Abstract
Background:
The level of high-density lipoprotein cholesterol (HDL-C) is an important risk indicator and used in risk factor counting and quantitative risk assessment; however, the effect of HDL-C in young male patients with acute myocardial infarction (AMI) is unclear. The aim of this study was to investigate the effect of HDL-C in young male patients.
Methods:
We recruited 267 consecutive young male patients (≤44 years) diagnosed with AMI. Other 247 participants free from coronary heart disease were enrolled as controls. HDL-C levels of AMI patients and controls were evaluated to analyze the predictive value on AMI. According to the cutoff point of 1.04 mmol/L HDL-C, patients of AMI were divided into two subgroups (normal HDL-C group and low HDL-C group) and were followed up for 2 years. Clinical end points included all major adverse coronary events (MACEs): the main cause of death, nonfatal myocardial infarction, readmissions for acute coronary syndrome, arrhythmias, or revascularization. The prognostic value of HDL-C was evaluated using Cox regression according to MACE.
Results:
Patients of AMI had decreased proportion in normal HDL-C group compared to controls (47.2% vs. 57.9%; P = 0.017). Logistic regression analysis showed that there was an inverse relationship between HDL-C and AMI in young males. In the low HDL-C subgroup of AMI patients (n = 141), 34 (24.1%) patients experienced a MACE during the 2-year follow-up, compared with 15 (11.9%) patients in normal HDL-C subgroup (n = 126). The Cox regression analysis showed that HDL-C was an independent predictor of a MACE during the follow-up period (hazard ratio = 0.354, P = 0.006).
Conclusion:
HDL-C was an important parameter for predicting the risk and the clinical outcomes of AMI in young male patients.
Keywords: Acute Myocardial Infarction, High-density Lipoprotein Cholesterol, Male, Prognosis
Introduction
Acute myocardial infarction (AMI) is unusual among young people. With an upward trend in changing to unhealthy lifestyle, an increased incidence of juvenescence with AMI is getting more concerns from clinical research recently. Various issued studies showed that premature myocardial infarction (MI) individuals were predominantly males, characterized by smoking, obesity, and dyslipidemia (an essential key contributing to atherosclerosis).[1,2,3,4] As a component of lipid metabolism disorders, the abnormal of level of high-density lipoprotein cholesterol (HDL-C) has been gradually being valued recently. Decreased HDL-C levels are often associated with increased atherosclerotic cardiovascular disease (ASCVD) risk. However, in human studies, low HDL-C levels are not consistently associated with premature ASCVD.[5] In this study, we aimed to evaluate the role of HDL-C in young AMI males compared to other risk factors of coronary heart disease (CHD), including the predictive effect at the onset of AMI in a case-control study and the prognostic effect assessed in a cohort study with a follow-up period of two years.
Methods
According to the World Health Organization's definition of youth, we recruited male patients aged from 18 to 44 years. All patients were admitted to the Department of Cardiology at Beijing Anzhen Hospital and hospitalized for the treatment of AMI from January 2013 to January 2014. The diagnosis of AMI was based on the following criteria: elevated myocardial enzyme levels (cardiac troponin I [cTnI], creatine kinase [CK], and CK-MB), typical electrocardiogram changes, and typical chest pain >30 min. There were 299 patients altogether from January 2013 to January 2014, and we excluded 32 patients from this study. Patients were excluded if they presented with the following clinical conditions: history of prior MI and history of percutaneous coronary intervention (PCI) or coronary artery bypass grafting, valvular heart disease, acute stroke, serious liver or kidney disease, chronic consumption disease, thyroid dysfunction, or cancer. Patients taking lipid-lowering drug therapy recently were also excluded from the study.
All patients underwent coronary angiography using the Judkins or Sones technique during hospitalization. Coronary artery stenosis was defined as >50% reduction in lumen diameter of any of the three coronary arteries or their primary branches.
Other 247 males as controls, also undergoing coronary angiography and diagnosed free from CHD (diagnosed as esophagitis, pneumonia, cholecystitis, cervical spine disease before discharge), were recruited and matched on age at the same period. In addition, all individuals in the study were divided into two subgroups, according to the cutoff point of 1.04 mmol/L HDL-C.[5] The normal HDL-C group had a level ≥1.04 mmol/L and the low HDL-C group with a level <1.04 mmol/L.
