Abstract
Pyranose 2-oxidase (POX) was recovered from Phanerochaete chrysosporium BKM-F-1767 solid substrate culture using mild extraction conditions and was purified. 13C-nuclear magnetic resonance confirmed production of d-arabino-hexos-2-ulose (glucosone) from d-glucose with the oxidase. Peptide fingerprints generated by liquid chromatography-tandem mass spectrometry of tryptic digests and analysis of the corresponding cDNA revealed a structurally unusual sequence for the P. chrysosporium POX. Relatively high levels of pox transcript were detected under carbon-starved culture conditions but not under nutrient sufficiency. This regulation pattern is similar to that observed for lignin peroxidases, manganese peroxidases, and glyoxal oxidase of P. chrysosporium, supporting evidence that POX has a role in lignocellulose degradation.
Pyranose oxidase (POX) (EC 1.1.3.10) is believed to have roles in ligninolysis (8, 49), production of peroxide for peroxidases, and synthesis of the antibiotic cortalcerone (3). It also is of interest for numerous biotechnological applications, analytical methods, and synthesis of novel chemicals (16). Indications are that POX is the major glucose-oxidizing enzyme in Phanerochaete chrysosporium ligninolytic cultures, even with the fungal strain and culture conditions for which glucose 1-oxidase (GOX) (EC 1.1.3.4) was previously reported (49). P. chrysosporium produces another peroxide-producing enzyme, glyoxal oxidase, which is secreted and coordinately expressed with the lignin peroxidases and manganese peroxidases (22, 24). The cloning, genomic organization, heterologous expression, site-specific mutagenesis, active-site characterization, and regulation of glyoxal oxidase have been described previously (20, 21, 23, 25, 28, 54, 55).
Several wood decay fungi in addition to P. chrysosporium are reported to produce POX (16), including Oudemansiella mucida, Trametes versicolor (8), Polyporus obtusus (36), Phlebiopsis gigantea (37), and Trametes multicolor (51). In general, POX oxidizes various aldopyranoses and disaccharides to the corresponding 2-keto sugars concomitant with the reduction of O2 to H2O2 (16, 29). Substrates include d-glucose, d-xylose, l-sorbose, and d-glucono-1,5-lactone (2, 13). POX from T. versicolor oxidizes both alpha and beta anomers of glucose essentially equally well and therefore can be used in the microdetermination of glucose without the addition of mutarotase (43). POX is a FAD homotetramer with subunit molecular sizes of 68 to 76 kDa (10, 30, 48, 51), and in some instances it is glycosylated (16). The native POX purified from P. chrysosporium strain K3 mycelia, however, is not glycosylated, and three isoforms with pI 5.0, 5.05, and 5.15 are observed (2).
Ultrastructural and immunocytochemical studies of P. chrysosporium have demonstrated that the oxidase is concentrated within the periplasmic space, where it is associated with membrane-bound vesicles, other membrane structures, and extracellular slime (8, 9). Some POX activity is observed in culture fluid, but this appears to be related to cell lysis (9). POX can also be extracted from decayed birch (8, 9), and an extracellular activity is observed when the fungus is grown on the natural lignocellulosic substrate, wheat straw (52).
The purpose of the present study was to identify and characterize the glucose-oxidizing activity of P. chrysosporium grown on solid substrate. We used 13C-nuclear magnetic resonance (NMR) and 13C-labeled glucose to unambiguously distinguish GOX from POX. The extracellular POX was purified, the corresponding gene was identified, and a full-length cDNA was sequenced. Northern blots showed differential regulation of pox with transcript levels most abundant under carbon-starved ligninolytic conditions.
MATERIALS AND METHODS
Chemicals.
[1-13C]glucose, acetone-d6, and 13C-labeled acetone were purchased from Cambridge Isotope Laboratories, Inc. (Andover, Mass.). [1-13C]glucosone was synthesized from [1-13C]glucose (4, 35). d-glucose, d-cellobiose, d-xylose, and d-glucono-1,5-lactone were purchased from Sigma (St. Louis, Mo.).
Organism and culture conditions.
P. chrysosporium BKM-F-1767 was grown in 12-oz. wide-mouth jars (Ball Corp., Broomfield, Colo.) containing 10 g of wheat bran amended with 7 ml of 1% corn steep liquor (Sigma) and harvested after 2 weeks of growth at 30°C. P. chrysosporium RP78, a homokaryotic strain derived from BKM-F-1767 (40), was used for isolation of total RNA (see below).
Enzyme purification.
Combined cultures of P. chrysosporium BKM-F-1767 were extracted with 5 volumes of water (based on grams of original wheat bran) at 90 rpm on ice for 30 min. The extracted proteins were separated from the wheat bran by filtration through Miracloth (Calbiochem, La Jolla, Calif.). Particulates were removed by centrifugation at 12,000 × g for 30 min. The supernatant was saturated to 85% ammonium sulfate, protein was recovered by centrifugation at 4,000 × g for 10 min, and the pellet was dissolved in 10 mM phosphate buffer (pH 6.5). The sample was dialyzed against 10 mM phosphate buffer (pH 6.5). The POX was purified on an fast protein liquid chromatography unit (Pharmacia, Uppsala, Sweden) at room temperature with the sample in the appropriate buffer for each of four chromatography steps: (i) DEAE Sepharose (Sigma) eluting with 800 ml of a 0 to 0.5 M NaCl gradient in 10 mM potassium phosphate (pH 6.5); (ii) Hitrap affinity column with Zn (Amersham Biosciences, Piscataway, N.J.) eluting with 30 ml of a 0 to 30 mM imidazole gradient in a solution of 20 mM sodium phosphate (pH 7.2), 1 M NaCl; (iii) Sephacryl S-200-HR (Sigma) with a 20 mM sodium phosphate (pH 7.2), 1 M NaCl mobile phase; and (iv) MonoQ (Amersham Biosciences) eluting with 34 ml of a gradient from 0 to 0.5 M NaCl in 20 mM piperazine (pH 6.0). A representative purification gave yields of 100, 300, 408, 161, and 116% for the ammonium sulfate precipitation, DEAE Sepharose, Zn HiTrap, Sephacryl S-200-HR, and MonoQ purification steps, respectively. This provided approximately 1 mg of purified POX from 150 g of wheat bran culture.
