Abstract
Background:
Epigallocatechin-3-gallate (EGCG) has exhibited antitumor properties in several types of cancers, including nasopharyngeal carcinoma (NPC), but the molecular mechanisms underlying this function remain incompletely understood. The aim of the present study was to characterize the global impact of EGCG on the expression of microRNAs (miRNAs) in NPC cells.
Methods:
Using microarray analysis, the alterations of miRNA expression profiles were investigated in EGCG-treated CNE2 cells. Furthermore, the target genes and signaling pathways regulated by EGCG-specific miRNAs were identified using target prediction program and gene ontology analysis.
Results:
A total of 14 miRNAs exhibited >2-fold expression changes in a dose-dependent manner after treatment with 20 μmol/L and 40 μmol/L EGCG. Totally 43, 49, and 52 target genes from these differentially expressed miRNAs were associated with the apoptosis, cell cycle regulation, and cell proliferation, respectively. A total of 66 signaling pathways, primarily involved in cancer development and lipid and glucose metabolism, were shown to be regulated by EGCG-specific miRNAs.
Conclusion:
EGCG induces considerable alterations of miRNA expression profiles in CNE2 cells, which provides mechanistic insights into cellular responses and antitumor activity mediated by EGCG.
Keywords: Antitumor Agents, Epigallocatechin-3-gallate, MicroRNAs, Nasopharyngeal Carcinoma
Introduction
Green tea (Camellia sinensis) is widely consumed for its characteristic flavor and potential health benefits. Epidemiological studies have revealed that the consumption of green tea is associated with a reduced risk of various cancers, including breast cancer, lung cancer, colon cancer, and nasopharyngeal carcinoma (NPC).[1,2] The most abundant and active anticancer constituent in green tea is epigallocatechin-3-gallate (EGCG). The cancer preventive and inhibitory effects of EGCG have been extensively demonstrated in different tumor cell lines and animal models, and its mechanisms are involved in cell cycle arrest, apoptosis, angiogenesis, and anti-oxidant activity.[3] However, the molecular mechanisms underlying these effects are not fully understood.
NPC is one of the most common malignancies prevalent in South China and Southeast Asia.[4,5] The development of NPC involves cumulative genetic and epigenetic changes, among which oncogene activation and anti-oncogene inactivation play critical roles. MicroRNAs (miRNAs) are small noncoding RNAs involved in the posttranscriptional regulation of gene expression by binding to 3’-untranslated regions of their target miRNAs. The dysregulation of miRNAs has been closely correlated with the initiation and progression of various cancers, including NPC.[6] Moreover, there is evidence that the expression pattern of miRNAs in cancer cells was obviously influenced by anticancer agents, suggesting that some tumor-specific miRNAs may serve as the targets for cancer therapy.[7]
EGCG has been shown to inhibit proliferation, clonogenicity, and invasiveness of NPC cells in vivo and in vitro.[8,9] It remains to be determined whether the cellular changes in NPC cells treated with EGCG are associated with the alternations in miRNA expression. Here, we used miRNA microarray analysis to characterize the effects of EGCG on miRNA expression profiles in CNE2 cells. The mRNA targets of the miRNAs regulated by EGCG may contribute to the identification of new molecular mechanisms for the action of EGCG in NPC.
Methods
Reagents and cell line
EGCG (≥95%) was purchased from Sigma-Aldrich (St. Louis, MO, USA), dissolved in dimethyl sulfoxide (DMSO), and stored at −20°C. CNE2 cells, a poorly differentiated NPC cell line, were cultured in RPMI 1640 medium (GIBCO, Grand Island, NY, USA), supplemented with 10% fetal bovine serum (GIBCO), 100 U/ml penicillin, and 100 mg/ml streptomycin (Invitrogen, Carlsbad, CA, USA).
MicroRNA microarray assay
CNE2 cells were treated with 20 μmol/L or 40 μmol/L of EGCG dissolved in DMSO (final concentration in culture media, 0.1%) for 24 h. DMSO was also added to culture media as a vehicle control. Total RNA was isolated using the mirVana RNA isolation kit (Ambion, Austin, TX, USA). The miRNA expression profiles were determined at the Bioassay Laboratory of CapitalBio Corporation (Beijing, China) using Agilent Human miRNA 8*60K Microarray Release 16.0 (Agilent Technologies, Santa Clara, CA, USA).
Quantitative real-time polymerase chain reaction
To verify the results obtained from the microarray analysis, quantitative real-time polymerase chain reaction (qRT-PCR) was carried out to detect the expression levels of differentially expressed miRNAs. Total RNA from cells was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and then was subjected to reverse transcription reaction to synthesize cDNA using the MMLV reverse transcription kit (Promega, Madison, WI, USA). qRT-PCR was performed with FS Universal SYBR Green Master (Roche, Foster City, CA, USA) in the ABI PRISM 7500 System (Applied Biosystems, Foster City, CA, USA) according to the manufacture's protocol. The bulge-loop miRNA qRT-PCR primer sets (one reverse transcription primer and a pair of quantitative PCR primers for each set) were designed and synthesized by RiboBio (Guangzhou, China). U6 small nuclear RNA was used as an endogenous control. The fold change of miRNA in log 2 scale was calculated by the equation 2−ΔΔCt, where ΔCt = CtmiRNA– CtU6 and ΔΔCt = ΔCttreated samples−ΔCtuntreated controls. Comparisons between groups were done by one-way analysis of variance with Dunnett's post hoc corrections for multiple comparisons. Statistical analysis was performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered significant differences.
