Abstract
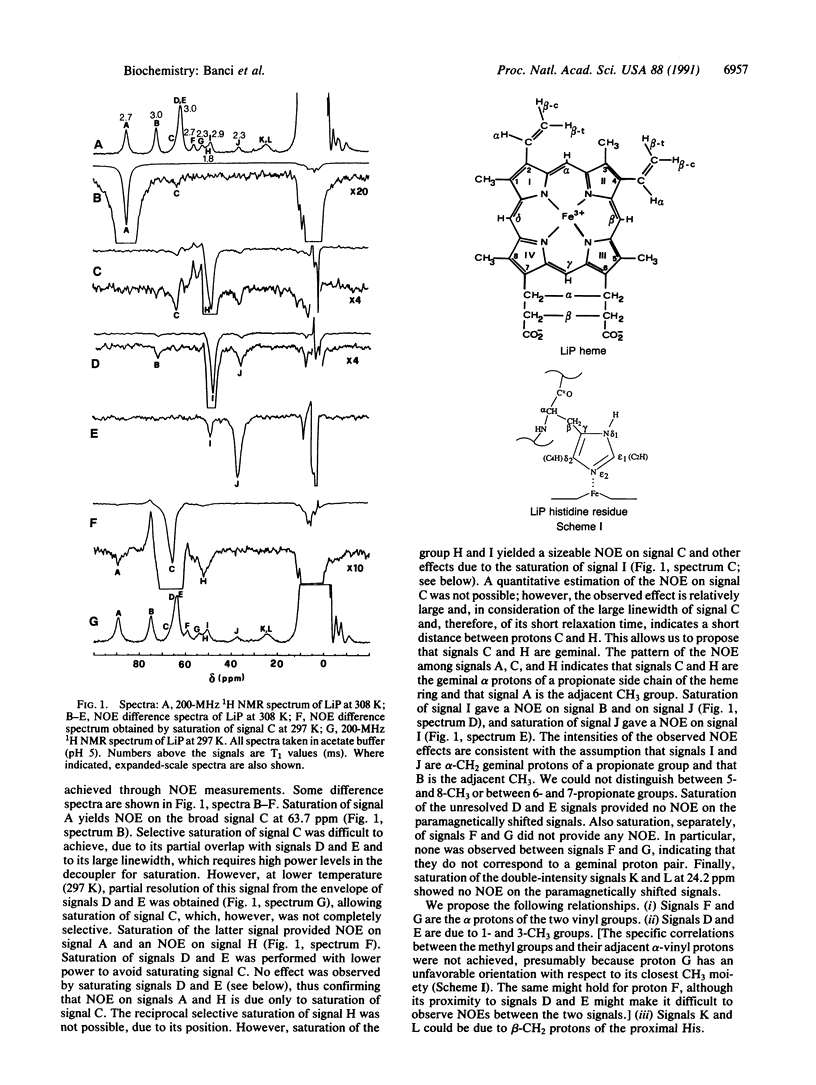
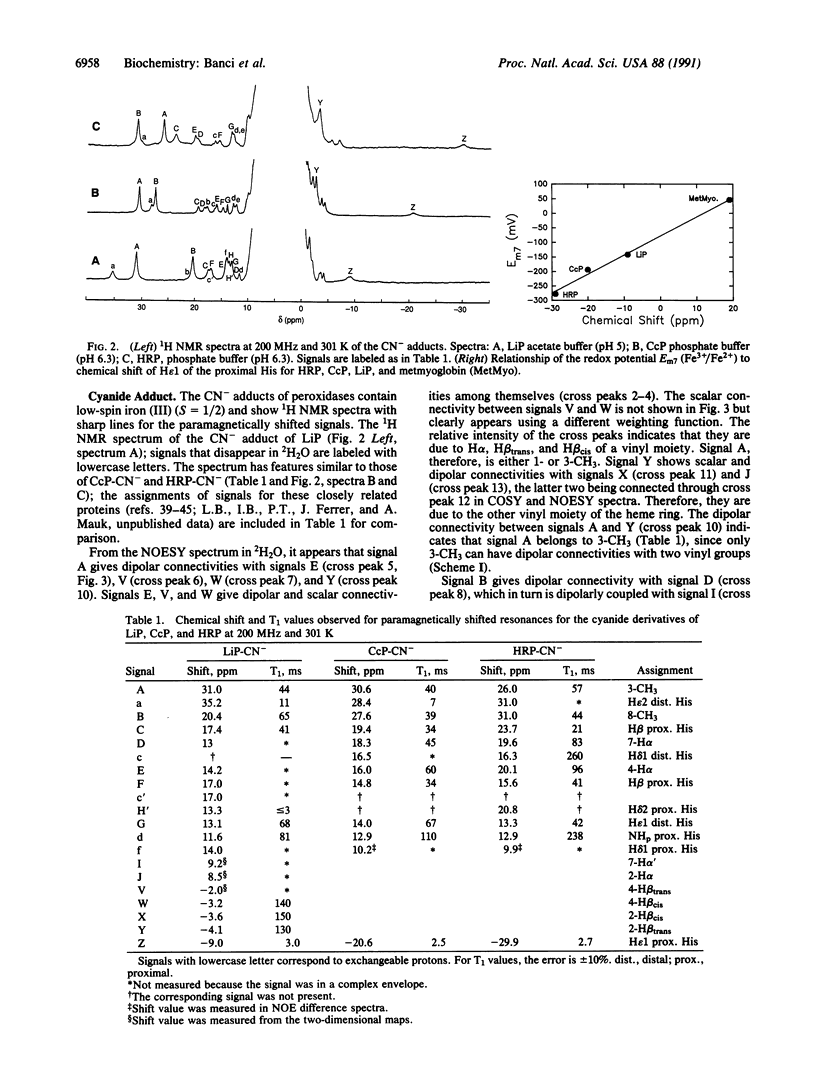
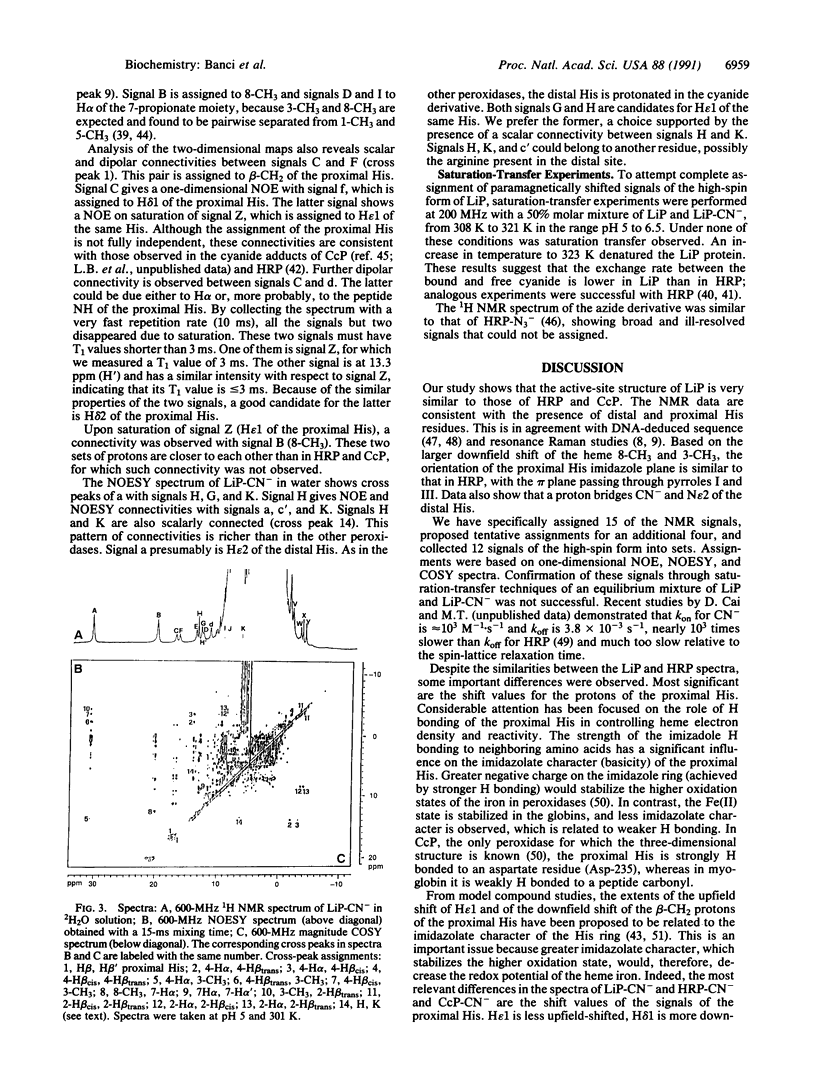
Lignin peroxidase shares several structural features with the well-studied horseradish peroxidase and cytochrome c peroxidase but carries a higher redox potential. Here the heme domain of lignin peroxidase and the lignin peroxidase cyanide adduct was examined by 1HNMR spectroscopy, including nuclear Overhauser effect and two-dimensional measurements, and the findings were compared with those for horseradish peroxidase and cytochrome c peroxidase. Structural information was obtained on the orientation of the heme vinyl and propionate groups and the proximal and distal histidines. The shifts of the epsilon1 proton of the proximal histidine were found to be empirically related to the Fe3+/Fe2+ redox potentials.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. A., Renganathan V., Loehr T. M., Gold M. H. Lignin peroxidase: resonance Raman spectral evidence for compound II and for a temperature-dependent coordination-state equilibrium in the ferric enzyme. Biochemistry. 1987 Apr 21;26(8):2258–2263. doi: 10.1021/bi00382a028. [DOI] [PubMed] [Google Scholar]
- Conroy C. W., Tyma P., Daum P. H., Erman J. E. Oxidation-reduction potential measurements of cytochrome c peroxidase and pH dependent spectral transitions in the ferrous enzyme. Biochim Biophys Acta. 1978 Nov 20;537(1):62–69. doi: 10.1016/0005-2795(78)90602-5. [DOI] [PubMed] [Google Scholar]
- DePillis G. D., Wariishi H., Gold M. H., Ortiz de Montellano P. R. Inactivation of lignin peroxidase by phenylhydrazine and sodium azide. Arch Biochem Biophys. 1990 Jul;280(1):217–223. doi: 10.1016/0003-9861(90)90539-b. [DOI] [PubMed] [Google Scholar]
