Abstract
Three active fractions of fructosyl-amino acid oxidase (FAOD-Ao1, -Ao2a, and -Ao2b) were isolated from Aspergillus oryzae strain RIB40. N-terminal and internal amino acid sequences of FAOD-Ao2a corresponded to those of FAOD-Ao2b, suggesting that these two isozymes were derived from the same protein. FAOD-Ao1 and -Ao2 were different in substrate specificity and subunit assembly; FAOD-Ao2 was active toward Nɛ-fructosyl Nα-Z-lysine and fructosyl valine (Fru-Val), whereas FAOD-Ao1 was not active toward Fru-Val. The genes encoding the FAOD isozymes (i.e., FAOAo1 and FAOAo2) were cloned by PCR with an FAOD-specific primer set. The deduced amino acid sequences revealed that FAOD-Ao1 was 50% identical to FAOD-Ao2, and each isozyme had a peroxisome-targeting signal-1, indicating their localization in peroxisomes. The genes was expressed in Escherichia coli and rFaoAo2 showed the same characteristics as FAOD-Ao2, whereas rFaoAo1 was not active. FAOAo2 disruptant was obtained by using ptrA as a selective marker. Wild-type strain grew on the medium containing Fru-Val as the sole carbon and nitrogen sources, but strain ΔfaoAo2 did not grow. Addition of glucose or (NH4)2SO4 to the Fru-Val medium did not affect the assimilation of Fru-Val by wild-type, indicating glucose and ammonium repressions did not occur in the expression of the FAOAo2 gene. Furthermore, conidia of the wild-type strain did not germinate on the medium containing Fru-Val and NaNO2 as the sole carbon and nitrogen sources, respectively, suggesting that Fru-Val may also repress gene expression of nitrite reductase. These results indicated that FAOD is needed for utilization of fructosyl-amino acids as nitrogen sources in A. oryzae.
There have been many reports for Maillard reaction in vivo since the finding of glycated hemoglobin (20, 29). Amadori products such as fructosyl-amino acids are formed by nonenzymatic glycation reaction, in which biological amines (i.e., free amino acids, amino acid residues of proteins, and amino groups of nucleic acids) react with reducing sugars at an early stage of the Maillard reaction. The resultant Amadori compounds repeat dehydration and condensation to form stable compound called advanced glycation end product (2, 3, 7, 18). Glycation affects the function of the proteins in vivo (29), and protein cross-links with advanced glycation end product cause the development of diabetic complication and aging (20). Thus, it is important to investigate the intervention against the Maillard reaction (17).
Two types of deglycation enzymes have been identified: fructosyl-amino acid oxidases (FAODs) (EC 1.5.3) (9) and fructosamine kinases (FNKs) (5, 26). Both enzymes act on fructosyl-amino acids, but the deglycation mechanisms are quite different. FAOD catalyzes the oxidative deglycation of fructosyl-amino acids, yielding corresponding amino acid, glucosone, and H2O2 (22, 32). Most FAODs show this type of oxidation, but the enzyme found in Pseudomonas sp. has a somewhat different reaction mechanism (25). This enzyme produces fructosamine residue from fructosyl-amino acid, indicating that C-N bonds suffered from the cleavage are different in these two types of enzymes are different. FNKs are found in mammals (26) and Escherichia coli (30) and phosphorylate the third and sixth carbons, respectively, in the fructosyl moiety of fructosyl-amino acids. The corresponding amino acid and 3-deoxyglucosone are formed from fructosyl-amino acid by the mammalian enzyme, whereas another hydrolase is needed for deglycation of the phosphorylated compound produced by bacterial FNK.
We have studied the application of fungal FAODs in the clinical diagnosis of diabetes mellitus (35), since the amounts of glycated proteins such as hemoglobin and albumin in blood reflect the level of blood glucose, and fructosyl-amino acids are model compounds for the glycated proteins. We also showed that FAOD is a peroxisomal enzyme and widely distributed in filamentous fungi, such as the genera Aspergillus, Fusarium, Penicillium, and Gibberella (23, 36). Therefore, we thought that FAOD plays an important role in fungal cells. However, the definite physiological role of the enzyme is still unknown.
Aspergillus oryzae is an important fungus in Japanese fermentation industries (11), and there is a lot of information on genetic and fermentation techniques related to this organism. In the present study, we found FAOD isozymes in A. oryzae strain RIB40 and used them as a model to elucidate the physiological role of FAOD in fungal cells. We purified and characterized FAOD isozymes from A. oryzae, and gene cloning and disruption were also studied.
MATERIALS AND METHODS
Materials.
Nɛ-Fructosyl Nα-benzyloxycarbonyl-l-lysine (Fru-Z-Lys) and fructosyl l-valine (Fru-Val) were kindly provided by Arkray, Inc., Kyoto, Japan. Fructosyl l-lysine (Fru-Lys) used in cultivation was synthesized according to the method of Hashiba et al. (6), and further purification was done with Bond Elute PBA column (100 mg; GL Sciences, Inc., Tokyo, Japan). Bacto Tryptone was from Nippon Becton Dickinson Co., Tokyo, Japan. Dried yeast extract (type S) and Polypepton were from Nihon Pharmaceutical Co., Tokyo, Japan.
Microorganisms and culture media.
Two minimal media (MM-1 and MM-2) were used for A. oryzae strains RIB40 and niaD-2 (ΔniaD), respectively. MM-1 contained 0.3% NaNO3, 0.1% KH2PO4, 0.2% KCl, 0.05% MgSO4 · 7H2O, 2% glucose, 0.1% trace element solution [0.1% FeSO4 · 7H2O, 0.88% ZnSO4 · 7H2O, 0.04% CuSO4 · 5H2O, 0.01% Na2B4O7 · 10H2O, and 0.005% (NH4)6Mo7O24 · 4H2O], and 1.5% agar. MM-2 used 0.3% NaNO2 as a sole nitrogen source in MM-1. MY medium was used as a complete medium. The pH of these media was adjusted to pH 5.5. E. coli strains HMS174(DE3), BL21(DE3), JM109, and NM522 were cultivated in 2X YT medium containing 1.6% of Bacto Tryptone, 1% yeast extract, and 0.5% NaCl (pH 7.0).
For sporulation, A. oryzae strain RIB40 was cultured in 100 ml of MM-1 medium at 30°C for about 2 weeks (strain niaD-2 was cultured in MY medium). Sporulating mycelia were suspended in 0.8% NaCl containing 0.1% Tween 80 and filtrated through an 11G-2 glass filter. The filtrate was centrifuged at 2,200 × g for 10 min. The pellet was washed twice with the same solution. For enzyme production, A. oryzae spores (7.0 × 108 spores) were incubated at 30°C for 26 h in 7 liters of the autoclave-browned medium described previously (36) by using a model MDN 10-liter jar fermentor (B.E. Marubishi Co., Tokyo, Japan) with agitation (300 rpm) and aeration (7 liters/min).
For the assimilation test, A. oryzae strains were cultivated at 30°C with the medium containing 0.1% KH2PO4, 0.2% KCl, 0.05% MgSO4 · 7H2O, a 0.1% volume of the trace elements solution, 1% carbon source, 0.3% nitrogen source, and 1.5% agar (pH 5.5). Spores (103) of each strain were spotted on the center of a plate and incubated at 30°C.
