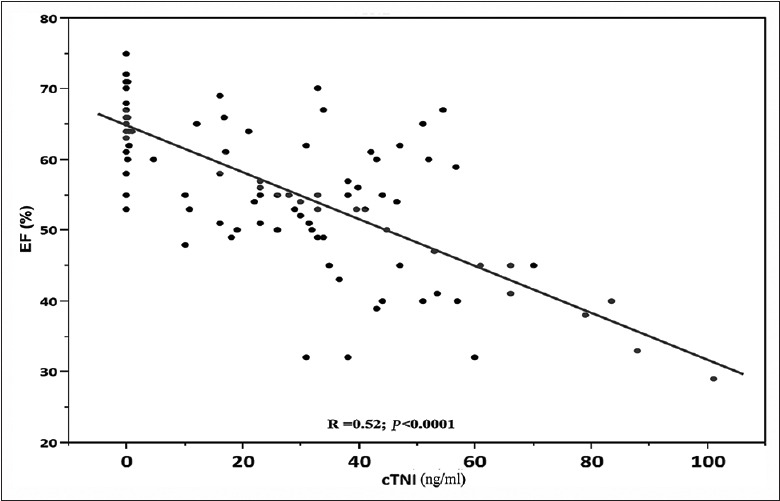
Correlation between cTNI levels and EF. Scatter diagram demonstrating the correlation between miRNA-145 levels and ejection fraction (n = 100). MiRNA-145: MicroRNA-145; cTnI: Cardiac troponin I; EF: Ejection fraction.
Figure 5.
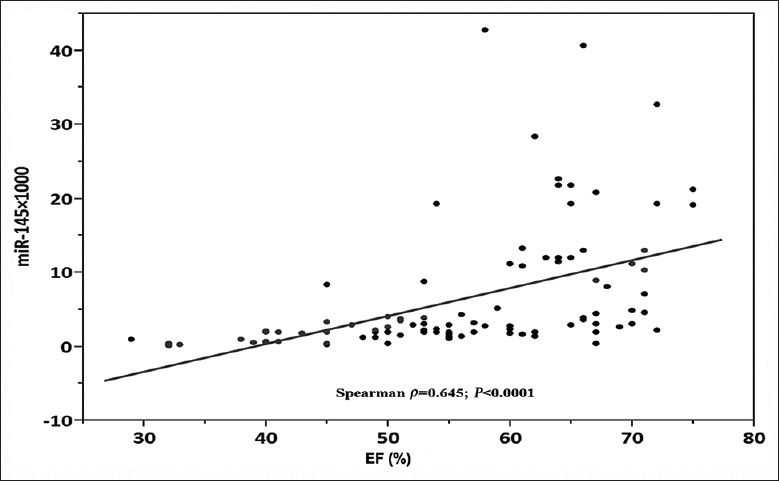
Correlation between EF and plasma miRNA-145. Scatter diagram demonstrating the correlation between miRNA-145 levels and EF (n = 100). EF: Ejection fraction; miRNA-145: MicroRNA-145.
Figure 6.
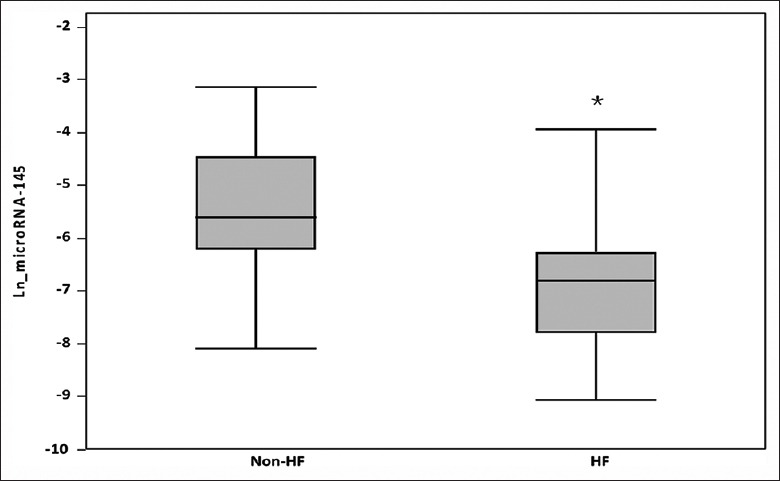
Plasma miRNA-145 levels and clinical presentation of HF. Bars represent average log miRNA-145 levels among patients with and without HF. T-bars indicate standard deviation. *Statistically different versus non-HF. HF: Heart failure; Ln: Logarithmic; miRNA-145: MicroRNA-145.
Correlation between levels of microRNA-145 and brain natriuretic peptide
The correlation between plasma brain natriuretic peptide (BNP) and miRNA-145 levels was also examined. Plasma BNP levels among patients with heart failure were significantly higher than those of patients without heart failure (336.8 ± 3.2 vs. 72.5 ± 18.0 pg/ml, P < 0.03). Moreover, plasma miRNA-145 levels were strongly correlated with plasma BNP concentration (Spearman ρ = −0.60, P < 0.0001) [Figure 7].