Bioinformatics analysis
The differentially expressed miRNAs that either increased or decreased by more than 2-fold following EGCG treatment were chosen for target prediction in online databases. Two independent searches were performed using TargetScan 6.2 (http://www.targetscan.org/) and miRDB (http://mirdb.org/miRDB/), and the results were compared. A common set of presumptive targets of each significant miRNA was identified and collected in a target pool. To determine the functions of the predicted target genes, the gene ontology (GO) of the target pool was analyzed using CapitalBio Molecule Annotation System 3.0 (MAS, http://bioinfo.capitalbio.com/mas3/). This database enables the assignment of predicted target genes to functional groups based on biological process, cellular component, and molecular function. The pathways modified by EGCG through putative targets of specific miRNAs were further analyzed using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. The significant GO terms and KEGG pathways were determined by P and Q < 0.001.
Results
MiRNA expression profiles in CNE2 cells treated with epigallocatechin-3-gallate
Previous studies have shown that EGCG inhibited the proliferation of CNE2 cells in time- and dose-dependent manners.[9] As the concentration increased and action time was prolonged, EGCG also induced apoptosis in CNE2 cells.[9] To examine whether miRNAs could be involved in cellular responses to EGCG treatment, we employed miRNA microarray analysis to compare EGCG-treated and untreated cells. The doses of EGCG used in this experiment were chosen by CCK-8 assay to evaluate dose-dependent cell viability (data not shown). Among the 1205 human miRNAs in the microarray, a total of 205 miRNAs (17.0%) exhibited differential expression between the EGCG-treated and untreated cells [Figure 1a]. We further identified the miRNAs that experienced >2-fold changes in expression. As shown in Table 1, 16 of the 205 miRNAs (7.8%) exhibited >2-fold expression changes in both 20 μmol/L and 40 μmol/L EGCG-treated groups compared with the control, and another 16 miRNAs (7.8%) showed >2-fold changes only in the 40 μmol/L EGCG-treated group. Among 32 miRNAs (>2-fold changes in 40 μmol/L EGCG-treated group), 29 miRNAs were upregulated and 1 miRNA was downregulated in a dose-dependent manner [Figure 1b and Table 2]. One miRNA (hsa-miR-4281) was shown to be upregulated at similar levels in 20 μmol/L and 40 μmol/L EGCG-treated cells. Interestingly, although the expression of hsa-miR-29b-1-5p was upregulated at both concentrations of EGCG treatment, the fold change was decreased in the higher concentration group (4.82–2.61-fold). These data strongly indicated that EGCG is able to influence the expression of specific miRNAs in CNE2 cells.
Figure 1.
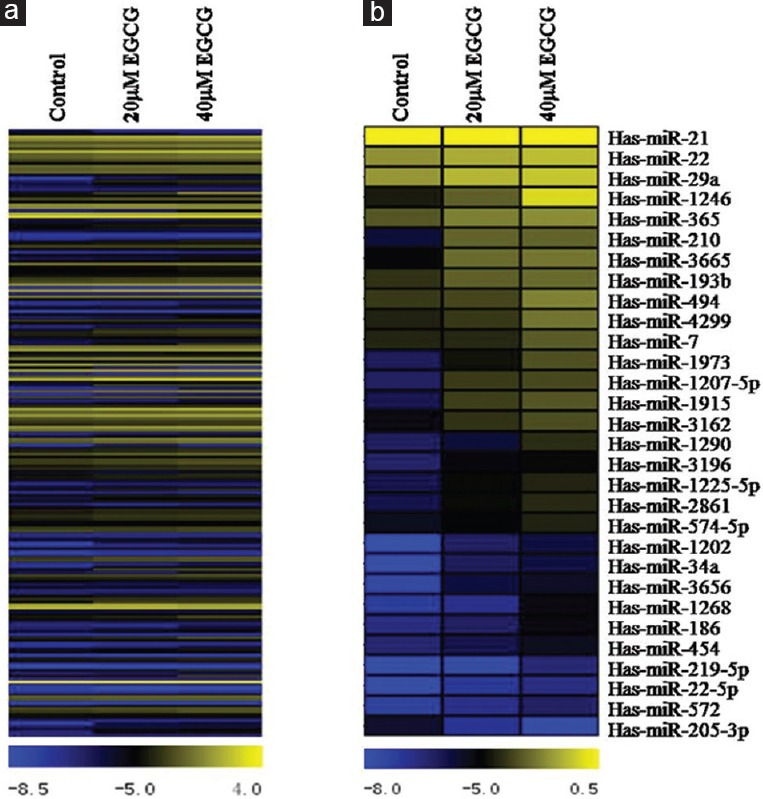
The microRNA expression profiles of CNE2 cells after epigallocatechin-3-gallate treatment. A total of 205 human microRNAs showed differential expression between the epigallocatechin-3-gallate-treated and untreated cells (a); 30 microRNAs exhibited >2-fold expression changes after 40 μmol/L epigallocatechin-3-gallate treatment in a dose-dependent manner (b). The relative expression levels of these microRNAs are represented in a color scale ranging from blue (downregulation) to yellow (upregulation) for each treatment group (control, 20 μmol/L and 40 μmol/L epigallocatechin-3-gallate).