- Glenn J. K., Morgan M. A., Mayfield M. B., Kuwahara M., Gold M. H. An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1077–1083. doi: 10.1016/0006-291x(83)90672-1. [DOI] [PubMed] [Google Scholar]
- Hammel K. E., Kalyanaraman B., Kirk T. K. Substrate free radicals are intermediates in ligninase catalysis. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3708–3712. doi: 10.1073/pnas.83.11.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten P. J., Kalyanaraman B., Hammel K. E., Reinhammar B., Kirk T. K. Comparison of lignin peroxidase, horseradish peroxidase and laccase in the oxidation of methoxybenzenes. Biochem J. 1990 Jun 1;268(2):475–480. doi: 10.1042/bj2680475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- La Mar G. N., De Ropp J. S., Chacko V. P., Satterlee J. D., Erman J. E. Axial histidyl imidazole non-exchangeable proton resonances as indicators of imidazole hydrogen bonding in ferric cyanide complexes of heme peroxidases. Biochim Biophys Acta. 1982 Nov 19;708(3):317–325. doi: 10.1016/0167-4838(82)90443-5. [DOI] [PubMed] [Google Scholar]
- La Mar G. N., de Ropp J. S. Assignment of exchangeable proximal histidine resonances in high-spin ferric hemoproteins: substrate binding in horseradish peroxidase. Biochem Biophys Res Commun. 1979 Sep 12;90(1):36–41. doi: 10.1016/0006-291x(79)91586-9. [DOI] [PubMed] [Google Scholar]
- La Mar G. N., de Ropp J. S., Smith K. M., Langry K. C. Proton nuclear magnetic resonance study of the electronic and molecular structure of the heme crevice in horseradish peroxidase. J Biol Chem. 1980 Jul 25;255(14):6646–6652. [PubMed] [Google Scholar]
- Lukat G. S., Rodgers K. R., Jabro M. N., Goff H. M. Magnetic resonance spectral characterization of the heme active site of Coprinus cinereus peroxidase. Biochemistry. 1989 Apr 18;28(8):3338–3345. doi: 10.1021/bi00434a032. [DOI] [PubMed] [Google Scholar]