Purification of FAOD isozymes.
All purification steps were done at 4°C, and column chromatography was done on a fast-performance liquid chromatography system (Amersham Biosciences K.K., Tokyo, Japan). Washed mycelia (520 g [wet weight]) were suspended in 1.3 liter of 0.1 M Tris-HCl buffer (pH 8.5) containing 2 mM dithiothreitol (DTT) and then disrupted in a glass bead mill (Bead-beater model 11079-S; Biospec Products, Bartlesville, Okla.). The homogenate was centrifuged at 18,000 × g for 75 min to remove unbroken cells and debris. (NH4)2SO4 powder was added to the supernatant to give 50% saturation. After being gently stirred for 60 min, the mixture was centrifuged at 18,000 × g for 75 min. The supernatant was adjusted to 75% saturation by (NH4)2SO4 powder. After another 60 min of stirring and centrifugation under the same conditions, the precipitate was dissolved in a minimum volume of buffer A (50 mM Tris-HCl buffer [pH 8.5] containing 2 mM DTT). The enzyme solution was then dialyzed against the same buffer for 35 h and centrifuged at 6,500 × g for 20 min. The supernatant was applied to a DEAE-Toyopearl column (2.2 by 20 cm; Tosoh Co., Tokyo, Japan) equilibrated with buffer A. The column was washed with 230 ml of the same buffer. The adsorbed protein was eluted with a 450-ml linear gradient of 0 to 0.1 M NaCl in buffer A. The active fractions were suspended in buffer A containing a 40% saturation of (NH4)2SO4. The enzyme solution was applied to a Butyl-Toyopearl column (1.6 by 10 cm; Tosoh) equilibrated with buffer A with 40%-saturated (NH4)2SO4. After the column was washed extensively with the equilibration buffer, elution was done with a 50-ml linear gradient of 40 to 0% saturation of (NH4)2SO4 in buffer A. The enzyme-active fractions were collected and dialyzed by Centricon YM-30 (Nihon Millipore Ltd., Tokyo, Japan) and then applied to a HiLoad 16/60 Superdex 200-pg column (1.6 by 60 cm; Amersham Biosciences) equilibrated with 0.1 M Tris-HCl buffer (pH 8.5) containing 0.1 M NaCl and 2 mM DTT. The active fractions were pooled, dialyzed against buffer A, and applied to a Resource Q column (0.64 by 3 cm; Amersham Biosciences). The column was washed with 20 ml of the same buffer. The adsorbed enzyme was eluted with a 35-ml linear gradient of 0 to 1 M NaCl in buffer A.
Preparation of genomic DNA.
Wet mycelia (0.1 g) were ground in a mortar with liquid N2. The powdered cells were transferred to a 50-ml conical tube, 8 ml of Tris-EDTA (TE) buffer, and 8 ml of the lysis buffer composed of 50 mM EDTA and 0.5% sodium dodecyl sulfate (SDS) was added, followed by mixing upside down. The mixture was shaken gently at 37°C for 30 min and centrifuged at 3,000 × g and 4°C for 5 min. The supernatant was purified by phenol-chloroform extraction, and the precipitate was dissolved in 1.6 ml of TE buffer. RNase A (50 μg; Nippon Gene Co., Tokyo, Japan) was added to the 400 μl of the DNA solution, and the mixture was incubated at 37°C for 30 min. After phenol-chloroform extraction, the precipitate was dissolved in an appropriate amount of TE buffer.
Cloning of the gene encoding FAOD isozymes.
Partial genes encoding FAOD isozymes (FAOAo1 and FAOAo2) were amplified by PCR with A. oryzae strain RIB40 genome as a template with an FAOD-specific primer set: 5′-GGBTTYTTCWTSGARCCNRAYGA-3′ and 5′-GTRTCVGYRYMCCAGCAVAT-3′ as the 5′- and 3′-primers, respectively. The FAOD-specific primer set was designed from the consensus sequences for FAODs (see the legend for Fig. 3). The PCR was done under the following conditions: 94°C for 1 min, followed by 35 cycles of 94°C for 1 min, 57°C for 1 min, and 72°C for 2 min, and finally by 72°C for 3 min. Purified PCR fragment was ligated with a pCR2.1-TOPO vector by using TOPO TA cloning kit (both from Invitrogen Corp., Carlsbad, Calif.). E. coli JM109 competent cells were transformed with the ligated product by electroporation with a BTX Electro Cell Manipulator ECM 600 (Genetronics, Inc., San Diego, Calif.) (13). 5′- and 3′-flanking regions of FAO genes were cloned by using an LA-PCR in vitro cloning kit (Takara Bio, Inc., Otsu, Shiga, Japan).
FIG. 3.
Amplification of partial FAO genes with an FAOD-specific primer set (A) and FAO cDNAs for expression in E. coli (B). In panel A, the FAOD-specific primer set was designed from the alignment of fungal FAODs reported previously. The nucleotide positions of these FAODs for 5′- and 3′-primer designs were as follows: FAOD of A. terreus at positions 784 to 806 and 997 to 1016, amadoriase I of A. fumigatus at positions 797 to 819 and 1016 to 1035, amadoriase II of A. fumigatus at positions 787 to 809 and 1000 to 1019; FOD of F. oxysporum at positions 796 to 818 and 1012 to 1031. Lanes in panel A: 1, φX174-HincII digest; 2, PCR product. Lanes in panel B: 1, λ-EcoT14 I digest; 2, FAOAo1; 3, FAOAo2.
Construction of expression systems for FAO genes.
Total RNA was prepared from 0.1 g of strain RIB40 mycelia by homogenization with ISOGEN (Nippon Gene Co.) in a glass bead mixer (Mini-Bead Beater; Biospec). First-strand cDNA was prepared with avian myeloblastosis virus reverse transcriptase by using a First-Strand cDNA synthesis kit (Life Sciences, Inc., St. Petersburg, Fla.). cDNA fragment attributed to FAOAo1 was amplified with the primers 5′-GGAATTCATGACATCCTCCAAGTTGACTC-3′ and 5′-AAACGCGTCGACTTAAAGCCGACTCTTATCGCCAATC-3′, and that for FAOAo2 was amplified with the primers 5′-CGGGATCCATGACTGTCGCCAAATCTTCC-3′ and 5′-AAACGCGTCGACCTACAGCTTCGCAGTATCC-3′. PCR was done under the following conditions: 95°C for 5 min, followed by 30 cycles of 94°C for 1 min, 56°C for 1 min, and 72°C for 2 min, and finally by 72°C for 10 min. These entire cDNA fragments were ligated to pGEX-6P-1 (Amersham Biosciences) and used to transform E. coli strain HMS174(DE3) competent cells by electroporation.
Purification of GST fusion proteins.