Table 1.
MiRNAs with >2-fold changes in CNE2 cells after EGCG treatment
| EGCG dose (µmol/L) | Number of miRNAs | MiRNAs | |
|---|---|---|---|
| Upregulated | Downregulated | ||
| 20 and 40 | 16 | hsa-miR-1202, hsa-miR-1207-5p | hsa-miR-205-3p |
| hsa-miR-1225-5p, hsa-miR-1246 | |||
| hsa-miR-1915, hsa-miR-1973 | |||
| hsa-miR-210, hsa-miR-2861 | |||
| hsa-miR-29b1-5p, hsa-miR-3162 | |||
| hsa-miR-3196, hsa-miR-34a | |||
| hsa-miR-3656, hsa-miR-3665 | |||
| hsa-miR-4281 | |||
| 40 only | 16 | hsa-miR-1268, hsa-miR-1290 | |
| hsa-miR-186, hsa-miR-193b | |||
| hsa-miR-21, hsa-miR-219-5p | |||
| hsa-miR-22, hsa-miR-22-5p | |||
| hsa-miR-29a, hsa-miR-365 | |||
| hsa-miR-4299, hsa-miR-454 | |||
| hsa-miR-494, hsa-miR-572 | |||
| hsa-miR-574-5p, hsa-miR-7 | |||
EGCG: Epigallocatechin-3-gallate; miRNAs: microRNAs.
Table 2.
Expression levels of miRNAs exhibiting >2-fold changes in response to both concentrations of EGCG
| MiRNAs | Accession number | Fold change | Up-/down-regulation | ||
|---|---|---|---|---|---|
| 20 versus 0 | 40 versus 0 | 40 versus 20 | |||
| hsa-miR-1246 | MIMAT0005898 | 2.86 | 17.51 | 6.12 | Up |
| hsa-miR-1973 | MIMAT0009448 | 2.79 | 7.28 | 2.60 | Up |
| hsa-miR-210 | MIMAT0000267 | 4.14 | 7.14 | 1.73 | Up |
| hsa-miR-1915 | MIMAT0007892 | 5.00 | 6.95 | 1.39 | Up |
| hsa-miR-1207-5p | MIMAT0005871 | 5.69 | 6.93 | 1.22 | Up |
| hsa-miR-3665 | MIMAT0018087 | 4.53 | 5.22 | 1.15 | Up |
| hsa-miR-1202 | MIMAT0005865 | 3.56 | 4.87 | 1.37 | Up |
| hsa-miR-34a | MIMAT0000255 | 3.30 | 4.21 | 1.28 | Up |
| hsa-miR-3656 | MIMAT0018076 | 3.38 | 4.16 | 1.23 | Up |
| hsa-miR-3162 | MIMAT0015036 | 2.45 | 3.55 | 1.45 | Up |
| hsa-miR-2861 | MIMAT0013802 | 2.09 | 3.35 | 1.60 | Up |
| hsa-miR-1225-5p | MIMAT0005572 | 2.09 | 3.19 | 1.53 | Up |
| hsa-miR-3196 | MIMAT0015080 | 2.23 | 2.70 | 1.21 | Up |
| hsa-miR-4281 | MIMAT0018087 | 4.70 | 4.30 | −1.10 | Up |
| hsa-miR-29b-1-5p | MIMAT0004514 | 4.82 | 2.61 | −1.84 | Up |
| hsa-miR-205-3p | MIMAT0009197 | −2.65 | −4.58 | −1.73 | Down |
EGCG: Epigallocatechin-3-gallate; miRNAs: microRNA
To validate the microarray results, we performed qRT-PCR to detect the expression levels of six differentially expressed miRNAs (upregulated: hsa-miR-1246, hsa-miR-1973, hsa-miR-210, hsa-miR-1915, and hsa-miR-34a; downregulated: hsa-miR-205-3p) after EGCG treatment [Figure 2]. The relative expression levels of these miRNAs between the EGCG-treated and untreated cells were shown to be comparable to those observed by microarray analysis. Overall, these results confirmed our findings of differential miRNAs expression by microarray.
Figure 2.
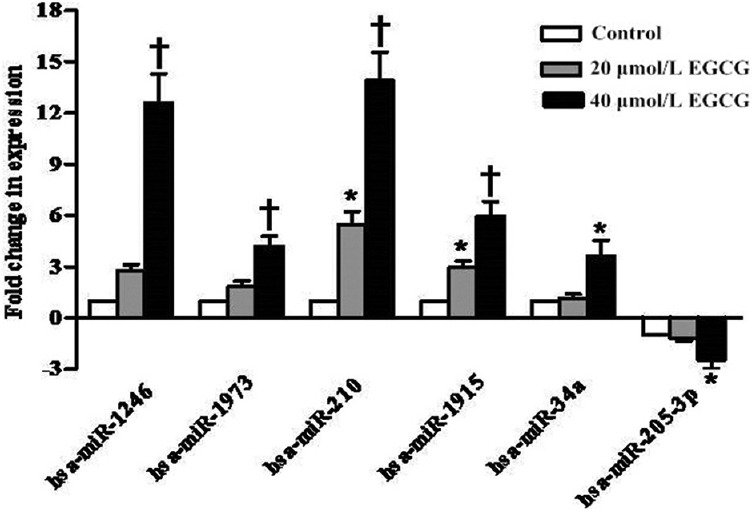
The effect of epigallocatechin-3-gallate on expression levels of the microRNAs was validated by quantitative real-time polymerase chain reaction. Fold changes in microRNA expression are expressed as mean ± standard deviation (n = 3). *P < 0.05, †P < 0.001 versus control group.