Enzyme reaction conditions.
Activity of POX was determined in 1-ml reaction mixtures containing 0.01% phenol red, 50 mM 2,2′-dimethysuccinic acid (DMS) buffer with pH adjusted to 6.0 with NaOH, 0.01 mg of horseradish peroxidase (type II; Sigma), and 50 mM d-glucose, d-cellobiose, d-xylose, or d-glucono-1,5-lactone. Reaction mixtures were incubated at 37°C, reactions were stopped with 50 μl of 2 N NaOH, and absorbances at 610 nm were determined (24).
For NMR characterizations, POX reaction solutions (1.8 ml) contained 5 mM concentrations of one of [1-13C]glucosone through [6-13C]glucosone, 2 mM phenol red, 0.01 mg of horseradish peroxidase type II (Sigma), and 10 mM DMS buffer (pH 6). The reaction solution was flushed with O2, and O2 consumption was monitored at 37°C with a Clark-type electrode. When O2 was depleted the solution was flushed again. After three cycles NMR spectra were obtained.
NMR analyses.
NMR spectra (62.9 MHz, 13C) were measured at 25°C on a Bruker DPX-250 spectrometer. Acetone-d6 (29.83 ppm) was used as reference signal with authentics. In enzymatic reactions, 13C-acetone (30.56 ppm) in D2O (1,3-13C2, 99%) was used as reference signal. Chemical shift assignments for glucosone, glucono-1,5-lactone, gluconic acid, and gluconate were determined from authentics. NMR reference data include those reported for glucose (58), aldono-1,5-lactones (53), monosaccharides (5), and glucosone (14).
Gel electrophoresis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and isoelectric focusing were performed using the Bio-Rad Criterion system (Hercules, Calif.) according to the manufacturer's instructions. Precast 10% Tris-HCl Ready Gel and a pH 3 to 10 isoelectric focusing (IEF) Ready Gel were used for SDS-PAGE and isoelectric focusing, respectively.
Tryptic digestion of protein and LC-MS/MS analysis.
The band containing POX was cut from an SDS-PAGE gel stained with Coomassie brilliant blue R-250 (Bio-Rad). The excised gel slice was washed three times with 400 μl of 50% acetonitrile in 25 mM NH4-bicarbonate (pH 8), dehydrated in 100% acetonitrile, and then dried in a speed-vac. The sample was submitted to the University of Wisconsin—Madison Biotechnology Center for LC-MS/MS (Agilent 1100 high-performance liquid chromatography [HPLC]/Micromass QTOF2 system; Waters, Milford, Mass.) of a tryptic digest. Data files were extracted and analyzed in Spectrum Mill MS Proteomics Workbench Rev. A.03.00.014 (Agilent Technologies, Palo Alto, Calif.).
cDNA preparation and sequencing.
Based on LC-MS/MS peptide sequences, a putative POX gene, pox, was identified by tBlastn searches of the P. chrysosporium genome database (http://www.jgi.doe.gov/). Following manual corrections of pox gene model genewise.62.7.1, suitable primers were designed for amplification of the corresponding cDNA. Two-week-old cultures of P. chrysosporium BKM-F-1767 grown on wheat bran and supplemented with corn steep liquor were snap frozen with liquid nitrogen, and total RNA was extracted (21, 46). mRNA was purified by magnetic capture using Dynabeads (Dynal Biotech, Oslo, Norway) following the manufacturer's instructions. cDNAs were synthesized as described previously (17, 21, 46).
Northern blots.
P. chrysosporium RP78, a haploid homokaryotic strain derived from BKM-F-1767 (40), was used for Northern blot hybridizations. Using QIAGEN′s RNeasy Plant Mini kit (Valencia, Calif.), total RNA was isolated from mycelium harvested from (i) log phase cultures grown in B3 medium (27); (ii) ligninolytic C-limited B3 medium (39); and (iii) defined mineral salts medium (12) with 0.4% Avicel cellulose as sole carbon source (47). Twenty micrograms of RNA was size fractionated on formaldehyde gels and transferred to nylon membranes (Nytran; Schleicher & Schuell Bioscience Inc., Keene, N.H.) following Ambion's Northern Max protocol (Austin, Tex.). PCR amplicons of pox and glyceraldehyde-3-phosphate dehydrogenase (gpd) cDNAs were partially purified (Wizard DNA Clean-Up System; Promega Corp., Madison, Wis.) and random primed (Stripable DNA Probe; Ambion) to specific activity of >5 × 108 cpm/μg. PCR was as described previously (38) using primer pairs 5′-CCCCGGCCAGGTTCCCATCC-3′ and 5′-ATTGTACTTACGCAGCACGAGGTT-3′ for pox and 5′-ATGCCGGTCAAAGCAGGAATCAA-3′ and 5′-CACCGCAGACAAACATGGGGGCA-3′ for gpd. Denatured probes were added to a final concentration of 5 × 106 cpm/ml of Ultrahyb solution (Ambion) and were hybridized overnight at 42°C.
Glycosylation analyses.
The glycosylation of POX protein, separated on an SDS-PAGE gel, was determined with the Gelcode Glycosylation staining kit (Pierce Biotechnology, Rockford, Ill.) following the instructions of the manufacturer. Horseradish peroxidase and soybean trypsin inhibitor proteins were used as positive and negative controls, respectively.
Phylogenetic analysis.
The POXs were aligned using ClustalX (45) and phylogenetic analyses of aligned amino acid sequences (TreeBase No. S1100; http://www.treebase.org/treebase/index.html) were performed with the Distance method as implemented in PAUP version 4.0*10b (42) using a neighbor-joining search. Stability of clades was assessed by 1,000 bootstrap replicates.
Nucleotide sequence accession number.
The pox cDNA nucleotide sequence of P. chrysosporium BKM-F-1767 has been deposited in the GenBank database under accession number AY522922.
RESULTS
Enzyme production and purification.
