Abstract
Background:
Q fever endocarditis, a chronic illness caused by Coxiella burnetii, can be fatal if misdiagnosed or left untreated. Despite a relatively high positive rate of Q fever serology in healthy individuals in the mainland of China, very few cases of Q fever endocarditis have been reported. This study summarized cases of Q fever endocarditis among blood culture negative endocarditis (BCNE) patients and discussed factors attributing to the low diagnostic rate.
Methods:
We identified confirmed cases of Q fever endocarditis among 637 consecutive patients with infective endocarditis (IE) in the Peking Union Medical College Hospital between 2006 and 2016. The clinical findings for each confirmed case were recorded. BCNE patients were also examined and each BCNE patient's Q fever risk factors were identified. The risk factors and presence of Q fever serologic testing between BCNE patients suspected and unsuspected of Q fever were compared using the Chi-squared or Chi-squared with Yates’ correction for continuity.
Results:
Among the IE patients examined, there were 147 BCNE patients, of whom only 11 patients (7.5%) were suspected of Q fever and undergone serological testing for C. burnetii. Six out of 11 suspected cases were diagnosed as Q fever endocarditis. For the remaining136 BCNE patients, none of them was suspected of Q fever nor underwent relevant testing. Risk factors for Q fever endocarditis were comparable between suspected and unsuspected patients, with the most common risk factors being valvulopathy in both groups. However, significantly more patients had consulted the Infectious Diseases Division and undergone comprehensive diagnostic tests in the suspected group than the unsuspected group (100% vs. 63%, P = 0.03).
Conclusions:
Q fever endocarditis is a serious yet treatable condition. Lacking awareness of the disease may prevent BCNE patients from being identified, despite having Q fever risk factors. Increasing awareness and guideline adherence are crucial in avoiding misdiagnosing and missed diagnosing of the disease.
Keywords: Blood Culture, Endocarditis, Q Fever
Introduction
Q fever endocarditis is a chronic infection caused by the zoonosis Coxiella burnetii. Q fever infections can be common in regions where people are in close contact with livestock. However, only a small proportion of those who have acute Q fever develop the chronic form. Chronic Q fever has nonspecific manifestations, and diagnosis often occurs after serological testing and nonspecific cardiac findings. If left untreated, Q fever endocarditis can be severe and potentially fatal.[1] Large numbers of patients with Q fever endocarditis have been reported in Europe. Reviews of Q fever endocarditis reported a prevalence of one in one million per year,[2] and of 5% in all infective endocarditis (IE).[3] Scott et al. reported that Q fever endocarditis accounts for about 1% of all cases of endocarditis in the United States.[4] The literature has reported numerous cases in Western Europe and the United States, and Australia; however, there are very few reports in the literature on Q fever endocarditis in China. This study summarizes cases of Q fever endocarditis diagnosed in blood culture negative endocarditis (BCNE) patients in the Peking Union Medical College Hospital (PUMCH), compares the risk factor for Q fever among suspected and unsuspected patients, and discusses factors that may contribute to the low diagnostic rate.
Methods
Patients and procedures
Six hundred and thirty-seven consecutive cases of IE, between January 2006 and August 2016, were identified from the PUMCH database and examined for cases of Q fever endocarditis. Before 2006, Q fever serological diagnostic testing available to our facility was the complement fixation method. The complement fixation method has been documented to be less specific, lack sensitivity, and be detected later than the immunofluorescence antibody assay (IFAA).[5] For this study, we focused on cases after 2006, where IFAA to detect Phase I and II C. burnetii antibodies from the serum was accessible. All IFAAs were done at the State Key Laboratory of Pathogen and Biosecurity of the Institute of Microbiology and Epidemiology at the Academy of Military Medical Sciences (Beijing, China). All Q fever endocarditis patients must satisfy the modified Duke criteria for IE[3] and have significant levels of immunoglobulin (Ig) G titer antibodies against C. burnetii (Phase I IgG titer >1:800).[6]
The PUMCH Institutional Review Board approved all procedures of this study, and each patient provided written informed consent for this study. The Q fever endocarditis patients’ demographics, initial presentation, cardiovascular manifestations, other clinical features, laboratory results, serology, treatment, and follow-up were collected from each patient's medical record. Since Q fever endocarditis patients are known to have uncommon presentations, the patients’ initial presentation and present history was particularly noted, and a short patient description was written for each patient. Echocardiograms, aerobic and anaerobic pathogen blood cultures were performed in all patients. No blood or resected cardiac tissue culture for C. burnetii was performed in these patients.