A total of 5 ml of an overnight culture of E. coli transformant was incubated at 30°C in 500 ml of 2× YT medium containing 50 μg of ampicillin/ml. After incubation for 4 h (to an optical density at 600 nm of 0.30), IPTG (isopropyl-β-d-thiogalactopyranoside) was added to the medium to a final concentration of 0.1 mM. The cells were harvested by centrifugation at 6,000 × g for 10 min after cultivation for 26 h. The cells were washed with 0.85% KCl, suspended in 25 ml of potassium phosphate buffer (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.8 mM KH2PO4, at pH 7.3), and then disrupted with a sonicator (model 450 Sonifier; Branson Ultrasonics Corp., Danbury, Conn.). The recombinant FAOD isozymes were purified by using Glutathione-Sepharose 4B and PreScission protease (both from Amersham Biosciences) according to the manufacturer's instructions.
Construction of FAOAo2 gene disruption cassette.
The FAOAo2 gene disruption cassette was constructed by replacing the middle region of the gene with the pyrithiamine resistance gene (ptrA) from A. oryzae. The pPTR I plasmid (Takara Bio) carrying ptrA was used for amplification of the gene. Disruption cassette was constructed by three-step PCR method (1, 15).
First, amplification of ptrA fragment (fragment 1) was done with 50 ng of pPTR I plasmid/ml as a template with the primers 5′-GGGGTGACGATGAGCCGCTC-3′ and 5′-GGGCAATTGATTACGGGATCCC-3′. PCR was done under the following conditions: 95°C for 5 min, followed by 30 cycles of 94°C for 1 min, 61°C for 1 min, and 72°C for 3 min and finally by 72°C for 10 min. Amplification of the 5′-flanking region of FAOAo2 (fragment 2; containing a 5′ part of the FAOAo2 structural gene) was performed by using 50 ng of A. oryzae strain niaD-2 genomic DNA/ml with the primers 5′-GGATAGTTAGCAAGACGACATGGTG-3′ and 5′-GAGCGGCTCATCGTCACCCCTGACTCTTCCTTGGGTGCCTG-3′ under the following conditions: 95°C for 5 min, followed by 30 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 5 min and finally by 72°C for 10 min. The 3′-flanking region of FAOAo2 (fragment 3; containing a 3′ part of the FAOAo2 structural gene) was amplified as described above with the primers 5′-ATGGGATCCCGTAATCAATTGCCCAGTTTGCCCTTTGAGAAGACCC-3′ and 5′-TTGACACCATAGAGATGCCTCGTAG-3′ (the underlined regions correspond to the 5′- or 3′-flanking region of the ptrA gene).
Second, the fusion PCR was done by using 133 ng of each of the fragments 1 to 3/ml with the primer 5′-ACAGCCACGACGATAACCTTTCCAC-3′ and the 3′ primer used for the amplification of 3′-flanking region of FAOAo2 under the following conditions: 95°C for 5 min, followed by 30 cycles of 94°C for 1 min, 60°C for 30 s, and 68°C for 8 min and finally by 68°C for 10 min. The PCR products were size fractionated by electrophoresis, and the expected band (fragment 4) was extracted from the gel.
Finally, the nested PCR was done by using 145 ng of fragment 4 as a template/ml with the primers 5′-GCAAGATTTGGTAACCTACGGGAGT-3′ and 5′-AGGATGGAGAAGTTTTCCGAGC-3′ under the following conditions: 95°C for 5 min, followed by 30 cycles of 94°C for 1 min and 68°C for 7 min and finally by 68°C for 10 min.
The final PCR product (fragment 5) was ligated with a pCR2.1-TOPO vector and used to transform E. coli BL21(DE3) competent cells by electroporation (13). Fragment 5 was reamplified in the same way and used to transform A. oryzae strain niaD-2 cells.
Disruption of the FAOAo2 gene.
A. oryzae strain niaD-2 mycelia (150 mg/ml) were incubated at 30°C for 3 h with protoplast solution containing 20 mg of Yatalase (Takara Bio)/ml, 5 mg of cellulase (Onozuka R-10; Yakult Pharmaceutical Industry Co., Tokyo, Japan)/ml, 0.8 M NaCl, 10 mM DTT, and 10 mM sodium phosphate buffer (pH 6.0). After filtration through an 11G-2 glass filter, the protoplasts were washed with 0.8 M NaCl by centrifugation at 700 × g for 5 min. The pellet was suspended in solution I composed of 0.8 M NaCl and 10 mM CaCl2 in 10 mM Tris-HCl (pH 8.0) to a final concentration of 2 × 108 cells/ml, and 0.2 volume of solution II containing 40% (wt/vol) PEG 4000 (Wako Pure Chemical Industries, Osaka, Japan), 50 mM CaCl2, and 50 mM Tris-HCl (pH 8.0) was then added to the mixture. The 0.2-ml portion of this protoplast suspension and 20 μg of the FAOAo2 disruption cassette were mixed on ice for 30 min. One milliliter of the solution II was added to the mixture, followed by incubation at room temperature for 15 min. After the addition of 8.5 ml of solution I, the mixture was centrifuged at 700 × g for 5 min. The pellet was suspended in 0.2 ml of solution I, and a small portion was spread on an MM-2 medium containing 0.8 M NaCl and 0.1 mg of pyrithiamine/ml. After incubation at 30°C for 3 days, each colony was picked up and subcultured three times.
Southern blot analysis.
A partial FAOAo2 open reading frame (the nucleotide positions 1 to 513) was amplified by PCR and used as a probe. The PCR fragment was labeled by DIG-High Prime (Roche Diagnostics K.K., Tokyo, Japan). The genomic DNA of A. oryzae strain niaD-2 transformants (7.5 μg) were digested with BamHI, and fragments were separated by electrophoresis. The fragments were transferred onto a Hybond-N+ membrane (Amersham Biosciences) with 0.4 N NaOH. The membrane was heated in an oven at 120°C for 30 min. The membrane was transferred to prehybridization solution (DIG Easy Hyb; Roche Diagnostics) and preincubated at 50°C for 1 h. Digoxigenin-labeled probe was added to the prehybridization solution to the final concentration of 50 ng/ml, and hybridization was done overnight at 50°C. The membrane was washed twice for 10 min at room temperature with 0.1% SDS in 2× SSC (0.3 M NaCl plus 30 mM trisodium citrate dihydrate [pH 7.0]) and washed twice for 15 min at 68°C with 0.1% SDS in 0.1× SSC. Detection was done by using the DIG-High Prime DNA labeling and detection starter kit II (Roche Diagnostics).
Analytical methods.
FAOD activity was measured spectrophotometrically (22). Fru-Z-Lys was used as a substrate throughout the purification at a concentration of 1.7 mM. For examination of the substrate specificity, 10 mM concentrations of various substrates were used. The amount of protein was determined with a Bio-Rad protein assay kit (Nippon Bio-Rad Laboratories, Tokyo, Japan) with bovine serum albumin (Bio-Rad) as the standard. Gel filtration was carried out with HiLoad 16/60 Superdex 200 pg equilibrated with 0.1 M Tris-HCl (pH 8.5) containing 0.1 M NaCl and 2 mM DTT to determine the molecular masses of proteins. To determine the accurate molecular masses, matrix-assisted laser desorption ionization-time of flight mass spectra were measured with a Voyager-DE PRO (Applied Biosystems Japan Ltd., Tokyo, Japan) with α-cyano-4-hydroxycinnamic acid as a matrix. The molecular mass of the subunit of the purified protein was determined by SDS-PAGE in a 12.5% gel by using a Phast System (Amersham Biosciences). Quantity and quality analyses of the flavin bound to the enzyme were determined by measuring AMP liberated after enzymatic digestion (35). For analysis of the N-terminal amino acid sequences, the purified FAODs were separated on a gel by SDS-PAGE, blotted electrophoretically onto a polyvinylidene difluoride membrane (Immobilon-P; Millipore) in a Trans-Blot SD Semi-Dry Transfer Cell apparatus (Bio-Rad), and stained with Coomassie brilliant blue R-250 (Sigma-Aldrich Japan K.K., Tokyo, Japan). N-terminal and internal amino acid sequences were determined by the Edman method with a model 476A protein sequencer (Applied Biosystems).