Our early attempts to isolate POX from P. chrysosporium grown on aspen sawdust were unsuccessful, although fungal growth appeared adequate (data not shown). Failure to detect activity could be due to wood extractives interfering with detection, or perhaps the enzyme was strongly associated with the natural growth substrate. We then tested wheat bran supplemented with corn steep liquor as a solid substrate, because the low extractive content would possibly minimize assay interferences and its nonwoody characteristics may allow an extracellular oxidase to be easily extracted. Accordingly, POX was recovered for purification by simple water extraction of fresh cultures of P. chrysosporium BKM-F-1767 (see Materials and Methods). There were dramatic increases in POX activity yields for the early chromatographic steps, suggesting that inhibitors were significant even with this isolation technique. SDS-PAGE gave a doublet band at about 70 kDa, results similar to that reported for the intracellular POX of P. chrysosporium K-3 (48).
Product identification.
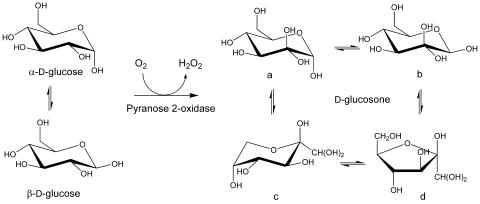
The NMR characterizations for the enzymatic reactions with d-glucose, 13C-labeled individually at each of the six positions, indicate the conversion of α- and β-glucose to glucosone having four tautomers, designated a to d (Fig. 1). The chemical shifts we observed for glucosone are consistent with that reported by Freimund et al. (14), and our data rely on their tautomeric designations. Accordingly, the chemical shifts (in parts per million) observed at each carbon for the conversion of α-glucose, β-glucose to a to d glucosone are the following: C-1, 92.51, 96.37 to 95.03, 95.48, 89.91, 90.53; C-2, 71.95, 74.62 to 94.01, 93.43, 97.67, 101.14; C-3, 73.25, 76.27 to 74.04, 76.85, 68.20, 76.27; C-4, 70.10, 70.06 to 69.22, 69.05, 70.34, 74.92; C-5, 71.79, 76.32 to 72.3, 76.3, 69.59, 80.87; C-6, 61.01, 61.21 to 61.37, 61.37, 63.74, 62.42, respectively. We observe relative tautomeric abundances of 46, 26, 18, and 10% for a to d, respectively, compared to values of 36, 31, 21, and 12% reported by Freimund et al. (14). A systematic offset of our data might be a result of solvent conditions and/or reference calibration. Based on these NMR results, the isolated oxidase is designated a POX.
FIG. 1.
Reaction catalyzed by POX. POX oxidizes both α- and β-d-glucose at C-2 to give d-glucosone with four isomeric forms in aqueous solution. The approximate proportions of the four isomers a to d under our experimental conditions are 46, 26, 18, and 10%, respectively, based on 13C-NMR peak intensities.
Enzyme characterization.
The purified oxidase showed the typical absorption maxima of flavoproteins at 275, 360, and 455 nm. Consistent with earlier reports (2, 16) of the POX from P. chrysosporium, the POX we isolated was not glycosylated, as indicated by the Gelcode glycosylation staining method. Three isoforms were observed at pIs of ca. 5.0, 5.05, and 5.15 by IEF electrophoresis. In contrast to a previous report that P. chrysosporium POX loses activity with repeated freezing and thawing without glycerol (2), the POX we isolated retained activity (data not shown). The relative activities of the isolated POX with 50 mM d-glucose, d-cellobiose, d-xylose, and d-glucono-1,5-lactone were 100, 17, 35, and 68%, respectively. The substrate specificity is similar to that of other pyranose oxidases, although the activity with cellobiose seems uncharacteristically high.
Protein-gene assignment.
Sixteen distinct peptides derived from the LC-MS/MS data were correlated to one protein in the P. chrysosporium database, covering 42% of the predicted protein. The corresponding gene model, genewise.62.7.1, features several inaccuracies, as is often the case for automated predictions in eukaryotic genomes. Several inaccuracies occurred at the 5′ and 3′ termini, in part due to short exons in these regions. Nevertheless, the genewise.62.7.1 model served as a framework for designing a series of flanking primers, and a full-length pox cDNA was ultimately amplified.
cDNA sequence and predicted protein.
The coding region of the pox cDNA is 1,866 bp long (GenBank accession no. AY522922), and alignment with the genomic sequence indicates that the gene is interrupted with 14 introns 48 to 67 bp long. Low-stringency BLAST of the Phanerochaete genome indicates a single POX gene in the genome.
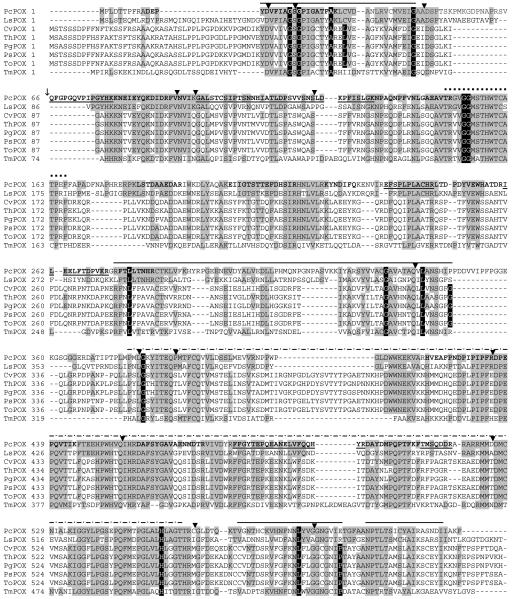
The translate tool from Expasy (http://www.expasy.org/) predicts a protein of 621 amino acids, molecular size of 69 kDa, and pI 6.03 from POX cDNA sequence. Reanalysis of the LC-MS/MS data against the predicted protein indicates 2 additional matching peptides, giving a total of 18 covering 40% of the protein. Notably, one of the peptides was 56 amino acids upstream of the N terminus reported by Artolozaga et al. (2) (Fig. 2). This is consistent with the POX that we isolated being slightly larger (70 kDa by SDS-PAGE) than the 65-kDa result reported by Artolozaga et al. (2).