Identification of putative targets of differentially expressed microRNAs
The miRNAs that showed a dose-dependent response to EGCG treatment might be involved to EGCG-mediated anticancer properties. To elucidate the biological functions of EGCG-specific miRNAs, the putative targets of 14 differentially expressed miRNAs which exhibited >2-fold expression changes in a dose-dependent manner were identified using TargetScan and miRDB databases. We identified 1767 targets of EGCG-specific upregulated miRNAs and 648 targets of EGCG-specific downregulated miRNAs (hsa-miR-205-3p was only present in the miRDB database). Subsequently, the functions of these potential targets were determined using GO classification based on gene annotation and summary information. As shown in Table 3, a total of 43, 49, and 52 target genes from EGCG-specific miRNAs were associated with the apoptosis, cell cycle regulation, and cell proliferation, respectively.
Table 3.
Functional analysis of the predicted targets of miRNAs exhibiting >2-fold changes in response to both concentrations of EGCG
| miRNA name | Apoptosis | Cell cycle | Cell proliferation |
|---|---|---|---|
| hsa-miR-1202 | PAK2 | ARL3, PAFAH1B1, EML4, UHMK1, SMAD3 | ETS1, SMAD3 |
| hsa-miR-1207-5p | ARC, MKL1, IGF1, MCL1, UBE2Z, TNS4, SH3KBP1, CSRNP1, BCAP31, RTN3, SARM1, MCL1 | FOXO4, PPP1R9B, GAS7, APBB1, IGF1, RBM14, CAMK2A, FOXN3 | SRC, LRP1, ERF, ELN, ACVRL1, DRD2, PTPRU, FGF9, NACC1, GNAI2, IGF1, PPP1R9B, APBB1, FOXO4 |
| hsa-miR-1225-5p | – | SH3BP4 | – |
| hsa-miR-1246 | SEMA6A | CREBL2 | – |
| hsa-miR-1915 | PDE1B, ZC3H12A, E2F2, SHB, SMAD3 | SET, RAD9A, NF2, GAS7, SMAD3, E2F2 | SMAD3, SOCS7, NRAS |
| hsa-miR-1973 | – | RPRM | – |
| hsa-miR-210 | BDNF | – | – |
| hsa-miR-2861 | ALX4, BMF, SARM1, MKL1, PPP1R13B, E2F2, SGPL1 | PPP1R13B, FOXN3, PPP1CA | FURIN, VSX2 |
| hsa-miR-3162 | AKTIP | – | – |
| hsa-miR-3196 | PIK3R2 | – | – |
| hsa-miR-34a | MET, MDM4, SGPP1, HSPA1B, PEA15, SYVN1, SRC, NOTCH1, IL6R, ERC1 | MTUS1, SIPA1, CCNE2, STRN3, CDK6, MAPK13, PDGFRA, KITLG, MDM4 | NOTCH1, MARCKSL1, KITLG, PDGFRB, SIPA1, ADIPOR2, MAP2K1, CSF1R, CBFA2T3, UHRF2, GAB1, SRC, RASGRP4, SIPA1, PDXK, CDK6, MDM4, TOB2 |
| hsa-miR-3656 | – | RPRM | – |
| hsa-miR-3665 | RYBP, PPARD | PPP1CB, FOXO4 | OSR2, GNAI2, FGFR1, TNN |
| hsa-miR-205-3p* | RRM2B, BCLAF1, BCL2L13, TGM2 | PPM1D, RIF1, RAD21, TFDP2, CDS1, DST, PTP4A1, PAFAH1B1, FBXO5, PPP6C, APC, NCAPG2, USP16, MPP7, MAP9, SESN3, APBB2, IL8 | TOB1, PPM1D, MNT, MDM4, NOX4, NUP62, IL8, ADAM10, CIAO1, PRDX3, GKN1, FXN |
*Predicted targets of miRNAs only come from miRDB database. EGCG: Epigallocatechin-3-gallate; miRNAs: microRNAs; –: Not applicable.
KEGG pathway analysis was used to illustrate all available pathways containing these potential genes targeted by differentially expressed miRNAs. We identified the significantly regulated pathways with both P and Q < 0.001. A total of 66 pathways were statistically enriched by target genes of EGCG-specific miRNAs. The top thirty of the most significant pathways are listed in Table 4. These pathways were primarily involved in tumor development and progression, lipid and glucose metabolism, inflammation, cytoskeleton and gap junction, and calcium signaling. Furthermore, we focused on the cancer-related pathways in KEGG system and observed that these targets were strongly associated with a wide variety of cancer pathways. The ten most significant pathways were mitogen-activated protein kinase (MAPK) signaling pathway, focal adhesion, cell adhesion molecules (CAMs), Wnt signaling pathway, ErbB signaling pathway, Adherens junction, phosphatidylinositol signaling system, p53 signaling pathway, toll-like receptor signaling pathway, and extracellular matrix (ECM)-receptor interaction.
