Abstract
Accumulative indirect evidence of the epidemiology of Mycobacterium ulcerans infections causing chronic skin ulcers (i.e., Buruli ulcer disease) suggests that the development of this pathogen and its transmission to humans are related predominantly to aquatic environments. We report that snails could transitorily harbor M. ulcerans without offering favorable conditions for its growth and replication. A novel intermediate link in the transmission chain of M. ulcerans becomes likely with predator aquatic insects in addition to phytophage insects. Water bugs, such as Naucoris cimicoides, a potential vector of M. ulcerans, were shown to be infected specifically by this bacterium after feeding on snails experimentally exposed to M. ulcerans.
Mycobacterium ulcerans is the causative agent of Buruli ulcer, one of the most common mycobacterial diseases of humans. This environmental mycobacterium has been found in swampy and wetland habitats that are home to aquatic insects implicated in the transmission of this pathogen (3-7, 10). Recently, Marsollier et al. found that certain aquatic macrophytes stimulated the growth of M. ulcerans and that the bacterium had a strong tendency to form biofilms on the plants' surfaces (2). In this report, we demonstrate the following results: (i) a few aquatic snails may harbor M. ulcerans after consuming aquatic macrophytes where an M. ulcerans biofilm has developed, (ii) no mycobacterial growth was detected in aquatic snails, so they may be a passive host, and (iii) after having eaten experimentally infected snails, the salivary glands of biting naucorid water bugs were found to contain M. ulcerans.
Experimental infection of snails.
Tropical aquatic Pomacea canaliculata (Ampullariidae) and Planorbis planorbis (Planorbidae) snails 0.5 to 0.8 cm in total shell length were housed in an aquarium with water at a temperature of 28°C without aquatic vegetation and starved for 10 days. For a 24-h period, 30 snails were placed in additional aquaria containing aquatic vegetation covered by an M. ulcerans biofilm (strain number 1G897, isolated from a skin biopsy sample from a patient from French Guiana [1]) as previously described (2). The presence of an M. ulcerans biofilm on aquatic plants was confirmed by scanning electron microscopy (2). The number of acid-fast bacilli (AFB) on aquatic plants was evaluated by the method described by Shepard and Rae (9) at 106 bacilli/g of plant tissue. After this period, the snails were removed and placed in a noncontaminated aquarium without mycobacteria. At different intervals, four snails were sacrificed, the shells were removed, and the individual tissues were ground and homogenized. A PCR was performed for the detection of M. ulcerans as previously described (3, 8). The AFB were counted and cultured with the methods described by Marsollier et al. (3). During the 72 h after feeding on vegetation-bound M. ulcerans cells, viable bacilli were found in the feces of the snails. Within the first 10 days of the experiment, a slight but nonsignificant increase in AFB was observed, whereas the number of AFB in snail tissues remained steady for 30 days after feeding on vegetation-bound M. ulcerans. Then the number decreased gradually, until AFB were no longer detected after 50 days (Fig. 1). M. ulcerans was isolated by culture only at 1, 7, and 25 days postfeeding. Some snails were also infected by other bacteria or fungi. By PCR, M. ulcerans was detected 50 days postfeeding. Negative controls were checked with snails feeding on the same kind of vegetation free of M. ulcerans.
FIG. 1.
Evolution of AFB in aquatic snail tissues (Pomacea canaliculata). The snails were experimentally infected after feeding for 24 h on aquatic vegetables covered by an M. ulcerans biofilm (day 0). Data are expressed as means ± standard deviations (n = 4).
On examination of 5-μm sections of sagittal tissue from infected snails, Ziehl-Neelsen staining showed that AFB were restricted to the digestive tract (Fig. 2). Twenty snails were placed in additional aquaria containing aquatic vegetation covered with Mycobacterium kansasii (strain number 11B0014, isolated from tap water) or with Mycobacterium szulgai (strain number 01CIMS02, isolated from an aquatic environment in Ivory Coast). Under our experimental conditions, no mycobacterial growth could be detected. Two months after the onset of experiments, no AFB were found. Moreover, AFB were rarely observed in the digestive tracts of control snails. All these data show that aquatic snails may be passive hosts of M. ulcerans and other environmental mycobacteria.
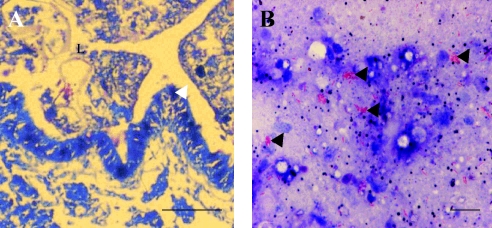
FIG. 2.
Localization of M. ulcerans in 5-μm sections of tissue from infected snails (Planorbis planorbis). (A) Ziehl-Neelsen-stained sagittal sections of an experimentally infected snail show a partial view of the lumen (L) of the digestive tract limited by epithelial cells (arrowhead) and filled by amorphous material (B) that at higher magnification contains aggregates of M. ulcerans cells (arrowheads). Scale bars: A, 200 μm; B, 40 μm.
Infection of aquatic bugs.
Adult biting water bugs, Naucoris cimicoides (Naucoridae), that were 2.5 cm long were collected from swamps in the west of France. The insects were maintained in an M. ulcerans-free aquarium as previously described (3). Ten bugs were placed in an aquarium with 20 specimens of Pomacea canaliculata experimentally infected by M. ulcerans. The snails were the exclusive food for the carnivorous water bugs. After 7 weeks, M. ulcerans was detected both by PCR and by histological studies in two insects, with a bacterial distribution localized only in the salivary glands.