FIG. 2.
Alignment of P. chrysosporium BKM-F-1767 POX (PcPOX; accession no. AY522922) translated from cDNA sequence, with the POXs from Lyophyllum shimeji (LsPOX; accession no. gi|44886072|), Coriolus versicolor (CvPOX; accession no. gi|25091018| [33]), T. hirsuta (ThPOX; accession no. gi|25091016| [7]), Peniophora gigantea (PgPOX; accession no. gi|34452037|), Peniophora sp. (PsPOX; accession no. gi|27436422|), Trametes ochracea (ToPOX; accession no. gi|31044224|) and Tricholoma matsutake (TmPOX; accession no. gi|25553433| [44]). Grey shaded amino acids indicate homology with PcPOX; black shaded amino acids with white typeface indicate CvPOX-GMC consensus sequences (see the text). Solid lines above sequences indicate FAD-binding domains; dotted lines above sequences indicate flavin attachment loops; dashed lines above sequences indicate substrate binding domains. Boldface indicates peptides identified by LC-MS/MS; underlined and boldface type distinguishes separate peptides within a continuous sequence. ▾, intron positions; ↓, the start of the N terminus reported by Artolozaga et al. (2) for the POX of P. chrysosporium K3.
Northern analysis.
Figure 3 shows that the relative transcript level of pox was highest in carbon-starved media, with very low transcript levels in log phase cultures in B3 media or media with Avicel cellulose as sole carbon source. These results suggest a basal level of expression of POX and activation due to carbon starvation in P. chrysosporium. This is consistent with POX production during idiophasic growth of P. chrysosporium (13).
FIG. 3.
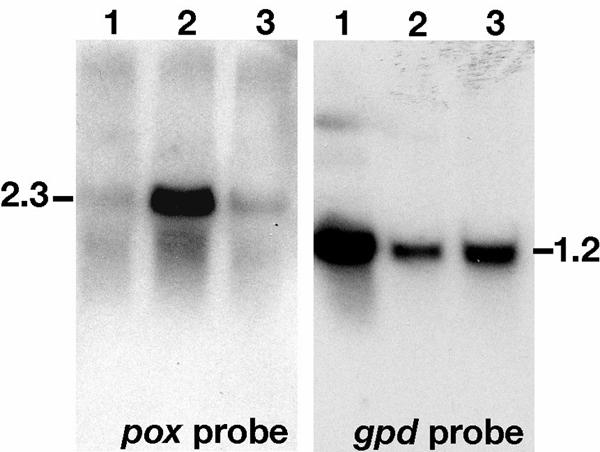
Northern blots showing differential regulation of POX. Lanes contain 20 μg of total RNA derived from log phase B3 medium (lanes 1), carbon starved B3 medium (lanes 2), and defined salts medium with Avicel cellulose as sole carbon source (lanes 3). Following washes, the blot probed with the gpd housekeeping probe was exposed to XAR film (Kodak) for 5 h without an amplifying screen. The pox-probed blot was exposed overnight with an amplifying screen. Autoradiograms were scanned, and the digitized images were labeled in Photoshop Illustrator (Adobe, San Jose, Calif.).
DISCUSSION
The glucose oxidase described here is classified as a POX based on the NMR analyses of the enzymatic product from glucose and on substrate specificity. Although GOX can be distinguished from POX by 13C-NMR using [1-13C]glucose as substrate (11), here we show that the products exactly match the four glucosone tautomers at all six carbons. Label at C-1 or C-2 alone is highly diagnostic, and the method has distinct advantages because no derivatization or product isolation is required. Relatively low concentration of glucose substrate is sufficient because of the sensitivity afforded by the enriched 13C label, and therefore oxygen limitation is not problematic for exhaustive consumption of the carbon substrate. This method not only allows the characterization of glucosone as the final product with purified oxidase but also as an intermediate in reactions with crude enzyme preparations (11).
The isolation technique (simple H2O extraction) that we used for POX suggests an extracellular location for the oxidase when grown on wheat bran. However, analysis of the cDNA sequence by SignalP V1.1 (31) (http://www.cbs.dtu.dk/services/SignalP) does not indicate a typical secretion signal. Consequently, although secretion of proteins without a secretion signal is known (for example, see reference 38), autolysis may best account for the easy extraction of the enzyme. The stability to freeze-thaw cycles of the POX described here, in contrast to that of the enzyme isolated from mycelia (2), prompted us to test for glycosylation as a possible maturation step, but no glycosylation was detected. However, the POX reported here has significant N-terminal sequence (see below) that apparently is lacking from freeze-thaw-sensitive POX. Whether this N-terminal peptide contributes to the stability of the enzyme is not known.
Recently, pyranose oxidase was identified as a member of the glucose-methanol-choline oxidoreductase (GMC-OXRED) family of proteins based on Coriolus versicolor POX (CvPOX) and Trametes hirsutus POX (ThPOX) sequence analyses (1). The POXs show only very low, nonsignificant identity to individual GMC-OXRED members, but structure-based multiple sequence alignment shows distant homology. Fifteen strictly conserved residues and three major conserved regions (discussed below) were identified. P. chrysosporium POX (PcPOX) shows only moderate identity with CvPOX (41%) and ThPOX (41%) according to National Center for Biotechnology Information tBlastn (http:/www.ncbi.nlm.nih.gov/BLAST/), and only 12 of the 15 strict consensus GMC-OXRED residues (1) are conserved (Fig. 2). Therefore, for the purposes of discussion, the strictly conserved residues identified by Albrecht and Langauer (1) are referenced here only in the restrictive context in which they were defined and termed CvPOX-GMC residues. Other POXs (Fig. 2) also show only moderate identity with PcPOX, e.g., Lyophyllum shimeji POX (LsPOX; 53%), Peniophora gigantea POX (PgPOX; 41%), Peniophora sp. POX (PsPOX; 41%), Trametes ochracea POX (ToPOX; 41%), and Tricholoma matsutake POX (TmPOX; 36%). As a subgroup, C. versicolor, T. hirsuta, P. gigantea, Peniophora sp., and T. ochracea POXs show high identity with each other (82 to 99%) and also conform to strict CvPOX-GMC consensus.