Figure 7.
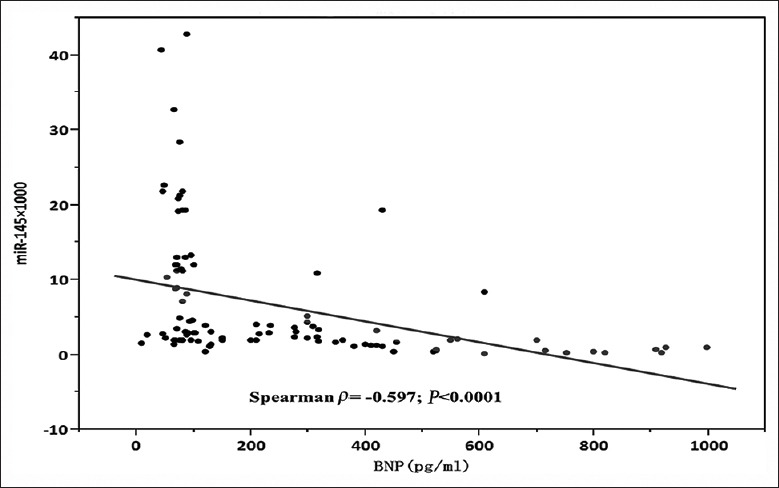
Correlation between BNP and plasma miRNA-145. Scatter diagram demonstrates the correlation between levels of miRNA-145 and BNP (n = 100). BNP: Brain natriuretic peptide; miRNA-145: MicroRNA-145.
Discussion
Although accumulating evidence suggests that miRNAs are important in cardiovascular disease, little is known about the relationship between levels of circulating miRNA-145 and the presence of AMI. MiRNA-145 has been shown to regulate coronary atherosclerosis.[13,14]
Cheng et al.[14] found that miRNA-145 is selectively expressed in VSMCs and is the most abundant miRNA in normal vascular walls. MiR-145 regulates the structure and function of VSMCs and influences their proliferation and migration.[14] Smooth muscle cell proliferation and migration into the intima are key mechanisms in the pathogenesis of atherosclerosis. The current study shows that miRNA-145 is negatively associated with the extent of coronary arterial stenotic lesions, such that levels of miRNA-145 in patients with three-vessel disease were significantly lower than those with one- or two-vessel disease. Levels of miRNA-145 may, therefore, reflect the degree of coronary atherosclerosis or may influence the progression of coronary atherosclerosis, though the precise role remains unclear and requires further study.
AMI not only induces cardiac myocyte death but also results in the death of other types.[17,18] Cardiac troponin is released by cardiac myocytes, is the pivotal marker in diagnosing AMI, and is related to prognosis among patients with HF.[19] In this study, we found that the levels of miRNA-145 correlated with infarct size as estimated by troponin T levels while peak levels of miRNA-145 correlated with peak levels of troponin T. In addition, our study also showed that miRNA-145 levels correlated negatively with EF, and peak troponin T levels correlated significantly with the EF. Collectively, these findings demonstrate a relationship between miRNA-145 levels and the degree of cardiac damage as well as the level of residual cardiac function. Levels of miRNA-145 may therefore be used as markers of acute cardiac myocyte death and in turn be used to estimate infarct size and residual cardiac function. To evaluate the relationship between miRNA-145 and cardiac function, in the current study, we analyzed whether levels of miRNA-145 correlated with HF. We found that levels of miRNA-145 were significantly lower in those with HF compared to those without HF. We further assessed the correlation between plasma BNP and miRNA-145 levels. BNP play an important role in regulating salt and water excretion and vasodilation and constitute a pivotal part of the endogenous compensatory response to HF and usually regarded as a conventional marker of heart failure.[20] In myocardial infarction and HF patients, BNP is independent sensitive and specific marker of both morbidity and mortality.[21] In this study, we found that plasma miRNA-145 levels correlated strongly with levels of BNP. These results suggest that plasma miRNA-145 levels may have a role in identifying patients at risk of developing HF and act as a biomarker of cardiac function and patient prognosis; however, this relationship requires further more studies to confirm.
Our study has several limitations. On the one hand, it is a single-center study involving a small sample size and therefore the results of the study should be interpreted with caution. On the another hand, we could not demonstrate the mechanisms behind the association between lower miRNA-145 levels in the plasma and AMI in this study, so large-scale, multicenter studies are required to further elucidate the role of miRNA-145 as a potential marker in acute coronary syndrome.