Aquatic snails from areas where M. ulcerans is endemic.
Ten snails from the Planorbidae family (Planorbis sp. and Bulinus sp.) were collected in the Daloa region (Ivory Coast), an area known to be heavily affected by Buruli disease. Collections were made from the Lobo River in March 2001, just before the rainy season. By PCR, M. ulcerans was detected in one planorbid and one bullin. After decontamination (3), the samples were inoculated onto Löwenstein medium and incubated at 30°C without any successful isolation of the M. ulcerans strain. The samples also contained other mycobacteria, such as Mycobacterium gordonae and M. szulgai, or fungi. Furthermore, along the same river, M. ulcerans has been shown to be associated with aquatic bugs and vegetation (2, 3). Study of another set of snails collected in Papua New Guinea in July 2003 yielded similar observations in line with our hypothesis (data not shown). Recently, on the banks of the Oueme River in Benin, M. ulcerans was detected by PCR in 4% of the aquatic snails (n = 86) collected (unpublished results).
In tropical or nontropical regions, aquatic snails are the hosts of numerous organisms responsible for human infections, such as schistosomiasis and fascioliasis. These aquatic consumers may concentrate microorganisms, such as environmental mycobacteria and M. ulcerans, thereby participating in the dissemination of the bacilli in the environment. To test the possibility that other animals, such as the larvae of insects and possibly small fish, might also play a role in dissemination, we examined such potential vectors. Using PCR, we detected M. ulcerans in 6% of 60 larvae of dragonflies (of the families Gomphidae and Libellulidae) and in 70 juvenile fishes (of the family Clariidae) in Benin near the Oueme River (unpublished data). A network of aquatic agents begins gradually to be delineated in the spread of M. ulcerans infections. Further studies are needed to appreciate the role of the M. ulcerans virulence plasmid in the chain of transmission to humans and its behavior in certain environmental niches (11).
REFERENCES
- 1.De Gentile, P. L., C. Mahaza, F. Rolland, B. Carbonnelle, J. L. Verret, and D. Chabasse. 1992. Cutaneous ulcer from Mycobacterium ulcerans. Apropos of 1 case in French Guyana. Bull. Soc. Pathol. Exot. 85:212-214. (In French.) [PubMed] [Google Scholar]
- 2.Marsollier, L., T. Stinear, J. Aubry, J. P. Saint Andre, R. Robert, P. Legras, A. L. Manceau, C. Audrain, S. Bourdon, H. Kouakou, and B. Carbonnelle. 2004. Aquatic plants stimulate the growth of and biofilm formation by Mycobacterium ulcerans in axenic culture and harbor these bacteria in the environment. Appl. Environ. Microbiol. 70:1097-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsollier, L., R. Robert, J. Aubry, P. Legras, A. L. Manceau, H. Kouakou, C. Mahaza, and B. Carbonnelle. 2002. Aquatic insects as a vector for Mycobacterium ulcerans. Appl. Environ. Microbiol. 68:4623-4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marston, B. J., M. O. Diallo, C. R. Horsburgh, Jr., I. Diomande, M. Z. Saki, J. M. Kanga, G. Patrice, H. B. Lipman, S. M. Ostroff, and R. C. Good. 1995. Emergence of Buruli ulcer disease in the Daloa region of Côte d'Ivoire. Am. J. Trop. Med. Hyg. 52:219-224. [DOI] [PubMed] [Google Scholar]
- 5.Portaels, F., P. Elsen, A. Guimaraes-Peres, P.-A. Fonteyne, and W. M. Meyers. 1999. Insects in the transmission of Mycobacterium ulcerans infection. Lancet 353:986. [DOI] [PubMed] [Google Scholar]
- 6.Roberts, B., and R. Hirst. 1997. Immunomagnetic separation and PCR for detection of Mycobacterium ulcerans. J. Clin. Microbiol. 35:2709-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross, B. C., P. D. Johnson, F. Oppedisano, L. Marino, A. Sievers, T. Stinear, J. A. Hayman, M. G. Veitch, and R. M. Robins-Browne. 1997. Detection of Mycobacterium ulcerans in environmental samples during an outbreak of ulcerative disease. Appl. Environ. Microbiol. 63:4135-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross, B. C., L. Marino, F. Oppedisano, R. Edwards, R. M. Robins-Browne, and P. D. R. Johnson. 1997. Development of a PCR assay for rapid diagnosis of Mycobacterium ulcerans infection. J. Clin. Microbiol. 35:1696-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shepard, C. C., and D. H. M. Rae. 1968. A method for counting acid fast bacteria. Int. J. Lepr. 36:78-82. [PubMed] [Google Scholar]
- 10.Stinear, T., J. K. Davies, G. A. Jenkin, J. A. Hayman, F. Oppedisano, and P. D. R. Johnson. 2000. Identification of Mycobacterium ulcerans in the environment from regions in southeast Australia in which it is endemic with sequence capture-PCR. Appl. Environ. Microbiol. 66:3206-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stinear, T., A. Mve-Obiang, P. Small, W. Frigui, M. J. Pryor, R. Brosch, G. A. Jenkin, P. D. R. Johnson, J. K. Davies, R. E. Lee, S. Adusumilli, T. Garnier, S. F. Haydock, P. F. Leadlay, and S. T. Cole. 2004. Giant plasmid-encoded polyketide synthases produce the macrolide toxin of Mycobacterium ulcerans. Proc. Natl. Acad. Sci. USA 101:1345-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]