The three conserved regions identified for CvPOX (1) were correlated with glucose 1-oxidase of Penicillium amagasakiense (PaGOX) because of obvious functional relatedness and because crystal X-ray diffraction data are available (57). The conserved regions are (i) the FAD-binding site with four subregions, (ii) the flavin attachment loop, and (iii) the substrate-binding region. Here, we deduce these structure-based regions from alignments of CvPOX with the other POXs (Fig. 2).
The first FAD-binding subregion is the ADP-binding βαβ-fold motif near the N terminus beginning at Pc-Y14 (Fig. 2). Five strictly conserved CvPOX-GMC residues were identified for this subregion (1) and are part of the GxGxxGxxxAxxL βαβ-fold motif (56). However, PcPOX, LsPOX, and TmPOX break from consensus at the fourth conserved residue, with Cv-L replaced by Pc-C, Ls-C, and Tm-I, respectively (Fig. 2).
The second FAD-binding subregion, corresponding to Pc-R275 to Pc-P347, has four strictly conserved CvPOX-GMC residues (1). Again, PcPOX, LsPOX, and TmPOX deviate from consensus (Fig. 2), with the last CvPOX-GMC residue G replaced with unconservative residues Pc-P, Ls-S, and Tm-R, respectively (Fig. 2). The G at Pc-G333 in this subregion is conserved by all POXs and CvPOX-GMC proteins, and this corresponding glycine in PaGOX is between the diphosphate and ribose of FAD (1, 26). This G is also the beginning of the GMC-OXRED signature 2 (Prosite PS00624) (6). PcPOX and CvPOX each mismatch 3 of the 10 consensus residues for a total of 5 mismatches, indicating that this signature, [GS]-[PSTA]-x(2)-[ST]-[PS]-x-[LIVM](2)-x(2)-S-G-[LIVM]-G, isnot very diagnostic in POXs.
The third FAD-binding subregion, from Pc-V567 to Pc-E589, has two strictly conserved CvPOX-GMC residues (1). However, the strict CvPOX-GMC consensus at Cv-P is broken by Pc-E and Ls-R, again not conservative substitutions. The fourth region has no strictly conserved CvPOX-GMC residues and forms the C-terminal helix. The POX sequences show strong homology at the beginning of this short subregion.
The flavin attachment loop, located from Pc-T149 to Pc-F166 (Fig. 2), is highly conserved among the POXs, and they all conform to the strict CvPOX-GMC consensus for this region (i.e., at PcG153-G154). The FAD of T. multicolor (synonymous with T. ochracea) was shown to be of the 8α-(N3-histidyl)-FAD linkage type attached through the histidine of the STHW domain (18). This region is part of the GMC-OXRED signature 1 given by [GA]-[RKNC]-x-[LIV]-G(2)-[GST](2)-x-[LIVM]-N-x(3)-[FYWA]-x(2)-[PAG]-x(5)-[DNESH] (Prosite PS00623) (6). However, both PcPOX and CvPOX deviate at 7 of the 12 consensus residues of GMC-OXRED signature 1.
The contiguous substrate-binding region is the least similar region among the GMCs (1, 26) and corresponds here to Pc-P377 to Pc-T557 (Fig. 2). Interestingly, there is a high degree of identity among the POXs in this region, but it is interrupted with a 21- to 26-amino-acid deletion in PcPOX, LsPOX, and TmPOX, starting at Pc-P410 in the case of PcPOX. Strictly conserved CvPOX-GMC residues are observed at Pc-G381 and Pc-H554. The conserved histidine is thought to be part of the active site involved in general base catalysis-accepting protons from the glucose substrate (26). POX alignments with PaGOX indicate only one other possible residue common in the active site (1). This residue corresponds to Pc-Y85 (Cv-Y97) and is in a region of high POX homology (Fig. 2). As with CvPOX and ThPOX, no other active-site residues are suggested for PcPOX as revealed by alignments with PaGOX (data not shown). Reliable structure-function analyses will require structure determinations of the POXs, for which there is only preliminary data available in the case of T. multicolor (19).
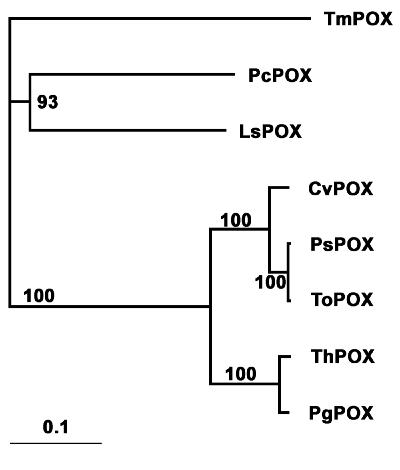
The distinctions based on GMC structure-function of the various POXs, as discussed above, are reflected in the corresponding phylogenetic tree (Fig. 4). As expected, the POXs with all 15 CvPOX-GMC consensus residues (CvPOX, PsPOX, ToPOX, ThPOX, and PgPOX) group as a separate clade. However, TmPOX, PcPOX, and LsPOX break from CvPOX-GMC consensus in the first and second FAD-binding subregions and are distinguished from the consensus group. PcPOX and LsPOX further deviate from consensus in the third FAD-binding subregion and form a separate clade (Fig. 4).
FIG. 4.
Phylogenetic analysis of POX. Distance analysis and tree construction of amino acid sequence data were done by the neighbor-joining method in PAUP version 4.0*10b. Numbers indicate the percent support for nodes after 1,000 replications of bootstrap analysis. The bar represents a 10% estimated sequence difference. Refer to the legend to Fig. 2 for abbreviations and gi numbers.