- Makino R., Chiang R., Hager L. P. Oxidation-reduction potential measurements on chloroperoxidase and its complexes. Biochemistry. 1976 Oct 19;15(21):4748–4754. doi: 10.1021/bi00666a033. [DOI] [PubMed] [Google Scholar]
- Marion D., Wüthrich K. Application of phase sensitive two-dimensional correlated spectroscopy (COSY) for measurements of 1H-1H spin-spin coupling constants in proteins. Biochem Biophys Res Commun. 1983 Jun 29;113(3):967–974. doi: 10.1016/0006-291x(83)91093-8. [DOI] [PubMed] [Google Scholar]
- Millis C. D., Cai D. Y., Stankovich M. T., Tien M. Oxidation-reduction potentials and ionization states of extracellular peroxidases from the lignin-degrading fungus Phanerochaete chrysosporium. Biochemistry. 1989 Oct 17;28(21):8484–8489. doi: 10.1021/bi00447a032. [DOI] [PubMed] [Google Scholar]
- Morishima I., Ogawa S., Inubushi T., Yonezawa T., Iizuka T. Nuclear magnetic resonance studies of hemoproteins. Acid-alkaline transition, ligand binding characteristics, and structure of the heme environments in horseradish peroxidase. Biochemistry. 1977 Nov 15;16(23):5109–5115. doi: 10.1021/bi00642a025. [DOI] [PubMed] [Google Scholar]
- Poulos T. L. Heme enzyme crystal structures. Adv Inorg Biochem. 1988;7:1–36. [PubMed] [Google Scholar]
- Poulos T. L., Kraut J. The stereochemistry of peroxidase catalysis. J Biol Chem. 1980 Sep 10;255(17):8199–8205. [PubMed] [Google Scholar]
- Satterlee J. D., Erman J. E., DeRopp J. S. Proton hyperfine resonance assignments in cyanide-ligated cytochrome c peroxidase using the nuclear Overhauser effect. J Biol Chem. 1987 Aug 25;262(24):11578–11583. [PubMed] [Google Scholar]
- Satterlee J. D., Erman J. E., LaMar G. N., Smith K. M., Langry K. C. Assignment of hyperfine shifted resonances in high-spin forms of cytochrome c peroxidase by reconstitutions with deuterated hemins. Biochim Biophys Acta. 1983 Mar 16;743(2):246–255. doi: 10.1016/0167-4838(83)90221-2. [DOI] [PubMed] [Google Scholar]