All participants were interviewed during hospitalization, and information about family history of premature CHD (<55 years of age in a male first-degree relative or <65 years in a female), hypertension (a cuff blood pressure ≥140/90 mmHg and/or the current use of anti-hypertensive medication), diabetes (a fasting plasma glucose was ≥7.0 mmol/L or 2 h postprandial glucose was >11.1 mmol/L or the current use of anti-diabetic medication), and smoking (cigarettes within one month of the index admission) were recorded. The body mass index (BMI) was calculated as weight divided by height squared (kg/m2).
All patients consented to this study. The study was approved by the Ethics Committee of Beijing Anzhen hospital of the Capital University of Medical Sciences and complies with the Declaration of Helsinki (No. 2016004X).
Venous blood samples were collected from the individuals after an overnight fast within 12 h after incident of AMI. Blood was drawn from the median cubital vein into ethylenediaminetetraacetic acid-containing vials. Plasma was separated within 2 h after collection. Total cholesterol (TC), HDL-C, and triglycerides (TGs) were measured enzymatically using Beckman Coulter AU5400 Clinical Chemistry analyzer. Moreover, low-density lipoprotein cholesterol (LDL-C) was measured with a homogeneous direct assay. Urea nitrogen, creatinine (Cr), uric acid (UA), and hemoglobin (Hb) were measured according to the standard procedure of Anzhen hospital laboratory.
Follow-up of the acute myocardial infarction patients
After discharge, AMI patients were followed up at every 2-month intervals for 2 years by a trained interviewer at Beijing Anzhen hospital. The evaluated follow-up information was feedback for all 267 AMI patients (100% completed). Data were obtained either from face-to-face appointment or telephone interview. End points were defined as major adverse cardiovascular events (MACEs) as the main cause of death, nonfatal MI, arrhythmias (ventricular tachycardia or ventricular fibrillation), readmissions for acute coronary syndrome, or revascularization (coronary angioplasty or coronary artery bypass graft).
Statistical analysis
Analyses were performed using SPSS version 22.0 software (IBM Corp., USA). Continuous data are expressed as the mean and standard deviations, and differences between groups were determined using the Student's t-test. Variables with a skewed normal distribution are presented as medians (interquartile range), and the between-group differences for these variables were determined using the Rank-Sum test. Categorical variables are presented as percentages, and the differences between groups were valued using the Chi-square test. The logistic model was used to evaluate the associations between AMI and clinical parameters. For all of the odds ratios, we calculated 95% confidence intervals (CI). MACE rate estimates were generated with the Kaplan–Meier method. Cox proportional hazards modeling was used to assess the relative importance of the baseline risk factors to the end points. Hazard ratios (HRs) are presented with 95% CIs to show the risk of an event when the factor is present. Significance was defined at the 5% level using a two-tailed test.
Results
In the case-control study, detailed baseline characteristics of the two groups are summarized in Table 1. The prevalence of the risk factors (e.g., hypertension, diabetes, smoking, and family history of premature CHD) was higher in AMI group than control group (P < 0.001, P < 0.001, P < 0.001, and P < 0.001, respectively). The AMI patients also had higher levels of LDL-C, TC, TG, and increased BMI (P < 0.001, P < 0.001, P < 0.001, and P = 0.002, respectively). At the time of incident AMI, AMI patients had decreased proportion in normal HDL-C subgroup (47.2%) compared to controls (57.9%, P = 0.017).
Table 1.