Besides the distinguishing features of PcPOX as it relates to other GMCs, there are several other aspects worth highlighting in comparison with the other POX sequences. For example, PcPOX has a 22-amino-acid insert at position 54 and a 14-amino-acid insert at position 345 which are not observed with the other POX sequences. The N terminus reported by Artolozaga et al. (2) for native POX from P. chrysosporium K3 (QFGPGQVPIPGYSKNNEIEYQKDIDRFVNVI) is within the 22-amino-acid insert and is encoded by the fourth and fifth exons starting at position 66 (Fig. 2). A 2-amino-acid discrepancy is observed (out of 31 amino acids) that may be due to fungal strain differences. The Spectrum Mill analysis of our LC-MS/MS data identified a peptide beginning at position 10, indicating that the protein we isolated has significant N-terminal sequence not observed by Artolozaga et al. (2). This would explain why Leitner et al. (29) observed no apparent homology of the N terminus of P. chrysosporium with those of T. multicolor and T. versicolor; i.e., the corresponding sequence was absent. P. chrysosporium POX has the smallest N-terminal sequence preceding the FAD-binding region 1. Specifically, there are only 13 amino acids with PcPOX, in contrast to 46 with CvPOX. The CvPOX is presumably synthesized as a preproenzyme and is localized in the periplasmic space after processing of 38 amino acids (33). The maturation mechanism for these different POXs is clearly different considering the lack of homology in this region.
Northern blots show that pox of P. chrysosporium is strongly regulated with relatively high transcript levels under C limitation (Fig. 3). This is consistent with POX activity profiles observed for P. chrysosporium in response to carbon limitation (13). In contrast, constitutive activity is observed in T. versicolor (32) and O. mucida (34) liquid cultures. This suggests different and/or multiple roles for POX in these various fungi. Importantly, the regulation pattern observed here with P. chrysosporium pox is similar to that observed for lignin peroxidases, manganese peroxidases, and glyoxal oxidase (41), supporting evidence that POX has a role in lignocellulose degradation.
One role for POX may be peroxide production for the extracellular peroxidases. However, extracellular glyoxal oxidase, for which a secretion signal has been identified (23), would seem to have a more direct role in extracellular metabolism. An alternate or additional role for POX could be related to further metabolism of the glucosone. P. chrysosporium has a pyranosone dehydratase (PYD) for subsequent conversion of glucosone to the antibiotic cortalcerone (15, 50). No PYD have been reported for the other fungi, for which there is POX cDNA sequence (Fig. 2). Many other wood decay fungi possessing POX apparently lack a PYD (3). This observation raises questions regarding the metabolic fate of glucosone during wood decay.
Acknowledgments
The research of P.J.K. was supported in part by the Cooperative State Research, Education, and Extension Service, U.S. Department of Agriculture, under agreement no. 2001-35103-11246. The research of D.C. was supported in part by U.S. Department of Energy grant DE-FG02-87ER13712.
The NMR analyses by Larry Landucci are acknowledged.
REFERENCES
- 1.Albrecht, M., and T. Lengauer. 2003. Pyranose oxidase identified as a member of the GMC oxidoreductase family. Bioinformatics 19:1216-1220. [DOI] [PubMed] [Google Scholar]
- 2.Artolozaga, M. J., E. Kubatova, J. Volc, and H. M. Kalisz. 1997. Pyranose 2-oxidase from Phanerochaete chrysosporium—further biochemical characterisation. Appl. Microbiol. Biotechnol. 47:508-514. [DOI] [PubMed] [Google Scholar]
- 3.Baute, M.-A., and R. Baute. 1984. Occurrence among macrofungi of the bioconversion of glucosone to cortalcerone. Phytochemistry 2:271-274. [Google Scholar]
- 4.Bayne, S. 1963. Aldouloses (osones), p. 421-423. In R. L. Whistler, M. L. Wolfrom, and J. N. BeMiller (ed.), Methods in carbohydrate metabolism, vol. II. Academic Press, New York, N.Y. [Google Scholar]
- 5.Bock, K., and C. Pedersen. 1983. Carbon-13 nuclear magnetic resonance spectroscopy of monosaccharides. In R. S. Tipson and D. Horton (ed.), Advances in carbohydrate chemistry and biochemistry, vol. 41. Academic Press, New York, N.Y.
- 6.Cavener, D. R. 1992. GMC oxidoreductases. A newly defined family of homologous proteins with diverse catalytic activities. J. Mol. Biol. 223:811-814. [DOI] [PubMed] [Google Scholar]
- 7.Christensen, S., S. F. Lassen, and P. Schneider. May 1999. U.S. patent 6,146,865.
- 8.Daniel, G., J. Volc, and E. Kubatova. 1994. Pyranose oxidase, a major source of H2O2 during wood degradation by Phanerochaete chrysosporium, Trametes versicolor, and Oudemansiella mucida. Appl. Environ. Microbiol. 60:2524-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniel, G., J. Volc, E. Kubatova, and T. Nilsson. 1992. Ultrastructural and immunocytochemical studies on the H2O2-producing enzyme pyranose oxidase in Phanerochaete chrysosporium grown under liquid culture conditions. Appl. Environ. Microbiol. 58:3667-3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danneel, H. J., E. Rossner, A. Zeeck, and F. Giffhorn. 1993. Purification and characterization of a pyranose oxidase from the basidiomycete Peniophora gigantea and chemical analyses of its reaction products. Eur. J. Biochem. 214:795-802. [DOI] [PubMed] [Google Scholar]
- 11.de Koker, T. H., M. D. Mozuch, and P. J. Kersten. 2003. Analysis of D-glucose metabolism of wood decay fungi using 13C-NMR and 13C-labeled substrate. document no. IRG34 03-10475. International Research Group on Wood Preservation, Stockholm, Sweden.