Particular attention was also paid to the BCNE patients as these patients may have risk factors warranting Q fever screening. Previous studies have identified the following presentations (1) cardiac valvulopathy or valvular prostheses defects, (2) fever without leukocytosis, (3) immunocompromised, (4) periprosthetic leak in the absence of vegetations - place BCNE patients at risk of Q fever endocarditis.[6,7] Patients suspected of BCNE but not fulfilling the modified Duke Criteria[3,8] were excluded. The presence of Q fever risk factors was analyzed in all BCNE patients from 2006 to 2016 [Figure 1]. The reasons why some but not all patients were selected for Q fever screening were noted to understand guideline adherence and daily practice.[3,8]
Figure 1.
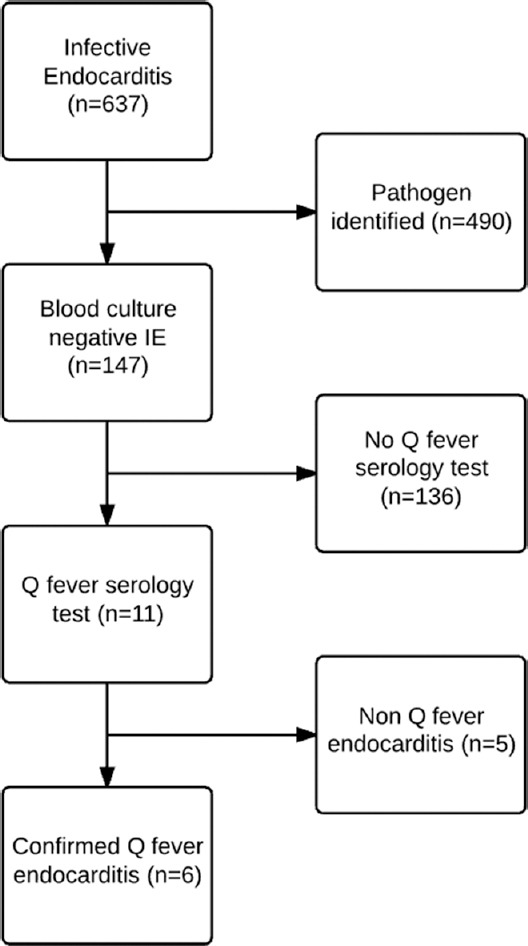
Flowchart of the infective blood culture negative endocarditis and Q fever endocarditis patient selection process.
Statistical analysis
Standard descriptive statistics (including mean and standard deviation) were used to describe Q fever endocarditis patients. The differences in clinical risk factors between suspected and unsuspected BCNE patients were analyzed with Chi-squared test or Chi-squared test with Yates’ correction for continuity. The level of significance was set at P < 0.05. Statistical analyses were performed with SPSS version 18.0 (SPSS Inc., Chicago, Illinois, USA).
Results
Patient demographics and initial presentation
Of the 637 cases of IE, six cases of Q fever endocarditis were found. All identified Q fever endocarditis patients were Han Chinese males and their ages ranged from 22 to 61 years old. Demographic and clinical data of the six patients were summarized in Tables 1 and 2.
Table 1.