Nucleotide sequence accession numbers.
The cDNA sequences of FAOD from A. oryzae reported here have been submitted to the GenBank database under accession numbers AB180732 (FAOAo1) and AB180733 (FAOAo2). The gene accession numbers for the other genes used in the present study are as follows: Y09020 for FAOD of Aspergillus terreus, AF035700 for amadoriase I of Aspergillus fumigatus, U82830 for amadoriase II of A. fumigatus, and E16562 for FOD of Fusarium oxysporum.
RESULTS
Purification and characterization of FAOD isozymes from A. oryzae strain RIB40.
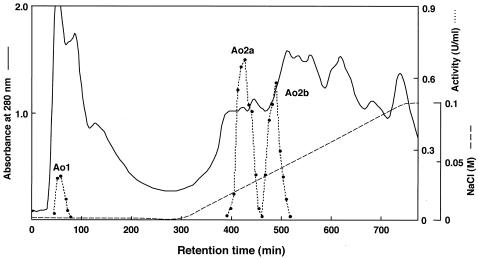
Three FAOD-active fractions were found on a DEAE-Toyopearl chromatogram. One of these enzyme activities was observed in a washing fraction (FAOD-Ao1), and the other fractions were eluted with NaCl (FAOD-Ao2a and -Ao2b) (Fig. 1). These fractions were further separately purified. The results of the purifications are shown in Table 1. Each enzyme showed a single band on SDS-polyacrylamide gel electrophoresis (PAGE) (data not shown). The molecular mass of FAOD-Ao1 was estimated to be 49 kDa, and those of FAOD-Ao2a and -Ao2b were estimated to be 48 kDa. Superdex 200 gel filtration showed that the molecular mass of FAOD-Ao1 was 39 kDa, and those of FAOD-Ao2a and -Ao2b were 98 kDa, suggesting that FAOD-Ao1 was monomeric and that the others were dimeric enzymes. FAOD-Ao1 and -Ao2a showed two maxima of absorption at 385 and 447 nm and 363 and 445 nm, respectively. One of the peaks of each protein around 450 nm had a shoulder around 480 nm. Such spectra are typical of flavoproteins. The supernatant after precipitation of these proteins with 1% trichloroacetic acid had no absorbance, whereas the residual pellets were yellow. Each flavin was identified as covalently bound FAD by the enzymatic method with adenylate kinase (35). N-terminal amino sequences of FAOD-Ao2a and -Ao2b were identical, but FAOD-Ao1 had a different N terminus from the other isozymes (Table 2). Furthermore, the fraction of FAOD-Ao2b was not observed in the chromatogram in some cases. Thus, FAOD-Ao1 and -Ao2a were used for further examination.
FIG.1.
Elution profile of FAOD isozymes in A. oryzae strain RIB40 from a DEAE-Toyopearl column.
TABLE 1.
Purification of FAOD isozymes from A. oryzae strain RIB40
| Enzyme | Purification step | Total activity (U) | Total protein (mg) | Sp act (U/mg of protein) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|---|
| None | Cell extract | 294 | 6,410 | 0.0459 | 1 | 100 |
| Ammonium sulfate (50-75% saturation) | 243 | 2,270 | 0.107 | 2 | 83 | |
| FAOD-Ao1 | DEAE-Toyopearl | 5.56 | 47.6 | 0.117 | 2.49 | 1.9 |
| Butyl-Toyopearl | 3.85 | 6.47 | 0.595 | 13 | 1.3 | |
| Superdex 200 | 1.46 | 0.59 | 2.47 | 54 | 0.5 | |
| Resource Q | 1.34 | 0.53 | 2.52 | 55 | 0.5 | |
| FAOD-Ao2a | DEAE-Toyopearl | 41.9 | 21.2 | 1.98 | 43 | 14 |
| Butyl-Toyopearl | 24.5 | 2.53 | 9.68 | 211 | 8.3 | |
| Superdex 200 | 10.1 | 0.566 | 17.8 | 388 | 3.4 | |
| Resource Q | 2.78 | 0.0913 | 30.4 | 662 | 0.9 | |
| FAOD-Ao2b | DEAE-Toyopearl | 14.5 | 16.2 | 0.895 | 19 | 4.9 |
| Butyl-Toyopearl | 7.4 | 1.38 | 5.36 | 117 | 2.5 | |
| Superdex 200 | 2.78 | 0.272 | 10.2 | 222 | 0.9 | |
| Resource Q | 0.947 | 0.0481 | 19.7 | 429 | 0.3 |
TABLE 2.
N-terminal amino acid sequences of purified FAOD isozymes
| Protein | N-terminal amino acid sequence |
|---|---|
| FAOD-Ao1 | TSSKLTPTSSILIVGAGT |
| FAOD-Ao2a | TVAKSSSILIIGAGT |
| FAOD-Ao2b | TVAKSSSILIIGA |
The isozymes were active toward Fru-Z-Lys, but FAOD-Ao1 was not active toward Fru-Val. l-Pipecolate, sarcosine, and l-proline were the substrates for sarcosine-related enzymes but were not the substrate for FAOD isozymes in A. oryzae. Furthermore, the isozymes were not active toward opines, such as mannopine. The Km values of FAOD-Ao1 and -Ao2a for Fru-Z-Lys were 0.51 and 0.22 mM, respectively, and that of FAOD-Ao2a for Fru-Val was 1.38 mM. The Vmax values of FAOD-Ao1 and -Ao2 for Fru-Z-Lys were 2.7 and 17.7 μmol min−1 mg−1, respectively, and that of FAOD-Ao2a for Fru-Val was 31.2 μmol min−1 mg−1.
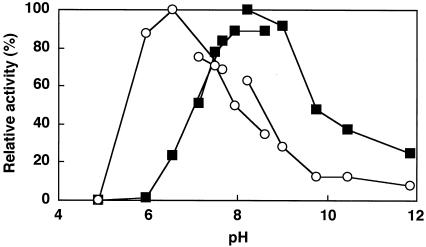
The optimal pH values of the enzyme reactions for FAOD-Ao1 and -Ao2a were 6.6 and 8.2, respectively (Fig. 2). FAOD-Ao2a did not have enzyme activity at pH 6.0, whereas FAOD-Ao1 demonstrated 85% of the initial activity at this pH. Both isozymes were inactivated gradually when incubated at >30°C in Tris-HCl buffer (pH 8.0) and completely lost the activity when incubated at 60°C for 10 min (data not shown).
FIG. 2.