- 12.Eriksson, K.-E., and S. G. Hamp. 1978. Regulation of endo-1,4-β-glucanase production in Sporotrichum pulverulentum. Eur. J. Biochem. 90:183-190. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson, K. E., B. Pettersson, J. Volc, and V. Musilek. 1986. Formation and partial characterization of glucose-2-oxidase, a hydrogen peroxide producing enzyme in Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 23:257-262. [Google Scholar]
- 14.Freimund, S., L. Baldes, A. Huwig, and F. Giffhorn. 2002. Enzymatic synthesis of D-glucosone 6-phosphate (D-arabino-hexos-2-ulose 6-(dihydrogen phosphate)) and NMR analysis of its isomeric forms. Carbohydr. Res. 337:1585-1587. [DOI] [PubMed] [Google Scholar]
- 15.Gabriel, J., J. Volc, P. Sedmera, G. Daniel, and E. Kubátová. 1993. Pyranosone dehydratase from the basidiomycete Phanerochaete chrysoporium: improved purification, and identification of 6-deoxy-D-glucosone and D-xylosone reaction products. Arch. Microbiol. 160:27-34. [DOI] [PubMed] [Google Scholar]
- 16.Giffhorn, F. 2000. Fungal pyranose oxidases: occurrence, properties and biotechnical applications in carbohydrate chemistry. Appl. Microbiol. Biotechnol. 54:727-740. [DOI] [PubMed] [Google Scholar]
- 17.Gilliland, G., S. Perrin, and H. Bunn. 1990. Competitive PCR for quanititation of mRNA. In M. Innis, D. Gelford, J. Sninsky, and T. White (ed.), PCR protocols. Academic Press, New York, N.Y.
- 18.Halada, P., C. Leitner, P. Sedmera, D. Haltrich, and J. Volc. 2003. Identification of the covalent flavin adenine dinucleotide-binding region in pyranose 2-oxidase from Trametes multicolor. Anal. Biochem. 314:235-242. [DOI] [PubMed] [Google Scholar]
- 19.Hallberg, B. M., C. Leitner, D. Haltrich, and C. Divne. 2004. Crystallization and preliminary X-ray diffraction analysis of pyranose 2-oxidase from the white-rot fungus Trametes multicolor. Acta Crystallogr. Sect. D. Biol. Crystallogr. 60:197-199. [DOI] [PubMed] [Google Scholar]
- 20.Hammel, K. E., M. D. Mozuch, K. A. Jensen, and P. J. Kersten. 1994. H2O2 recycling during oxidation of the arylglycerol β-aryl ether lignin structure by lignin peroxidase and glyoxal oxidase. Biochemistry 33:13349-13354. [DOI] [PubMed] [Google Scholar]
- 21.Janse, B. J. H., J. Gaskell, M. Akhtar, and D. Cullen. 1998. Expression of Phanerochaete chrysosporium genes encoding lignin peroxidases, manganese peroxidases, and glyoxal oxidase in wood. Appl. Environ. Microbiol. 64:3536-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kersten, P. J. 1990. Glyoxal oxidase of Phanerochaete chrysosporium: its characterization and activation by lignin peroxidase. Proc. Natl. Acad. Sci. USA 87:2936-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kersten, P. J., and D. Cullen. 1993. Cloning and characterization of a cDNA encoding glyoxal oxidase, a H2O2-producing enzyme from the lignin-degrading basidiomycete Phanerochaete chrysosporium. Proc. Natl. Acad. Sci. USA 90:7411-7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kersten, P. J., and T. K. Kirk. 1987. Involvement of a new enzyme, glyoxal oxidase, in extracellular H2O2 production by Phanerochaete chrysosporium. J. Bacteriol. 169:2195-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kersten, P. J., C. Witek, A. Vanden Wymelenberg, and D. Cullen. 1995. Phanerochaete chrysosporium glyoxal oxidase is encoded by two allelic variants: structure, genomic organization, and heterologous expression of glx1 and glx2. J. Bacteriol. 177:6106-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiess, M., H.-J. Hecht, and H. M. Kalisz. 1998. Glucose oxidase from Penicillium amagasakiense: primary structure and comparison with other glucose-methanol-choline (GMC) oxidoreductases. Eur. J. Biochem. 252:90-99. [DOI] [PubMed] [Google Scholar]
- 27.Kirk, T. K., E. Schultz, W. J. Connors, L. F. Lorenz, and J. G. Zeikus. 1978. Influence of culture parameters on lignin metabolism by Phanerochaete chrysosporium. Arch. Microbiol. 117:277-285. [Google Scholar]
- 28.Kurek, B., and P. J. Kersten. 1995. Physiological regulation of glyoxal oxidase from Phanerochaete chrysosporium by peroxidase systems. Enzyme Microb. Technol. 17:751-756. [Google Scholar]
- 29.Leitner, C., J. Volc, and D. Haltrich. 2001. Purification and characterization of pyranose oxidase from the white rot fungus Trametes multicolor. Appl. Environ. Microbiol. 67:3636-3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Machida, Y., and T. Nakanishi. 1984. Purification and properties of pyranose oxidase from Coriolus versicolor. Agric. Biol. Chem. 48:2463-2470. [Google Scholar]
- 31.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 32.Nishida, A., and K.-E. Eriksson. 1987. Formation, purification, and partial characterization of methanol oxidase, a H2O2-producing enzyme in Phanerochaete chrysosporium. Biotechnol. Appl. Biochem. 9:325-338. [Google Scholar]
- 33.Nishimura, I., K. Okada, and Y. Koyama. 1996. Cloning and expression of pyranose oxidase cDNA from Coriolus versicolor in Escherichia coli. J. Biotechnol. 52:11-20. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira, P., N. Rodeia, A. Clemente, and A. Karmali. 1992. Glucose-2-oxidase production by whiterot fungi, p. 33-40. In J. F. Kennedy, G. O. Phillips, and P. A. Williams (ed.), Lignocellulosics: science, technology, development and use. Ellis Horword, New York, N.Y.