Comparison of baseline clinical characteristics between AMI and controls
| Characteristics | AMI (n = 267) | Control (n = 247) | P |
|---|---|---|---|
| Age (years) | 38.68 ± 4.44 | 38.27 ± 4.48 | 0.255 |
| BMI (kg/m2) | 26.93 ± 2.34 | 26.05 ± 3.85 | 0.002 |
| Hb (g/L) | 148.00 ± 12.52 | 153.83 ± 10.89 | <0.001 |
| BUN (mmol/L) | 4.75 ± 1.41 | 5.64 ± 1.42 | <0.001 |
| Cr (mmol/L) | 83.82 ± 17.70 | 86.57 ± 13.79 | 0.061 |
| UA (mmol/L) | 372.21 ± 98.94 | 398.95 ± 86.23 | 0.001 |
| LDL-C (mmol/L) | 3.05 ± 0.87 | 2.66 ± 0.63 | <0.001 |
| TG (mmol/L) | 2.76 ± 2.17 | 2.01 ± 1.34 | <0.001 |
| TC (mmol/L) | 5.00 ± 1.46 | 4.37 ± 0.85 | <0.001 |
| HDL-C (mmol/L) | 1.01 ± 0.28 | 1.05 ± 0.21 | 0.062 |
| Normal HDL-C | 126 (47.2) | 143 (57.9) | 0.017 |
| Hypertension | 123 (46.1) | 44 (17.8) | <0.001 |
| Diabetes | 91 (34.1) | 4 (1.6) | <0.001 |
| Smoking | 221 (82.8) | 165 (66.8) | <0.001 |
| Alcohol | 86 (32.2) | 74 (30.0) | 0.634 |
| Family history of premature CHD | 87 (32.6) | 28 (11.3) | <0.001 |
Data were presented as mean ± SD or n (%). BMI: Body mass index; Hb: Hemoglobin; BUN: Blood urea nitrogen; Cr: Creatinine; UA: Uric acid; LDL-C: Low-density lipoprotein cholesterol; TG: Triglyceride; TC: Total cholesterol; HDL-C: High-density lipoprotein cholesterol; CHD: Coronary heart disease; AMI: Acute myocardial infarction; SD: Standard deviation.
In the logistic regression analysis, which considered the occurrence of AMI as the binary dependent variable, age, BMI, Hb, blood urea nitrogen (BUN), Cr, UA, LDL-C, TG, TC, normal HDL-C, hypertension, diabetes, smoking, alcohol, and family history of premature CHD were considered independent variables. It was found that AMI in young males was significantly and positively associated with family history of premature CHD, smoking, diabetes, hypertension, LDL-C, and TG while inversely associated with HDL-C and Hb. The regression coefficient of HDL-C was −0.754 [Table 2].
Table 2.
Predictive value of HDL-C using logistic regression analysis
| Items | B | Exp(B) | 95% CI for Exp(B) | P | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Normal HDL-C | −0.754 | 0.471 | 0.297 | 0.746 | 0.001 |
| Family history of premature CHD | 1.119 | 3.062 | 1.755 | 5.340 | 0.000 |
| Smoking | 0.693 | 1.999 | 1.200 | 3.329 | 0.008 |
| Diabetes | 2.988 | 19.848 | 6.805 | 57.899 | 0.000 |
| Hypertension | 1.277 | 3.586 | 2.227 | 5.774 | 0.000 |
| LDL-C | 0.529 | 1.697 | 1.246 | 2.312 | 0.001 |
| Hb | −0.035 | 0.966 | 0.948 | 0.984 | 0.000 |
| TG | 0.232 | 1.261 | 1.076 | 1.477 | 0.004 |
CI: Confidence interval; HDL-C: High-density lipoprotein cholesterol; CHD: Coronary heart disease; LDL-C: Low-density lipoprotein cholesterol; Hb: Hemoglobin; TG: Triglyceride.
The baseline characteristics of prognostic study groups (normal and low HDL-C groups) are summarized in Table 3. There were no significant differences between the low HDL-C and normal HDL-C group, with regard to age, non-ST segment elevated MI, PCI, BMI, Hb, BUN, Cr, TC, LDL-C, ejection fraction of left ventricular, hypertension, smoking, and family history of premature CHD. The patients of normal HDL-C group had higher level of TG, decreased proportion of single-vessel lesion and alcohol, and increased proportion of alcohol.
Table 3.