Effect of pH on the activities of FAOD-Ao1 and -Ao2. The enzyme activities were measured by using the following buffers: McIlvaine (pH 4.97 to 7.85), Tris-HCl (pH 7.00 to 8.50), and glycine-NaOH (pH 8.30 to 12.0). Fru-Z-Lys (1.7 mM) was used as a substrate. Symbols: ○, activity of FAOD-Ao1; ▪ activity of FAOD-Ao2.
Cloning of FAOAo1 and FAOAo2 genes.
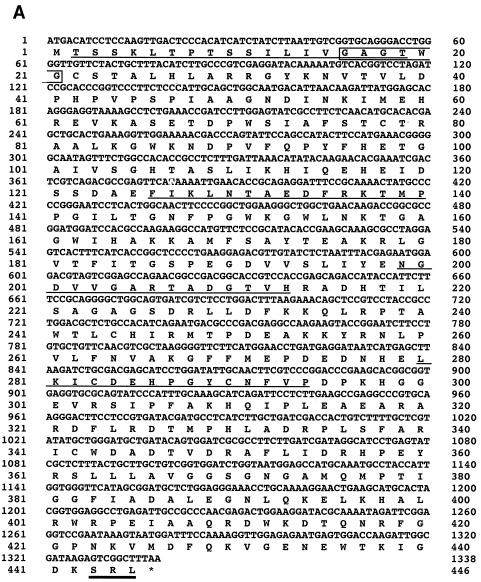
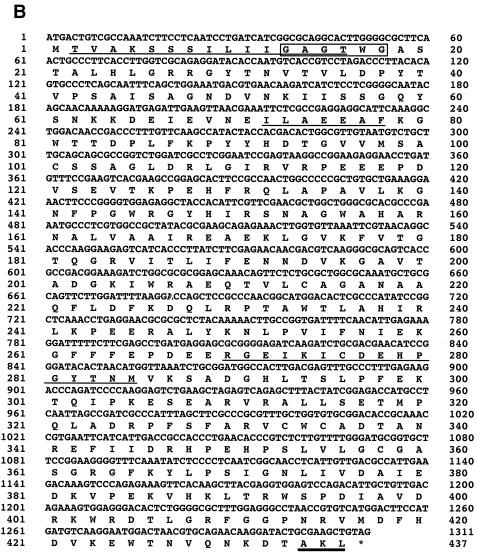
The 230-bp fragment was amplified by PCR from A. oryzae strain RIB40 genome with an FAOD-specific primer set (Fig. 3A). Only two sequences, which showed high similarity to the genes encoding various FAODs (FAOs), were obtained from this band. These two FAO genes were cloned by extension of 5′- and 3′-flanking regions of the PCR products (Fig. 3B). Reverse transcription-PCR analysis showed that FAOAo1 and FAOAo2 consisted of 1,338- and 1,311-bp open reading frames, which encode 445 and 436 amino acids, respectively, and both genes contained single intron regions (194 to 246 bp and 1091 to 1147 bp), respectively (Fig. 4). The N-terminal and internal amino acid sequences determined from the purified isozymes were also found in the deduced amino acid sequences (Fig. 4). Thus, it was confirmed that the two genes (FAOAo1 and FAOAo2) encode FAOD-Ao1 and -Ao2a, respectively. FAOD-Ao1 showed 78% identity with amadoriase I from A. fumigatus, and FAOD-Ao2a was 83 and 82% identical with FAOD from A. terreus strain GP1 and amadoriase II from A. fumigatus, respectively. All FAODs contained a cysteine residue (Cys342 in FAOD-Ao1, Cys334 in FAOD-Ao2a, Cys334 in A. terreus FAOD, Cys342 in amadoriase I, Cys335 in amadoriase II, Cys347 in A. nidulans FAOD, and Cys347 in P. janthinellum FAOD), to which FAD may be bound covalently (35, 33). Furthermore, the two isozymes of A. oryzae had an AMP-binding motif, GXGXXG (31), at their N termini in the structures. These results corresponded to the fact that these isozymes were flavoproteins. Peroxisome-targeting signal 1 was found at the C termini of the deduced amino acid sequences, suggesting that both isozymes are located in peroxisomes.
FIG. 4.
Nucleotide and the deduced amino acid sequences of FAOAo1 cDNA (A) and FAOAo2 cDNA (B). The N-terminal and internal amino acid sequences from the purified FAOD-Ao1 and FAOD-Ao2 are underlined. The AMP-binding motif (GXGXXG) is boxed. The bold line indicates the peroxisome-targeting signal-1. The stop codon is indicated by an asterisk.
Expression of FAOAo1 and FAOAo2 genes.
FAO genes of A. oryzae were expressed in E. coli as GST fusion proteins, which were purified to apparent homogeneity on SDS-PAGE (data not shown). The physicochemical properties of these recombinant proteins (e.g., molecular mass and flavin binding) were almost the same as those of purified enzymes from A. oryzae. The Km values of rFaoAo2 for Fru-Z-Lys and Fru-Val were 0.30 and 1.4 mM, respectively. The Vmax values of rFaoAo2 for Fru-Z-Lys and Fru-Val were 11.6 and 15.7 μmol min−1 mg−1, respectively. The FAOD activity of rFaoAo1 could not be detected when various expression systems were used. Since the molecular mass of rFaoAo1 predicted from the amino acid sequence corresponded to that determined by matrix-assisted laser desorption ionization-time of flight mass spectrometry analysis (m/z 50,677.32), protease digestion may not have occurred when rFaoAo1 was expressed in E. coli. Refolding of the protein with use of any surface-active reagents did not restore FAOD activity (data not shown).
Disruption of FAOAo2 gene.
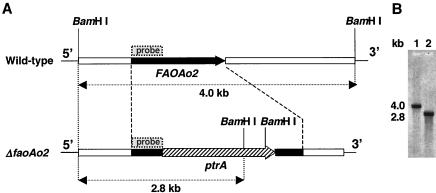
A gene disruption cassette containing ptrA gene (5.5 kb) was constructed by three-step PCR. The cassette was used to transform protoplasts of A. oryzae, and the pyrithiamine-resistant transformants were obtained. The disruption was confirmed by Southern blot analysis. As shown in Fig. 5B, the disruptant gave a 2.8-kb band with BamHI-digested genome as the template. A 4.0-kb band was observed in the wild-type strain, suggesting there was no insertion of ptrA. This result showed that a single copy of disruption cassette was integrated into the FAOAo2 locus of the wild-type strain.
FIG. 5.
Physical map (A) and Southern blot analysis (B) of the FAOAo2 loci in the wild-type and disruptant strains. In panel A, solid boxes show FAOAo2 gene containing its 5′- and 3′-flanking regions. The upper map represents the physical map of FAOAo2 locus in the wild-type strain, and the lower map represents the result of homologous recombination of the gene disruption cassette. The hatched arrow shows the ptrA gene. In panel B, genomic DNA was digested with BamHI and separated by electrophoresis on a 1% agarose gel. A part of the FAOAo2 gene (513 bp; shown in panel A) was used as a probe. Lane 1, the wild-type strain; lane 2, strain ΔfaoAo2.