- 35.Richtmyer, N. K. 1963. Phenylosazones, p. 127-131. In R. L. Whistler, M. L. Wolfrom, and J. N. BeMiller (ed.), Methods in carbohydrate chemistry, vol. II. Academic Press, New York, N.Y. [Google Scholar]
- 36.Ruelius, H. W., R. M. Kerwin, and F. W. Janssen. 1968. Carbohydrate oxidase, a novel enzyme from Polyporus obtusus. I. Isolation and purification. Biochim. Biophys. Acta 167:493-500. [DOI] [PubMed] [Google Scholar]
- 37.Schäfer, A., S. Bieg, A. Huwig, G. W. Kohring, and F. Giffhorn. 1996. Purification by immunoaffinity chromatography, characterization, and structural analysis of a thermostable pyranose oxidase from the white rot fungus Phlebiopsis gigantea. Appl. Environ. Microbiol. 62:2586-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simons, B. H., P. Barnett, E. G. Vollenbroek, H. L. Dekker, A. O. Muijsers, A. Messerschmidt, and R. Wever. 1995. Primary structure and characterization of the vanadium chloroperoxidase from the fungus Curvularia inaequalis. Eur. J. Biochem. 229:566-574. [DOI] [PubMed] [Google Scholar]
- 39.Stewart, P., and D. Cullen. 1999. Organization and differential regulation of a cluster of lignin peroxidase genes of Phanerochaete chrysosporium. J. Bacteriol. 181:3427-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stewart, P., J. Gaskell, and D. Cullen. 2000. A homokaryotic derivative of a Phanerochaete chrysosporium strain and its use in genomic analysis of repetitive elements. Appl. Environ. Microbiol. 66:1629-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart, P., P. J. Kersten, A. Vanden Wymelenberg, J. Gaskell, and D. Cullen. 1992. Lignin peroxidase gene family of Phanerochaete chrysosporium: complex regulation by carbon and nitrogen limitation and identification of a second dimorphic chromosome. J. Bacteriol. 174:5036-5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swofford, D. L. 2002. PAUP*: phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer Associates, Sunderland, Mass.
- 43.Taguchi, T., K. Ohwaki, and J. Okuda. 1985. Glucose 2-oxidase (Coriolus versicolor) and its application to D-glucose colorimetry. J. Appl. Biochem. 7:289-295. [Google Scholar]
- 44.Takakura, Y., and S. Kuwata. 2003. Purification, characterization, and molecular cloning of a pyranose oxidase from the fruit body of the basidiomycete, Tricholoma matsutake. Biosci. Biotechnol. Biochem. 67:2598-2607. [DOI] [PubMed] [Google Scholar]
- 45.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vallim, M. A., B. J. H. Janse, J. Gaskell, K. A. A. Pizzirani, and D. Cullen. 1998. Phanerochaete chrysosporium cellobiohydrolase and cellobiose dehydrogenase transcripts in wood. Appl. Environ. Microbiol. 64:1924-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanden Wymelenberg, A., S. Denman, D. Dietrich, J. Bassett, X. Yu, R. Atalla, P. Predki, U. Rudsander, T. T. Teeri, and D. Cullen. 2002. Transcript analysis of genes encoding a family 61 endoglucanase and a putative membrane-anchored family 9 glycosyl hydrolase from Phanerochaete chrysosporium. Appl. Environ. Microbiol. 68:5765-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volc, J., and K.-E. Erikksson. 1988. Pyranose 2-oxidase from Phanerochaete chrysosporium. Methods Enzymol. 161:316-322. [Google Scholar]
- 49.Volc, J., E. Kubátová, G. Daniel, and V. Prikrylová. 1996. Only C-2 specific glucose oxidase activity is expressed in ligninolytic cultures of the white rot fungus Phanerochaete chrysosporium. Arch. Microbiol. 165:421-424. [DOI] [PubMed] [Google Scholar]
- 50.Volc, J., E. Kubatova, P. Sedmera, G. Daniel, and J. Gabriel. 1991. Pyranose oxidase and pyranosone dehydratase: enzymes responsible for conversion of D-glucose to cortalcerone by the basidiomycete Phanerochaete chrysosporium. Arch. Microbiol. 156:297-301. [Google Scholar]
- 51.Volc, J., C. Leitner, P. Sedmera, P. Halada, and D. Haltrich. 1999. Enzymatic formation of dicarbonyl sugars: C-2 oxidation of 1,6 disaccharides gentiobiose, isomaltose and melibiose by pyranose 2-oxidase from Trametes multicolor. J. Carbohydr. Chem. 18:999-1007. [Google Scholar]
- 52.Vyas, B. R. M., J. Volc, and V. Sasek. 1994. Ligninolytic enzymes of selected white rot fungi cultivated on wheat straw. Folia Microbiol. 39:235-240. [Google Scholar]
- 53.Walaszek, Z., D. Horton, and I. Ekiel. 1982. Conformational studies on aldonolactones by NMR spectroscopy: conformations of D-glucono-1,5-lactone and D-mannono-1,5-lactone in solution. Carbohydr. Res. 106:193-202. [Google Scholar]
- 54.Whittaker, M. M., P. J. Kersten, D. Cullen, and J. W. Whittaker. 1999. Identification of catalytic residues in glyoxal oxidase by targeted mutagenesis. J. Biol. Chem. 274:36226-36232. [DOI] [PubMed] [Google Scholar]
- 55.Whittaker, M. M., P. J. Kersten, N. Nakamura, J. Sanders-Loehr, E. S. Schweizer, and J. W. Whittaker. 1996. Glyoxal oxidase from Phanerochaete chrysosporium is a new radical-copper oxidase. J. Biol. Chem. 271:681-687. [DOI] [PubMed] [Google Scholar]
- 56.Wierenga, R. K., P. Terpstra, and W. G. J. Hol. 1986. Prediction of the occurrence of the ADP-binding βαβ-fold in proteins, using an amino acid sequence fingerprint. J. Mol. Biol. 187:101-107. [DOI] [PubMed] [Google Scholar]
- 57.Wohlfahrt, G., S. Witt, J. Hendle, D. Schomburg, H. M. Kalisz, and H. J. Hecht. 1999. 1.8 and 1.9 Å resolution structures of the Penicillium amagasakiense and Aspergillus niger glucose oxidases as a basis for modelling substrate complexes. Acta Crystallogr. Sect. D. Biol. Crystallogr. 55:969-977. [DOI] [PubMed] [Google Scholar]
- 58.Zhu, Y., J. Zajicek, and A. S. Serianni. 2001. Acyclic forms of [1-13C] aldohexoses in aqueous solution: quantitation by 13C NMR and deuterium isotope effects on tautomeric equilibria. J. Org. Chem. 66:6244-6251. [DOI] [PubMed] [Google Scholar]