Baseline characteristics of prognostic study groups (normal and low HDL-C groups)
| Characteristics | Low HDL-C (n = 141) | Normal HDL-C (n = 126) | P |
|---|---|---|---|
| MACE | 34 (24.1) | 15 (11.9) | 0.011 |
| NSTEMI | 13 (10.6) | 12 (9.5) | 0.548 |
| PCI | 130 (92.2) | 118 (93.7) | 0.414 |
| Age (years) | 38.72 ± 3.89 | 38.63 ± 5.01 | 0.871 |
| BMI (kg/m2) | 26.96 ± 2.68 | 26.89 ± 1.89 | 0.819 |
| Hb (g/L) | 146.60 ± 12.28 | 149.57 ± 12.65 | 0.054 |
| BUN (mmol/L) | 4.90 ± 1.47 | 4.57 ± 1.30 | 0.061 |
| Cr (mmol/L) | 82.83 ± 16.34 | 84.92 ± 19.10 | 0.338 |
| UA (mmol/L) | 384.01 ± 106.55 | 359.00 ± 88.22 | 0.039 |
| TG (mmol/L) | 3.37 ± 2.78 | 2.07 ± 0.70 | 0.000 |
| TC (mmol/L) | 5.04 ± 1.72 | 4.96 ± 1.11 | 0.661 |
| LDL-C (mmol/L) | 3.03 ± 0.81 | 3.06 ± 0.93 | 0.787 |
| HDL-C (mmol/L) | 0.84 ± 0.16 | 1.26 ± 0.19 | 0.000 |
| EF (%) | 57.00 ± 9.47 | 56.25 ± 8.81 | 0.507 |
| Hypertension | 69 (48.9) | 54 (42.9) | 0.328 |
| Diabetes | 61 (43.3) | 30 (23.8) | 0.001 |
| Smoking | 117 (83.0) | 104 (82.5) | 1.000 |
| Single-vessel lesion | 60 (42.6) | 90 (71.4) | 0.000 |
| Alcohol | 32 (22.7) | 54 (42.9) | 0.001 |
| Family history of premature CHD | 51 (36.2) | 36 (28.6) | 0.194 |
| Drug therapy of during follow-up period | |||
| Dual antiplatelet treatment | 133 (94.3) | 120 (95.2) | 0.479 |
| Lipid-lowing medication | 119 (84.4) | 105 (83.3) | 0.472 |
| β-blockers | 108 (76.6) | 92 (73.0) | 0.297 |
| ACE inhibitors or AT1 blockers | 91 (64.5) | 83 (65.9) | 0.461 |
Data were presented as mean ± SD or n (%). MACE: Major adverse cardiovascular event; NSTEMI: Non-ST segment elevated myocardial infarction; PCI: Percutaneous coronary intervention; BMI: Body mass index; Hb: Hemoglobin; BUN: Blood urea nitrogen; Cr: Creatinine; UA: Uric acid; TG: Triglyceride; TC: Total cholesterol; LDL-C: Low-density lipoprotein cholesterol; HDL-C: High-density lipoprotein cholesterol; EF: Ejection fraction; CHD: Coronary heart disease; SD: Standard deviation; ACE: Angiotensin-converting enzyme; AT1: Angiotensin II Type 1.
All of AMI patients were followed up for up to two years. The Kaplan-Meier analyses for MACE showed that there were significant differences between the low and normal HDL-C groups after AMI [Figure 1]. MACEs during the study period occurred in 49 patients. There were 34 (24.1%) patients in low HDL-C group which was more than 2-fold increase compared with 15 (11.9%) in normal HDL-C group (P < 0.05). Among these patients, 31 developed ACS (20 in low HDL-C group and 11 in normal HDL-C group), 16 had a revascularization procedure (11 in low HDL-C group and 5 in normal HDL-C group), 2 had arrhythmic events (2 in low HDL-C group), and no death occurred.
Figure 1.