Table 4.
KEGG pathway associating with predicted targets of miRNAs exhibiting >2-fold changes in response to both concentrations of EGCG
| Pathway | Count | P | Q |
|---|---|---|---|
| MAPK signaling pathway | 52 | 2.25E-29 | 3.93E-27 |
| Focal adhesion | 37 | 1.44E-20 | 2.47E-19 |
| Regulation of actin cytoskeleton | 38 | 1.55E-20 | 2.47E-19 |
| Insulin signaling pathway | 29 | 2.62E-18 | 3.53E-17 |
| Axon guidance | 26 | 5.25E-16 | 3.83E-15 |
| CAMs | 26 | 1.14E-15 | 7.67E-15 |
| Wnt signaling pathway | 27 | 3.25E-15 | 1.84E-14 |
| Colorectal cancer | 20 | 3.61E-14 | 1.66E-13 |
| ErbB signaling pathway | 19 | 8.38E-13 | 2.99E-12 |
| Adherens junction | 18 | 1.54E-12 | 5.38E-12 |
| Prostate cancer | 18 | 1.08E-11 | 2.97E-11 |
| Gap junction | 18 | 2.89E-11 | 7.12E-11 |
| Melanoma | 16 | 3.19E-11 | 7.75E-11 |
| Glioma | 15 | 9.14E-11 | 1.97E-10 |
| Neuroactive ligand-receptor interaction | 28 | 1.93E-10 | 3.93E-10 |
| Epithelial cell signaling in Helicobacter pylori infection | 15 | 2.82E-10 | 5.54E-10 |
| Pancreatic cancer | 15 | 5.28E-10 | 1.02E-09 |
| Endometrial cancer | 13 | 5.60E-10 | 1.06E-09 |
| Nonsmall cell lung cancer | 13 | 9.31E-10 | 1.73E-09 |
| Phosphatidylinositol signaling system | 15 | 9.59E-10 | 1.77E-09 |
| Leukocyte transendothelial migration | 18 | 2.56E-09 | 4.39E-09 |
| Calcium signaling pathway | 22 | 3.16E-09 | 5.32E-09 |
| Melanogenesis | 16 | 1.19E-08 | 1.81E-08 |
| Fc epsilon RI signaling pathway | 14 | 1.50E-08 | 2.26E-08 |
| Type II diabetes mellitus | 11 | 1.54E-08 | 2.31E-08 |
| Glycerophospholipid metabolism | 13 | 2.27E-08 | 3.33E-08 |
| B-cell receptor signaling pathway | 13 | 6.43E-08 | 9.15E-08 |
| GnRH signaling pathway | 15 | 1.20E-07 | 1.65E-07 |
| Adipocytokine signaling pathway | 12 | 1.41E-07 | 1.89E-07 |
| T-cell receptor signaling pathway | 15 | 1.55E-07 | 2.02E-07 |
EGCG: Epigallocatechin-3-gallate; miRNAs: microRNAs; MAPK: Mitogen-activated protein kinase; CAMs: Cell adhesion molecules; KEGG: Kyoto Encyclopedia of Genes and Genomes.
Discussion
Numerous miRNAs are known to function as oncogenes or tumor suppressors, regulating tumor initiation and progression at all levels.[6] The knockdown or re-expression of specific miRNAs could inhibit cell proliferation and transformation, suppress cell invasion and metastasis, and induce drug sensitivity.[10] Recently, many studies have shown that natural agents, including curcumin, resveratrol, EGCG, etc., could cause the alterations of specific miRNAs expression, which may play critical roles in their chemoprotective and therapeutic effects against tumors.[7] It is reasonable to believe that a specific or a cluster of miRNAs in NPC cells may serve as the targets for the anticancer agents. To date, the global impact of EGCG on the expression of miRNAs in NPC cells has seldomly been reported.
In the present study, we investigated the effects of EGCG on miRNA expression profiles in CNE2 cells using microarray analysis. Among the 1205 human miRNAs in the microarray, 16 miRNAs in the 20 μmol/L EGCG-treated group and 32 miRNAs in the 40 μmol/L EGCG-treated group exhibited >2-fold expression changes compared with the control. Most of these miRNAs responded to EGCG in a dose-dependent manner. Of note, the microarray results showed that the number of upregulated miRNAs was considerably higher than the number of downregulated miRNAs, indicating that the EGCG-mediated effects on CNE2 cells were associated more with miRNA upregulation. This result was contradicted to the EGCG-treated liver cancer HepG2 cells, in which the number of upregulated miRNAs was lower than that of downregulated miRNAs.[11,12] There was no overlap between the differentially expressed miRNAs in response to EGCG in HepG2 cells and those in CNE2 cells, suggesting that there may be different mechanisms of action in NPC, depending on differences in cellular contexts.