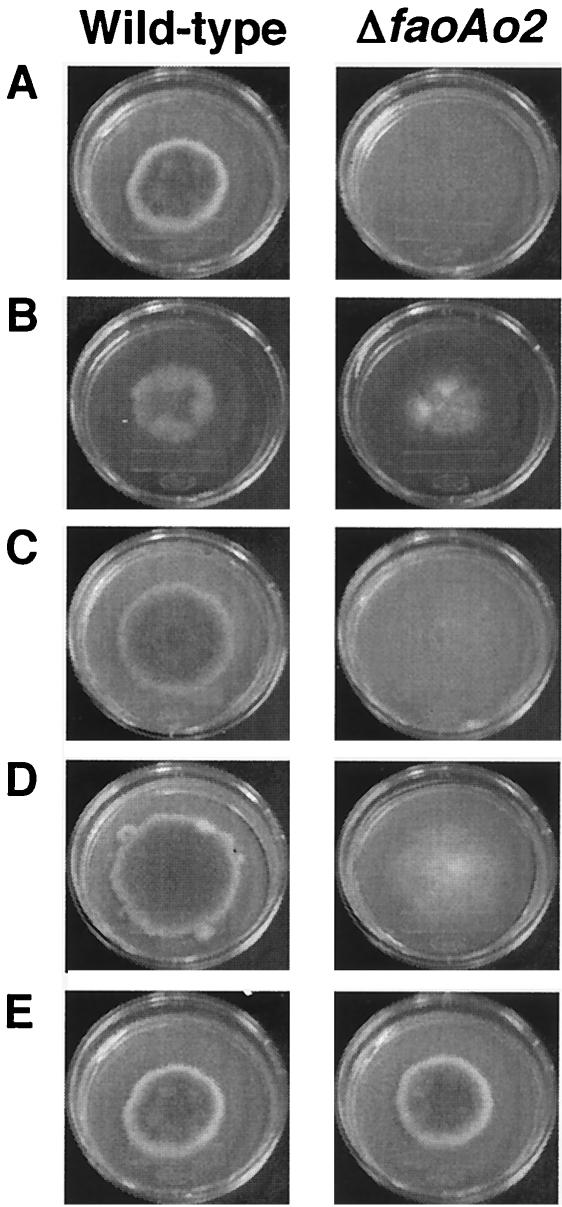
Table 3 and Fig. 6 show the growth of the wild-type and ΔfaoAo2 strains on various solid media. When Fru-Val was used as carbon source, (NH4)2SO4 was used as a nitrogen source. The wild-type strain niaD-2 (ΔniaD) grew on the medium containing Fru-Val as the sole carbon and/or nitrogen sources to the same level as that found with the medium containing glucose and NaNO2 as the sole carbon and nitrogen sources, indicating that catabolite repression did not affect the expression of FAOAo2. Maximum growth was observed after 11 to 12 days in all of the cases described above. The faoAo2 disruptant strain did not grow on Fru-Val as the sole carbon and/or nitrogen sources. The wild-type strain and the faoAo2 disruptant strain did not grow on Fru-Val as the sole carbon source when NaNO2 was used as the nitrogen source. Furthermore, the wild-type strain and the disruptant did not grow when both NaNO2 and (NH4)2SO4 were present in the media.
TABLE 3.
Growth of wild-type and ΔfaoAo2 strains of A. oryzae on various solid mediaa
| Source
|
Growth
|
||
|---|---|---|---|
| C | N | Wild type | ΔfaoAo2 mutant |
| Fru-Val | Fru-Val | +++ | − |
| Fru-Lys | Fru-Lys | +++ | ++ |
| Fru-Val | NaNO2 | − | − |
| Fru-Val | (NH4)2SO4 | +++ | − |
| Glc | Fru-Val | +++ | − |
| Glc | NaNO2 | +++ | +++ |
| Glc | (NH4)2SO4 | + | + |
| Glc | NaNO2, (NH4)2SO4 | − | − |
| Glc | Fru-Val, NaNO2 | − | − |
| Glc | NaNO3 | − | − |
The wild-type (niaD-2) and ΔfaoAo2 strains of A. oryzae were cultivated at 30°C on media for an assimilation test containing various carbon and nitrogen sources. Growth (as measured by the mycelial diameter): +++, >5 cm; ++, 3 to 5 cm; +, <3 cm; −, no growth.
FIG. 6.
Growth of the wild-type and ΔfaoAo2 strains of A. oryzae on various solid media. The photographs related to Table 3 are shown. (A) Fru-Val was used carbon and nitrogen sources; (B) Fru-Lys was used carbon and nitrogen sources; (C) Fru-Val and (NH4)2SO4 were used as the carbon and nitrogen sources, respectively; (D) Glc and Fru-Val were used as the carbon and nitrogen sources, respectively; (E) Glc and NaNO2 were used as the carbon and nitrogen sources, respectively.
DISCUSSION
Three active fractions of FAOD were found in A. oryzae. FAOD-Ao2a and -Ao2b were thought to be derived from the same origin by biochemical and structural analyses. Only two genes, FAOAo1 and -Ao2, were cloned when an FAOD-specific primer set was used. Properties of rFaoAo2 were well consistent with those of FAOD-Ao2a and -Ao2b, and it was also confirmed that rFaoAo1 corresponded with FAOD-Ao1 by various analyses, although the activity of rFaoAo1 was not detected. Thus, it should be concluded that there are two types of FAOD in A. oryzae. Takahashi et al. also reported that there are four FAOD-active fractions in A. fumigatus (27, 28). In this case, three of them gave almost the same enzymatic properties and N-terminal sequences, and Takahashi et al. suggested that this resulted from partial proteolytic degradation during isolation.
FAOD showed similarity to monomeric sarcosine oxidases from various origins (14, 16, 21), which are highly active toward sarcosine and significantly active toward l-pipecolate and l-proline. Recently, the genes encoding FAOD-homologous proteins were found in the genome of Schizosaccharomyces pombe (34). In that study, it was shown that two homologous proteins did not have oxidative activity toward Fru-Z-Lys and Fru-Val. However, these recombinant proteins had oxidase activities toward l-pipecolate, sarcosine, and l-saccharopine. The substrates of FAOD homologs have a common partial structure, and the enzymes cleave the C-N bonds of the imino group. FAOD isozymes of A. oryzae did not catalyze oxidation of these compounds, whereas phylogenetic analysis revealed that the deduced amino acid sequences of these isozymes showed striking similarity with that of the fission yeast saccharopine oxidase. Corynebacterium sp. strain 2-4-1 is known to be the FAOD producer in prokaryotes (9, 24). Corynebacterium FAOD showed high similarity to AgaE-like protein of Agrobacterium sp. (8). AgaE-like protein is concerned with opine metabolism during infection in plants and has activities with opines such as mannopine as the substrates (8). The origin of prokaryotic FAOD is thought to be the Agrobacterium sp. protein. AgaE-like protein has FAOD activity, but FAOD isozymes from A. oryzae were not active toward any opines used, indicating that the physiological role of eukaryotic FAOD is different from that of prokaryotic FAOD.