Clinical findings and relative incidence of finding in Q fever endocarditispatients
| Findings | Normal value or range (unit) | Number of patients with indicated findings (%)* | Reference reports[9] |
|---|---|---|---|
| Clinical findings | |||
| Fever | – | 6 (100) | N/A |
| Cardiac failure | – | 4 (67) | N/A |
| Clubbing | – | 4* | N/A |
| Arterial embolism | – | 1 (17) | N/A |
| Hepatomegaly | – | 2 (33) | N/A |
| Splenomegaly | – | 3 (50) | N/A |
| Purpuric rash | – | 1 (17) | N/A |
| Valvulopathy | |||
| Aortic | – | 5 (83) | N/A |
| Mitral | – | 3 (50) | N/A |
| Laboratory findings | |||
| Leukocyte | |||
| Leukocytosis | 4.0–10.0 (×109/L) | 0 | N/A |
| Leukopenia | 1 (17) | N/A | |
| Anemia | 110–160 (g/L) | 3 (50) | 40–55% with anemia |
| Elevated creatine | 53–132 (µmol/L) | 1 (17) | 65–73% elevated |
| Thrombocytopenia | 100–360 (×109/L) | 1 (1) | 26–56% with thrombocytopenia |
| Elevated transaminase | |||
| AST | 5–37 (U/L) | 4 (67) | 40–83% elevated |
| ALT | 5–40 (U/L) | 3 (50) | N/A |
| Hyperbilirubinemia (TBIL) | 5.1–22.2 (µmol/L) | 0 | N/A |
| ALP | 27–107 (U/L) | 3 (50) | 88% elevated |
| Elevated ESR | 0–20 (mm/h) | 5 (83) | N/A |
| Elevated LDH | 97–270 (U/L) | 3 (50) | 94% elevated |
| Elevated gamma globulin | 7–17 (g/L) | 1* | 35% present |
| Antinuclear antibody | <1:40 | 0* | 60% present |
| Rheumatoid factor | 0–20 (U/ml) | 2* | 40% present |
| Smooth muscle antibodies | <1:20 | 0* | N/A |
*Some patient data unavailable; exact percentages not calculated. AST: Aspartate transaminase; ALT: Alanine transaminsase; ALP: Alkaline phosphatase; LDH: Lactate dehydrogenase; ESR: Erythrocyte sedimentation rate; TBIL: Total bilirubin; N/A: Not available.
Table 2.
Cases of Q fever endocarditis reported in PUMCH from 2006 to 2016
| Items | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
|---|---|---|---|---|---|---|
| Gender | Male | Male | Male | Male | Male | Male |
| Age (years) | 41 | 22 | 61 | 54 | 55 | 58 |
| NYHA classification | 2 | 3 | 1 | 2 | N/A | 3 |
| Symptoms | Fever, cough, jaundice | Fever, cough, chest pain | Fever, erythema | Fever, arterial embolism | Fever, weight loss, cough | Fever, chills, weakness, weight loss |
| Cardiac findings | Mitral aortic valve deformity, aortic valve vegetation | Vegetations on chordae tendineae of the anterior leaflet of the mitral valve | Aortic valve thickening with vegetations | Vegetations on bioprosthetic aortic valve, mild to moderate aortic perivalvular leakage | Stenosis and Regurgitation of aortic and mitral valves. Aortic valve vegetation | Atrial perivalvular abscess and vegetation. Mitral valve regurgitation |
| Phase I IgG | 1:800 | 1:1600 | 1:>5120 | 1:3200 | >1:1600 | 1:6400 |
| Phase II IgG | 1:800 | 1:800 | 1:>5120 | 1:800 | >1:1600 | N/A |
| Antimicrobial therapy | Minocycline | Doxycycline + SMZ–TMP | Doxycycline | Minocycline + HCQ | Amikacin + vancomycin | Minocycline + HCQ |
| Operation | No | No | No | Yes, due to congestive heart failure | Yes | No |
| Follow–up (months) | 53 | 30 | 37 | 36 | 24 | 48 |
| Outcome | Cure | Cure | Cure | Bioprostheticvalvuloplasty and cure | Cure | Stable* |
*Reported to be stable upon discharge, further details unknown. NYHA: New York Heart Association; SMZ–TMP: Sulfamethoxazole–trimethoprim; HCQ: Hydroxycholoroquine; PUMCH: Peking Union Medical College Hospital; N/A: Not available.