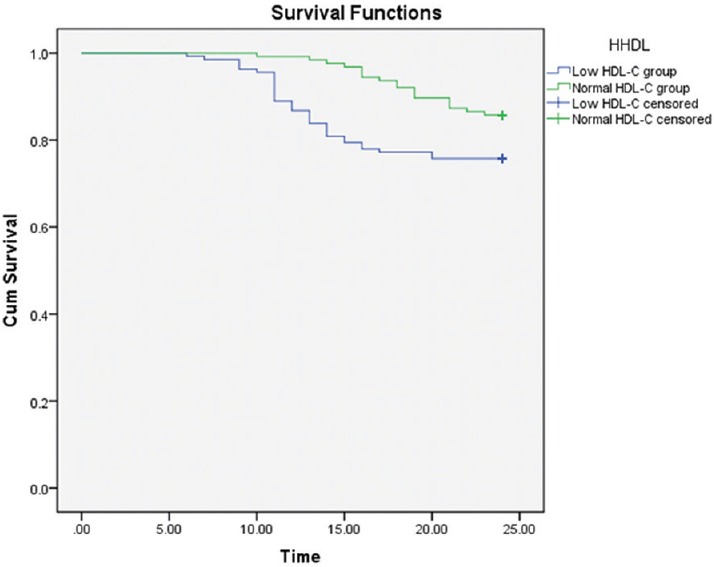
The Kaplan–Meier survival curves of the two groups of acute myocardial infarction patients: Cumulative occurrence of major adverse coronary event. Patients were divided into normal and low high-density lipoprotein cholesterol subgroups using the cutoff level of 1.04 mmol/L (P = 0.006) (Log Rank, χ2 = 7.610).
Multivariate Cox regression analysis showed that hypertension, diabetes, LDL-C, family history of premature CHD, single-vessel lesion, and normal HDL-C predicted the occurrence of MACE in the study participants. The regression coefficient was −1.014, with HR 0.354 (95% CI = 0.181–0.726, P = 0.006) [Table 4].
Table 4.
Prognostic value of HDL-C using multivariate Cox regression analysis
| Items | B | Exp(B) | 95% CI for Exp(B) | P | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Hypertension | 0.696 | 2.006 | 1.026 | 3.923 | 0.042 |
| Diabetes | 2.120 | 8.334 | 4.087 | 16.995 | 0.000 |
| Single-vessel lesion | −1.888 | 0.151 | 0.066 | 0.347 | 0.000 |
| LDL-C | 1.282 | 3.605 | 2.488 | 5.222 | 0.000 |
| Family history of premature CHD | 0.747 | 2.111 | 1.143 | 3.898 | 0.017 |
| Normal HDL-C | −1.014 | 0.354 | 0.181 | 0.726 | 0.006 |
HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; CHD: Coronary heart disease; CI: Confidence interval.
Discussion
In this study, we investigated two relevant researches to evaluate the effect of HDL-C for predicting the risk of developing AMI and the outcomes of AMI after discharge. The results indicated a strong relationship between HDL-C level and both the beginning and 2-year prognosis of AMI in young males.
A reduced HDL-C level is an ASCVD risk factor used in ASCVD risk factor counting and quantitative risk assessment.[5] Epidemiologically, HDL-C is an independent cardiovascular risk factor[6] and has an inverse relationship with ASCVD risk, irrespective of gender, race, and ethnicity.[5] HDL-C has been known to be protective against atherosclerosis by several mechanisms. HDL-C takes an anti-atherosclerotic role by the reverse transport of cholesterol from the peripheral tissues.[7] Moreover, HDL-C may possess antioxidant and anti-inflammatory actions[8] and inhibit the cytokine-induced expression of endothelial cell adhesion molecules, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1.[9] In addition, HDL can inhibit thrombus formation.[10] HDL-C particles include many surface proteins and lipids that influence the function of HDL and may provide atheroprotection via favorable effects upon atherosclerotic mechanisms, including modulation of inflammation, oxidation, endothelial function, and insulin secretory capacity.
We found that the blood lipids of young males with AMI were different from controls, which have a high level of TC, TG, LDL-C (P < 0.05), and HDL-C (1.01 ± 0.28 vs. 1.05 ± 0.21, P > 0.05). The levels of HDL-C of the two groups have not yet reached statistical significance, but there have been changes in the results. Moreover, when using the cutoff point of 1.04 mmol/L HDL-C, AMI patients had decreased proportion in normal HDL-C group (47.2%) compared to controls (57.9%). This result suggested that dyslipidemia in young AMI males was prevalent and closely associated to the development of AMI. In addition, the characteristics of this particular patients were more closely aligned with metabolic syndrome diagnostic criteria of Adult Treatment Panel III,[11] including increased BMI and abnormal level of TG, HDL-C. The decrease in HDL-C and hypertriglyceridemia as important features of the metabolic syndrome play an important role in the development of atherosclerotic CHD.