Jeong et al. reported that the disruption of faoA gene encoding FAOD in A. nidulans did not affect its phenotype (e.g., growth and physiological appearance) (12). They suggested that the putative role of FAOD in A. nidulans could be the provision of a carbon and/or a nitrogen source necessary for sexual development since the faoA gene expression was induced by fructosyl amines and depended on the veA gene (a sexual development regulator gene) (12). However, these authors did not use the medium containing a fructosyl-amino acid as the sole carbon and/or nitrogen sources. FAOD activity in cell extract of A. nidulans was low, and the biochemical and physicochemical characterizations of the enzyme have not been examined. In the present study we showed, using the imperfect fungus A. oryzae, that FAOAo2 gene disruptant had a different phenotype from the wild-type strain on the medium containing Fru-Val as the sole carbon source and/or nitrogen sources (Table 3 and Fig. 6). FAOAo2 was necessary for the growth on Fru-Val as the sole carbon and/or nitrogen sources. The ΔfaoAo2 disruptant grew on the medium containing Fru-Lys as the sole carbon and nitrogen sources, however, more slowly than the wild-type strain. It was suggested that the gene products of FAOAo1 in the disruptant was involved in the degradation of Fru-Lys. It is consistent with the substrate specificity of the two FAOD isozymes in A. oryzae.
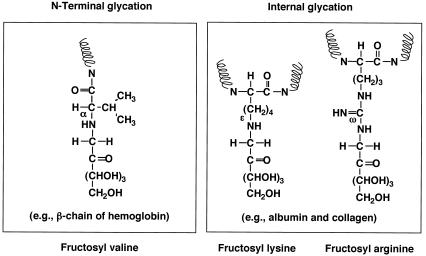
The two FAOD isozymes in A. oryzae were different in substrate specificities for α- and ɛ-glycated compounds. The glycation sites of Fru-Z-Lys and Fru-Val are ɛ- and α-amino groups, respectively (22). It is noteworthy that FAOD-Ao1 was not active toward Fru-Val. This is the same for amadoriase isozymes in A. fumigatus; one of the isozymes, amadoriase I, prefers Nɛ-fructosyl Nα-t-Boc-lysine (ɛ-glycated) to Nα-fructosyl Nɛ-acetyllysine (α-glycated), while another, amadoriase II, is active toward both compounds to the same extent (27). In general, the amino groups in the side chains of Lys and Arg residues in proteins are susceptible to glycation (Fig. 7) (29). Thus, we suggest that fructosyl lysine and fructosyl arginine in nature are derived from the glycated proteins. Actually, we detected fructosyl lysine and fructosyl arginine in fungal cells cultivated for the long period in the minimum medium not containing any amino acids (N. Yoshida et al., submitted for publication). Preliminary study of FAO gene expression showed that the induction levels of FAOAo1 were relatively low in various autoclave-browned media, compared to those of FAOAo2. We have thought that FAOAo2 is used for utilization of exogenous fructosyl-amino acids, whereas FAOAo1 is used for deglycation of indigenously produced fructosyl-amino acid residues in fungal cells. Human erythrocyte has a unique deglycation system, in which fructosamine kinase (FNK) phosphorylates glycated hemoglobin produced in blood, followed by spontaneous deglycation from the phosphorylated protein molecule (4). There is no report for fungal FNK and A. oryzae did not have such a kinase activity (data not shown). It was suggested that FAOD-Ao1 has a function similar to that of human FNK. We are now examining FAOAo1 disruption.
FIG. 7.
Possible glycation sites of proteins.
In the nitrogen catabolism of fungi, ammonium and glutamine are known as primary nitrogen sources (10). Ammonium and glutamine repress the enzymes involved in utilization of secondary nitrogen sources such as nitrate, methylamine, and other amino acids. This reaction is called ammonium repression. However, ammonium did not inhibit the utilization of Fru-Val as a nitrogen source. During the present study, the wild-type strain could not grow on the medium containing glucose, nitrite, and ammonium and on the medium containing nitrite and Fru-Val. However, wild-type strain could grow normally on the medium containing Fru-Val and ammonium. It is known that a high level of nitrite exhibits cytotoxicity in fungi (19). When the medium containing nitrite as the sole nitrogen source was used, both the wild-type strain and the disruptant could grow normally, probably due to the consumption of toxic nitrite. Thus, it was suggested that, when the medium contained both ammonium and nitrite, expression of nitrite reductase was repressed by ammonium, and high concentration of nitrite remained, where both strains could not grow. Fru-Val may also repress nitrite reductase and ammonium may not repress FAOD induction. These results indicate that FAOD-Ao2 is needed for utilization of fructosyl-amino acids such as Fru-Val as nitrogen sources in A. oryzae and significant amounts of fructosyl-amino acids are found in nature.
REFERENCES
- 1.Amberg, D. C., D. Botstein, and E. M. Beasley. 1995. Precise gene disruption in Saccharomyces cerevisiae by double fusion polymerase chain reaction. Yeast 11:1275-1280. [DOI] [PubMed] [Google Scholar]
- 2.Arai, K., S. Maguchi, S. Fujii, H. Ishibashi, K. Oikawa, and N. Taniguchi. 1987. Glycation and inactivation of human Cu-Zn-superoxide dismutase. Identification of the in vivo glycated sites. J. Biol. Chem. 262:16969-16972. [PubMed] [Google Scholar]
- 3.Blakytny, R., and J. J. Harding. 1992. Glycation (non-enzymic glycosylation) inactivates glutathione reductase. Biochem. J. 288:303-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delpierre, G., F. Collard, J. Fortpied, and E. V. Schaftingen. 2002. Fructosamine 3-kinase is involved in an intracellular deglycation pathway in human erythrocytes. Biochem. J. 365:801-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delpierre, G., M. H. Rider, F. Collard, V. Stroobant, F. Vanstapel, H. Santos, and E. V. Schaftingen. 2000. Identification, cloning, and heterologous expression of a mammalian fructosamine-3-kinase. Diabetes 49:1627-1634. [DOI] [PubMed] [Google Scholar]
- 6.Hashiba, H. 1976. Participation of Amadori rearrangement products and carbonyl compounds in oxygen-dependent browning of soy sauce. J. Agric. Food Chem. 24:70-73. [Google Scholar]
- 7.Heath, M. M., K. C. Rixon, and J. J. Harding. 1996. Glycation-induced inactivation of malate dehydrogenase protection by aspirin and a lens molecular chaperone, alpha-crystallin. Biochim. Biophys. Acta 1315:176-184. [DOI] [PubMed] [Google Scholar]
- 8.Hirokawa, K., and N. Kajiyama. 2002. Recombinant Agrobacterium AgaE-like protein with fructosyl amino acid oxidase activity. Biosci. Biotechnol. Biochem. 66:2323-2329. [DOI] [PubMed] [Google Scholar]