Patient 1
A 41-year-old male cattle and sheep herdsman presenting with fever and jaundice was admitted in March 2008. The patient previously had splenectomy due to hepatosplenomegaly. He occasionally consumed moderate amounts of alcohol (approximately 100 ml of liquor daily) over the past 15 years. His hepatitis screening, for the hepatitis B, C, and D virus antibodies, returned negative.
Patient 2
A 22-year-old male college student with intermittent cough, fever, and episodic chest pain for 1 year was admitted in March 2010. He was diagnosed at a local hospital with viral myocarditis, acute hepatitis, and pneumonia. The patient denied having a history of heart disease or viral hepatitis.
Patient 3
A 61-year-old male civil servant was admitted in July 2009 for a fever and rash lasting 4 months. He began experiencing afternoon fever accompanied by chills, fatigue, sleep hyperhidrosis, and weight loss.
Patient 4
A 54-year-old taxicab driver was admitted in May 2012 for intermittent fever. He had undergone bioprosthetic aortic valve replacement due to chronic fever and congenital bicuspid aortic malformation 2 years prior in a local hospital. However, his fever relapsed 10 months after the operation, and five routine blood cultures were all negative.
Patient 5
A 55-year-old veterinarian with chronic exposure to livestock (including cattle, sheep, and swine) was admitted in February 2015 due to fever, low appetite and weight loss for 6 months. On admission, he was also diagnosed with rheumatic heart disease. Aortic and mitral stenosis and regurgitation were found.
Patient 6
A 58-year-old man admitted in March 2014 due to recurring fever lasting over four years. In 2010 he underwent anti-tuberculosis treatment. Over the next few years, vegetations were found, and he was placed on various antibiotics. He underwent aortic and mitral valvuloplasty, however, 8 months after the operation his recurring fever continued, and perivalvular leaks was found.
Cardiovascular manifestations
Four patients had congestive heart failure, ranging from Class I to III, according to the New York Heart Association classification. One patient had previous rheumatic heart disease. Transthoracic echocardiography was performed in all patients. In five patients, the vegetation was seen on the aortic leaflet while in one patient the vegetation was seen on the papillary muscle of the mitral valve. For all patients, the vegetation was also accompanied by some degree of insufficiency and regurgitation.
Other clinical features and laboratory results
Other clinical features were noted and can be found in Table 1. All patients presented with fever and two patients had a high-grade fever. All patients repeatedly had negative blood cultures, on average 4.4 times per person. Elevated liver enzymes, leukocytosis, leukopenia, anemia, and an increased erythrocyte sedimentation rate were observed as well. One patient (case 3) had impaired T-cell function with weight loss, hepatosplenomegaly, acropachy, and rash.
Serology, treatment, and follow-up
To confirm the diagnosis of Q fever endocarditis, all six patients received serological testing for C. burnetii antigens by IFAA. All patients showed elevated IgG Phase I and Phase II antibody titers. The values upon initial diagnosis can be found in Table 1.
Upon diagnosis, all patients were treated with antimicrobial therapy. The specific drugs administered can be found in Table 2. All patients responded well except for patient 4 who continued to exhibit symptoms and high levels of Phase I and II antibodies. The patient experienced decompensated heart failure and later experienced periprosthetic valvular regurgitation and partial dehiscence [Figure 2a]. He underwent repeat valvuloplasty with a mechanical aortic valve. Histological examination of the bioprosthetic valve revealed fibrosis, inflammatory cell infiltration, and myxomatous degeneration. Histological staining, including hematoxylin and eosin staining, gram staining, periodic acid-Schiff staining, Grocott's methenamine silver staining and acid-fast staining, all returned negative. Two weeks after receiving mechanical valvuloplasty, his titer of Phase I antibody dropped from 1:3200 to 1:800 and the titer of Phase II antibody from 1:1600 to 1:200 [Figure 2b]. He was asymptomatic at the 3-year follow-up after surgery.
Figure 2.