- Satterlee J. D., Erman J. E., Mauro J. M., Kraut J. Comparative proton NMR analysis of wild-type cytochrome c peroxidase from yeast, the recombinant enzyme from Escherichia coli, and an Asp-235----Asn-235 mutant. Biochemistry. 1990 Sep 18;29(37):8797–8804. doi: 10.1021/bi00489a042. [DOI] [PubMed] [Google Scholar]
- Schalch H., Gaskell J., Smith T. L., Cullen D. Molecular cloning and sequences of lignin peroxidase genes of Phanerochaete chrysosporium. Mol Cell Biol. 1989 Jun;9(6):2743–2747. doi: 10.1128/mcb.9.6.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanabal V., La Mar G. N., de Ropp J. S. A nuclear Overhauser effect study of the heme crevice in the resting state and compound I of horseradish peroxidase: evidence for cation radical delocalization to the proximal histidine. Biochemistry. 1988 Jul 26;27(15):5400–5407. doi: 10.1021/bi00415a003. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T. K., Bull C., Fee J. A. Steady-state and transient-state kinetic studies on the oxidation of 3,4-dimethoxybenzyl alcohol catalyzed by the ligninase of Phanerocheate chrysosporium Burds. J Biol Chem. 1986 Feb 5;261(4):1687–1693. [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983 Aug 12;221(4611):661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H(2)O(2)-requiring oxygenase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Tu C. P. Cloning and sequencing of a cDNA for a ligninase from Phanerochaete chrysosporium. Nature. 1987 Apr 2;326(6112):520–523. doi: 10.1038/326520a0. [DOI] [PubMed] [Google Scholar]
- Yamada H., Makino R., Yamazaki I. Effects of 2,4-substituents of deuteropheme upon redox potentials of horseradish peroxidases. Arch Biochem Biophys. 1975 Jul;169(1):344–353. doi: 10.1016/0003-9861(75)90350-1. [DOI] [PubMed] [Google Scholar]