In conclusion, the current study demonstrates that levels of miRNA-145 are associated with AMI and that low miRNA-145 levels correlate inversely with the severity of AMI. Circulating miRNA-145 might not only be of use in diagnosing myocardial infarction but also could potentially be helpful in prognosticating cardiac function and the risk of developing HF. However, the significance of these finding needs to be confirmed in a larger sample of patients.
Financial support and sponsorship
This study was supported by a grant from the Basic and Clinical Research Cooperation Fund of Capital Medical University (No. 2014A18).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
References
- 1.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–65. doi: 10.1056/NEJMoa0908610. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 2.He J, Gu D, Wu X, Reynolds K, Duan X, Yao C, et al. Major causes of death among men and women in China. N Engl J Med. 2005;353:1124–34. doi: 10.1056/NEJMsa050467. doi: 10.1056/NEJMsa050467. [DOI] [PubMed] [Google Scholar]
- 3.Keller T, Zeller T, Peetz D, Tzikas S, Roth A, Czyz E, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868–77. doi: 10.1056/NEJMoa0903515. doi: 10.1056/NEJMoa0903515. [DOI] [PubMed] [Google Scholar]
- 4.Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–67. doi: 10.1056/NEJMoa0900428. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 5.Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He J, et al. Circulating microRNA: A novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J. 2010;31:659–66. doi: 10.1093/eurheartj/ehq013. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- 6.Li C, Pei F, Zhu X, Duan DD, Zeng C. Circulating microRNAs as novel and sensitive biomarkers of acute myocardial Infarction. Clin Biochem. 2012;45:727–32. doi: 10.1016/j.clinbiochem.2012.04.013. doi: 10.1016/j.clinbiochem.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 8.Sonkoly E, Ståhle M, Pivarcsi A. MicroRNAs and immunity: Novel players in the regulation of normal immune function and inflammation. Semin Cancer Biol. 2008;18:131–40. doi: 10.1016/j.semcancer.2008.01.005. doi: 10.1016/j.semcancer.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Schober A, Thum T, Zernecke A. MicroRNAs in vascular biology – Metabolism and atherosclerosis. Thromb Haemost. 2012;107:603–4. doi: 10.1160/TH12-02-0122. doi: 10.1160/TH12-02-0122. [DOI] [PubMed] [Google Scholar]
- 10.Schroen B, Heymans S. Small but smart – MicroRNAs in the centre of inflammatory processes during cardiovascular diseases, the metabolic syndrome, and ageing. Cardiovasc Res. 2012;93:605–13. doi: 10.1093/cvr/cvr268. doi: 10.1093/cvr/cvr268. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–8. doi: 10.1073/pnas.0804549105. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: Novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2012;110:483–95. doi: 10.1161/CIRCRESAHA.111.247452. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 13.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–10. doi: 10.1038/nature08195. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105:158–66. doi: 10.1161/CIRCRESAHA.109.197517. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pleister A, Selemon H, Elton SM, Elton TS. Circulating miRNAs: Novel biomarkers of acute coronary syndrome? Biomark Med. 2013;7:287–305. doi: 10.2217/bmm.13.8. doi: 10.2217/bmm.13.8. [DOI] [PubMed] [Google Scholar]
- 16.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–84. doi: 10.1161/CIRCRESAHA.109.215566. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 17.Meder B, Keller A, Vogel B, Haas J, Sedaghat-Hamedani F, Kayvanpour E, et al. MicroRNA signatures in total peripheral blood as novel biomarkers for acute myocardial infarction. Basic Res Cardiol. 2011;106:13–23. doi: 10.1007/s00395-010-0123-2. doi: 10.1007/s00395-010-0123-2. [DOI] [PubMed] [Google Scholar]
- 18.Zhu H, Fan GC. Whether circulating miRNAs or miRNA-carriers serve as biomarkers for acute myocardial infarction. J Biomark Drug Dev. 2012;1:1–3. doi: 10.4172/jbdd.1000e103. doi: 10.4172/jbdd.1000e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bøhmer E, Hoffmann P, Abdelnoor M, Seljeflot I, Halvorsen S. Troponin T concentration 3 days after acute ST-elevation myocardial infarction predicts infarct size and cardiac function at 3 months. Cardiology. 2009;113:207–12. doi: 10.1159/000201991. doi: 10.1159/000201991. doi: 10.1159/000201991. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki T, Yamazaki T, Yazaki Y. The role of the natriuretic peptides in the cardiovascular system. Cardiovasc Res. 2001;51:489–94. doi: 10.1016/s0008-6363(01)00238-3. [DOI] [PubMed] [Google Scholar]
- 21.Richards AM, Nicholls MG, Yandle TG, Frampton C, Espiner EA, Turner JG, et al. Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin: New neurohormonal predictors of left ventricular function and prognosis after myocardial infarction. Circulation. 1998;97:1921–9. doi: 10.1161/01.cir.97.19.1921. doi: 10.1161/01.CIR.97.19.1921. [DOI] [PubMed] [Google Scholar]