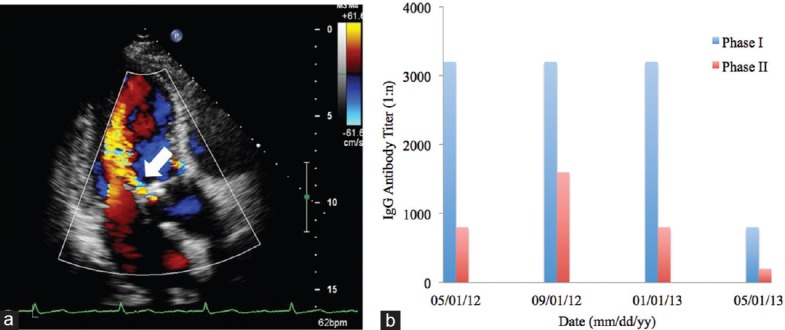
Laboratory results from patient 4. (a) Transthoracic echocardiogram showing a severe perivalvular regurgitation of the bioprosthetic aortic valve (arrow). No valvular vegetations were found. (b) Serial serologic testing of immunoglobulin G against Coxiella burnetii showed consistently high levels of Phase I and II antibodies prior to the valvuloplasty. The Phase I antibody drop dramatically from 1:3200 to 1:800 and the Phase II from 1:1600 to 1:200 two weeks after the aortic bioprosthesis replacement.
Patient follow-up ranged from 24 to 53 months. A follow-up examination was performed every 3–6 months; each time a routine blood test, liver and kidney function test, echocardiogram and serological test were administered.
Blood culture-negative endocarditis cases
There were 637 cases of IE during the period. Four hundred and ninety cases had causative microorganisms identified through blood culturing or through tissue culturing. The remaining 147 (23.1%) cases were BCNE on unknown cause, of which 11 patients (7.5% of BCNE cases) were suspected of Q fever and underwent Q fever serological analysis by IFAA. The remaining 136 patients were unsuspected of Q fever and did not undergo relevant testing.
To understand the reasoning behind the low Q fever endocarditis diagnostic rate, we examined the clinical characteristics of the BCNE patients. It was found that among the 136 unsuspected patients, many possess risks warranting Q fever suspicion and thus serological testing. Fifty percent of the patients suspected of Q fever had two or more risk factors, and this percentage was nearly identical (54%, n = 74) in the unsuspected patients. The most and second most common risk factors were valvulopathy and fever without leukocytosis, respectively, in both groups. Apart from perivalvular leakage, both groups had a comparable proportion of each Q fever risk factor. Furthermore, all patients suspected of Q fever endocarditis were either admitted to or were consulted by the Infectious Diseases Division while only 63% of the unsuspected patients had consulted the Infectious Diseases Division (P = 0.03). None of the unsuspected patients had undergone C. burnetii serological testing [Table 3].
Table 3.
Clinical characteristics of blood culture–negative endocarditis patients based on suspicion of Q fever (from 2006 to 2012)
| Characteristics | Patients suspected of Q fever (n = 11) | Patients not suspected of Q fever (n = 136) | χ2 | P |
|---|---|---|---|---|
| Q fever risk factors, n (%) | ||||
| Valvulopathy | 8 (55) | 96 (71) | 0.02 | 0.88 |
| Fever without leukocytosis | 7 (73) | 79 (58) | 0.13 | 0.72 |
| Immunocompromised | 1 (64) | 23 (17) | 0.46 | 0.50 |
| Perivalvular leak | 3 (27) | 8 (6) | 6.73 | 0.01 |
| Number of risk factors present (%) | ||||
| ≤1 | 5 (45) | 62 (46) | 0.00 | 0.99 |
| ≥2 | 6 (55) | 74 (54) | ||
| Contact with infectious disease division and guideline adherence, n (%) | 11 (100) | 86 (63) | 4.6 | 0.03 |
Discussion
To the best of our knowledge, our study reported the most cases of Q fever endocarditis in the mainland of China. The patient characteristics were similar to those reported from Western countries. The patients’ were predominately male and mostly over 40-year-old.[9] The patients had a high incidence of fever, abnormal liver function as exhibited by elevated liver enzymes, the telltale sign of valvular defects, and routine blood cultures were negative in all the patients.[7,10] Vegetative growth seen by echocardiography was observed in all patients despite being present in only about 12% of chronic Q fever patients.[11]
In this study, patient 4 failed to respond to combined antimicrobial therapy. Along with his persistently elevated Phase I IgG levels, the patient experienced deterioration in cardiac function and worsening of valve regurgitation. Intriguingly, not only did his symptoms improve rapidly, but his Phase I and II IgG levels fell dramatically after bioprosthesis replacement, suggesting that high levels of IgG may be associated with C. burnetii residing in the bioprosthesis.