According to the results, it was estimated that 82.8% of AMI young males smoked, even though 66.8% in controls, as in other series that have reported that the proportion of smokers among premature patients was between 76% and 92%.[12,13,14,15] Smoking can decrease in serum antioxidant activity, which further promotes the composition of blood lipids (lowering HDL-C and aggravating LDL-C).[16]
Based on the comparisons of two groups, we can see that when risk factors such as BMI, smoking, family history of premature CHD, hypertension, and diabetes mellitus accumulated together, these risk factors formed in a positive way of interaction and coordination. Hence, nonlipid ASCVD risk factors should also be managed appropriately, particularly hypertension, smoking, and diabetes mellitus in young males.
In the logistic regression analysis, traditional risk factors (hypertension, diabetes mellitus, family history of premature CHD, and smoking) still play a pivotal role in young males with AMI and should be controlled as the elderly. HDL-C has been showed an inverse correlation in MI, which suggested that the change of HDL-C level was closely related to AMI in young men, and normal level of HDL-C was a protective factor from AMI.
As using the cutoff point of 1.04 mmol/L level of HDL-C, there were differences in the two subgroups. The proportion of the single-vessel lesions and alcohol consumption reduced in low HDL-C with the increase of diabetes, which indicated that coronary artery lesions are correlated with level of HDL-C. When young males have lower levels of HDL-C, it was not only indicated that the incidence of MI will be increased but also indicated that the degree of coronary artery lesions will be more serious. It was also suggested that patients with diabetes have lower level of HDL-C, and appropriate alcohol intake can increase the level of HDL-C.[5] As reported, HDL-C levels increase with increasing intake of alcohol for both sexes in agreement with the established role of HDL-C as a biomarker of alcohol intake.[17] According to Rimm et al.,[18] HDL-C concentrations will increase by 0.133 mg/dl, i.e., 0.0035 mmol·L−1·g−1 of alcohol consumed a day.
We found that hypertension, diabetes, LDL-C, family history of premature CHD, single-vessel lesion, and HDL-C were independent predictors of MACE after AMI. All of the first four factors are well-known predictors of long-term mortality in middle-aged or elderly patients with CAD.[19,20,21] However, there are few data regarding their prognostic impact in young males of AMI. The impressive finding of our study was that HDL-C was the protective predictor for recurrent cardiac events in young males with AMI. Patients with low HDL-C were associated with 2-fold increase for MACE compared to patients with normal HDL-C (24.1% vs. 11.9%, P < 0.05) during the follow-up period. The HDL-C level at the onset of AMI in young males was closely related to the prognosis of this particular people. Qun Lu also reported that the HDL-C level was an independent predictor of a major cardiovascular event in PCI patients with a follow-up of 120 weeks. It is plausible to speculate that young males might have more benefits from high level of HDL-C at the onset of AMI. Therefore, it is important to evaluate the HDL-C level of young males who may benefit from an increased HDL-C level for the prevention of AMI and prediction in prognosis.
Our study has some potential limitations needed to be considered. First, all patients enrolled were hospitalized in one center. The sample sizes were relatively small. Second, the study objectives were selected from patients completing coronary angiography during hospitalization. We might lost few AMI patients who could not undergo angiography before discharge for contraindications. Third, the time of follow-up was not long enough for this specific population. Although MACEs occurred in 49 patients, mortality rate in our study (0.0%) is discordant to other studies in young coronary patients for long-term study (one 15-year mortality rate was 25.5% among 108 patients aged ≤40 years with AMI;[22] another 15-year mortality of 30% in 843 patients diagnosed as having CAD under the age of 40 years, of whom 55% had a history of AMI[23]). However, these studies were performed 30–40 years ago, and the perfusion treatment of AMI and drugs (i.e., ACE inhibitors, statins, and clopidogrel) were not widely applied. Therefore, the low mortality rate in our study is mainly due to the improved pattern of treatment during the acute phase of MI and after patients’ hospital discharge.