Hsa-miR-1246, the most significantly upregulated miRNA observed in the present study, was dramatically decreased in cervical cancer tissues and negatively correlated with clinical stage and HPV16E6-infected status.[13] However, Piepoli et al. showed that the relative expression of miR-1246 was significantly higher in colorectal cancer compared with matched adjacent normal tissues.[14] These data suggested a contradictory role of miR-1246 in different cancers with various cellular contexts. Hsa-miR-210 is one of the hypoxia-regulated miRNAs and functions as a tumor suppressor in regulating tumor cell growth, angiogenesis, and apoptosis.[15] In NPC CNE cells, the overexpression of miR-210 disturbed mitotic progression and reduced tumor formation through targeting a group of mitosis-related genes.[16] Similar to the results in CNE2 cells, EGCG could increase the level of miR-210 in human and mouse lung cancer cells.[17] Hsa-miR-205-3p, which was downregulated to the greatest extent in the present study, was reported to be significantly upregulated in the tissues and serum of non-small cell lung carcinoma (NSCLC) patients compared with the control groups, indicating that miR-205-3p may be a valuable biomarker for diagnosis and prognosis of NSCLC patients.[18] Overall, the modification of miRNA expression profiles may imply the potential roles of miRNAs in the anticancer effects of EGCG on NPC.
MiRNAs function by engaging with their target miRNAs, resulting in either the degradation or the inhibition of transcription. A single miRNA can regulate the expression of multiple target miRNAs, and conversely, a single target miRNA may be modulated by several miRNAs. To assess the global impact of these EGCG-specific miRNAs, we conducted the bioinformatics analysis to identify putative target genes of differently expressed miRNAs, analyze their GO, and categorize them into cancer-related biological processes including apoptosis, cell cycle regulation, and cell proliferation. E2F2, a member of E2F transcription factor family, was identified as the target gene of hsa-miR-1915 and hsa-miR-2861. Interestingly, E2F2 has either positive or negative effects on tumor development depending on the experimental contexts. Chen and Wells's studies indicated that E2F2 has strong oncogenic capacity in NIH/3T3 fibroblasts and promotes cell cycle progression and malignant transformation.[19] However, Opavsky et al. demonstrated that E2F2 inactivation in T-cells accelerated lymphomagenesis in transgenic mice expressing Myc.[20] Our data will provide useful clues to further explore the roles of E2F2 in the tumorigenesis of NPC. Hsa-miR-34a, a direct transcriptional target of p53, is downregulated in many types of cancers and acts as a tumor suppressor.[21] It has been revealed that the hypofractionated radiotherapy increased the expression of p53/miR-34a and enhanced the apoptosis in NPC cells.[22] In silico and experimental studies have validated that Notch1 is a direct and functional target of miR-34a.[23] Notch1 signaling was aberrantly activated in NPC and its overexpression promoted NPC cell proliferation, migration, and invasion.[24] It is, therefore, hypothesized that p53/miRNA-34a/Notch1 signaling may play a critical role in EGCG-mediated inhibitory effect on CNE2 cells. Among those miRNAs upregulated by EGCG, some of their target genes are also known to have oncogenic activity, for example, Src, FGFR1, Met, and PDGFRA. These data suggested that the anti-cancerogenic activity of EGCG may be derived from the effects on the expression of miRNAs targeting important regulators of cell survival.
In the view of pathway analysis, the genes targeted by differentially expressed miRNAs are most significantly associated with cancer-related signal transduction involved in regulating cell survival, cell cycle, apoptosis, invasion, and migration. The pathway most preferentially targeted by EGCG-specific miRNAs is MAPK signaling pathway. MAPK pathway is crucial in numerous stimulated cellular processes, and its dysregulation was involved in the carcinogenesis and progression of NPC.[25] EGCG exhibited strong inhibition on MAPKs activities in ras-transformed fibroblasts and lung cancer cells, without affecting the kinases in the normal cells.[26,27] Wnt signaling pathway, specifically enriched with target genes, controls a wide variety of cellular processes such as determination of cell fate, proliferation, migration, and polarity.[28] Although the roles of the Wnt signaling in NPC have not been fully explored, there is abundant evidence that the aberrant expression of Wnt pathway components, including CKIIβ, β-catenin, and CREB-binding protein, was associated with NPC development and progression.[29] EGCG has been shown to suppress Wnt signaling in colon cancer and breast cancer cells by promoting β-catenin phosphorylation and degradation.[30,31] Our data suggested that MAPK and Wnt signaling pathways might play crucial roles in the anticancer activity of EGCG against NPC cells. In addition, pathway analysis revealed that EGCG was involved in the regulation of the lipid and glucose metabolism, including insulin signaling pathway, Type II diabetes mellitus, glycerophospholipid metabolism, and adipocytokine signaling pathway. Extensive investigations have demonstrated that EGCG has positive health effects on a variety of metabolic disorders such as obesity, diabetes, insulin resistance, and dyslipidemia.[32,33] However, the molecular mechanisms by which EGCG modulates these metabolic processes remain largely unknown. Nevertheless, the view of pathway analysis in the present study provides trustworthy data to elucidate the global impact of EGCG-specific miRNAs on NPC cells.