Compared to tetracycline and rifampin-cotrimoxazole or its combination, doxycycline plus hydroxychloroquine is an optimal therapeutic regimen with a lower relapse rate.[12] In this study, three patients responded well to minocycline, doxycycline or doxycycline-cotrimoxazole therapy and had no symptoms at the 30-month follow-up. It is suggested that follow-up should be conducted every month for the 1st year, then every 3 months for 18 months to 4 years,[13] and a lifelong treatment of doxycycline may be appropriate. However, whether the serial serological testing can be used as a prognostic index is still controversial.[9,14] In a report from Spain, IgG Phase I antibody levels were monitored in patients with Q fever endocarditis to see the association between the IgG titers and prognosis. After three years of follow-up, the reduction of IgG Phase I antibody was rare and not related with cure or relapse.[14]
The first confirmed case of Q-fever in China was reported in 1950.[15] However, very few cases of Q fever endocarditis from the mainland of China have been reported in the literature. Since the 1980s, there were approximately 28,000 cases of IE in the mainland of China, and only four other cases of Q fever endocarditis were reported.[13,16] Thus far, estimating the incidence of Q fever endocarditis in the mainland of China cannot be accurately made, but it is clear that the number of confirmed cases is very low. Despite the few reported Q fever endocarditis cases in the literature, reports have shown that Q fever may be fairly common. Among the 34 Chinese provinces and municipalities, 24 have reported cases of Q fever and outbreaks have also been reported in the Inner Mongolia, Sichuan, Xinjiang, Yunnan, and Tibet Provinces.[17] In these regions of China, many individuals’ livelihoods involve working with livestock. A serological screening of over 3000 livestock found that the seroprevalence of Q fever was 24.9% in cattle and 13.5% in sheep.[18] Based on a seroepidemiological survey, the positive rate of Q fever serology ranges from 1.6% to 28.7% in healthy people living in the Southwestern Chinese Provinces of Guizhou, Sichuan, Yunnan, and Tibet.[15,19] The high positive rate of Q fever serology and the low number of Q fever endocarditis cases suggest that Q fever endocarditis may be an underreported and under-identified disease in China.
A few factors may attribute to the low diagnostic rate of Q fever endocarditis among physicians at our facility and even throughout China. First, in our review of BCNE patients, the presence of Q fever risk factors was similar across unsuspected and suspected patient groups, suggesting special attention should be paid to BCNE patients as a whole. Fournier et al. reported that among 740 patients with BCNE, 37% had C. burnetti as the causative agent.[20] In clinical guidelines, serological analysis for C. burnetti has been recommended in patients with IE, especially in patients with BCNE.[3,8] This study showed that when Q fever endocarditis was suspected among BCNE patients, the diagnostic rate through serological analysis was 55%, comparable to that, 48%, reported in the literature.[20] However, in the present study, the serological testing for C. burnetii is performed in very few patients with BCNE. Of note, all BCNE cases that underwent serological testing were consulted by physicians in the Infectious Diseases Division. Thus, guideline adherence and awareness may not be as stringent among physicians in divisions other than infectious diseases. This suggests that awareness of Q fever endocarditis presentation, risk factors, and general guidelines is critical in the approach to BCNE patients.