- 9.Horiuchi, T., T. Kurokawa, and N. Saito. 1989. Purification and properties of fructosyl-amino acid oxidase from Corynebacterium sp. 2-4-1. Agric. Biol. Chem. 53:103-110. [Google Scholar]
- 10.Hynes, M. J. 1974. Effects of ammonium, l-glutamate, and l-glutamine on nitrogen catabolism in Aspergillus nidulans. J. Bacteriol. 120:1116-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishida, H., K. Matsumura, Y. Hata, A. Kawato, K. Suginami, Y. Abe, S. Imayasu, and E. Ichishima. 2001. Establishment of a hyper-protein production system in submerged Aspergillus oryzae culture under tyrosinase-encoding gene (melO) promoter control. Appl. Microbiol. Biotechnol. 57:131-137. [DOI] [PubMed] [Google Scholar]
- 12.Jeong, H. Y., M. H. Song, J. H. Back, D. M. Han, X. Wu, V. Monnier, K. Y. Jahng, and K. S. Chae. 2002. The veA gene is necessary for the inducible expression by fructosyl amines of the Aspergillus nidulans faoA gene encoding fructosyl amino acid oxidase (amadoriase, EC 1.5.3). Arch. Microbiol. 178:344-350. [DOI] [PubMed] [Google Scholar]
- 13.Kobori, M., and H. Nojima. 1993. A simple treatment of DNA in a ligation mixture prior to electroporation improves transformation frequency. Nucleic Acids Res. 21:2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koyama, Y., H. Yamamoto-Otake, M. Suzuki, and E. Nakano. 1991. Cloning and expression of the sarcosine oxidase gene from Bacillus sp. NS-129 in Escherichia coli. Agric. Biol. Chem. 55:1259-1263. [PubMed] [Google Scholar]
- 15.Kuwayama, H., S. Obara, T. Morio, M. Katoh, H. Urushihara, and Y. Tanaka. 2002. PCR-mediated generation of a gene disruption construct without the use of DNA ligase and plasmid vectors. Nucleic Acids Res. 30:E2. [DOI] [PMC free article] [PubMed]
- 16.Mihalik, S. J., M. McGuinness, and P. A. Watkins. 1991. Purification and characterization of peroxisomal l-pipecolic acid oxidase from monkey liver. J. Biol. Chem. 266:4822-4830. [PubMed] [Google Scholar]
- 17.Monnier, V. M. 2003. Intervention against the Maillard reaction in vivo. Arch. Biochem. Biophys. 419:1-15. [DOI] [PubMed] [Google Scholar]
- 18.Monnier, V. M., and A. Cerami. 1981. Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science 211:491-493. [DOI] [PubMed] [Google Scholar]
- 19.Pombeiro, S. R. C., N. M. Martines-Rossi, and A. Rossi. 1983. Nitrite toxicity in Aspergillus nidulans: a new locus in a proA1 pabaA6 yA2 strain. Genet. Res. 41:203-207. [DOI] [PubMed] [Google Scholar]
- 20.Rahbar, S. 1968. An abnormal hemoglobin in red cells of diabetics. Clin. Chim. Acta 22:296-298. [DOI] [PubMed] [Google Scholar]
- 21.Reuber, B. E., C. Karl, S. A. Reimann, S. J. Mihalik, and G. Dodt. 1997. Cloning and functional expression of a mammalian gene for a peroxisomal sarcosine oxidase. J. Biol. Chem. 272:6766-6776. [DOI] [PubMed] [Google Scholar]
- 22.Sakai, Y., N. Yoshida, A. Isogai, Y. Tani, and N. Kato. 1995. Purification and properties of fructosyl lysine oxidase from Fusarium oxysporum S-1F4. Biosci. Biotechnol. Biochem. 59:487-491. [DOI] [PubMed] [Google Scholar]
- 23.Sakai, Y., N. Yoshida, H. Yurimoto, K. Takabe, and N. Kato. 1999. Subcellular localization of fructosyl amino acid oxidases in peroxisomes of Aspergillus terreus and Penicillium janthinellum. J. Biosci. Bioeng. 87:108-111. [DOI] [PubMed] [Google Scholar]
- 24.Sakaue, R., M. Hiruma, N. Kajiyama, and Y. Koyama. 2002. Cloning and expression of fructosyl-amino acid oxidase gene from Corynebacterium sp. 2-4-1 in Escherichia coli. Biosci. Biotechnol. Biochem. 66:1256-1261. [DOI] [PubMed] [Google Scholar]
- 25.Saxena, A. K., P. Saxena, and V. M. Monnier. 2002. Purification and characterization of a membrane-bound deglycating enzyme (1-deoxyfructosyl alkyl amino acid oxidase, EC 1.5.3) from a Pseudomonas sp. soil strain. J. Biol. Chem. 271:32803-32809. [DOI] [PubMed] [Google Scholar]
- 26.Szwergold, S., S. Howell, and P. J. Beisswenger. 2001. Human fructosamine-3-kinase: purification, sequencing, substrate specificity, and evidence of activity in vivo. Diabetes 50:2139-2147. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi, M., M. Pischetsrieder, and V. M. Monnier. 1997. Isolation, purification, and characterization of amadoriase isoenzymes (fructosyl amine-oxygen oxidoreductase EC 1.5.3) from Aspergillus sp. J. Biol. Chem. 272:3437-3443. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi, M., M. Pischetsrieder, and V. M. Monnier. 1997. Molecular cloning and expression of amadoriase isoenzyme (fructosyl amine:oxygen oxidoreductase, EC 1.5.3) from Aspergillus fumigatus. J. Biol. Chem. 272:12505-12507. [DOI] [PubMed] [Google Scholar]
- 29.Ulrich, P., and A. Cerami. 2001. Protein glycation, diabetes, and aging. Recent Prog. Horm. Res. 56:1-21. [DOI] [PubMed] [Google Scholar]
- 30.Wiame, E., G. Delpierre, F. Collard, and E. V. Schaftingen. 2002. Identification of a pathway for the utilization of the Amadori product fructoselysine in Escherichia coli. J. Biol. Chem. 277:42523-42529. [DOI] [PubMed] [Google Scholar]
- 31.Wierenga, R. K., P. Terpstra, and W. G. J. Hol. 1986. Prediction of the occurrence of the ADP-binding βαβ-fold in proteins, using an amino acid sequence fingerprint. J. Mol. Biol. 187:101-107. [DOI] [PubMed] [Google Scholar]
- 32.Wu, X., B. A. Palfey, V. V. Mossine, and V. M. Monnier. 2001. Kinetic studies, mechanism, and substrate specificity of amadoriase I from Aspergillus sp. Biochemistry 40:12886-12895. [DOI] [PubMed] [Google Scholar]
- 33.Wu, X., M. Takahashi, S. G. Chen, and V. M. Monnier. 2000. Cloning of amadoriase I isoenzyme from Aspergillus sp.: evidence of FAD covalently linked to Cys342. Biochemistry 39:1515-1521. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida, N., S. Akazawa, T. Katsuragi, and Y. Tani. 2004. Characterization of two fructosyl-amino acid oxidase homologs of Schizosaccharomyces pombe. J. Biosci. Bioeng. 97:278-280. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida, N., Y. Sakai, A. Isogai, H. Fukuya, M. Yagi, Y. Tani, and N. Kato. 1996. Primary structures of fungal fructosyl amino acid oxidases and their application to the measurement of glycated proteins. Eur. J. Biochem. 242:499-505. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida, N., Y. Sakai, M. Serata, Y. Tani, and N. Kato. 1995. Distribution and properties of fructosyl amino acid oxidase in fungi. Appl. Environ. Microbiol. 61:4487-4489. [DOI] [PMC free article] [PubMed] [Google Scholar]