In conclusion, we found that the levels of HDL-C were the lipid parameter strongly associated with premature AMI. In addition, the HDL-C levels at the onset of AMI could predict the cardiovascular events of 2-year follow-up in young males. This study suggests that more attention is needed to evaluate HDL-C levels in young males, and it might serve as a powerful risk predictor in young males (≤44 years).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
References
- 1.Doughty M, Mehta R, Bruckman D, Das S, Karavite D, Tsai T, et al. Acute myocardial infarction in the young – The University of Michigan experience. Am Heart J. 2002;143:56–62. doi: 10.1067/mhj.2002.120300. doi: 10.1067/mhj.2002.120300. [DOI] [PubMed] [Google Scholar]
- 2.Pineda J, Marín F, Marco P, Roldán V, Valencia J, Ruiz-Nodar JM, et al. Premature coronary artery disease in young (age <45) subjects: Interactions of lipid profile, thrombophilic and haemostatic markers. Int J Cardiol. 2009;136:222–5. doi: 10.1016/j.ijcard.2008.04.020. doi: 10.1016/j.ijcard.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 3.van Loon JE, de Maat MP, Deckers JW, van Domburg RT, Leebeek FW. Prognostic markers in young patients with premature coronary heart disease. Atherosclerosis. 2012;224:213–7. doi: 10.1016/j.atherosclerosis.2012.06.067. doi: 10.1016/j.atherosclerosis.2012.06.067. [DOI] [PubMed] [Google Scholar]
- 4.Schoenenberger AW, Radovanovic D, Stauffer JC, Windecker S, Urban P, Niedermaier G, et al. Acute coronary syndromes in young patients: Presentation, treatment and outcome. Int J Cardiol. 2011;148:300–4. doi: 10.1016/j.ijcard.2009.11.009. doi: 10.1016/j.ijcard.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Bays HE, Jones PH, Brown WV, Jacobson TA National Lipid Association. National Lipid Association Annual Summary of Clinical Lipidology 2015. J Clin Lipidol. 2014;8(6 Suppl):S1–36. doi: 10.1016/j.jacl.2014.10.002. doi: 10.1016/j.jacl.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Natarajan P, Ray KK, Cannon CP. High-density lipoprotein and coronary heart disease: Current and future therapies. J Am Coll Cardiol. 2010;55:1283–99. doi: 10.1016/j.jacc.2010.01.008. doi: 10.1016/j.jacc.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Kapur NK, Ashen D, Blumenthal RS. High density lipoprotein cholesterol: An evolving target of therapy in the management of cardiovascular disease. Vasc Health Risk Manag. 2008;4:39–57. doi: 10.2147/vhrm.2008.04.01.39. doi: 10.2147/VHRM.S1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deakin SP, Bioletto S, Bochaton-Piallat ML, James RW. HDL-associated paraoxonase-1 can redistribute to cell membranes and influence sensitivity to oxidative stress. Free Radic Biol Med. 2011;50:102–9. doi: 10.1016/j.freeradbiomed.2010.09.002. doi: 10.1016/j.freeradbiomed.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Zhong QQ, Zhao SP, Wang X, Yu BL, Dong J. Effect of high-density lipoprotein on interleukin-8 secretion in 3T3-L1 adipocytes (in Chinese) Chin J Endocrinol Metab. 2010;26:888–90. doi: 10.7150/ijbs.10258. [Google Scholar]
- 10.Kaneko T, Wada H, Wakita Y, Minamikawa K, Nakase T, Mori Y, et al. Enhanced tissue factor activity and plasminogen activator inhibitor-1 antigen in human umbilical vein endothelial cells incubated with lipoproteins. Blood Coagul Fibrinolysis. 1994;5:385–92. [PubMed] [Google Scholar]