Second, notifiable or reportable disease surveillance is crucial for infectious disease prevention and control. In countries such as the United States,[6] and Australia,[21] Q fever infection is a notifiable disease with each country's Center for Disease Control or equivalent. In the People's Republic of China, according to the Chinese Infectious Diseases Prevention Act (2013 update),[22] there are 39 notifiable diseases and Q fever is not among the list. In addition, Q fever serology testing through IFAA is not accessible to all hospitals. For instance, only one clinical facility, the Academy of Military Medical Sciences, in Northern China, covering a population of approximately 160 million, can conduct serological testing for C. burnetti.
Third, empirical antibiotic therapy may have also contributed to the low diagnostic rate of Q fever. Of the 147 cases with BCNE of unknown cause, empirical antibiotic therapy was given before admission. Empirical therapy consists mainly of gentamicin or vancomycin to target the common etiologies of IE,[23] thus, often rendering the diagnostic culturing and sampling inconclusive.[24] If there lack systematic testing protocols for uncommon pathogens, proper diagnosis of Q fever can easily be overlooked.
Due to the limited cases of Q fever endocarditis found and few BCNE patients undergoing Q fever serological testing, definitive conclusions cannot be made from this study. However, with the available data, we compared the risk factors to the literature including the IE guidelines released by the United States Center for Diseases Control.[6] We found that many patients had clinical indications where a Q fever serological test was recommended but failed to receive one.
In conclusion, this study found a low diagnostic rate of Q fever among BCNE patients. According to serological surveying and screening, Q fever or Q fever endocarditis is underestimated in China. The array of presentations and non-specific symptoms across patients remain the key challenge in diagnosing the disease. We noted limitations, specific to the mainland of China, hindering the identification, and management of this disease, among which we believe physician awareness of the risk factors and adherence to clinical guidelines play crucial roles.
Financial support and sponsorship
This work was supported by grants from the National Natural Science Foundation of China (No. 81470426), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (SRF for ROCS, and SEM), Elite Class, and PUMCH Young and Middle-aged Investigation Fund, Key Project (No. PUMCH-2016-1.12).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Min Chen
References
- 1.Million M, Lepidi H, Raoult D. Q fever: Current diagnosis and treatment options. Med Mal Infect. 2009;39:82–94. doi: 10.1016/j.medmal.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Tissot Dupont H, Raoult D, Brouqui P, Janbon F, Peyramond D, Weiller PJ, et al. Epidemiologic features and clinical presentation of acute Q fever in hospitalized patients: 323 French cases. Am J Med. 1992;93:427–34. doi: 10.1016/0002-9343(92)90173-9. [DOI] [PubMed] [Google Scholar]
- 3.Fournier PE, Casalta JP, Habib G, Messana T, Raoult D. Modification of the diagnostic criteria proposed by the Duke Endocarditis Service to permit improved diagnosis of Q fever endocarditis. Am J Med. 1996;100:629–33. doi: 10.1016/s0002-9343(96)00040-x. [DOI] [PubMed] [Google Scholar]
- 4.Scott JW, Baddour LM, Tleyjeh IM, Moustafa S, Sun YG, Mookadam F. Q fever endocarditis: The Mayo Clinic experience. Am J Med Sci. 2008;336:53–7. doi: 10.1097/MAJ.0b013e31815cff75. [DOI] [PubMed] [Google Scholar]
- 5.Péter O, Dupuis G, Burgdorfer W, Peacock M. Evaluation of the complement fixation and indirect immunofluorescence tests in the early diagnosis of primary Q fever. Eur J Clin Microbiol. 1985;4:394–6. doi: 10.1007/BF02148690. [DOI] [PubMed] [Google Scholar]