Overall, we demonstrated that EGCG induces considerable miRNA changes in CNE2 cells. Putative targets and signaling pathways regulated by differentially expressed miRNAs were further elucidated. The characterization of EGCG-specific miRNA changes will provide mechanistic insight into the cellular responses and antitumor activity mediated by EGCG. Targeting miRNAs could be a novel strategy for cancer prevention and treatment. Further studies on the validation of putative miRNA targets and in vivo experiments are under consideration.
Financial support and sponsorship
The present study was supported by grants from the National Natural Science Foundation of China (No. 81502411), Science and Technology Planning Project of Guangdong Province (No. 2014A020212560), and PhD Start-up Fund of Guangdong Medical University (No. B2014001).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
References
- 1.Yuan JM. Cancer prevention by green tea: Evidence from epidemiologic studies. Am J Clin Nutr. 2013;98(6 Suppl):1676S–81S. doi: 10.3945/ajcn.113.058271. doi: 10.3945/ajcn.113.058271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruan HL, Xu FH, Liu WS, Feng QS, Chen LZ, Zeng YX, et al. Alcohol and tea consumption in relation to the risk of nasopharyngeal carcinoma in Guangdong, China. Front Med China. 2010;4:448–56. doi: 10.1007/s11684-010-0280-6. doi: 10.1007/s11684-010-0280-6. [DOI] [PubMed] [Google Scholar]
- 3.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–39. doi: 10.1038/nrc2641. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765–77. doi: 10.1158/1055-9965.EPI-06-0353. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 5.Wang CQ, He J. Ubiquitous distribution of Epstein-Barr virus and the Highly uneven distribution of nasopharyngeal carcinoma. Chin Med J. 2016;129:2506–7. doi: 10.4103/0366-6999.191827. doi: 10.4103/0366-6999.191827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iorio MV, Croce CM. MicroRNAs in cancer: Small molecules with a huge impact. J Clin Oncol. 2009;27:5848–56. doi: 10.1200/JCO.2009.24.0317. doi: 10.1200/JCO.2009.24.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phuah NH, Nagoor NH. Regulation of microRNAs by natural agents: New strategies in cancer therapies. Biomed Res Int 2014. 2014:804510. doi: 10.1155/2014/804510. doi: 10.1155/2014/804510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang CY, Wu CC, Hsu HY, Chuang HY, Huang SY, Tsai CH, et al. EGCG inhibits proliferation, invasiveness and tumor growth by up-regulation of adhesion molecules, suppression of gelatinases activity, and induction of apoptosis in nasopharyngeal carcinoma cells. Int J Mol Sci. 2015;16:2530–58. doi: 10.3390/ijms16022530. doi: 10.3390/ijms16022530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Yang P, Gao F, Yang J, Yao K. Effects of epigallocatechin gallate on the proliferation and apoptosis of the nasopharyngeal carcinoma cell line CNE2. Exp Ther Med. 2014;8:1783–8. doi: 10.3892/etm.2014.2020. doi: 10.3892/etm.2014.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong AW, Nemunaitis J. Modulation of miRNA activity in human cancer: A new paradigm for cancer gene therapy? Cancer Gene Ther. 2008;15:341–55. doi: 10.1038/cgt.2008.8. doi: 10.1038/cgt.2008.8. [DOI] [PubMed] [Google Scholar]
- 11.Arola-Arnal A, Bladé C. Proanthocyanidins modulate microRNA expression in human HepG2 cells. PLoS One. 2011;6:e25982. doi: 10.1371/journal.pone.0025982. doi: 10.1371/journal.pone.0025982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsang WP, Kwok TT. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells. J Nutr Biochem. 2010;21:140–6. doi: 10.1016/j.jnutbio.2008.12.003. doi: 10.1016/j.jnutbio.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Xie YJ, Xu Q, Chen JX, Shan NC, Zhang Y. Down-regulation of miR-1246 in cervical cancer tissues and its clinical significance. Gynecol Oncol. 2015;138:683–8. doi: 10.1016/j.ygyno.2015.06.015. doi: 10.1016/j.ygyno.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Piepoli A, Tavano F, Copetti M, Mazza T, Palumbo O, Panza A, et al. Mirna expression profiles identify drivers in colorectal and pancreatic cancers. PLoS One. 2012;7:e33663. doi: 10.1371/journal.pone.0033663. doi: 10.1371/journal.pone.0033663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin Q, Furong W, Baosheng L. Multiple functions of hypoxia-regulated miR-210 in cancer. J Exp Clin Cancer Res. 2014;33:50. doi: 10.1186/1756-9966-33-50. doi: 10.1186/1756-9966-33-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He J, Wu J, Xu N, Xie W, Li M, Li J, et al. MiR-210 disturbs mitotic progression through regulating a group of mitosis-related genes. Nucleic Acids Res. 2013;41:498–508. doi: 10.1093/nar/gks995. doi: 10.1093/nar/gks995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Bian S, Yang CS. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1a. Carcinogenesis. 2011;32:1881–9. doi: 10.1093/carcin/bgr218. doi: 10.1093/carcin/bgr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang M, Zhang P, Hu G, Xiao Z, Xu F, Zhong T, et al. Relative expressions of miR-205-5p, miR-205-3p, and miR-21 in tissues and serum of non-small cell lung cancer patients. Mol Cell Biochem. 2013;383:67–75. doi: 10.1007/s11010-013-1755-y. doi: 10.1007/s11010-013-1755-y. [DOI] [PubMed] [Google Scholar]