- 11.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 12.Barbash GI, White HD, Modan M, Diaz R, Hampton JR, Heikkila J, et al. Acute myocardial infarction in the young – The role of smoking. The investigators of the international tissue plasminogen activator/streptokinase mortality trial. Eur Heart J. 1995;16:313–6. [PubMed] [Google Scholar]
- 13.von Eyben FE, Bech J, Madsen JK, Efsen F. High prevalence of smoking in young patients with acute myocardial infarction. J R Soc Health. 1996;116:153–6. doi: 10.1177/146642409611600305. [DOI] [PubMed] [Google Scholar]
- 14.Panagiotakos DB, Rallidis LS, Pitsavos C, Stefanadis C, Kremastinos D. Cigarette smoking and myocardial infarction in young men and women: a case-control study. Int J Cardiol. 2007;116:371–5. doi: 10.1016/j.ijcard.2006.04.051. doi: 10.1016/j.ijcard.2006.04.051. [DOI] [PubMed] [Google Scholar]
- 15.Al-Khadra AH. Clinical profile of young patients with acute myocardial infarction in Saudi Arabia. Int J Cardiol. 2003;91:9–13. doi: 10.1016/s0167-5273(02)00579-x. doi: 10.1016/S0167-5273(02)00579-X. [DOI] [PubMed] [Google Scholar]
- 16.Rallidis LS, Sakadakis EA, Tympas K, Varounis C, Zolindaki M, Dagres N, et al. The impact of smoking on long-term outcome of patients with premature (≤35 years) ST-segment elevation acute myocardial infarction. Am Heart J. 2015;169:356–62. doi: 10.1016/j.ahj.2014.12.003. doi: 10.1016/j.ahj.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Magnus P, Bakke E, Hoff DA, Høiseth G, Graff-Iversen S, Knudsen GP, et al. Controlling for high-density lipoprotein cholesterol does not affect the magnitude of the relationship between alcohol and coronary heart disease. Circulation. 2011;124:2296–302. doi: 10.1161/CIRCULATIONAHA.111.036491. doi: 10.1161/CIRCULATIONAHA.111.036491. [DOI] [PubMed] [Google Scholar]
- 18.Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: Meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319:1523–8. doi: 10.1136/bmj.319.7224.1523. doi: 10.1136/bmj.319.7224.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rea TD, Heckbert SR, Kaplan RC, Smith NL, Lemaitre RN, Psaty BM. Smoking status and risk for recurrent coronary events after myocardial infarction. Ann Intern Med. 2002;137:494–500. doi: 10.7326/0003-4819-137-6-200209170-00009. [DOI] [PubMed] [Google Scholar]
- 20.Starling MR, Crawford MH, Henry RL, Lembo NJ, Kennedy GT, O’Rourke RA. Prognostic value of electrocardiographic exercise testing and noninvasive assessment of left ventricular ejection fraction soon after acute myocardial infarction. Am J Cardiol. 1986;57:532–7. doi: 10.1016/0002-9149(86)90830-1. doi: 10.1016/0002-9149(86)90830-1. [DOI] [PubMed] [Google Scholar]
- 21.Herlitz J, Abrahamsson P, Dellborg M, Karlson BW, Karlsson T, Lindqvist J. Long-term survival after development of acute myocardial infarction has improved after a more widespread use of thrombolysis and aspirin. Cardiology. 1999;91:250–5. doi: 10.1159/000006919. doi: 10.1159/000006919. [DOI] [PubMed] [Google Scholar]
- 22.Cole JH, Miller JI, 3rd, Sperling LS, Weintraub WS. Long-term follow-up of coronary artery disease presenting in young adults. J Am Coll Cardiol. 2003;41:521–8. doi: 10.1016/s0735-1097(02)02862-0. doi: 10.1016/S0735-1097(02)02862-0. [DOI] [PubMed] [Google Scholar]
- 23.Fournier JA, Cabezón S, Cayuela A, Ballesteros SM, Cortacero JA, Díaz De La Llera LS. Long-term prognosis of patients having acute myocardial infarction when ≤40 years of age. Am J Cardiol. 2004;94:989–92. doi: 10.1016/j.amjcard.2004.06.051. doi: 10.1016/j.amjcard.2004.06.051. [DOI] [PubMed] [Google Scholar]