- 6.Anderson A, Bijlmer H, Fournier PE, Graves S, Hartzell J, Kersh GJ, et al. Diagnosis and management of Q fever – United States, 2013: Recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep. 2013;62:1–30. [PubMed] [Google Scholar]
- 7.Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–53. doi: 10.1128/cmr.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Jr, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 9.Million M, Thuny F, Richet H, Raoult D. Long-term outcome of Q fever endocarditis: A 26-year personal survey. Lancet Infect Dis. 2010;10:527–35. doi: 10.1016/S1473-3099(10)70135-3. [DOI] [PubMed] [Google Scholar]
- 10.Raoult D, Tissot-Dupont H, Foucault C, Gouvernet J, Fournier PE, Bernit E, et al. Q fever 1985-1998. Clinical and epidemiologic features of 1,383 infections. Medicine (Baltimore) 2000;79:109–23. doi: 10.1097/00005792-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Fenollar F, Fournier PE, Carrieri MP, Habib G, Messana T, Raoult D. Risks factors and prevention of Q fever endocarditis. Clin Infect Dis. 2001;33:312–6. doi: 10.1086/321889. [DOI] [PubMed] [Google Scholar]
- 12.Subramanya NI, Wright JS, Khan MA. Failure of rifampicin and co-trimoxazole in Q fever endocarditis. Br Med J (Clin Res Ed) 1982;285:343–4. doi: 10.1136/bmj.285.6338.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang LJ, Fu XP, Zhang JS. Q fever endocarditis with multi-organ complication: A case report. Chin Med J. 2006;119:1580–2. [PubMed] [Google Scholar]
- 14.Mogollón MV, Anguita MP, Aguado JM, Tornos P, Miró JM, Gálvez-Acebal J, et al. Q fever endocarditis in Spain. Clinical characteristics and outcome. Enferm Infecc Microbiol Clin. 2011;29:109–16. doi: 10.1016/j.eimc.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Yu S. The progress of Q fever investigations in China (in Chinese) Chin J Epidemiol. 2000;21:456–9. [Google Scholar]
- 16.Wang X, Zhang S. Q fever endocarditis: A case report (in Chinese) Chin J Intern Med. 2002;41:490. [Google Scholar]
- 17.Wu XB, Na RH, Wei SS, Zhu JS, Peng HJ. Distribution of tick-borne diseases in China. Parasit Vectors. 2013;6:119. doi: 10.1186/1756-3305-6-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cong W, Meng QF, Shan XF, Sun WW, Kang YH, Chen L, et al. Coxiella burnetii (Q fever) infection in farmed ruminants in three northeastern provinces and inner mongolia autonomous region, China. Vector Borne Zoonotic Dis. 2015;15:512–4. doi: 10.1089/vbz.2015.1789. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Zhang F. The study progress of Q fever and ehrlichiosis. Med Recapitul. 2008;14:4. [Google Scholar]
- 20.Fournier PE, Thuny F, Richet H, Lepidi H, Casalta JP, Arzouni JP, et al. Comprehensive diagnostic strategy for blood culture-negative endocarditis: A prospective study of 819 new cases. Clin Infect Dis. 2010;51:131–40. doi: 10.1086/653675. doi: 10.1086/653675. [DOI] [PubMed] [Google Scholar]
- 21.Australian Government Department of Health. Q Fever Case Definition. 2004. [Last accessed on 2016 May 09]. Available from: http://www.health.gov.au/internet/main/publishing.nsf/content/cda-surveil-nndss-casedefs-cd-qfev.htm .
- 22.12thStanding Committee of the National People's Congress. Chinese Infectious Diseases Prevention Act. 2013. [Last accessed on 2016 May 01]. Available from: http://www.jxga.gov.cn/zwgk/zhengfucaigou/2014-04-16/1337.html .
- 23.Horstkotte D, Follath F, Gutschik E, Lengyel M, Oto A, Pavie A, et al. Guidelines on prevention, diagnosis and treatment of infective endocarditis executive summary;the task force on infective endocarditis of the European society of cardiology. Eur Heart J. 2004;25:267–76. doi: 10.1016/j.ehj.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Lamas C. Diagnostic strategy for blood culture-negative endocarditis. Clin Infect Dis. 2010;51:141–2. doi: 10.1086/653676. doi: 10.1086/653676. [DOI] [PubMed] [Google Scholar]