- 19.Chen C, Wells AD. Comparative analysis of E2F family member oncogenic activity. PLoS One. 2007;2:e912. doi: 10.1371/journal.pone.0000912. doi: 10.1371/journal.pone.0000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Opavsky R, Tsai SY, Guimond M, Arora A, Opavska J, Becknell B, et al. Specific tumor suppressor function for E2F2 in Myc-induced T cell lymphomagenesis. Proc Natl Acad Sci U S A. 2007;104:15400–5. doi: 10.1073/pnas.0706307104. doi: 10.1073/pnas.0706307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lodygin D, Tarasov V, Epanchintsev A, Berking C, Knyazeva T, Körner H, et al. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle. 2008;7:2591–600. doi: 10.4161/cc.7.16.6533. doi: 10.4161/cc.7.16.6533. [DOI] [PubMed] [Google Scholar]
- 22.Long Z, Wang B, Tao D, Huang Y, Tao Z. Hypofractionated radiotherapy induces miR-34a expression and enhances apoptosis in human nasopharyngeal carcinoma cells. Int J Mol Med. 2014;34:1388–94. doi: 10.3892/ijmm.2014.1937. doi: 10.3892/ijmm.2014.1937. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Guessous F, Zhang Y, Dipierro C, Kefas B, Johnson E, et al. MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes. Cancer Res. 2009;69:7569–76. doi: 10.1158/0008-5472.CAN-09-0529. doi: 10.1158/0008-5472.CAN-09-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Peng J, Zhang H, Zhu Y, Wan L, Chen J, et al. Notch1 signaling is activated in cells expressing embryonic stem cell proteins in human primary nasopharyngeal carcinoma. J Otolaryngol Head Neck Surg. 2010;39:157–66. doi: 10.2310/7070.2009.090150. [PMC free article] [PubMed] [Google Scholar]
- 25.Wu M, Li X, Li X, Li G. Signaling transduction network mediated by tumor suppressor/susceptibility genes in NPC. Curr Genomics. 2009;10:216–22. doi: 10.2174/138920209788488481. doi: 10.2174/138920209788488481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang YC, Bachrach U. The specific anti-cancer activity of green tea (-)-epigallocatechin-3-gallate (EGCG) Amino Acids. 2002;22:131–43. doi: 10.1007/s007260200002. doi: 10.1007/s007260200002. [DOI] [PubMed] [Google Scholar]
- 27.Lu G, Liao J, Yang G, Reuhl KR, Hao X, Yang CS. Inhibition of adenoma progression to adenocarcinoma in a 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis model in A/J mice by tea polyphenols and caffeine. Cancer Res. 2006;66:11494–501. doi: 10.1158/0008-5472.CAN-06-1497. doi: 10.1158/0008-5472.CAN-06-1497. [DOI] [PubMed] [Google Scholar]
- 28.Luo J, Chen J, Deng ZL, Luo X, Song WX, Sharff KA, et al. Wnt signaling and human diseases: What are the therapeutic implications? Lab Invest. 2007;87:97–103. doi: 10.1038/labinvest.3700509. doi: 10.1038/labinvest.3700509. [DOI] [PubMed] [Google Scholar]
- 29.Zeng ZY, Zhou YH, Zhang WL, Xiong W, Fan SQ, Li XL, et al. Gene expression profiling of nasopharyngeal carcinoma reveals the abnormally regulated Wnt signaling pathway. Hum Pathol. 2007;38:120–33. doi: 10.1016/j.humpath.2006.06.023. doi: 10.1016/j.humpath.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 30.Oh S, Gwak J, Park S, Yang CS. Green tea polyphenol EGCG suppresses Wnt/ß-catenin signaling by promoting GSK-3ß- and PP2A-independent ß-catenin phosphorylation/degradation. Biofactors. 2014;40:586–95. doi: 10.1002/biof.1185. doi: 10.1002/biof.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J, Zhang X, Rieger-Christ KM, Summerhayes IC, Wazer DE, Paulson KE, et al. Suppression of Wnt signaling by the green tea compound (-)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells. Requirement of the transcriptional repressor HBP1. J Biol Chem. 2006;281:10865–75. doi: 10.1074/jbc.M513378200. doi: 10.1074/jbc.M513378200. [DOI] [PubMed] [Google Scholar]
- 32.Liu G, Zheng X, Xu Y, Lu J, Chen J, Huang X. Long non-coding RNAs expression profile in HepG2 cells reveals the potential role of long non-coding RNAs in the cholesterol metabolism. Chin Med J. 2015;128:91–7. doi: 10.4103/0366-6999.147824. doi: 10.4103/0366-6999.147824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Legeay S, Rodier M, Fillon L, Faure S, Clere N. Epigallocatechin gallate: A review of its beneficial properties to prevent metabolic syndrome. Nutrients. 2015;7:5443–68. doi: 10.3390/nu7075230. doi: 10.3390/nu7075230. [DOI] [PMC free article] [PubMed] [Google Scholar]


